Abstract
The release of lipoteichoic acid (LTA) and teichoic acid (TA) from a Streptococcus pneumoniae type 3 strain during exposure to ceftriaxone, meropenem, rifampin, rifabutin, quinupristin-dalfopristin, and trovafloxacin in tryptic soy broth was monitored by a newly developed enzyme-linked immunosorbent assay. At a concentration of 10 μg/ml, a rapid and intense release of LTA and TA occurred during exposure to ceftriaxone (3,248 ± 1,688 ng/ml at 3 h and 3,827 ± 2,133 ng/ml at 12 h) and meropenem (2,464 ± 1,081 ng/ml at 3 h and 2,900 ± 1,364 ng/ml at 12 h). Three hours after exposure to rifampin, rifabutin, quinupristin-dalfopristin, and trovafloxacin, mean LTA and TA concentrations of less than 460 ng/ml were observed (for each group, P < 0.01 versus the concentrations after exposure to ceftriaxone). After 12 h of treatment, the LTA and TA concentrations were 463 ± 126 ng/ml after exposure to rifampin, 669 ± 303 ng/ml after exposure to rifabutin, and 1,236 ± 772 ng/ml after exposure to quinupristin-dalfopristin (for each group, P < 0.05 versus the concentrations after exposure to ceftriaxone) and 1,745 ± 1,185 ng/ml after exposure to trovafloxacin (P = 0.12 versus the concentration after exposure to ceftriaxone). At 10 μg/ml, bactericidal antibacterial agents that do not primarily affect cell wall synthesis reduced the amount of LTA and TA released during their cidal action against S. pneumoniae in comparison with the amount released after exposure to β-lactams. Larger quantities of LTA and TA were released after treatment with low concentrations (1× the MIC and 1× the minimum bactericidal concentration) than after no treatment for all antibacterial agents except the rifamycins. This does not support the concept of using a low first antibiotic dose to prevent the release of proinflammatory cell wall components.
The release of bacterial cell wall components into the cerebrospinal fluid (CSF) during antibiotic-induced bacterial lysis may cause a burst of meningeal inflammation subsequent to the initiation of antibiotic therapy (8, 16).
Pneumococcal cell wall components stimulate the synthesis of tumor necrosis factor alpha (TNF-α) and interleukin-6 by human monocytes (4). Heat-inactivated pneumococci and pneumococcal cell walls exert a cytotoxic effect in cultures of microglia and astrocytes. No neuronal damage was observed when neurons were cultured alone, whereas coculture of neurons and glial cells exposed to pneumococcal cell walls resulted in the death of neurons (6). These findings raise the possibility that pneumococcal cell wall components mediate the damage of brain tissue during meningitis not only by inducing the invasion of leukocytes but also by directly stimulating glial cells.
Teichoic acid (TA) and lipoteichoic acid (LTA) are the most potent proinflammatory constituents of the membrane and the cell wall of Streptococcus pneumoniae. They are composed of repetitive oligosaccharide units conjugated to phosphorylcholine (1). LTA additionally has a hydrophobic trihexosyldiacylglycerol residue. When injected into the subarachnoid space, TA and LTA cause profound meningeal inflammation (16).
In order to attenuate subarachnoid space inflammation, adjunctive anti-inflammatory drugs have been introduced into the therapy for bacterial meningitis. Yet, although different regimens have been proven to be effective in animal models, only dexamethasone therapy has been successfully established in clinical practice. Dexamethasone, however, has been shown to decrease the concentrations of hydrophilic antibiotics in CSF and to affect CSF sterilization for those pathogens with a decreased sensitivity to antibiotics (10). Furthermore, dexamethasone may promote neuronal damage in the dentate gyrus of the hippocampal formation by inhibiting the uptake of the neurotoxic excitatory amino acid glutamate by astrocytes (17).
A therapeutic approach which circumvents immunosuppression and the associated problems is to decrease the release of bacterial proinflammatory cell wall products into the CSF. This may be possible by using bactericidal antibacterial agents that do not directly affect cell wall synthesis. Such compounds may terminate the ability of bacteria to grow, replicate, and release cell wall components several hours before bacterial lysis occurs (7). Quinolones, rifamycins, and pristinamycins are promising in this regard: they are rapidly bactericidal against pneumococci (2, 9, 12). At lower concentrations quinolones inhibit the topoisomerase II, and at high concentrations they also affect protein synthesis and RNA synthesis. Rifamycins inhibit RNA synthesis, and pristinamycins affect protein synthesis.
In the present study we addressed the question of whether different modes of action of antibacterial agents influence the amount of proinflammatory cell wall components released from S. pneumoniae. For this purpose we studied the release of LTA and TA from pneumococci during in vitro treatment with the quinolone trovafloxacin, the rifamycins rifampin and rifabutin, and the combination of streptogramins quinupristin and dalfopristin (RP59500) in comparison with the release during treatment with the cephalosporin and carbapenem antibiotics ceftriaxone and meropenem using a newly developed enzyme-linked immunosorbent assay (ELISA).
(The study has been presented in part at the 8th European Congress of Clinical Microbiology and Infectious Diseases, Lausanne, Switzerland, 25 to 28 May 1997.)
MATERIALS AND METHODS
Reagents and bacterial strains.
Rifabutin was kindly provided by Pharmacia Upjohn (Milano, Italy), and RP59500, consisting of 30% quinupristin and 70% dalfopristin, was provided by Rhone-Poulenc Rorer (Cologne, Germany). Trovafloxacin was a gift from Pfizer (Karlsruhe, Germany), and ceftriaxone was provided by Hoffmann-La Roche (Grenzach Wyhlen, Germany). Rifampin and meropenem were purchased from Grünenthal (Stolberg, Germany).
The mouse immunoglobulin A (IgA) monoclonal antibody (MAb) TEPC-15, which recognizes the phosphorylcholine residues of pneumococcal LTA and TA, was purchased from Sigma, Deisenhofen, Germany.
Bacteria were grown at 37°C in tryptic soy broth (Difco, Detroit, Mich.). The unencapsulated R6 strain (a gift from W. Fischer, Erlangen, Germany) was used for the preparation of LTA, and a penicillin-sensitive S. pneumoniae type 3 strain (15) was used to investigate the release of cell wall components. The MICs and minimal bactericidal concentrations (MBCs) for this strain were determined by the broth macrodilution method according to the guidelines of the National Commitee for Clinical Laboratory Standards, and the respective values were as follows: ceftriaxone, 0.03, and 0.06 μg/ml; meropenem, 0.008 and 0.03 μg/ml; quinupristin-dalfopristin, 0.06 and 0.12 μg/ml; rifabutin, 0.008 and 0.06 μg/ml; rifampin, 0.008μ and 0.06 μg/ml; and trovafloxacin, 0.06 and 0.12 μg/ml.
Release of proinflammatory components as a result of exposure to antibacterials.
S. pneumoniae type 3 was grown to an optical density at 578 nm of approximately 0.1. Cells were collected by centrifugation and were resuspended in fresh tryptic soy broth to a final concentration of approximately 108 CFU/ml. Control cultures were grown after resuspension without antibacterial agents. The resuspension in fresh broth ensured growth in the logarithmic phase for at least 3 h, i.e., drugs were added during the logarithmic phase of growth. At the end of the logarithmic phase, bacterial titers approximated 1010 CFU/ml (see Fig. 1G).
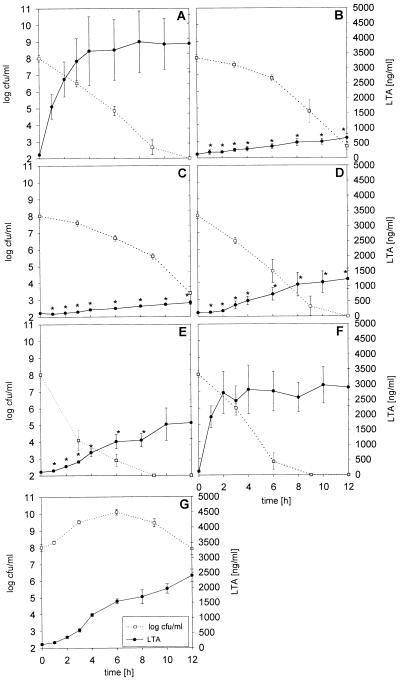
FIG. 1.
Release of LTA and TA by S. pneumoniae type 3 during treatment with 10 μg of ceftriaxone (A), rifabutin (B), rifampin (C), quinupristin-dalfopristin (D), trovafloxacin (E), and meropenem (F) per ml and by untreated cultures (G) (closed circles). The means ± standard errors of the means of five experiments are shown. The asterisks denote significant differences versus the results for ceftriaxone-treated bacteria. The open squares represent the bacterial titers (means ± standard errors of means).
In the first set of experiments, ceftriaxone, rifampin, rifabutin, quinupristin-dalfopristin, trovafloxacin, and meropenem at concentrations of 10 μg/ml each were added to 15-ml aliquots of the bacterial suspension. The uniform concentration of 10 μg/ml was used to ensure a maximum bactericidal effect for all drugs studied. Samples (1 ml) for the detection of free LTA and free TA were collected before and at 1, 2, 3, 4, 6, 8, 10, and 12 h after the addition of drugs. For each antibacterial agent, this experiment was repeated five times on separate days.
In the second set of experiments, the release of LTA and TA after 1, 2, 3, 4, 6, 8, 10, and 12 h of exposure to ceftriaxone, rifampin, rifabutin, quinupristin-dalfopristin, trovafloxacin, and meropenem was studied at four different concentrations: the MIC and the MBC, as determined previously, and 0.5 and 2 μg/ml (n = 3 for each antibacterial agent at each concentration).
Samples were centrifuged at 10,000 × g for 10 min, and the supernatants were frozen at −20°C for the measurement of LTA and TA. Bacterial counts were determined at 0, 3, 6, 9, and 12 h by plating 10-μl samples of 10-fold dilutions of bacteria onto blood agar plates. The bactericidal rates (δlog CFU per milliliter per hour) were determined by log-linear regression analysis of bacterial titers (CFU per milliliter) versus time, and the coefficient of total determination (r2) was given in brackets.
Sandwich ELISA for detection of pneumococcal LTA and TA.
LTA was prepared from S. pneumoniae R6 (1). Bacteria were grown in 10 liters of tryptic soy broth overnight at 37°C. The bacterial cells were disrupted with a French pressure cell and were then extracted with methanol and chloroform at different concentration ratios. LTA was detected in the aqueous layer. Following the removal of methanol, the suspension was further purified by hydrophobic interaction chromatography on octyl-Sepharose (Pharmacia Biotech, Freiburg, Germany). The purification grade was determined by using the choline/phosphate ratio, which is 0.66 in pure LTA (1).
Polyclonal antibodies were raised in two New Zealand White rabbits subcutaneously immunized with 500 μg of LTA mixed with an equal volume of incomplete Freund’s adjuvant. Immunization was repeated every 4 weeks until high titers (≥1:32,000) were obtained. The sera were preserved at −20°C.
The ELISA developed for the quantification of LTA and TA release from bacterial cultures used MAb TEPC-15 as the capture antibody and the polyclonal antiserum raised against LTA as the detector antibody. MAb TEPC-15 was coated overnight on 96-well microtiter plates (Nunc GmbH, Wiesbaden, Germany) at a concentration of 5 μg/ml. Blocking as well as the following steps were carried out with 5% fetal calf serum in phosphate-buffered saline. Appropriate dilutions of samples or twofold dilutions of purified LTA as a standard were incubated for 2 h, followed by a 2-h incubation with polyclonal antiserum (1:4,000). Bound rabbit antibodies were quantified with peroxidase-conjugated goat anti-rabbit IgG antibodies (1:8,000; Dianova, Hamburg, Germany). Enzyme activity was determined with a 1-mg/ml solution of 2,2′-azino-di(3-ethylbenzthiazolinsulfonate in 50 mM phosphate-citrate-buffer (pH 4.4) containing 3 mM sodium perborate. Absorption was determined after 30 min of incubation in an ELISA reader (Titertek Multiskan Plus MK II; Flow Laboratories GmbH, Meckenheim, Germany) at 405 nm.
Standard curves were constructed for each assay. Intra-assay and interday coefficients of variation determined by repeatedly measuring quality control samples spiked with LTA were 8.4 and 10.9%, respectively, at 300 ng/ml and 7.1 and 7.1%, respectively, at 1,800 ng/ml. No cross-reactions were observed with supernatants from cultures of Listeria monocytogenes, Haemophilus influenzae, Staphylococcus epidermidis, viridans group streptococci, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, Corynebacterium xerosis, Salmonella enteritidis, Proteus spp., Enterobacter sakazakii, and Enterobacter cloacae grown in tryptic soy broth and killed by exposure to ceftriaxone or vancomycin. The ELISA did recognize components released by Staphylococcus aureus. This reaction could easily be eliminated by adding human serum or solutions containing human IgG and was assumed to be due to protein A, a cell wall component of S. aureus known to bind specifically to the Fc portion of IgG (14). The addition of IgG in excess did not diminish the ELISA reaction in response to purified LTA or samples containing pneumococcal cell wall products. Ceftriaxone, meropenem, quinupristin-dalfopristin, rifabutin, rifampin, and trovafloxacin did not bind to TA or LTA or interfere with the assay.
Electron microscopy.
For scanning electron microscopy, S. pneumoniae type 3 was grown to the late logarithmic phase in tryptic soy broth and was then treated for 3 or 6 h with 10 μg of ceftriaxone or rifampin per ml. Thereafter, the bacteria were transferred to poly-l-lysine-coated coverslips and fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer for 24 h. After dehydration in graded ethanol, samples were dried in a critical-point dryer (Polaron, Watford, United Kingdom). After being mounted on stubs, the specimens were coated with gold-palladium in a cool sputter coater (Fisons Instruments, Uckfield, United Kingdom) and were examined in an electron microscope (DSM 960; Zeiss, Oberkochen, Germany).
Statistics.
Ceftriaxone (10 μg/ml) was used as the standard antibacterial agent, and the results obtained with ceftriaxone were compared with those obtained with the other drugs at the same concentration by unpaired analysis of variance. The P values were adjusted for repeated testing by the Bonferroni method.
RESULTS
The bactericidal rates during exposure to 10 μg of ceftriaxone (−0.60 ± 0.08 Δlog CFU/ml/h; r2 = 0.98 ± 0.004), meropenem (−0.87 ± 0.15 Δlog CFU/ml/h; r2 = 0.98 ± 0.02), quinupristin-dalfopristin (−0.68 ± 0.22 Δlog CFU/ml/h; r2 = 0.93 ± 0.06), and trovafloxacin (−0.79 ± 0.16 Δlog CFU/ml/h; r2 = 0.96 ± 0.08) per ml were slightly higher than those during exposure to 10 μg of rifabutin (−0.45 ± 0.05 Δlog CFU/ml/h; r2 = 0.85 ± 0.05) and rifampin (−0.37 ± 0.04 Δlog CFU/ml/h; r2 = 0.91 ± 0.05) per ml (values are means ± standard deviations). The differences in the bactericidal rates did not reach statistical significance. Twelve hours after the initiation of treatment, the concentrations of viable bacteria were below the detection limit of 102 CFU/ml after exposure to ceftriaxone, meropenem, quinupristin-dalfopristin, and trovafloxacin, whereas in the rifabutin- and rifampin-treated cultures the mean bacterial counts were 4.8 × 102 and 2.6 × 103/ml, respectively (Fig. 1).
A rapid and intense release of LTA and TA occurred in the first hours of exposure to 10 μg of ceftriaxone per ml (means ± standard deviations, 3,248 ± 1,688 ng/ml at 3 h and 3,827 ± 2,133 ng/ml at 12 h) and 10 μg of meropenem per ml (2,464 ± 1081 ng/ml at 3 h and 2,900 ± 1,364 ng/ml at 12 h) (the difference was not significant). Three hours after rifampin and rifabutin exposure (10 μg/ml each), mean LTA and TA concentrations of 166 ± 65 and 267 ± 121 ng/ml, respectively, were observed (for each group, P < 0.01 versus the concentrations after exposure to ceftriaxone). Three hours of exposure to 10 μg of quinupristin-dalfopristin per ml resulted in LTA and TA concentrations of 380 ± 285 ng/ml, and 10 μg of trovafloxacin per ml released 455 ± 89 ng of LTA and TA per ml during the first 3 h (P < 0.01 versus the concentrations after exposure to ceftriaxone). After 12 h of treatment with 10 μg of the various drugs per ml, the LTA and TA concentrations were 463 ± 126 ng/ml after exposure to rifampin, 669 ± 303 ng/ml after exposure to rifabutin, and 1,236 ± 772 ng/ml after exposure to quinupristin-dalfopristin (for each group, P < 0.05 versus the concentrations after exposure to ceftriaxone) and 1,745 ± 1,185 ng/ml after exposure to trovafloxacin (P = 0.12 versus the concentration after exposure to ceftriaxone) (Fig. 1). Meropenem at 10 μg/ml did not cause a significant reduction in the release of LTA and TA at any time (2,464 ± 1,081 ng/ml at 3 h and 2,900 ± 1,363 ng/ml at 12 h) when compared to the release resulting from exposure to ceftriaxone.
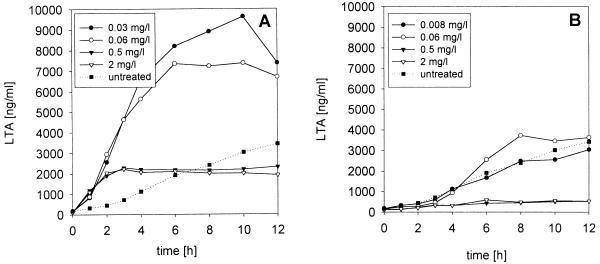
For all antibacterial agents studied, the release of LTA and TA from S. pneumoniae type 3 was dose dependent. At low concentrations (1× the MIC and 1× the MBC), the LTA and TA levels in the supernatants were higher than those at higher concentrations of the antibacterial agents. At 1× the MIC and 1× the MBC, more LTA and TA were released from S. pneumoniae cultures in the presence of ceftriaxone, meropenem, trovafloxacin, and quinupristin-dalfopristin than from nontreated cultures, although ceftriaxone and meropenem were bactericidal and trovafloxacin and quinupristin-dalfoprisitin were bacteriostatic at these concentrations (Table 1; Fig. 2A). The LTA and TA concentrations released from bacteria exposed to rifampin at 1× the MIC and 1× the MBC and to rifabutin at 1× the MIC were close to the concentrations observed in the supernatants of untreated cultures (Table 1; Fig. 2B).
TABLE 1.
Release of LTA and TA by S. pneumoniae type 3 during treatment with antibacterial agents at different concentrations
| Antibacterial agent | Concn (μg/ml) | Bactericidal rate (Δlog CFU/ml) | Concn (μl/ml) of LTA at the following times:
|
|
|---|---|---|---|---|
| 3 h | 12 h | |||
| Ceftriaxone | MIC | −0.29 | 4,640 | 7,382 |
| MBC | −0.47 | 4,625 | 6,693 | |
| 0.5 | −0.55 | 2,282 | 2,313 | |
| 2 | −0.68 | 2,217 | 1,916 | |
| Rifabutin | MIC | −0.21 | 482 | 2,673 |
| MBC | −0.37 | 319 | 627 | |
| 0.5 | −0.39 | 507 | 756 | |
| 2 | −0.40 | 378 | 803 | |
| Rifampin | MIC | −0.13 | 623 | 3,050 |
| MBC | −0.36 | 470 | 3,641 | |
| 0.5 | −0.36 | 352 | 542 | |
| 2 | −0.49 | 332 | 536 | |
| Trovafloxacin | MIC | 0.00 | 2,138 | 9,512 |
| MBC | 0.01 | 2,341 | 13,732 | |
| 0.5 | −0.42 | 1,551 | 7,357 | |
| 2 | −0.50 | 729 | 1,176 | |
| Quinupristin-dalfopristin | MIC | −0.03 | 607 | 4,063 |
| MBC | −0.12 | 801 | 5,191 | |
| 0.5 | −0.37 | 453 | 2,777 | |
| 2 | −0.63 | 415 | 800 | |
| Meropenem | MIC | −0.17 | 1,796 | 8,022 |
| MBC | −0.47 | 3,312 | 5,354 | |
| 0.5 | −0.54 | 1,807 | 2,416 | |
| 2 | −0.69 | 1,799 | 2,213 | |
| No antibacterial agent | 0.34 | 720 | 3,433 | |
FIG. 2.
Release of LTA and TA by S. pneumoniae type 3 during treatment with ceftriaxone (A) and rifampin (B) at different concentrations. Note that at low ceftriaxone concentrations (1× the MIC and 1× the MBC), the concentrations of LTA and TA in the supernatant exceeded the levels observed in the untreated cultures.
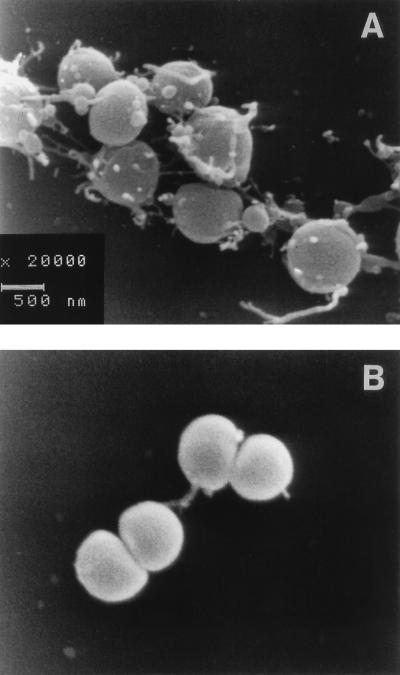
Scanning electron microscopy revealed differences in bacterial morphology following treatment with ceftriaxone and rifampin (both at a concentration of 10 μg/ml) for 3 and 6 h. Rifampin induced no remarkable morphological changes. Occasionally, blebs were seen on the bacterial surface, and blebs were also found on a few bacteria receiving no treatment (Fig. 3B). In contrast, treatment with ceftriaxone resulted in the abundant formation of blebs of different sizes on the cell surface (Fig. 3A). Furthermore, ceftriaxone-treated pneumococci developed filaments with lengths of up to 1 μm (Fig. 3A).
FIG. 3.
Representative scanning electron micrographs of S. pneumoniae type 3 after in vitro exposure to 10 μg of ceftriaxone (A) or rifampin (B) per ml for 6 h. The horizontal bar represents 500 nm. Note the numerous defects of the pneumococcal cell wall after exposure to ceftriaxone. Magnifications, ×15,600.
DISCUSSION
In the initial phase of treatment with β-lactam antibiotics, a brisk increase in meningeal inflammation occurs. This increase is caused by the release of proinflammatory bacterial cell wall components and may be responsible, in part, for the early mortality and long-term sequelae seen in patients with bacterial meningitis (8, 16). For gram-negative bacteria, the Limulus lysate assay is a sensitive method of measuring endotoxin activity in vitro and in vivo (3, 5, 11). To date, no method is capable of quantifying proinflammatory compounds of S. pneumoniae, the predominant pathogen in bacterial meningitis since the introduction of vaccination against H. influenzae type b. Although several approaches have been successful in detecting antigens of S. pneumoniae in patients with meningitis and respiratory tract infections (13, 14), these assays are unsuitable for quantitative measurements.
The ELISA that we developed with MAb TEPC-15, which recognizes phosphorylcholine residues, is able to quantitate both TA and LTA, the most active proinflammatory components of the cell wall of S. pneumoniae (16). The assay was calibrated with a standard LTA preparation (1) and does not differentiate between LTA and TA. It was used to measure the release of LTA and TA in response to several antibacterial agents with divergent modes of action at high (10 μg/ml) and low (1× the MIC, 1× the MBC, and 0.5 and 2 μg/ml) concentrations.
At 10 μg/ml, all drugs investigated were rapidly bactericidal (Fig. 1). The mean bactericidal rate was slightly lower for rifampin and rifabutin than for ceftriaxone, meropenem, trovafloxacin, and quinupristin-dalfopristin (the differences in δlog CFU per milliliter per hour were not significant). Despite similar bactericidal rates, the release of LTA and TA from the bacteria was slower in the presence of rifampin, rifabutin, trovafloxacin, and quinupristin-dalfopristin than in the presence of ceftriaxone and meropenem (Fig. 1). The rifamycins and quinupristin-dalfopristin led to distinct reductions in LTA and TA release. After 12 h of exposure to trovafloxacin and ceftriaxone, however, the LTA and TA concentrations were not significantly different. No morphological changes in pneumococci treated with 10 μg of rifampin per ml for 6 h were seen by scanning electron microscopy, whereas exposure to 10 μg of ceftriaxone per ml for an equal interval induced numerous defects in the pneumococcal cell wall (Fig. 3).
In the rabbit model of pneumococcal meningitis, high-dose trovafloxacin delayed the antibiotic-induced increase in TNF-α and interleukin-1β levels but did not reduce the final maximum concentrations (9). Treatment with rifabutin at high doses, in contrast, led to significantly lower concentrations of TNF-α in CSF compared with those in CSF during treatment with ceftriaxone in experimental pneumococcal meningitis (12).
Although the mechanisms of release of LTA and TA and endotoxin likely differ, our data are in accordance with observations with E. coli and the measurement of endotoxin levels. At high concentrations of antibacterial agents, the absolute amount of endotoxin released by ciprofloxacin was similar to that set free by ceftazidime. In serial investigations, however, ciprofloxacin released only 12.7% of the endotoxin within the first hour of exposure, whereas ceftazidime released 61.9% (3).
In an attempt to reduce the level of release of endotoxin from gram-negative bacteria, some physicians have advocated the initial use of bactericidal antibiotics below their recommended dose (5). In our in vitro study, however, for all antibacterial agents studied, the level of release of LTA and TA increased with decreasing drug concentrations. At low concentrations of antibacterial agents (1× the MIC and 1× the MBC), the levels of LTA and TA in S. pneumoniae cultures treated with ceftriaxone, meropenem, trovafloxacin, and quinupristin-dalfopristin were higher than those in cultures not treated with the antibacterial agents (Table 1; Fig. 2A). Similarly, concentrations of ceftazidime below and 2× above the MIC induced a strong lipopolysaccharide release in vitro, and these levels exceeded the levels released by untreated controls (5). In our study, only in the presence of low doses of rifamycins were the quantities of LTA and TA released into the medium not higher than those observed in the supernatants of untreated cultures (Table 1; Fig. 2B).
In conclusion, bactericidal antibacterial agents without a primary influence upon cell wall synthesis delayed the release of LTA and TA in comparison with the time of release after treatment with ceftriaxone. Furthermore, treatment with rifampin, rifabutin, and quinupristin-dalfopristin at concentrations of 10 μg/ml significantly decreased the amount of LTA and TA released during bacterial killing compared with the amount released after treatment with ceftriaxone. Further research with animal models may help in evaluating whether a delayed or decreased level of release of these proinflammatory compounds may reduce overall mortality or long-term sequelae in patients with life-threatening pneumococcal infections. At low antibacterial agent concentrations close to the MIC, larger quantities of LTA and TA were released during treatment with all drugs except the rifamycins than during no treatment. For this reason, our data do not support the concept of using a low first antibiotic dose to prevent the release of proinflammatory cell wall components.
ACKNOWLEDGMENT
This work was supported by the Deutsche Forschungsgemeinschaft (grant Na 165/2-2).
REFERENCES
- 1.Behr T, Fischer W, Peter-Katalinic J, Egge H. The structure of pneumococcal lipoteichoic acid. Eur J Biochem. 1992;207:1063–1075. doi: 10.1111/j.1432-1033.1992.tb17143.x. [DOI] [PubMed] [Google Scholar]
- 2.Dever L, Tarasi A, Tomasz A. The 3rd International Conference on the Macrolides, Azalides and Streptogramins. 1996. Bactericidal activity of RP59500 against Streptococcus pneumoniae in the rabbit model of experimental meningitis. [Google Scholar]
- 3.Evans M E, Pollack M. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J Infect Dis. 1993;167:1336–1343. doi: 10.1093/infdis/167.6.1336. [DOI] [PubMed] [Google Scholar]
- 4.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson J J, Kropp H. β-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J Infect Dis. 1992;165:1033–1041. doi: 10.1093/infdis/165.6.1033. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y S, Kennedy S, Täuber M G. Toxicity of Streptococcus pneumoniae in neurons, astrocytes, and microglia in vitro. J Infect Dis. 1995;171:1363–1368. doi: 10.1093/infdis/171.5.1363. [DOI] [PubMed] [Google Scholar]
- 7.Mason D J, Power E G M, Talsania H, Phillips I, Gant V A. Antibacterial action of ciprofloxacin. Antimicrob Agents Chemother. 1995;39:2752–2758. doi: 10.1128/aac.39.12.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustafa M M, Ramilo O, Mertsola J, Risser R C, Beutler B, Hansen E J, McCracken G H. Modulation of inflammation and cachectin activity in relation to treatment of experimental Haemophilus influenzae type b meningitis. J Infect Dis. 1989;160:818–825. doi: 10.1093/infdis/160.5.818. [DOI] [PubMed] [Google Scholar]
- 9.Nau R, Zysk G, Schmidt H, Fischer F R, Stringaris A, Stuertz K, Brück W. Trovafloxacin delays the antibiotic-induced inflammatory response in experimental pneumococcal meningitis. J Antimicrob Chemother. 1997;39:781–788. doi: 10.1093/jac/39.6.781. [DOI] [PubMed] [Google Scholar]
- 10.Paris M M, Hickey S M, Uscher M I, Shelton S, Olsen K D, McCracken G H. Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1994;38:1320–1324. doi: 10.1128/aac.38.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prins J M, Kuijper E J, Mevissen M L, Speelman P, van Deventer S J. Release of tumor necrosis factor alpha and interleukin 6 during antibiotic killing of Escherichia coli in whole blood: influence of antibiotic class, antibiotic concentration, and presence of septic serum. Infect Immun. 1995;63:2236–2242. doi: 10.1128/iai.63.6.2236-2242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt H, Zysk G, Reinert R R, Brück W, Stringaris A K, Fischer F R, Stuertz K, Bartels R, Schaper K, Weinig S, Nau R. Rifabutin for experimental pneumococcal meningitis. Chemotherapy (Basel) 1997;43:264–271. doi: 10.1159/000239577. [DOI] [PubMed] [Google Scholar]
- 13.Sippel J E, Hider P A, Controni G, Eisenach K D, Hill H R, Rytel M W, Wasilauskas B L. Use of the Directigen latex agglutination test for detection of Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis antigens in cerebrospinal fluid from meningitis patients. J Clin Microbiol. 1984;20:884–886. doi: 10.1128/jcm.20.5.884-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjögren A M, Holmberg H, Krook A. Etiologic diagnosis of pneumonia by antigen detection: crossreactions between pneumococcal C-polysaccharide and oral microorganisms. Diagn Microbiol Infect Dis. 1987;6:239–248. doi: 10.1016/0732-8893(87)90018-6. [DOI] [PubMed] [Google Scholar]
- 15.Täuber M G, Burroughs M, Niemöller U M, Kuster H, Borschberg U, Tuomanen E. Differences of pathophysiology in experimental meningitis caused by three strains of Streptococcus pneumoniae. J Infect Dis. 1991;163:806–811. doi: 10.1093/infdis/163.4.806. [DOI] [PubMed] [Google Scholar]
- 16.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 17.Zysk G, Brück W, Gerber J, Brück Y, Prange H W, Nau R. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol. 1996;55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]