Abstract
Wear particle-induced osteolysis is a serious complication that occurs in individuals with titanium (Ti)-based implants following long-term usage due to loosening of the implants. The control of excessive osteoclast differentiation and inflammation is essential for protecting against wear particle-induced osteolysis. The present study evaluated the effect of britanin, a pseudoguaianolide sesquiterpene isolated from Inula japonica, on osteoclastogenesis in vitro and Ti particle-induced osteolysis in vivo. The effect of britanin was examined in the osteoclastogenesis of mouse bone marrow-derived macrophages (BMMs) using TRAP staining, RT-PCR, western blotting and immunocytochemistry. The protective effect of britanin was examined in a mouse calvarial osteolysis model and evaluated using micro-CT and histomorphometry. Britanin inhibited osteoclast differentiation and F-actin ring formation in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kB ligand in BMMs. The expression of osteoclast-specific marker genes, including tartrate-resistant acid phosphatase, cathepsin K, dendritic cell-specific transmembrane protein, matrix metallopeptidase 9 and nuclear factor of activated T-cells cytoplasmic 1, in the BMMs was significantly reduced by britanin. In addition, britanin reduced the expression of B lymphocyte-induced maturation protein-1, which is a transcriptional repressor of negative osteoclastogenesis regulators, including interferon regulatory factor-8 and B-cell lymphoma 6. Conversely, britanin increased the expression levels of anti-oxidative stress genes, namely nuclear factor erythroid-2-related factor 2, NAD(P)H quinone oxidoreductase 1 and heme oxygenase 1 in the BMMs. Furthermore, the administration of britanin significantly reduced osteolysis in a Ti particle-induced calvarial osteolysis mouse model. Based on these findings, it is suggested that britanin may be a potential therapeutic agent for wear particle-induced osteolysis and osteoclast-associated disease.
Keywords: britanin, osteoclast, osteolysis, titanium particles, B lymphocyte-induced maturation protein-1, nuclear factor of activated T-cells, cytoplasmic 1
Introduction
Osteolysis is a pathological bone disorder primarily caused by the abnormal activation of osteoclasts and is widely reported in patients with dental implants and orthopedic prostheses. The long-term use of titanium (Ti)-based implants may produce wear debris, which subsequently causes osteolysis and aseptic loosening associated with orthopedic implant failure and dental peri-implantitis (1).
Wear particles are phagocytosed by monocytes/ macrophages around the prosthesis, which, in turn, release pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, resulting in the proliferation and differentiation of bone marrow-derived macrophages (BMMs) (2,3). In addition, wear particles can disrupt the balance of antioxidant systems via the generation of large amounts of reactive oxygen species (ROS) and the promotion of osteoclast formation. Endogenous ROS generated following receptor activator of nuclear factor kB (NF-κB) ligand (RANKL)-induced stimulation can activate the NF-κB/mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase pathway. Conversely, ROS also activate the nuclear factor erythroid-2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 pathway to relieve oxidative stress (4,5) and suppress osteoclast formation and activity (6). In summary, these findings demonstrate that wear particles promote osteoclast differentiation via the secretion of inflammatory cytokines and the disruption of the antioxidant system balance.
Osteoclasts are multinucleated cells derived from the bone marrow macrophage/monocyte lineage (7). Two essential cytokines regulate osteoclastogenesis: Macrophage colony-stimulating factor (M-CSF) and RANKL. M-CSF plays a critical role in the survival and proliferation of osteoclast progenitor cells. The binding of RANKL to RANK in BMMs activates downstream targets, including MAPK, NF-κB and c-Fos (8). Activation of NF-κB and c-Fos induces the expression of nuclear factor of activated T-cells cytoplasmic 1 (Nfatc1); subsequently, Nfatc1 functions in conjunction with other transcription factors such as PU.1 activator protein 1, microphthalmia-associated transcription factor and cAMP-responsive element binding protein (9). Nfatc1 is a master transcriptional regulator of osteoclast differentiation, which upregulates osteoclast-specific genes such as cathepsin K (Ctsk), tartrate-resistant acid phosphatase 5 (Acp5), matrix metallopeptidase 9 (Mmp9) and dendritic cell-specific transmembrane protein (Dc-stamp). Moreover, Nfatc1 regulates the expression of B lymphocyte-induced maturation protein-1 (Blimp1), which is a transcriptional repressor of negative regulators of osteoclastogenesis, including interferon regulatory factor-8 (Irf8), v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B (MafB) and B-cell lymphoma 6 (Bcl6), during osteoclast formation (10,11). Therefore, precise control of bone turnover is pivotal for bone health and is an efficient strategy for the inhibition of abnormal osteoclast formation and activity.
Britanin is a natural product that has a pseudoguaianolide sesquiterpene structure with an exomethylene group, and has been shown to exert preventive effects against inflammation, asthma and allergy (12–14). Britanin also inhibits lipopolysaccharide-induced nitric oxide production, prostaglandin E2 and pro-inflammatory cytokines via the inactivation of NF-κB and MAPK in macrophages (15), and exerts antitumor effects by inhibiting the proliferation and migration of breast, gastric and pancreatic cancer cell lines (16,17). In addition, britanin demonstrated the ability to inhibit tumor growth in a pancreatic cancer xenograft mouse model (17). Furthermore, britanin has been reported to protect organs against myocardial ischemia/reperfusion injury and cerebral ischemic reperfusion injury via the activation of Nrf2 signaling (18,19). These reports suggest that britanin exhibits multiple biological activities, including anti-inflammatory and anti-oxidative effects.
In the present study, the effects of britanin on in vitro osteoclastogenesis and in vivo Ti particle-mediated osteolysis were investigated in a mouse model. The mechanism underlying the effect of britanin on osteoclast formation was also investigated.
Materials and methods
Chemicals and reagents
α-modified Eagle's medium (α-MEM) was obtained from Gibco; Thermo Fisher Scientific, Inc. Recombinant murine M-CSF and RANKL were purchased from R&D Systems, Inc. The Leukocyte Acid Phosphatase kit [tartrate-resistant acid phosphatase (TRAP)] kit, 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) and all other unspecified reagents were purchased from Sigma-Aldrich; Merck KGaA. Specific antibodies against phospho-extracellular signal-regulated kinase (ERK; cat. no. 9101S), EKR1/2 (cat. no. 9102S) phospho-c-Jun N-terminal kinase (JNK; cat. no. 9251S), JNK (cat. no. 9252S), phospho-p38 (cat. no. 9211S), p38 (cat. no. 9212S), phospho-AKT (cat. no. 4058S), AKT (cat. no. 9272S) and phospho-inhibitor of nuclear factor kΒα (IκBα; cat. no. 2859S) were purchased from Cell Signaling Technology, Inc. Antibodies against IκBα (cat. no. sc-847), Irf8 (cat. no. sc-6058) and Nfatc1 (cat. no. sc-7294) were purchased from Santa Cruz Biotechnology, Inc. Antibodies against cathepsin K (cat. no. MAB3324) and β-actin (cat. no. A5441) were purchased from MilliporeSigma. Britanin, which is also known by the International Union of Pure and Applied Chemistry name of 9-acetyloxy-8-hydroxy-5,8a-dimethyl-1-methylidene-2-oxo-4,5,5a,6,7,8,9,9a-octahydro-3aH-azuleno[6,5-b]furan-6-yl) acetate, was isolated from Inula japonica (Inulae Flos), as described previously (20) and its purity was confirmed by one-dimensional nuclear magnetic resonance data comparison. Britanin was dissolved in DMSO for further experiments. Ti particles (purity, 99.9%; diameter, 30–50 nm; US Research Nanomaterials, Inc.) for in vivo experiments were prepared as previously described (21).
Cell culture and osteoclast differentiation
BMMs were isolated from mouse bone marrow as described previously (22). Briefly, cells were extracted from the tibiae and femurs of 4- to 6-week-old ICR male mice. The mice were purchased from Dae Han Bio Link Co., Ltd., and maintained under conventional housing conditions at 22–24°C and 50–60% humidity, with a 12-h light/12-h dark cycle and free access to water and food pellets. The mice were euthanized by cervical dislocation following CO2 treatment. The flow rate of CO2 was maintained at 40–60% chamber volume/min. Subsequently, the isolated cells were cultured in α-MEM (cat. no. 11900) containing 10% fetal bovine serum (cat. no. 16000; Gibco; Thermo Fisher Scientific, Inc.) for 24 h on a Petri dish. Non-adherent cells were centrifuged on a Histopaque density gradient medium (Sigma-Aldrich; Merck KGaA) and cultured for 3 days in the presence of M-CSF (30 ng/ml) to obtain BMMs. The BMMs were cultured with RANKL (20 ng/ml) and M-CSF (10 ng/ml) in the presence of different concentrations of britanin (1 or 5 µM) in 96-well plates. After 4 days, the cells were fixed in 4% paraformaldehyde for 15 min at room temperature and then stained using a TRAP staining kit according to the manufacturer's instructions. TRAP-positive multinucleated cells with ≥3 nuclei were considered osteoclasts. To examine signaling pathway in response to britanin, BMMs were stimulated with DMSO or 5 µM of britanin for 0, 5, 15, and 30 min, then the reaction was terminated using ice-cold PBS.
Cell viability assay
The cytotoxic effects of britanin were evaluated using the MTT assay. BMMs were cultured with M-CSF (10 ng/ml) with or without britanin (1 or 5 µM) for 3 days at 37°C. MTT reagent was then added to each well, and the cells were incubated at 37°C for 2 h. The formazan was subsequently solubilized with DMSO. The absorbance at 570 nm was measured using an Epoch 96-well microplate reader (BioTek Instruments, Inc.).
Western blotting
BMMs were lysed using RIPA lysis buffer (Pro-Prep™; Intron Biotechnology, Inc.) containing protease and phosphatase inhibitors. Protein concentrations were determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). Proteins (30 µg/lane) were separated on 10% gels using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to 0.2-µm nitrocellulose membranes (Whatman plc; Cytiva). Before incubation with the primary antibody, the membranes were blocked with a blocking buffer [3% non-fat milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T)] for 1 h at room temperature. The membranes were incubated with specific primary antibodies (1:1,000 by volume) overnight at 4°C in blocking buffer. The membranes were then washed with TBS-T and incubated with a secondary antibody (HRP-conjugated; cat. no. PA1-74362; 1:5,000 by volume; Thermo Fisher Scientific, Inc.) in blocking buffer for 1 h at room temperature. Immunoreactive bands were analyzed using a WesternBright ECL kit (Advansta, Inc.) and recorded using a chemiluminescent imager (cSeries Capture Software, version 1.9.5.0606; Azure Biosystems, Inc.).
Immunofluorescence staining
BMMs were plated on glass coverslips and cultured with RANKL (20 ng/ml) and M-CSF (10 ng/ml) in the presence or absence of britanin (5 µM). After 4 days of culture, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature and then treated with 0.25% Triton X-100 in phosphate-buffered saline (PBS). The cells were blocked with 5% normal goat serum (S-1000; Vector Laboratories, Inc.) in PBS for 1 h at room temperature, after which the cells were incubated with Nfatc1 primary antibody (1:500) overnight at 4°C, followed by Alexa Fluor 488-conjugated secondary antibody (1:100; cat. no. A-11059; Thermo Fisher Scientific, Inc.) for 1 h at room temperature. Rhodamine-conjugated phalloidin (1:1,000; Cytoskeleton, Inc.) and 4′,6-diamidino-2-phenylindole dihydrochloride (1:10,000; Santa Cruz Biotechnology, Inc.) were used to stain actin and nuclei, respectively. Fluorescence images were obtained using a Leica DM2500 microscope (Leica Microsystems GmbH). The images were aligned using ImageJ software (version 1.52a; National Institutes of Health).
Bone resorption pit assay
Briefly, 2.5×104 BMMs were placed on each bone slice (IDS Nordic) and incubated with RANKL (20 ng/ml) and M-CSF (10 ng/ml) for 3 days. After osteoclast formation, the cells were treated with or without 5 µM britanin for 2 days at 37°C. The bone slices were washed and soaked in hematoxylin solution for 30 sec at room temperature to visualize the resorption pits. The pit area was quantified using ImageJ software (version 1.52a).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from britanin or DMSO-treated cells for 1 day using TRI-Solution™ (Bio Science Technology), and 1 µg RNA was converted into cDNA using SuperScript II reverse transcriptase according to the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc.). The cDNA and each primer set were mixed with SYBR Premix Ex Taq (Takara Bio, Inc.). qPCR was performed using a LightCycler 1.5 Real-Time PCR System (Roche Diagnostics). The amplification conditions consisted of an initial denaturation step at 95°C, followed by 40 cycles of denaturation at 95°C, annealing at 60°°C and extension at 72°C. The primers used for the qPCR are listed in Table I and included Gapdh as the reference gene. The relative gene expression data were calculated using the 2−ΔΔCq method (23).
Table I.
PCR primer sequences.
| Gene | Primer sequence (5′ to 3′) | Amplicon size (bp) | RefSeq NCBI no. | Amplification factor (E) |
|---|---|---|---|---|
| Nfatc1 | F: ACCACCTTTCCGCAACCA | 72 | NM_001164112.1 | 2.03 |
| R: TTCCGTTTCCCGTTGCA | ||||
| Ctsk | F: GGCTGTGGAGGCGGCTAT | 66 | NM_007802.4 | 1.94 |
| R: AGAGTCAATGCCTCCGTTCTG | ||||
| Mmp9 | F: AAAGACCTGAAAACCTCCAACCT | 77 | NM_013599.5 | 1.97 |
| R: GCCCGGGTGTAACCATAGC | ||||
| Acp5 | F: TCCCCAATGCCCCATTC | 63 | NM_001102405.1 | 2.10 |
| R: CGGTTCTGGCGATCTCTTTG | ||||
| Dc-stamp | F: CTTCCGTGGGCCAGAAGTT | 64 | NM_029422.4 | 2.13 |
| R: AGGCCAGTGCTGACTAGGATGA | ||||
| TNF-α | F: GGTGCCTATGTCTCAGCCTCTT | 139 | NM_013693.3 | 2.03 |
| R: GCCATAGAACTGATGAGAGGGAG | ||||
| Nrf2 | F: CAGCATAGAGCAGGACATGGAG | 107 | NM_010902.5 | 1.89 |
| R: GAACAGCGGTAGTATCAGCCAG | ||||
| Nqo1 | F: GCCGAACACAAGAAGCTGGAAG | 120 | NM_008706.5 | 2.08 |
| R: GGCAAATCCTGCTACGAGCACT | ||||
| HO-1 | F: CACTCTGGAGATGACACCTGAG | 115 | NM_010442.2 | 2.14 |
| R: GTGTTCCTCTGTCAGCATCACC | ||||
| TRAF6 | F: AACTGTGCTGTGTCCATGGC | 246 | NM_001303273.1 | 1.89 |
| R: CAGTCTCATGTGCAACTGGG | ||||
| Blimp1 | F: TTCTTGTGTGGTATTGTCGGGACT | 148 | NM_001405929.1 | 1.90 |
| R: TTGGGGACACTCTTTGGGTAGAGTT | ||||
| Irf8 | F: GATCGAACAGATCGACAGCA | 214 | NM_001301811.1 | 1.91 |
| R: AGCACAGCGTAACCTCGTCT | ||||
| Bcl6 | F: ATGAGATTGCCCTGCATTTC | 202 | NM_009744.5 | 1.89 |
| R: TTCTTCCAGTTGCAGGCTTT | ||||
| Gapdh | F: ATGACATCAAGAAGGTGGTG | 177 | NM_001411843.1 | 1.90 |
| R: CATACCAGGAAATGAGCTTG |
Nfatc1, nuclear factor of activated T-cells, cytoplasmic 1; Ctsk, cathepsin K; Mmp9, matrix metallopeptidase 9; Acp5, tartrate-resistant acid phosphatase 5; Dc-stamp, dendritic cell-specific transmembrane protein; TNF-a, tumor necrosis factor-a; Nrf2, nuclear factor erythroid-2-related factor 2; Nqo1, NAD(P)H quinone oxidoreductase 1; HO-1, heme oxygenase 1; TRAF6, TNF receptor-associated factor 6; Blimp1, B lymphocyte-induced maturation protein-1; Irf8, interferon regulatory factor-8; Bcl6, B-cell lymphoma 6; NCBI, National Center for Biotechnology Information.
Ti particle-induced calvarial osteolysis model
Animal experiments were approved by the Committee on the Care and Use of Animals in Research at Kyungpook National University (Daegu, Korea; approval no. 2021-0071). As previously described, a mouse calvarial osteolysis model was established to determine the protective effect of britanin on osteolysis in vivo (24). Briefly, 6-week-old C57BL/J6 male mice (n=20) were divided into four groups: Sham (DMSO control; n=4), Ti + vehicle (DMSO) (15 mg Ti particles; n=6), Ti + 2 mg britanin (15 mg Ti particles plus 2 mg/kg/day britanin; n=4) and Ti + 25 mg britanin (15 mg Ti particles plus 25 mg/kg/day britanin; n=6). The mice were purchased from Dae Han Bio Link Co., Ltd., and maintained under conventional housing conditions at 22–24°C, with 50–60% humidity, a 12-h light/12-h dark cycle, and free access to water and food pellets. For mouse anesthesia, 240 mg/kg avertin was injected intraperitoneally. The mouse heads were shaved and disinfected using a 10% povidone-iodine solution under anesthesia. A periosteal pocket was created using a 28-gauge needle, followed by an injection of pure Ti particles (15 mg) dissolved in 30 µl PBS and embedded in the center of the calvarium under the periosteum. Britanin (2 or 25 mg/kg) was administered intraperitoneally daily for 9 days, while DMSO was administered daily to the mice in the sham and vehicle groups. No adverse events were detected during the animal experiments, and the mice were sacrificed at the end of the experimental period (10 days). To euthanize the mice, 480 mg/kg avertin was injected intraperitoneally, and cervical dislocation was performed under anesthesia. The calvariae were isolated and fixed for 1 day in 4% paraformaldehyde at 4°C for micro-computed tomography (CT) and histological analyses.
Micro-CT scanning
Harvested mouse calvariae were fixed and then analyzed using a high-resolution micro-CT scanner (Skyscan 1272; Bruker Corporation). The scan was set to a resolution of 10 µm with a voltage source of 70 kV and current of 142 µA. The three-dimensional image was reconstructed using CTvox software (version 3.0.0 r1114; Bruker), and a rectangular region of interest (3.2×2.1×0.1 mm) around the midline suture was assessed for further quantitative analysis as previously described (25). Bone volume as a percentage of tissue volume and the number of pores in each sample were measured as previously reported (26).
Histological and histomorphometric analysis
Calvarial samples were decalcified in 10% EDTA for 10 days and then embedded in paraffin. The paraffinized samples were cut in the coronal direction to a section thickness of 6 µm. Histological sections were stained with hematoxylin and eosin (H&E; hematoxylin for 5 min and eosin for 1 min at room temperature) or a TRAP kit (cat. no. 387A; MilliporeSigma) according to the manufacturer's instructions. The stained area was examined using a bright-field light microscope (Leica Microsystems GmbH), and the number of TRAP-positive osteoclasts in each sample was quantified using ImageJ software.
Statistical analysis
Experiments were performed in triplicate, and all data are presented as the mean ± standard deviation. Statistical analyses were performed using a two-tailed unpaired Student's t-test or one-way analysis of variance with Tukey's multiple comparison post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Britanin inhibits RANKL-induced osteoclastogenesis
Britanin was originally identified through the screening of natural product-derived compounds in an osteoclast differentiation model, with testing conducted at a concentration of 5 µM (22,27,28). Once its inhibitory effect on osteoclast differentiation was confirmed, its cytotoxicity was assessed across a range of concentrations from 1 to 10 µM. No cytotoxic effects were observed on BMMs at these concentrations, as determined by MTT assay. In addition, a 5-µM concentration of britanin significantly inhibited osteoclast differentiation in the presence of M-CSF (30 ng/ml) when applied for 3 days in vitro. As a result, concentrations of 1 and 5 µM were selected for further investigation to demonstrate the inhibitory effect on osteoclasts in vitro in the present study. Moreover, 5 µM britanin treatment significantly increased cell growth by 19% at 24 h (data not shown) and 11.5% at 72 h in BMMs when compared with that in the control group (Fig. S1).
The effect of britanin on osteoclast differentiation in BMMs was evaluated using TRAP staining. The BMMs were incubated with RANKL (20 ng/ml) and M-CSF (10 ng/ml) in the presence or absence of britanin. Multinucleated osteoclasts were formed in the control group, whereas britanin significantly inhibited osteoclast formation in a concentration-dependent manner, even when cell growth was enhanced. Compared with the control group, treatment with 1 and 5 µM britanin significantly reduced the number of TRAP-positive multinucleated cells by 25 and 98.7%, respectively (Fig. 1). These results suggest that britanin directly suppressed osteoclast formation without affecting cell viability.
Figure 1.
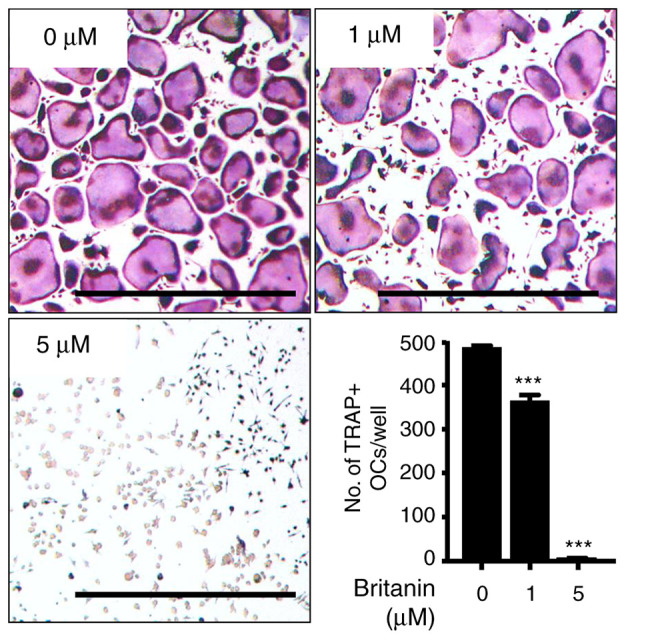
Britanin inhibits RANKL-induced osteoclast formation. Bone marrow-derived macrophages were cultured with macrophage colony-stimulating factor (10 ng/ml) and RANKL (20 ng/ml) in the presence of 0, 1 and 5 µM concentrations of britanin for 4 days. The cells were stained for TRAP, and TRAP-positive multinucleated cells (≥3 nuclei) were counted as OCs. Scale bar, 100 µm. ***P<0.001 vs. the vehicle-treated control as analyzed by one-way analysis of variance with Tukey's multiple comparison post hoc test. RANKL, receptor activator of nuclear factor kB ligand; TRAP, tartrate-resistant acid phosphatase; OC, osteoclast.
Britanin suppresses the expression of osteoclast-specific genes but induces the expression of antioxidant marker genes
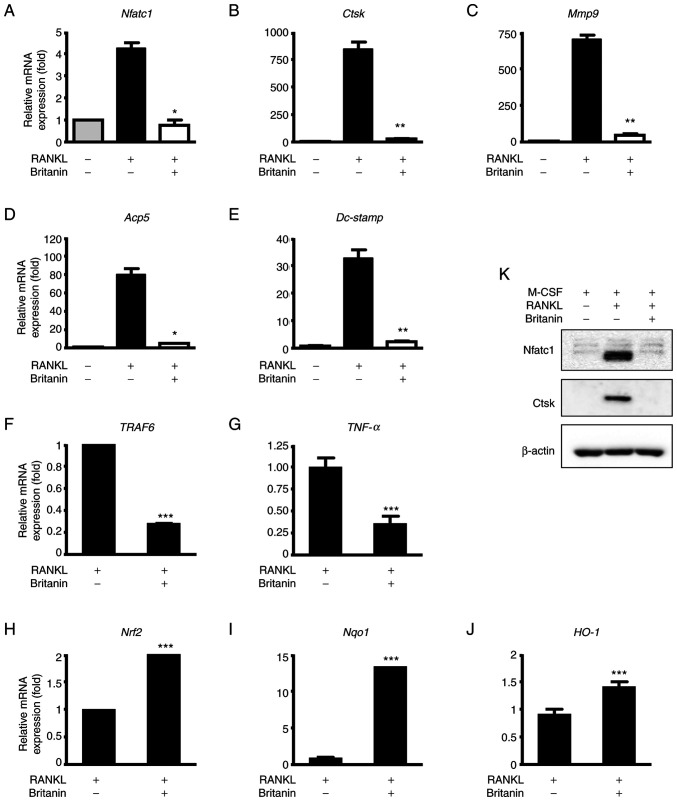
To further confirm that britanin inhibits osteoclast differentiation, the mRNA expression levels of osteoclast-associated genes were examined by RT-qPCR. The results revealed that the expression of Nfatc1 was increased in the RANKL-treated group, and britanin significantly suppressed this expression (Fig. 2A). Likewise, britanin significantly suppressed the RANKL-induced expression of osteoclast marker genes, namely Acp5, Dc-stamp, Mmp9 and Ctsk (Fig. 2B-E). Following 3 days of treatment, britanin markedly decreased the protein levels of Nfatc1 and Ctsk when compared with those in the osteoclastogenesis control (RANKL-treated) group (Fig. 2K). Inflammation and oxidative stress are major events that mediate osteolysis. Therefore, the effect of britanin on the expression of genes associated with inflammation and oxidative stress, namely TNF receptor-associated factor 6 (TRAF6) and TNF-α, was examined. The results demonstrated a significant reduction in the mRNA expression levels of TRAF6 and TNF-α in the RANKL-induced cells upon treatment with britanin (Fig. 2F and G).
Figure 2.
Britanin suppresses the expression of osteoclast and Nrf2-associated markers during osteoclastogenesis. (A-E) BMMs were cultured with M-CSF (10 ng/ml) and RANKL (20 ng/ml) in the absence or presence of britanin for 4 days and RT-qPCR was performed to analyze the expression of (A) Nfatc1, (B) Ctsk, (C) Mmp9, (D) Acp5 and (E) Dc-stamp. (K) BMMs were incubated in an osteoclastogenic medium with vehicle or britanin for 3 days and the protein expression levels of Nfatc1 and Ctsk were determined using western blotting. BMMs were cultured with M-CSF (10 ng/ml) and RANKL (20 ng/ml) in the absence or presence of britanin for (F and G) 1 day and (H and I) 4 days, and RT-qPCR was performed to analyze the expression of (F) TRAF6, (G) TNF-α, (H) Nrf2, (I) Nqo1 and (J) HO-1. *P<0.05, **P<0.01 and ***P<0.001 vs. RANKL-treated control as analyzed by (A-E) ANOVA with Tukey's multiple comparison post hoc test and (F-J) two-tailed unpaired Student's t-test. Nrf2, nuclear factor erythroid-2-related factor 2; BMMs, bone marrow-derived macrophages; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor kB ligand; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; Nfatc1, nuclear factor of activated T-cells, cytoplasmic 1; Ctsk, cathepsin K; Mmp9, matrix metallopeptidase 9; Acp5, tartrate-resistant acid phosphatase 5; Dc-stamp, dendritic cell-specific transmembrane protein; TRAF6, tumor necrosis factor receptor-associated factor 6; TNF-α, tumor necrosis factor-α; Nqo1, NAD(P)H quinone oxidoreductase 1; HO-1, heme oxygenase 1.
Nrf2 is a transcription factor that regulates cellular defense against oxidative stress, and its deficiency has been shown to promote RANKL-induced osteoclast differentiation (29,30). In BMMs undergoing osteoclastogenesis, britanin increased the mRNA expression of Nrf2, NAD(P)H quinone oxidoreductase 1 (Nqo1) and heme oxygenase 1 (HO-1) on day 4 (Fig. 2H-J). Taken together, these findings suggest that britanin may inhibit osteoclast differentiation via downregulation of the expression levels of genes associated with inflammatory cytokines and oxidative stress in vitro.
Britanin suppresses F-actin ring formation
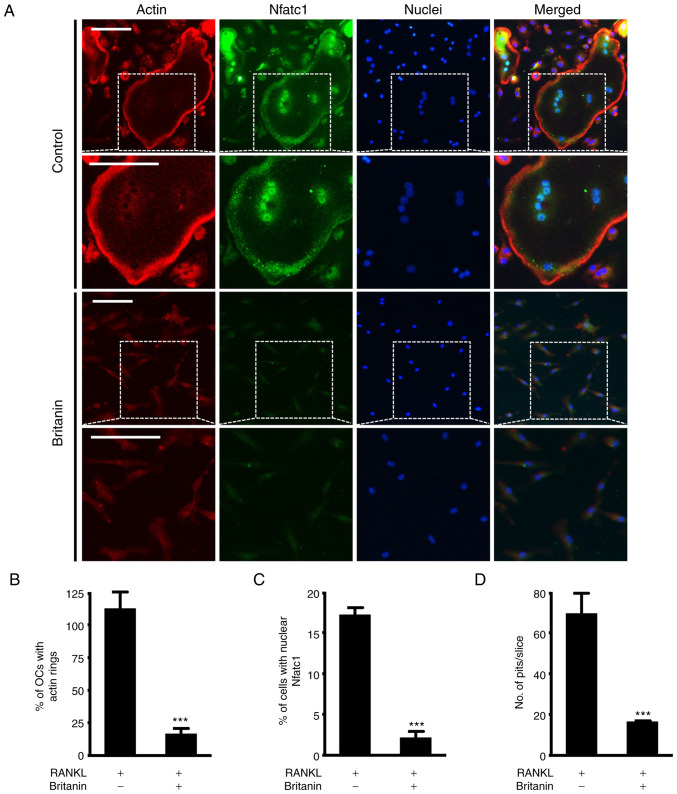
RANKL-induced mature osteoclasts attach to the bone matrix, reorganize the cytoskeleton and form circular actin ring structures that provide a sealing zone for osteoclast bone resorption (31,32). Frequently, large osteoclasts indicate more bone resorption, but in certain cases, small osteoclasts can actively resorb bone (33). Therefore, the effect of britanin on F-actin ring formation was investigated using immunofluorescence staining. While the control group exhibited large F-actin rings, the formation of F-actin rings was impaired in the britanin-treated group (Figs. 3A and S2). Compared with the RANKL control group, britanin significantly reduced the number of F-actin rings and nuclear Nfatc1-positive cells by 85.1 and 87.1%, respectively (Fig. 3B and C). In addition, bone resorption was monitored to determine whether it was influenced by the effect of britanin on F-actin ring formation. Britanin treatment was performed after initial osteoclast formation, and under these conditions, britanin markedly reduced the resorption ability of bone slices compared with that in the RANKL control group (Fig. 3D). Collectively, these findings suggest that britanin may attenuate osteoclast function.
Figure 3.
Britanin attenuates the formation of actin rings and resorption pits. (A) BMMs were incubated on glass coverslips with M-CSF (10 ng/ml) and RANKL (20 ng/ml) for 4 days in the absence or presence of britanin. Actin, nuclei and Nfatc1 were stained with rhodamine-phalloidin (red), 4′,6-diamidino-2-phenylindole dihydrochloride (blue) and anti-Nfatc1 antibody (green), respectively. The white dashed box indicates the magnified region. Scale bar, 50 µm. The percentage of (B) OCs displaying actin rings and (C) nuclear Nfatc1-positive cells was assessed. (D) BMMs were seeded on bone slices and cultured with an OC-inducing medium for 3 days. The cells were then treated with or without britanin for 2 days. Bone slices were stained with hematoxylin to observe the resorption pits. The number of resorption pits per bone slice was counted using ImageJ software. ***P<0.001 vs. RANKL-treated control as analyzed using two-tailed unpaired Student's t-test. BMMs, bone marrow-derived macrophages; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor kB ligand; Nfatc1, nuclear factor of activated T-cells, cytoplasmic 1; OC, osteoclast.
Britanin inhibits osteoclast differentiation via the repression of transcriptional negative regulators
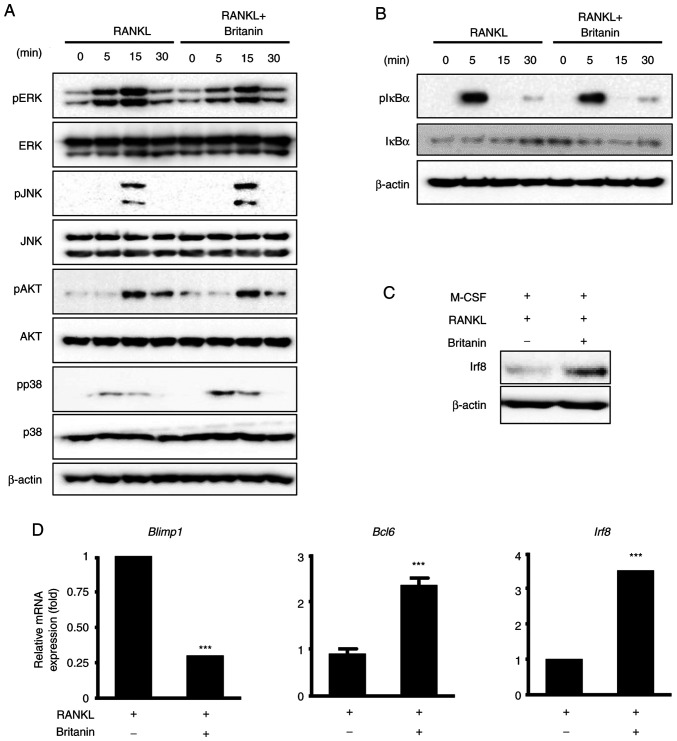
To elucidate the molecular mechanism underlying the inhibitory effect of britanin on RANKL-induced osteoclastogenesis, RANKL downstream signaling pathways, including ERK, JNK, p38, AKT and NF-κB, were analyzed using western blotting. The phosphorylation of ERK was notably decreased by britanin at 5 and 15 min; however, the phosphorylation levels of p38, JNK, AKT and IκBα were not markedly altered (Fig. 4A and B). These data suggest that britanin suppresses the ERK signaling pathway, which may underlie its ability to attenuate osteoclast differentiation and function.
Figure 4.
Britanin inhibits the suppression of negative mediators of RANKL-induced osteoclast differentiation. (A and B) BMMs incubated in serum-free medium were pretreated with britanin or vehicle for 1 h. Next, several time points were examined following treatment with RANKL (50 ng/ml). Phosphorylation levels of ERK, JNK, Akt, p38 and IκBα were determined using western blotting. (C) BMMs were incubated in an osteoclastogenic medium with vehicle or britanin for 3 days. The protein expression of Irf8 was examined using western blotting. (D) BMMs were cultured with M-CSF (10 ng/ml) and RANKL (20 ng/ml) in the absence or presence of britanin for 4 days. Reverse transcription-quantitative PCR was performed to analyze the expression of Blimp1, Bcl6 and Irf8. ***P<0.001 vs. RANKL-treated control as analyzed using two-tailed unpaired Student's t-test. RANKL, receptor activator of nuclear factor kB ligand; BMMs, bone marrow-derived macrophages; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; IκBα, inhibitor of nuclear factor kBa; Irf8, interferon regulatory factor-8; M-CSF, macrophage colony-stimulating factor; Blimp1, B lymphocyte-induced maturation protein-1; Bcl6, B-cell lymphoma 6; p-, phosphorylated.
The expression of Nfatc1 can also be suppressed by anti-osteoclastogenic genes such as Irf8, Bcl6 and MafB, and the expression levels of these anti-osteoclastic genes can be downregulated by Blimp1 (11–13). Britanin treatment for 4 days was confirmed to induce Irf8 protein expression associated with M-CSF and RANKL (Fig. 4C). In the case of mRNA expression, compared with the control group, britanin significantly decreased the expression of Blimp1 and significantly increased the expression of Bcl6 and Irf8 (Fig. 4D). These findings suggest that britanin may suppress osteoclast differentiation via the upregulation of negative regulators, including Irf8 and Bcl6.
Britanin exhibits inhibitory effects on a Ti particle-induced mouse calvarial osteolysis model
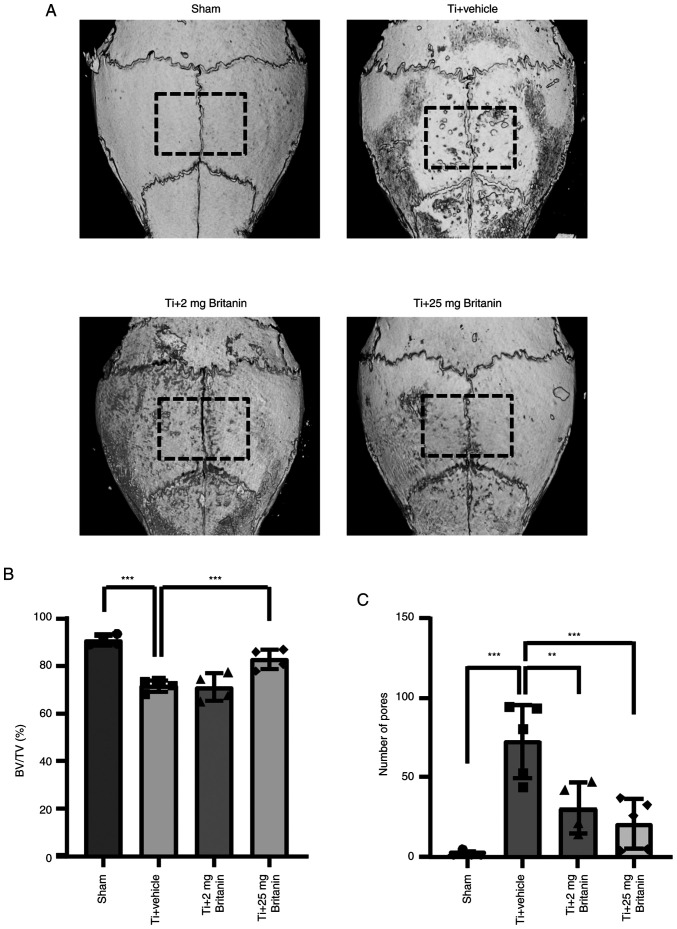
After confirming that britanin reduced osteoclast formation and function in vitro, the effect on osteolysis was investigated in a Ti particle-induced osteolysis mouse model (Fig. 5). In previous experiments (21), compounds that affected cell responses at a concentration of ~5 µM in vitro also demonstrated effective results in vivo within the range of 20–30 mg/kg. Consequently, an initial dose of 25 mg/kg was selected for in vivo testing, whereas an additional dose of 5 mg/kg was selected to assess the dose-dependent efficacy of britanin. To induce osteolysis, Ti particles were implanted into mouse calvaria. This model allowed the measurement of bone resorption in vivo owing to Ti particle-induced activated osteoclasts, which are typically generated in the Ti-implanted sites of patients (34). Despite excluding the load-bearing effect that can induce additional osteoclast-mediated bone resorption, Ti-particle-induced inflammation and osteoclast differentiation were detected. Micro-CT analysis was performed in Ti particle-loaded sites, revealing the presence of extensive bone resorption in the Ti particle-implanted group (vehicle). However, britanin appeared to reduce bone resorption in a dose-dependent manner (Fig. 5A). Based on quantitative analysis, the administration of high-dose britanin (Ti + britanin; 25 mg/kg/day) significantly preserved bone volume compared with that in the Ti-loading group (Fig. 5B). The number of bone-resorbing pits was significantly elevated in the Ti group, and the effect of Ti was reduced in the britanin-treated groups in a dose-dependent manner (Fig. 5C).
Figure 5.
Britanin prevents Ti particle-induced mouse calvarial osteolysis. (A) Representative micro-computed tomography three-dimensional reconstructed images from each group. (B) BV/TV percentage and (C) the number of pores were measured for each sample within the area in the black dashed boxes. **P<0.01 and ***P<0.001 as analyzed by one-way ANOVA with Tukey's multiple comparison post hoc tests. Ti, titanium; BV, bone volume; TV, tissue volume.
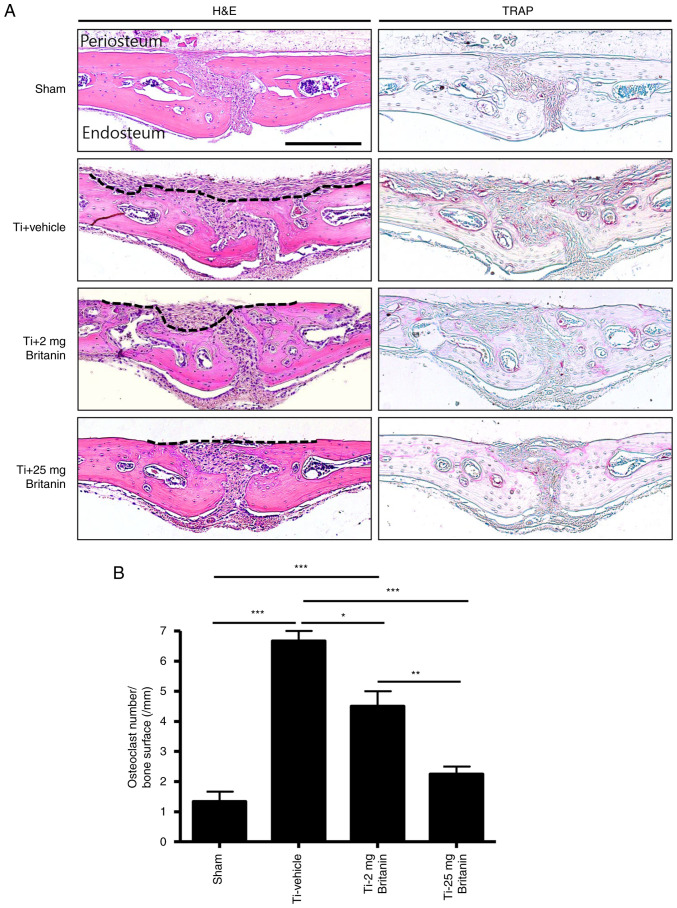
Histochemical analysis demonstrated that britanin protected bone from Ti particle-induced resorption in a dose-dependent manner (Fig. 6A). In the H&E stained tissue, the periosteum layers of the calvarial bone were clean and flat in the sham group. By contrast, a deep and wide area of periosteum side bone was resorbed in the Ti + vehicle group. Treatment with britanin substantially and dose-dependently reduced the Ti particle-induced resorbed area. Moreover, TRAP staining showed that the number of TRAP-positive osteoclasts was reduced by 29 and 56% in the Ti + britanin 2 mg (2 mg/kg/day) and Ti + britanin 25 mg (25 mg/kg/day) groups, respectively, compared with that in the Ti-vehicle group (Fig. 6B). These results suggest that britanin inhibits in vivo osteoclast formation and indicate that britanin may protect the bone against Ti particle-induced osteolysis via the suppression of osteoclast formation in mice.
Figure 6.
Britanin attenuates Ti particle-induced mouse calvarial osteolysis, assessed using histological and histomorphometric analysis. (A) H&E and TRAP staining were performed on the calvarial bone sections. The resorbed bone area is marked with a black dashed line. Scale bar, 250 µm. (B) Histomorphometric analysis of the number of TRAP-positive cells. *P<0.05 **P<0.01 and ***P<0.001 as analyzed by one-way ANOVA with Tukey's multiple comparison post hoc tests. Ti, titanium; H&E, hematoxylin and eosin; TRAP, tartrate-resistant acid phosphatase.
Discussion
Inflammation and oxidative stress are known to mediate periprosthetic osteolysis (24). Ti is widely used in medical devices, including dental and joint implants, which are used to recover the function of damaged tissues. However, weight or load bearing can gradually generate wear particles of various sizes from the implants over time. These nano- to micro-sized wear particles induce inflammation, oxidative stress and periprosthetic osteolysis, and may subsequently result in implant failure (34–36). To the best of our knowledge, the present study is the first to demonstrate that britanin exerts antiosteoclastic activity, indicating its therapeutic potential against wear particle-induced osteolysis.
Oxidative stress is typically involved in osteoclast differentiation (37). Wear particles induce ROS-mediated immune responses (24) and are crucial for RANKL-induced osteoclast differentiation (38). Elevated ROS levels decrease Nrf2 expression, thereby contributing to increased Nfatc1 expression during osteoclast formation (39,40). Accordingly, ROS signaling is indicated to be a suitable target for the pharmacological treatment of bone loss (30). The pathogenesis of periprosthetic osteolysis involves several inflammatory events. Specifically, Ti particles induce inflammatory cytokines such as TNF-α and ILs (1). TNF-α promotes osteoclast differentiation in the presence of ROS, and the expression of TNF-α is increased by ROS in the presence of large quantities of iron-like wear particles. In the present study, britanin not only upregulated the expression of ROS defense molecules, including Nrf2, Nqo1 and HO-1, in the absence of excess iron in vitro, but also significantly inhibited the RANKL-induced expression of TNF-α.
Considering the anti-inflammatory and antioxidative properties of britanin, it was speculated that britanin would be able to suppress abnormal osteoclast differentiation effectively, in vitro and in vivo. The inhibition of inflammation and ROS can suppress osteoclastogenesis; however, the regulation of osteoclast function can be challenging. Therefore, osteoclastogenesis is an important therapeutic target in periprosthetic osteolysis. The present study examined the effects of britanin on the differentiation and function of osteoclasts to explore its potential mode of action. The binding of RANK to RANKL leads to osteoclast differentiation and activation. The RANK-RANKL interaction activates the NF-κB and MAPK downstream signaling pathways by recruiting the signaling molecule TRAF6 and subsequently inducing the expression of the master osteoclast regulator Nfatc1 (41–44). In the present study, britanin significantly reduced the expression of Nfatc1 and its target genes, as well as ERK phosphorylation, without impacting NF-κB activity. In previous studies, britanin was found to exhibit antitumor effects via the inhibition of NF-κB activity in pancreatic, gastric and prostate cancer cell lines (16,17,45). NF-κB inhibition has also been shown to mediate mast cell-mediated inflammatory responses (12,13). However, in the present study, the results indicated that britanin inhibited osteoclast differentiation via suppression of the ERK pathway. Accordingly, these results suggest that the effects of britanin on NF-κB and MAPK may differ according to the cell type or environment.
Blimp1 is a transcriptional repressor that suppresses the expression of anti-osteoclastogenic genes, including Irf8, MafB and Bcl6. Blimp1 deletion has been shown to increase Bcl6 expression, resulting in osteopetrosis due to failure of osteoclast function (10,46). Conversely, Irf8 binds to TRAF6 through physical interactions and induces TRAF6 ubiquitination (47). Irf8 deficiency activates the expression of Nfatc1, resulting in osteoporosis in vivo owing to excessive osteoclast formation (48). In the present study, britanin inhibited Blimp1 gene expression and increased the expression of Bcl6 and Irf8, suggesting that its inhibitory effect on osteoclast differentiation may be partially associated with an attenuating effect on the Blimp1-mediated repression of anti-osteoclastogenic genes.
In addition, the present study evaluated the therapeutic potential of britanin in periprosthetic osteolysis in a mouse calvarial model. Ti particles significantly induced osteolysis in the examined mouse model, whereas britanin reduced bone resorption. Histological analysis revealed that britanin prevented Ti particle-induced osteolysis. In addition, TRAP staining indicated that britanin suppressed osteoclast formation in vivo. These results strongly suggest that britanin not only inhibits osteoclast differentiation but also suppresses inflammatory signals, such as those mediated by TNF-α and ROS. These britanin-mediated activities effectively inhibited osteolysis in vivo. Although the inhibitory effect of britanin on osteolysis in vivo was examined using a mouse calvaria osteolysis model, this may not be the most suitable model for fully mimicking human osteolysis. In this model, the absence of load-bearing prevents the gradual generation of titanium particles. Moreover, it does not replicate physiological dynamic environments such as the movement of joints in activities such as running and jumping.
In summary, the present study indicated that britanin regulates inflammation and oxidative stress signaling and inhibits osteoclast differentiation by the downregulation of Blimp1-Nfatc1 in vitro, thereby suppressing osteoclast differentiation and the expression of osteoclast-specific marker genes. In addition, britanin was demonstrated to protect bone from Ti particle-induced calvarial osteolysis in vivo. These results strongly suggest that britanin is a potential candidate for the treatment of osteoclast-associated diseases and wear particle-induced osteolysis.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This study was supported by a National Research Foundation of Korea grant funded by the Korean government (MSIT) (grant nos. 2017R1A5A2015391 and 2020M3A9I4039539).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JAK, SL and EKP were responsible for conceptualization. JAK, SL and HJI designed the study methodology. Validation was performed by JAK, SL and HJI. Formal analysis was performed by JAK, SL and EKP. JEK, KY and HC carried out the investigation. JM and HC contributed resources, and extracted and analyzed the britanin. The original draft of the manuscript was prepared and written by JAK and SL, and was reviewed and edited by HC and EKP. HC and EKP supervised the study. HC and EKP confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Animal experiments were approved by the Committee on the Care and Use of Animals in Research at Kyungpook National University (Daegu, Korea; approval no. 2021-0071).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Eger M, Hiram-Bab S, Liron T, Sterer N, Carmi Y, Kohavi D, Gabet Y. Mechanism and prevention of titanium particle-induced inflammation and osteolysis. Front Immunol. 2018;9:2963. doi: 10.3389/fimmu.2018.02963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holding CA, Findlay DM, Stamenkov R, Neale SD, Lucas H, Dharmapatni AS, Callary SA, Shrestha KR, Atkins GJ, Howie DW, Haynes DR. The correlation of RANK, RANKL and TNFalpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials. 2006;27:5212–5219. doi: 10.1016/j.biomaterials.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 3.Landgraeber S, Jäger M, Jacobs JJ, Hallab NJ. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediators Inflamm. 2014;2014:185150. doi: 10.1155/2014/185150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tao H, Ge G, Liang X, Zhang W, Sun H, Li M, Geng D. ROS signaling cascades: Dual regulations for osteoclast and osteoblast. Acta Biochim Biophys Sin (Shanghai) 2020;52:1055–1062. doi: 10.1093/abbs/gmaa098. [DOI] [PubMed] [Google Scholar]
- 5.Samelko L, Caicedo MS, Lim SJ, Della-Valle C, Jacobs J, Hallab NJ. Cobalt-Cobalt-alloy implant debris induce HIF-1α hypoxia associated responses: A mechanism for metal-specific orthopedic implant failure. PLoS One. 2013;8:e67127. doi: 10.1371/journal.pone.0067127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun YX, Xu AH, Yang Y, Li J. Role of Nrf2 in bone metabolism. J Biomed Sci. 2015;22:101. doi: 10.1186/s12929-015-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udagawa N. The mechanism of osteoclast differentiation from macrophages: Possible roles of T lymphocytes in osteoclastogenesis. J Bone Miner Metab. 2003;21:337–343. doi: 10.1007/s00774-003-0439-1. [DOI] [PubMed] [Google Scholar]
- 8.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 9.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa K, Nakashima T, Hayashi M, Fukunaga T, Kato S, Kodama T, Takahashi S, Calame K, Takayanagi H. Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proc Natl Acad Sci USA. 2010;107:3117–3122. doi: 10.1073/pnas.0912779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SJ, Huh JE, Shin J, Park DR, Ko R, Jin GR, Seo DH, Kim HS, Shin HI, Oh GT, et al. Sirt6 cooperates with Blimp1 to positively regulate osteoclast differentiation. Sci Rep. 2016;6:26186. doi: 10.1038/srep26186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Li X, Park YN, Kwon O, Piao D, Chang YC, Kim CH, Lee E, Son JK, Chang HW. Britanin suppresses IgE/Ag-induced mast cell activation by inhibiting the Syk pathway. Biomol Ther (Seoul) 2014;22:193–199. doi: 10.4062/biomolther.2014.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HH, Kim SG, Park YN, Lee J, Lee YJ, Park NY, Jeong KT, Lee E. Suppressive effects of britanin, a sesquiterpene compound isolated from Inulae flos, on mast cell-mediated inflammatory responses. Am J Chin Med. 2014;42:935–947. doi: 10.1142/S0192415X14500591. [DOI] [PubMed] [Google Scholar]
- 14.Kim SG, Lee E, Park NY, Park HH, Jeong KT, Kim KJ, Lee YJ, Jin M, Lee E. Britanin attenuates ovalbumin-induced airway inflammation in a murine asthma model. Arch Pharm Res. 2016;39:1006–1012. doi: 10.1007/s12272-016-0783-z. [DOI] [PubMed] [Google Scholar]
- 15.Park HH, Kim MJ, Li Y, Park YN, Lee J, Lee YJ, Kim SG, Park HJ, Son JK, Chang HW, Lee E. Britanin suppresses LPS-induced nitric oxide, PGE2 and cytokine production via NF-κB and MAPK inactivation in RAW 264.7 cells. Int Immunopharmacol. 2013;15:296–302. doi: 10.1016/j.intimp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Shi K, Liu X, Du G, Cai X, Zhan Y. In vivo antitumour activity of britanin against gastric cancer through nuclear factor-κB-mediated immune response. J Pharm Pharmacol. 2020;72:607–618. doi: 10.1111/jphp.13230. [DOI] [PubMed] [Google Scholar]
- 17.Li K, Zhou Y, Chen Y, Zhou L, Liang J. A novel natural product, britanin, inhibits tumor growth of pancreatic cancer by suppressing nuclear factor-κB activation. Cancer Chemother Pharmacol. 2020;85:699–709. doi: 10.1007/s00280-020-04052-w. [DOI] [PubMed] [Google Scholar]
- 18.Wu G, Zhu L, Yuan X, Chen H, Xiong R, Zhang S, Cheng H, Shen Y, An H, Li T, et al. Britanin ameliorates cerebral ischemia-reperfusion injury by inducing the Nrf2 protective pathway. Antioxid Redox Signal. 2017;27:754–768. doi: 10.1089/ars.2016.6885. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Xiao H, Dai M, Xue Y, Zhao R. Britanin relieves ferroptosis-mediated myocardial ischaemia/reperfusion damage by upregulating GPX4 through activation of AMPK/GSK3β/Nrf2 signalling. Pharm Biol. 2022;60:38–45. doi: 10.1080/13880209.2021.2007269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piao D, Kim T, Zhang HY, Choi HG, Lee CS, Choi HJ, Chang HW, Woo MH, Son JK. DNA topoisomerase inhibitory activity of constituents from the flowers of Inula japonica. Chem Pharm Bull (Tokyo) 2016;64:276–281. doi: 10.1248/cpb.c15-00780. [DOI] [PubMed] [Google Scholar]
- 21.Lee YE, Park KS, Park EK, Im SU, Choi YH, Song KB. Polycan suppresses osteoclast differentiation and titanium particle-induced osteolysis in mice. J Biomed Mater Res B Appl Biomater. 2016;104:1170–1175. doi: 10.1002/jbm.b.33415. [DOI] [PubMed] [Google Scholar]
- 22.Ihn HJ, Lee T, Kim JA, Lee D, Kim ND, Shin HI, Bae YC, Park EK. OCLI-023, a novel pyrimidine compound, suppresses osteoclastogenesis in vitro and alveolar bone resorption in vivo. PLoS One. 2017;12:e0170159. doi: 10.1371/journal.pone.0170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Li Z, Guo Y, Zhou Y, Zhang Z, Zhang Y, Luo G, Yang X, Liao W, Li C, et al. Wear particles promote reactive oxygen species-mediated inflammation via the nicotinamide adenine dinucleotide phosphate oxidase pathway in macrophages surrounding loosened implants. Cell Physiol Biochem. 2015;35:1857–1867. doi: 10.1159/000373996. [DOI] [PubMed] [Google Scholar]
- 25.Ihn HJ, Kim K, Cho HS, Park EK. Pentamidine inhibits titanium particle-induced osteolysis in vivo and receptor activator of nuclear factor-κB ligand-mediated osteoclast differentiation in vitro. Tissue Eng Regen Med. 2019;16:265–273. doi: 10.1007/s13770-019-00186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H, Ouyang Z, Xu X, Wang W, Fan Q, et al. The effect of enoxacin on osteoclastogenesis and reduction of titanium particle-induced osteolysis via suppression of JNK signaling pathway. Biomaterials. 2014;35:5721–5730. doi: 10.1016/j.biomaterials.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Deng W, Huang Y, Li H, Chen C, Lin Y, Wang M, Huang H, Liu T, Qin Q, Shao Y, et al. Dehydromiltirone inhibits osteoclast differentiation in RAW264.7 and bone marrow macrophages by modulating MAPK and NF-κB activity. Front Pharmacol. 2022;13:1015693. doi: 10.3389/fphar.2022.1015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q, Zhan P, Li X, Mo F, Xu H, Liu Y, Lai Q, Zhang B, Dai M, Liu X. Bisphosphonate-enoxacin inhibit osteoclast formation and function by abrogating RANKL-induced JNK signalling pathways during osteoporosis treatment. J Cell Mol Med. 2021;25:10126–10139. doi: 10.1111/jcmm.16949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyeon S, Lee H, Yang Y, Jeong W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic Biol Med. 2013;65:789–799. doi: 10.1016/j.freeradbiomed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Agidigbi TS, Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int J Mol Sci. 2019;20:3576. doi: 10.3390/ijms20143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyamoto T. Regulators of osteoclast differentiation and cell-cell fusion. Keio J Med. 2011;60:101–105. doi: 10.2302/kjm.60.101. [DOI] [PubMed] [Google Scholar]
- 32.Matsubara T, Kinbara M, Maeda T, Yoshizawa M, Kokabu S, Takano Yamamoto T. Regulation of osteoclast differentiation and actin ring formation by the cytolinker protein plectin. Biochem Biophys Res Commun. 2017;489:472–476. doi: 10.1016/j.bbrc.2017.05.174. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D, Jing J, Lou F, Li R, Ping Y, Yu F, Wu F, Yang X, Xu R, Li F, et al. Evidence for excessive osteoclast activation in SIRT6 null mice. Sci Rep. 2018;8:10992. doi: 10.1038/s41598-018-28716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarpada SP, Loloi J, Schwechter EM. A case of titanium pseudotumor and systemic toxicity after total hip arthroplasty polyethylene failure. Arthroplast Today. 2020;6:710–715. doi: 10.1016/j.artd.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tribst JPM, Werner A, Blom EJ. Failed dental implant: When titanium fractures. Diagnostics (Basel) 2023;13:2123. doi: 10.3390/diagnostics13122123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim KT, Eo MY, Nguyen TTH, Kim SM. General review of titanium toxicity. Int J Implant Dent. 2019;5:10. doi: 10.1186/s40729-019-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suda N, Morita I, Kuroda T, Murota S. Participation of oxidative stress in the process of osteoclast differentiation. Biochim Biophys Acta. 1993;1157:318–323. doi: 10.1016/0304-4165(93)90116-P. [DOI] [PubMed] [Google Scholar]
- 38.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 39.Lee HI, Lee GR, Lee J, Kim N, Kwon M, Kim HJ, Kim NY, Park JH, Jeong W. Dehydrocostus lactone inhibits NFATc1 via regulation of IKK, JNK, and Nrf2, thereby attenuating osteoclastogenesis. BMB Rep. 2020;53:218–222. doi: 10.5483/BMBRep.2020.53.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W, Liang X, Liu X, Bai J, Zhang W, Li W, Wang T, Li M, Wu Z, Chen L, et al. NOX4 blockade suppresses titanium nanoparticle-induced bone destruction via activation of the Nrf2 signaling pathway. J Nanobiotechnology. 2022;20:241. doi: 10.1186/s12951-022-01413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomomura M, Suzuki R, Shirataki Y, Sakagami H, Tamura N, Tomomura A. Rhinacanthin C inhibits osteoclast differentiation and bone resorption: Roles of TRAF6/TAK1/MAPKs/NF-κB/NFATc1 signaling. PLoS One. 2015;10:e0130174. doi: 10.1371/journal.pone.0130174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 43.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 44.Shi JH, Sun SC. Tumor tumor necrosis factor receptor-associated factor regulation of nuclear factor κB and mitogen-activated protein kinase pathways. Front Immunol. 2018;9:1849. doi: 10.3389/fimmu.2018.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng Q, Zeng Y, Nie X, Guo Y, Zhan Y. Britanin exhibits potential inhibitory activity on human prostate cancer cell lines through PI3K/Akt/NF-κB signaling pathways. Planta Med. 2020;86:1401–1410. doi: 10.1055/a-1211-4656. [DOI] [PubMed] [Google Scholar]
- 46.Miyauchi Y, Ninomiya K, Miyamoto H, Sakamoto A, Iwasaki R, Hoshi H, Miyamoto K, Hao W, Yoshida S, Morioka H, et al. The Blimp1-Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med. 2010;207:751–762. doi: 10.1084/jem.20091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao J, Kong HJ, Li H, Huang B, Yang M, Zhu C, Bogunovic M, Zheng F, Mayer L, Ozato K, et al. IRF-8/interferon (IFN) consensus sequence-binding protein is involved in Toll-like receptor (TLR) signaling and contributes to the cross-talk between TLR and IFN-gamma signaling pathways. J Biol Chem. 2006;281:10073–10080. doi: 10.1074/jbc.M507788200. [DOI] [PubMed] [Google Scholar]
- 48.Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med. 2009;15:1066–1071. doi: 10.1038/nm.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.