Key Points
Question
What are the prognostic implications of intraoperative margin sampling methods with recurrence among patients with oral cavity squamous cell carcinoma (OCSCC) after surgical resection?
Findings
This retrospective cohort study of 223 patients with OCSCC found no recurrence differences between tumor bed vs resection specimen sampling. Subgroup analyses of patients with both localized and advanced tumors did not reveal differences in recurrence rates, and specimen-driven sampling tended to have lower rates of positive frozen and final margins.
Meaning
These findings indicate that specimen-driven sampling may reduce margin positivity rates; however, this method was not associated with recurrence in surgically resected oral cancers, and adjuvant radiation therapy may obscure some of its potential prognostic benefits.
Abstract
Importance
Positive margins and margin clearance are risk factors for recurrence in oral cavity squamous cell carcinoma (OCSCC), and these features are used to guide decisions regarding adjuvant radiation treatment. However, the prognostic value of intraoperative tumor bed vs resection specimen sampling is not well defined.
Objective
To determine the prognostic implications of intraoperative margin assessment methods (tumor bed vs resection specimen sampling) with recurrence among patients who undergo surgical resection for OCSCC.
Design, Setting, and Participants
This was a retrospective study of patients who had undergone surgical resection of OCSCC between January 1, 2000, and December 31, 2021, at a tertiary-level academic institution. Patients were grouped by margin assessment method (tumor bed [defect] or resection specimen sampling). Of 223 patients with OCSCC, 109 patients had localized tumors (pT1-T2, cN0), 154 had advanced tumors, and 40 were included in both cohorts. Disease recurrence after surgery was estimated by the cumulative incidence method and compared between cohorts using hazard ratios (HRs). Data analyses were performed from January 5, 2023, to April 30, 2023.
Main Outcome and Measures
Recurrence-free survival (RFS).
Results
The study population comprised 223 patients (mean [SD] age, 62.7 [12.0] years; 88 (39.5%) female and 200 [90.0%] White individuals) of whom 158 (70.9%) had defect-driven and 65 (29.1%) had specimen-driven margin sampling. Among the 109 patients with localized cancer, intraoperative positive margins were found in 5 of 67 (7.5%) vs 8 of 42 (19.0%) for defect- vs specimen-driven sampling, respectively. Final positive margins were 3.0% for defect- (2 of 67) and 2.4% for specimen-driven (1 of 42) margin assessment. Among the 154 patients with advanced cancer, intraoperative positive margins were found in 29 of 114 (25.4%) vs 13 of 40 (32.5%) for defect- and specimen-driven margins, respectively. Final positive margins were higher in the defect-driven group (9 of 114 [7.9%] vs 1 of 40 [2.5%]). When stratified by margin assessment method, the 3-year rates of local recurrence (9.7% vs 5.1%; HR, 1.37; 95% CI, 0.51-3.66), regional recurrence (11.0% vs 10.4%; HR, 0.85; 95% CI, 0.37-1.94), and distant recurrence (6.4% vs 5.0%; HR, 1.10; 95% CI, 0.36-3.35) were not different for defect- vs specimen-driven sampling cohorts, respectively. The 3-year rate of any recurrence was 18.9% in the defect- and 15.2% in the specimen-driven cohort (HR, 0.93; 95% CI, 0.48-1.81). There were no differences in cumulative incidence of disease recurrence when comparing defect- vs specimen-driven cases.
Conclusions and Relevance
The findings of this retrospective cohort study indicate that margin assessment methods using either defect- or specimen-driven sampling did not demonstrate a clear association with the risk of recurrence after OCSCC resection. Specimen-driven sampling may be associated with reduced surgical margin positivity rates, which often necessitate concurrent chemotherapy with adjuvant radiation therapy.
This retrospective study of margin assessment methods after oral cavity squamous cell carcinoma resection compares tumor bed vs resection specimen sampling and the risk of recurrence.
Introduction
After undergoing surgical resection, patients with oral cavity squamous cell carcinoma (OCSCC) frequently require adjuvant radiation therapy that considers several known risk factors, such as distance to margins and positive surgical margins.1,2 However, the optimal method for accomplishing intraoperative margin assessment, either from the tumor bed (defect-driven sampling) vs from the resected en bloc specimen (specimen-driven sampling), is not well defined.
In a 2005 survey of members of the American Head and Neck Society, 76% of surgeons reported exclusively evaluating intraoperative defect-driven margins.3 However, there are several drawbacks to defect-driven sampling: tumor bed margins have a low sensitivity of 15% to 32% for identifying positive margins, resolving disagreements between the findings of frozen vs permanent margins is unclear, and fragmentation of pieces prevents spatial orientation to the specimen.4,5,6 Revision of intraoperative positive frozen margins from the tumor bed to achieve negative margins has not been associated with lower rates of recurrence.7,8,9,10
Intraoperative specimen-driven margin sampling has been proposed as a more accurate method of determining margin status by providing oriented information on margin positivity and by allowing more accurate margin depth assessment.11 The specimen-driven approach permits the measurement of margin clearance, whereas defect-driven margins are only reportable as positive or negative.4 Given these advantages, surgical practice at our institution has shifted in recent years toward more intraoperative specimen-driven margins.
There are discordant reports in the literature regarding the efficacy of specimen-driven sampling in reducing recurrence rates. Several studies have suggested that specimen-driven margin sampling is associated with lower recurrence rates,8,11,12,13,14,15 whereas others have not been able to demonstrate similar prognostic benefits.16,17 This study aimed to assess the prognostic implications of defect- vs specimen-driven margin assessment methods on recurrence in OCSCC. Most studies that evaluate the effects of margin assessment have focused on early-stage oral tongue cancers treated with surgery with or without radiation.7,13 In this study, we evaluated a cohort of patients with OCSCC, including some patients with advanced disease treated with adjuvant radiation therapy, to assess the prognostic implications of margin assessment methods and to validate whether specimen-driven margin sampling was associated with improved overall outcomes.
Methods
This study was reviewed and approved by the institutional review board of the Cleveland Clinic. Informed consent was waived because the study used only deidentified patient data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patient Selection
This retrospective cohort study included patients with OCSCC treated between January 1, 2000, and December 31, 2020, at a tertiary-level academic institution. Patient records were extracted from a head and neck cancer registry approved by the Cleveland Clinic. Patients were included if they had OCSCC treated by primary surgical resection with curative intent, were 18 years or older at the time of surgery, had at least 3 months of follow-up, and had sufficient operative and pathologic data to evaluate margin assessment method. Patients with non-SCC morphology, inadequate reports to determine margin assessment method, and those with second primary tumors of the head and neck were excluded. The 7th or 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual18,19 was used for this analysis based on year of diagnosis. Patients with pathologic T1 to T2 (pT1-T2) and clinically node-negative (cN0) disease were grouped into a localized tumor cohort to mirror prior studies. Patients were also grouped into an advanced tumor cohort defined as those receiving adjuvant external beam radiation therapy (RT) at a dose more than 50 Gy. Adjuvant RT was typically chosen for patients with aggressive histopathologic features such as close final margins, elevated depth of invasion (DOI), lymphovascular space invasion (LVSI), perineural invasion (PNI), and nodal disease, consistent with national guidelines and institutional practices.20
Data Collection
Demographic, tumor, treatment, and outcome characteristics were collected from electronic health records. Tumor characteristics included oral cavity subsite, pT classification, pathologic N (pN) classification, primary tumor diameter, DOI, LVSI, and PNI. Treatment variables included margin assessment method (defect- or specimen-driven sampling), lymph node dissection, adjuvant RT, and adjuvant chemotherapy. Margin status variables included intraoperative frozen margin status (positive or negative), whether further margin revision at the same surgical encounter was performed, revision margin status on frozen (positive or negative), and final margin status on permanent pathology (positive or negative). Negative margins were defined by absence of tumor cells at inked border. Tumor cells that were within 5 mm of the inked border were considered close margins. Margins were analyzed as a binary outcome (positive or negative) for both intraoperative frozen margins and final pathology results.
Margin Assessment Method
Pathology reports and operative notes were assessed for determination of margin assessment method. Defect-driven cases were determined if there were multiple separately submitted samples indicating frozen margins derived from the tumor bed (ie, anterior margin, posterior margin, inferior margin), and the primary specimen was not submitted for frozen section. Defect-driven margins from the tumor bed were sampled from peripheral margins, typically at anterior, posterior, superior, inferior, and deep positions relative to the tumor. Importantly, defect-driven margins were not guided by formal margin assessment on the resection specimen intraoperatively. Nerve, lymph node, and bone structures were not considered tumor bed margins. Specimen-driven cases were determined if frozen specimens were performed on the primary resection specimen. In these cases, the resection specimen was oriented and inked for frozen sections jointly by the head and neck surgeon and pathologist in real time.
Margin assessment methods were mutually exclusive; if the resection specimen was intraoperatively evaluated for margins, tumor bed margins were not assessed. Both defect- and specimen-driven cases were eligible for revisions in the same surgical encounter based on the frozen margins. Only surgeon preference dictated the selection of either defect- or specimen-driven techniques for each case, and there were no tumor characteristics that influenced margin assessment. Per institutional practices, all patients who had intraoperative frozen margins helped guide clinical decision-making regarding revisions and final margin status and influenced selection of adjuvant therapies.
Statistical Analysis
The primary end point was disease recurrence, defined as time between surgery and first local, regional, or distant recurrence, or last clinical follow-up. Univariate analysis for disease recurrence was performed using competing risk regression with all-cause mortality treated as a competing event. The following variables, patient’s age at surgery, smoking history, pT classification, oral cavity subsite, DOI, PNI, LVSI, frozen margin status, and margin assessment method, were included in the univariate analysis. Rates of recurrence were calculated using the cumulative incidence method, and differences in survival curves between margin assessment methods were assessed using hazard ratios (HRs) and 95% CIs. Differences in rates of overall survival were assessed using HRs and 95% CIs. Patients who were alive or were lost to follow-up at the end of the follow-up period were censored.
All statistical analyses were performed using SAS, version 9.4 (SAS Institute). Statistical tests were 2-tailed and P values < .05 were considered statistically significant. Data analyses were performed from January 5, 2023, to April 30, 2023.
Results
Study Population
The total study population consisted of 223 patients (mean [SD] age, 62.7 [12.0] years; 135 [60.5%] male and 88 [39.5%] female; and 1 [0.4%] American Indian/Alaska Native/Pacific Islander, 3 [1.3%] Asian, 11 [4.9%] Black, 8 [3.6%] Hispanic/Latine; 200 [90.0%] White individuals). More than half (65.1%) were current or former smokers (n = 145). Median follow-up time was 42.0 months (IQR, 17.3-63.8). Oral cavity subsite was most commonly oral tongue (n = 153; 68.5%), followed by floor of mouth (n = 27; 12.1%), alveolar ridge (n = 10; 4.5%), retromolar trigone (n = 10; 4.5%), and gingiva (n = 9; 4.0%). Of the 223 patients, 109 (48.9%) had localized tumors (pT1-T2, cN0) and 154 (69.1%) had advanced tumors and received adjuvant RT; 40 (17.9%) patients with localized tumors also had adjuvant RT, and thus were included in both cohorts (eFigure 1 in Supplement 1). There were 158 (70.9%) patients who had defect- and 65 (29.1%) who had specimen-driven margin assessment. There was a higher frequency of advanced pT classification, heavy alcohol consumption, and greater DOI in the defect- than in the specimen-driven cohort (Table 1).
Table 1. Demographic, Tumor, and Treatment Characteristics of the Total Study Population, by Margin Assessment Method.
| Variablea | Margin assessment method | Difference (95% CI) | |
|---|---|---|---|
| Defect-driven sampling (n = 158) | Specimen-driven sampling (n = 65) | ||
| Demographic characteristics | |||
| Age, mean (SD) | 62.6 (11.4) | 63.0 (13.5) | −0.4 (−3.9 to 3.1) |
| Female sex | 58 (36.7) | 30 (46.1) | NA |
| Male sex | 100 (63.3) | 35 (53.9) | 9.4 (−4.8 to 23.7) |
| Smoking (%) | |||
| Current/former | 106 (67.1) | 39 (60.0) | NA |
| Never | 52 (32.9) | 26 (40.0) | −7.1 (−21.1 to 6.9) |
| Heavy alcohol use | 42 (26.6) | 7 (10.8) | 15.8 (5.6 to 26.0) |
| Tumor characteristics | |||
| Oral cavity subsite | |||
| Oral tongue | 99 (62.7) | 54 (83.1) | NA |
| Other subsite | 59 (37.3) | 11 (16.9) | 20.4 (8.6 to 32.3) |
| pT classification (%) | |||
| pT1-pT2 | 98 (62.0) | 56 (86.2) | NA |
| pT3-pT4 | 60 (38.0) | 9 (13.8) | 24.2 (12.8 to 35.4) |
| Primary diameter, mean (SD), cm | 2.67 (1.79) | 2.18 (1.48) | 0.49 (0 to 0.99) |
| Depth of invasion, mean (SD), cm | 1.07 (0.89) | 0.86 (1.20) | 0.21 (−0.08 to 0.50) |
| LVSI (%) | 50 (32.3) | 22 (33.9) | −1.6 (−15.2 to 12.1) |
| PNI (%) | 75 (48.1) | 32 (50.0) | −1.9 (−16.5 to 12.6) |
| Lymph node dissection (%) | 132 (83.5) | 52 (80.0) | 3.5 (−7.8 to 14.9) |
| pN classification (%) | |||
| pN0 | 88 (66.7) | 39 (75.0) | NA |
| pN1-pN2 | 44 (33.3) | 13 (25.0) | 8.3 (5.9 to 22.6) |
| Treatment characteristics | |||
| Radiation therapy (%) | 114 (72.2) | 40 (61.5) | 10.6 (−3.1 to 24.4) |
| Concurrent chemotherapy (%) | 9 (5.7) | 2 (3.1) | 2.6 (−2.9 to 8.2) |
| Margin status | |||
| Intraoperative margin status | |||
| Negative | 127 (80.4) | 49 (75.4) | NA |
| Positive | 31 (19.6) | 16 (24.6) | −5.0 (−17.2 to 7.8) |
| Margin revision performed | |||
| No | 111 (70.3) | 40 (61.5) | NA |
| Yes | 47 (29.7) | 25 (38.5) | −8.7 (−22.5 to 5.1) |
| Revision margin status | |||
| Negative | 39 (83.0) | 23 (92.0) | NA |
| Positive | 8 (17.0) | 2 (8.0) | 9.0 (−6.1 to 24.1) |
| Final margin status | |||
| Negative | 147 (93.0) | 63 (96.9) | NA |
| Positive | 11 (7.0) | 2 (3.1) | 3.9 (−1.9 to 9.7) |
| Months to first recurrence median (IQR) | 12.9 (6.9 to 32.0) | 10.0 (5.9 to 23.1) | NA |
| 3-y rates (95% CI) | HR (95% CI)b | ||
| Local recurrence | 9.7 (5.6 to 15.3) | 5.1 (1.3 to 12.9) | 1.37 (0.51 to 3.66) |
| Regional recurrence | 11.0 (6.5 to 16.7) | 10.4 (4.2 to 19.9) | 0.85 (0.37 to 1.94) |
| Distant recurrence | 6.4 (3.1 to 11.2) | 5.0 (1.3 to 12.7) | 1.10 (0.36 to 3.35) |
| Any recurrence | 18.9 (12.1 to 24.6) | 15.2 (7.4 to 25.5) | 0.93 (0.48 to 1.81) |
| Survival | 79.9 (72.5 to 85.5) | 86.6 (74.9 to 91.1) | 1.34 (0.71 to 2.53)c |
Abbreviations: LVSI, lymphovascular space invasion; NA, not applicable; PNI, perineural invasion.
Patients with missing values excluded from analysis.
HRs and 95% CIs from competing risk regression with specimen margins as the reference group.
HRs and 95% CIs from Cox proportional hazards regression with specimen margins as the reference group.
Recurrence
For all patients, the 3-year local, regional, and distant recurrence rates were 8.5%, 10.9%, and 6.1%, respectively. The 3-year overall recurrence rate was 17.3% (Figure 1A). When stratified by margin assessment method, the 3-year rates of local recurrence (9.7% vs 5.1%; HR, 1.37; 95% CI, 0.51-3.66), regional recurrence (11.0% vs 10.4%; HR, 0.85; 95% CI, 0.37-1.94), and distant recurrence (6.4% vs 5.0%; HR, 1.10; 95% CI, 0.36-3.35) were not different for defect- vs specimen-driven cohorts, respectively. The 3-year rate of any recurrence was 18.9% in the defect-driven cohort and 15.2% in the specimen-driven cohort (HR, 0.93; 95% CI, 0.48-1.81) (Figure 1B).
Figure 1. Cumulative Incidence of Disease Recurrence Among Patients With OCSCC Who Underwent Resection, by Margin Assessment Method (Tumor Bed/Defect-Driven or Resection Specimen-Driven Sampling) and Tumor Severity.
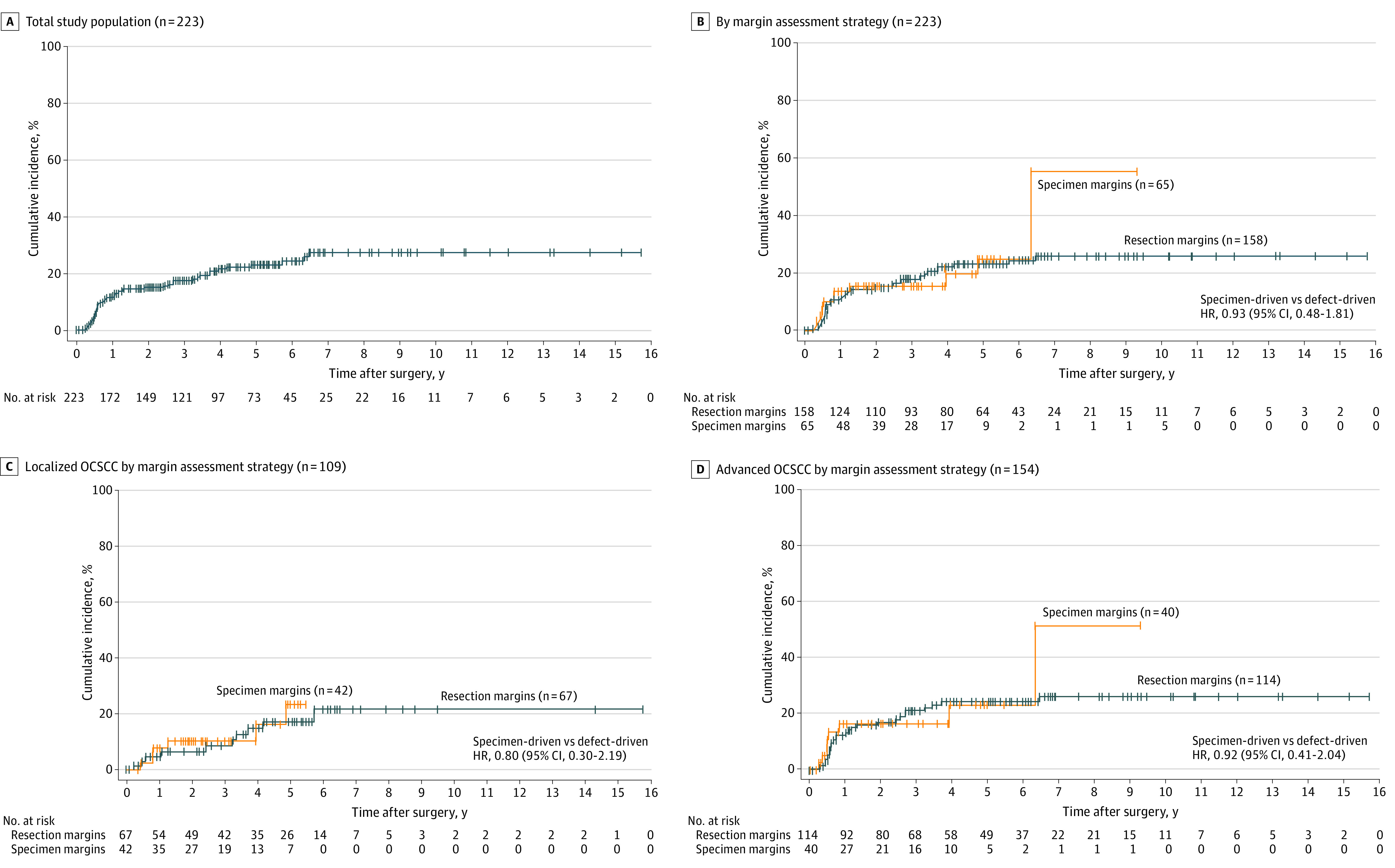
A, Both study groups and both margin assessment methods. B, Both study groups, stratified by margin assessment method. C, Patients with localized (pT1-T2, cN0) OCSCC, stratified by margin assessment method. D, Patients with OCSCC requiring adjuvant radiation therapy (pT1-T4, cN0-2), stratified by margin assessment strategy. OCSCC refers to oral cavity squamous cell carcinoma.
Localized Tumor Cohort
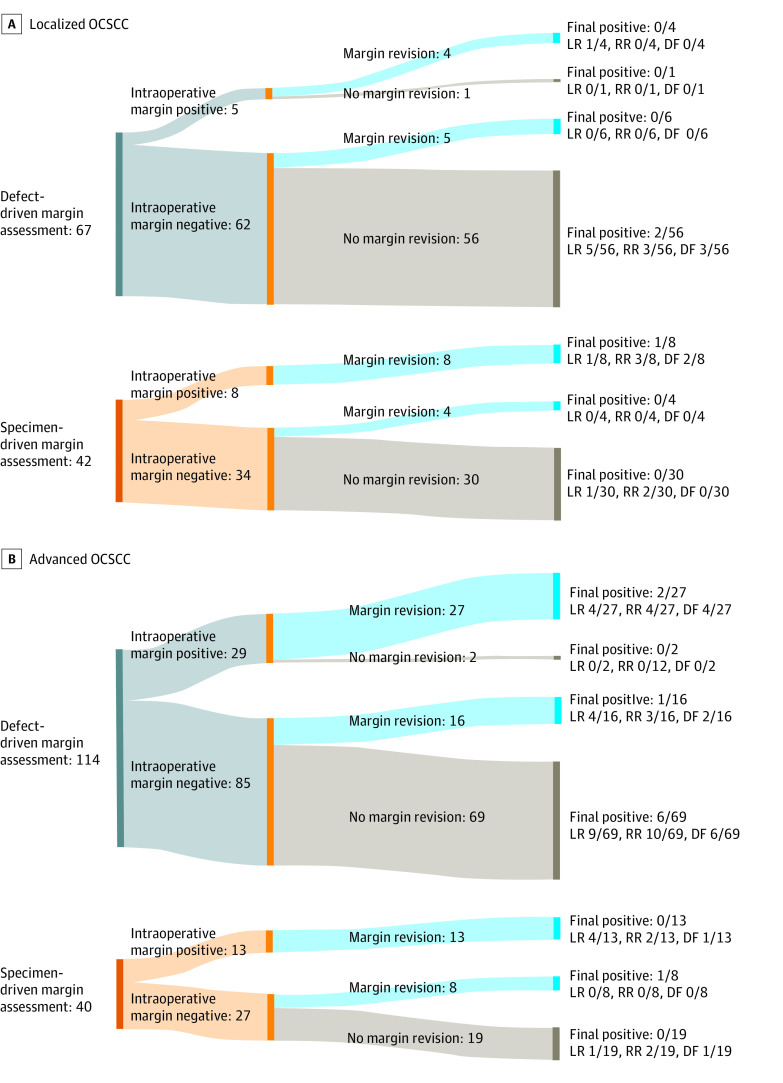
Of the patients with localized tumors, 67 (61.5%) had defect-driven and 42 (38.5%) had specimen-driven margin sampling (Table 2). There were no differences in age, sex, smoking status, oral cavity subsite, pT classification, pN classification, LVSI, or PNI between the 2 margin assessment groups. There were 13 (11.9%) patients who had intraoperative positive margins on frozen pathology, and 3 (2.8%) patients who had final positive margins on permanent pathology (Figure 2A). Intraoperative positive margins were 5 of 67 (7.5%) vs 8 of 42 (19.0%) for defect- vs specimen-driven, respectively. Final positive margins were 3.0% (2/67) in the defect-driven and 2.4% (1/42) in the specimen-driven group. The 3-year rates for local (3.0%), regional (5.2%), and distant (4.4%) recurrence were not different between margin assessment groups in the early-stage cohort. There were no differences in cumulative incidence of disease recurrence when comparing defect- vs specimen-driven cases (Figure 1C). On univariate analysis, never smokers (HR, 0.24; 95% CI, 0.09-0.66) and DOI greater than 5 mm (HR 0.14; 95% CI, 0.03-0.65) were associated with any recurrence among the localized tumor cohort (eTable 1 in Supplement 1).
Table 2. Demographic, Tumor, and Treatment Characteristics of the Localized Tumor Cohort, Stratified by Margin Assessment Method.
| Variablesa | Early-stage tumor (n = 109) | Margin assessment method | Difference (95% CI) | |
|---|---|---|---|---|
| Defect-driven sampling (n = 67) | Specimen-driven sampling (n = 42) | |||
| Demographic characteristics | ||||
| Age at diagnosis, mean (SD) | 62.5 (13.2) | 62.2 (13.0) | 62.8 (13.6) | −0.6 (−5.8 to 4.5) |
| Female sex | 41 (37.6) | 23 (34.3) | 18 (42.9) | NA |
| Male sex | 68 (62.4) | 44 (65.7) | 24 (57.1) | 8.6 (−10.3 to 27.3) |
| Smoking status | ||||
| Current/former | 23 (21.1) | 47 (70.1) | 23 (54.8) | NA |
| Never | 39 (35.8) | 20 (29.9) | 19 (45.2) | −15.3 (−34.0 to 3.2) |
| Heavy alcohol use | 16 (14.7) | 13 (19.4) | 3 (7.1) | 12.3 (0 to 24.5) |
| Tumor and treatment characteristics | ||||
| Oral cavity subsite | ||||
| Oral tongue | 95 (87.2) | 56 (83.6) | 39 (92.9) | NA |
| Other subsite | 1 (0.9) | 11 (16.4) | 3 (7.1) | 9.3 (−2.3 to 21.1) |
| Pathologic T stage | ||||
| T1 | 68 (62.4) | 47 (70.1) | 21 (50.0) | NA |
| T2 | 41 (37.6) | 20 (29.9) | 21 (50.0) | −20.1 (−38.8 to −1.5) |
| Pathologic N stage | ||||
| N0 | 68 (62.4) | 40 (97.6) | 28 (96.6) | NA |
| N1 | 2 (1.8) | 1 (2.4) | 1 (3.4) | −1 (−9.2 to 7.1) |
| Primary diameter, mean (SD), cm | 1.57 (1.03) | 1.56 (1.05) | 1.59 (0.99) | −0.02 (−0.43 to 0.38) |
| Depth of invasion, mean (SD), cm | 0.54 (0.47) | 0.58 (0.52) | 0.49 (0.38) | 0.08 (−0.10 to 0.27) |
| Positive LVSI | 20 (18.3) | 11 (16.4) | 9 (21.4) | −5.0 (−20.3 to 10.2) |
| Positive PNI | 36 (33.0) | 20 (30.8) | 16 (38.1) | −7.3 (−25.8 to 11.2) |
| Lymph node dissection | 70 (64.2) | 41 (61.2) | 29 (69.0) | −7.8 (−26.1 to 10.4) |
| Radiation therapy | 40 (36.7) | 23 (34.3) | 17 (40.5) | −6.2 (−24.9 to 12.6) |
| Concurrent chemotherapy | 5 (4.6) | 3 (4.5) | 2 (4.8) | −0.3 (−8.4 to 7.8) |
| Margin status | ||||
| Initial margin status | ||||
| Negative | 96 (88.1) | 62 (92.5) | 34 (81.0) | NA |
| Positive | 13 (11.9) | 5 (7.5) | 8 (19.0) | −11.5 (−25.0 to 1.9) |
| Margin revision performed | ||||
| No | 87 (79.8) | 57 (85.1) | 30 (71.4) | NA |
| Yes | 22 (20.2) | 10 (14.9) | 12 (28.6) | −13.7 (−29.8 to 2.5) |
| Revision margin status | ||||
| Negative | 21 (95.5) | 10 (100) | 11 (91.7) | NA |
| Positive | 1 (4.5) | 0 (0.0) | 1 (8.3) | −8.3 (−24.0 to 7.3) |
| Final margin status | ||||
| Negative | 106 (97.2) | 65 (97.0) | 41 (97.6) | NA |
| Positive | 3 (2.8) | 2 (3.0) | 1 (2.4) | 0.6 (−5.6 to 6.8) |
| 3-y rates (95% CI) | HR (95% CI)b | |||
| Local recurrence | 3.0 (0.8 to 7.8) | 3.2 (0.6 to 9.9) | 2.6 (0.2 to 11.7) | 1.4 (0.3 to 60.0) |
| Regional recurrence | 5.2 (1.9 to 10.9) | 3.4 (0.6 to 10.5) | 7.8 (2.0 to 19.2) | 0.3 (0.1 to 1.3) |
| Distant recurrence | 4.4 (1.4 to 10.1) | 5.4 (1.4 to 13.5) | 2.7 (0.2 to 12.3) | 0.9 (0.1 to 5.5) |
| Any recurrence | 9.5 (4.6 to 16.4) | 8.6 (3.1 to 17.6) | 10.4 (3.2 to 22.5) | 0.8 (0.3 to 2.2) |
| Survival | 90.8 (83.0 to 95.1) | 90.3 (79.7 to 95.6) | 91.3 (76.5 to 97.3) | 1.2 (0.4 to 3.4)c |
Abbreviations: LVSI, lymphovascular space invasion; NA, not applicable; PNI, perineural invasion.
Patients with missing values excluded from analysis.
Other aggressive features included lymphovascular invasion, perineural invasion, depth of invasion, and poorly differentiated squamous cell carcinoma.
HRs and 95% CIs from Cox proportional hazards regression with specimen margins as the reference group.
Figure 2. Margin Revision and Final Margin Status Among Patients With OCSCC Who Underwent Resection, by Tumor Severity and Margin Assessment Method.
A, Localized (pT1-T2, cN0) OCSCC. B, Advanced (pT1-T4, cN0-2) OCSCC. DF refers to distant failure; LR, local recurrence; OCSCC, oral cavity squamous cell carcinoma; and RR, regional recurrence.
Advanced Tumor Cohort
Of 154 patients with advanced tumors, 114 (74.0%) had defect-driven and 40 (26.0%) had specimen-driven margin assessment (Table 3). Age, sex, and smoking status were not different between margin methods, although patients in the defect-driven group were more likely to have floor of mouth subsite, higher pT classification, and increased depth of invasion. Intraoperative frozen margins were positive in 42 (27.3%) cases, and 64 (41.6%) cases had margin revision, with 10 (15.6%) cases having positive margins on revision (Figure 2B). Intraoperative positive margins were 29 of 114 (25.4%) vs 13 of 40 (32.5%) for defect- and specimen-driven, respectively. Final margins on permanent pathology were positive in 10 (6.5%) patients. Final positive margins were higher in the defect-driven group (9 of 114 [7.9%] vs 1 of 40 [2.5%]). The 3-year rates for local (11.4%), regional (11.9%), and distant (7.1%) recurrence were not different between margin assessment groups in the advanced tumor cohort. There were no differences in cumulative incidence of disease recurrence when comparing defect- vs specimen-driven cases (Figure 1D). On univariate analysis, the cohort with advanced tumors, PNI and pT classification T3 to T4 (HR, 2.30; 95% CI, 1.16-4.53) were associated with any recurrence (HR, 2.15; 95% CI, 1.01-4.54) (eTable 2 in Supplement 1).
Table 3. Demographic, Tumor, and Treatment Characteristics of the Advanced Tumor Cohort, Stratified by Margin Assessment Method.
| Variablesa | Advanced tumor (n = 154) | Margin assessment method | Difference (95% CI) | |
|---|---|---|---|---|
| Defect-driven sampling (n = 114) | Specimen-driven sampling (n = 40) | |||
| Demographic characteristics | ||||
| Age at diagnosis, mean (SD) | 62.5 (11.1) | 62.7 (10.5) | 61.9 (12.8) | 0.9 (−3.2 to 4.9) |
| Female sex | 64 (41.6) | 45 (39.5) | 19 (47.5) | NA |
| Male sex | 90 (58.4) | 69 (60.5) | 21 (52.5) | 8 (−9.9 to 25.9) |
| Smoking status | ||||
| Current/former | 106 (68.8) | 78 (68.4) | 28 (70.0) | NA |
| Never | 48 (31.2) | 36 (31.6) | 12 (30.0) | 1.6 (−15.0 to 18.2) |
| Heavy alcohol use | 41 (26.6) | 35 (30.7) | 6 (15.0) | 15.7 (1.8 to 29.6) |
| Tumor and treatment characteristics | ||||
| Oral cavity subsite | ||||
| Oral tongue | 89 (57.8) | 60 (52.6) | 29 (72.5) | NA |
| Other | 65 (42.2) | 54 (47.4) | 11 (27.5) | 19.9 (3.3 to 36.5) |
| Pathologic T stage | ||||
| T1-T2 | 85 (55.2) | 54 (47.4) | 31 (77.5) | NA |
| T3-T4 | 69 (44.8) | 60 (52.6) | 9 (22.5) | 30.1 (14.3 to 46.0) |
| Pathologic N stage | ||||
| N0 | 95 (61.7) | 69 (61.1) | 26 (65.7) | NA |
| N1-N2 | 57 (37.0) | 44 (38.9) | 13 (33.3) | 5.6 (−11.7 to 22.9) |
| Primary diameter, mean (SD), cm | 3.12 (1.65) | 3.28 (1.68) | 2.69 (1.50) | 0.59 (0 to 1.19) |
| Depth of invasion, mean (SD), cm | 1.31 (1.05) | 1.36 (0.89) | 1.18 (1.42) | 0.18 (−0.20 to 0.58) |
| Positive LVSI | 62 (40.3) | 44 (39.6) | 18 (45.0) | −5.4 (−23.3 to 12.5) |
| Positive PNI | 95 (61.7) | 69 (61.6) | 26 (66.7) | −5.6 (−22.3 to 11.7) |
| Lymph node dissection | 152 (98.7) | 113 (99.1) | 39 (97.5) | 1.6 (−3.5 to 6.8) |
| Concurrent chemotherapy | 7 (5.1) | 9 (7.9) | 2 (5.0) | 2.9 (−5.5 to 11.3) |
| Margin status | ||||
| Intraoperative margin status | ||||
| Negative | 112 (72.7) | 85 (74.6) | 27 (67.5) | NA |
| Positive | 42 (27.3) | 29 (25.4) | 13 (32.5) | −7.1 (−23.6to 9.5) |
| Margin revision performed | ||||
| No | 90 (58.4) | 71 (62.3) | 19 (47.5) | NA |
| Yes | 64 (41.6) | 43 (37.7) | 21 (52.5) | −14.8 (−32.6 to 3.1) |
| Revision margin status | ||||
| Negative | 54 (84.4) | 35 (81.4) | 19 (90.5) | NA |
| Positive | 10 (15.6) | 8 (18.6) | 2 (9.5) | 9.1 (−8.03 to 26.20) |
| Final margin status | ||||
| Negative | 144 (93.5) | 105 (92.1) | 39 (97.5) | NA |
| Positive | 10 (6.5) | 9 (7.9) | 1 (2.5) | 5.4 (−1.5 to 12.3) |
| 3-y rates (95% CI) | HR (95% CI)b | |||
| Local recurrence | 11.4 (6.9 to 17.3) | 12.1 (6.8 to 19.1) | 8.3 (2.1 to 22.3) | 0.9 (0.3 to 2.5) |
| Regional recurrence | 11.9 (7.2 to 17.8) | 12.8 (7.3 to 19.8) | 8.6 (2.2 to 21.0) | 1.2 (0.4 to 3.58) |
| Distant recurrence | 7.1 (3.6 to 12.2) | 7.5 (3.5 to 13.6) | 5.3 (0.9 to 15.7) | 1.6 (0.4 to 7.2) |
| Any recurrence | 20.3 (14.1 to 27.3) | 21.1 (14.0 to 29.2) | 16.4 (6.5 to 30.2) | 0.9 (0.4 to 2.0) |
| Survival | 77.9 (70.1 to 83.8) | 76.0 (66.8 to 83.0) | 84.2 (68.1 to 92.6) | 1.22 (0.59 to 2.51)c |
Abbreviations: LVSI, lymphovascular space invasion; NA, not applicable; PNI, perineural invasion.
Patients with missing values excluded from analysis.
HRs and 95% CIs from competing risk regression with specimen margins as the reference group.
HRs and 95% CIs from Cox proportional hazards regression with specimen margins as the reference group.
Discussion
Among 223 patients with OCSCC, margin sampling either by the defect- or specimen-driven method was not associated with local, regional, or distant recurrence. Further subgroup analyses revealed that neither localized tumors (pT1-T2, cN0) nor advanced tumors had a divergent recurrence profile when stratified by margin assessment method. Despite no difference in recurrence rates, however, defect-driven sampling had higher rates of intraoperative positive margins in localized cancers and higher rates of final positive margins in both localized tumors and advanced tumors requiring adjuvant RT.
The specimen-driven approach has been proposed to be the criterion standard method of margin sampling in oral tongue cancer because of its potentially improved prognostic value on local recurrence.11 Among 280 patients with early (pT1-T2, cN0) oral tongue SCC, Maxwell et al13 reported that specimen-driven but not defect-driven margins correlated with more favorable local recurrence, and 3-year locoregional RFS was superior in the specimen-driven group (90% vs 80%). Similarly, among 126 patients with early (pT1-T2, cN0) oral tongue SCC, Chang et al14 showed that local progression-free survival was 90% in specimen-driven and 73% in defect-driven margins.14 In contrast, our analysis of all oral cavity subsites shows that neither localized nor advanced oral cancers had any association between type of margin sampling and any recurrence.
Our negative findings corroborate those of 2 recent studies. Maharaj et al16 assessed 104 patients with T1 to T4 OCSCC and found no substantial difference in locoregional RFS (defect-driven, 62% vs specimen-driven sampling, 75%) or 18-month overall survival (defect, 76% vs specimen, 78%). Similar to our study findings, PNI (HR, 5.14; 95% CI, 1.60-16.52) was a prominent predictor of local RFS.16 MacKay et al17 assessed 153 patients with early T1 to T2 oral cavity and oropharyngeal SCC, and did not detect an association between margin sampling technique and 2-year disease-specific survival (specimen-driven HR, 1.32; 95% CI, 0.30-5.73) or local control (specimen-driven HR, 0.41; 95% CI, 0.08-2.10).
There may be several explanations for the lack of differential prognostic value of margin sampling sources on recurrence. First, intraoperative margin assessment can have limited clinical value given its low sensitivity for the margin final status.10,15 Amit et al15 reported that specimen-driven margins had a 91% sensitivity for predicting final clear margins, whereas defect-driven margins had a sensitivity of only 22%. Because final margin status has been shown to be associated with recurrence,7,21,22,23 intraoperative margin status may have an indirect relationship with recurrence. Second, intraoperative margin status may guide the decision to pursue revisions, but margin revision has not been associated with improved outcomes.14,24,25 Furthermore, positive margins may reflect more aggressive tumor biology that may confound the effects of margin sampling. The prognostic value of positive margins should be further explored with adjunct techniques such as circulating tumor DNA and genomic studies.26,27,28 Another explanation for our negative finding is that prudent patient selection for adjuvant RT may have obscured some of the potential prognostic benefit of specimen-driven margin evaluation.
Despite the absence of added prognostic value in our study, the specimen-driven approach may still hold merit in surgical practice. Sampling of tumor bed margins requires both operating room time and anesthesia expenses given the 15-minute processing time for each margin, which has been shown to average $1548 per patient.10 Each tumor bed margin requires pathologist review, which has averaged $1575 per patient.10 Specimen-driven margins may reduce the margin positivity rate and be associated with a lower number of re-resections for positive or close margins.17 De-escalation of patients from adjuvant RT can ameliorate both financial burden and potential treatment toxic effects. The estimated cost avoidance of adjuvant RT from the specimen-driven method is $31 639 per patient according to Horwich et al.22 However, the specimen-driven approach may be technically infeasible in some oral cavity subsites, such as floor of mouth, alveolar ridge, gingiva, and retromolar trigone. Furthermore, intraoperative pathologic evaluation of resection specimens can be challenging due to anatomic complexity, tissue distortion, and the notable time allotment needed for these cases.29,30 The contribution of experienced head and neck pathologists to the process is an important factor in this multidisciplinary effort, including in the evaluation of the cases presented in this article.
Our study findings corroborate those of prior studies, which showed lower rates of positive margins with the specimen-driven technique. Amit et al15 reported that among 71 patients with OCSCC, specimen-driven sampling had an improved rate of negative margins (84% vs 55%) and reduced need for adjuvant treatment (62% vs 90%) compared with defect-driven sampling. Similarly, Maharaj et al16 found that positive final margins were more frequent in defect- (11.8%) than specimen-driven (7.5%) margins. Horwich et al22 reported lower rates of positive final margins in specimen-driven (0.9%) than defect-driven (12.9%) margins. Yahalom et al12 reported a 40% positive margin rate from the tumor bed, and a 17% positive margin rate from the specimen. In our cohort, similar patterns were seen for both the early-stage and adjuvant RT cohorts.
Limitations
Although the strengths of this study lie in the large sample size with long-term follow-up, there are several limitations. Overall, there were few patients with positive intraoperative frozen margins, and even fewer with subsequently negative margins on permanent pathology. Thus, we had limited power to compare whether positive to subsequent negative margins behave more similarly vs intraoperatively positive or intraoperatively negative tumors. Second, our study was performed at a tertiary care institution with a high level of subspecialty expertise in oral cavity pathology, surgical resection, and RT, which may narrow the potential benefit of the margin method and limit the generalizability of our findings. Furthermore, distance to closest margin as a continuous variable was not assessed, although prior studies have noted recurrence rates dependent on distance to nearest margin.23,31
Conclusions
This retrospective cohort study at a tertiary-level academic institution found that margin assessment method using either defect-driven sampling from the tumor bed or specimen-driven margins did not demonstrate a clear association with risk of recurrence after OCSCC resection. Further subgroup analyses of both localized tumor (pT1-T2, cN0) and advanced tumor cohorts did not reveal differences in rates of recurrence. Although specimen-driven margin sampling may reduce surgical margin positivity rates, our study was not able to demonstrate an ultimate difference in oncologic outcome. Adjuvant RT may obscure some of the potential prognostic benefit of specimen margin evaluation. Future multi-institutional, randomized prospective studies are needed to further evaluate margin assessment in both localized and advanced oral cancers.
eFigure 1. Diagram of Patient Population Stratified by Localized vs Advanced Tumors
eTable 1. Competing Risk Regression for Recurrence Among Patients With Localized Oral Cavity Squamous Cell Carcinoma
eTable 2. Competing Risk Regression for Recurrence Among Patients With Advanced Oral Cavity Squamous Cell Carcinoma
Data Sharing Statement
References
- 1.Varvares MA, Walker RJ, Chiosea S. Does a specimen-based margin analysis of early tongue cancer better predict local control? Laryngoscope. 2016;126(11):2426-2427. doi: 10.1002/lary.26081 [DOI] [PubMed] [Google Scholar]
- 2.Liu SW, Woody NM, Wei W, et al. Evaluating compliance with process-related quality metrics and survival in oral cavity squamous cell carcinoma: multi-institutional oral cavity collaboration study. Head Neck. 2021;43(1):60-69. doi: 10.1002/hed.26454 [DOI] [PubMed] [Google Scholar]
- 3.Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice; the results of an International American Head and Neck Society Member Survey. Head Neck. 2005;27(11):952-958. doi: 10.1002/hed.20269 [DOI] [PubMed] [Google Scholar]
- 4.Hinni ML, Ferlito A, Brandwein-Gensler MS, et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35(9):1362-1370. doi: 10.1002/hed.23110 [DOI] [PubMed] [Google Scholar]
- 5.Prabhu AV, Sturgis CD, Lai C, et al. Improving margin revision: characterization of tumor bed margins in early oral tongue cancer. Oral Oncol. 2017;75:184-188. doi: 10.1016/j.oraloncology.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seethala RR, Weinreb I, Bullock MJ, et al. Protocol for the Examination of Specimens from Patients with Cancers of the Oral Cavity; 2021. Accessed August 22, 2023. https://documents.cap.org/protocols/HN.LipOral_4.1.0.0.REL_CAPCP.pdf
- 7.Buchakjian MR, Tasche KK, Robinson RA, Pagedar NA, Sperry SM. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142(12):1191-1198. doi: 10.1001/jamaoto.2016.2329 [DOI] [PubMed] [Google Scholar]
- 8.Ettl T, El-Gindi A, Hautmann M, et al. Positive frozen section margins predict local recurrence in R0-resected squamous cell carcinoma of the head and neck. Oral Oncol. 2016;55:17-23. doi: 10.1016/j.oraloncology.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 9.Du E, Ow TJ, Lo Y-T, et al. Refining the utility and role of Frozen section in head and neck squamous cell carcinoma resection. Laryngoscope. 2016;126(8):1768-1775. doi: 10.1002/lary.25899 [DOI] [PubMed] [Google Scholar]
- 10.DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110(10 Pt 1):1773-1776. doi: 10.1097/00005537-200010000-00039 [DOI] [PubMed] [Google Scholar]
- 11.Chiosea SI. Intraoperative margin assessment in early oral squamous cell carcinoma. Surg Pathol Clin. 2017;10(1):1-14. doi: 10.1016/j.path.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 12.Yahalom R, Dobriyan A, Vered M, Talmi YP, Teicher S, Bedrin L. A prospective study of surgical margin status in oral squamous cell carcinoma: a preliminary report. J Surg Oncol. 2008;98(8):572-578. doi: 10.1002/jso.21034 [DOI] [PubMed] [Google Scholar]
- 13.Maxwell JH, Thompson LDR, Brandwein-Gensler MS, et al. Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1104-1110. doi: 10.1001/jamaoto.2015.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang AMV, Kim SW, Duvvuri U, et al. Early squamous cell carcinoma of the oral tongue: comparing margins obtained from the glossectomy specimen to margins from the tumor bed. Oral Oncol. 2013;49(11):1077-1082. doi: 10.1016/j.oraloncology.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 15.Amit M, Na’ara S, Leider-Trejo L, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: a prospective randomized controlled study. Head Neck. 2016;38(suppl 1):E1803-E1809. doi: 10.1002/hed.24320 [DOI] [PubMed] [Google Scholar]
- 16.Maharaj DD, Thaduri A, Jat B, Poonia DR, Durgapal P, Rajkumar KS. Performance and survival outcomes of defect-driven versus specimen-driven method of frozen section intraoperative margin assessment in oral cancers. Int J Oral Maxillofac Surg. 2022;51(9):1131-1137. doi: 10.1016/j.ijom.2021.11.010 [DOI] [PubMed] [Google Scholar]
- 17.MacKay C, Turner B, Bullock M, et al. Margin sampling and survival outcomes in oral cavity and p16-positive oropharyngeal squamous cell carcinoma. OTO Open. 2022;6(3):2473974X221101024. doi: 10.1177/2473974X221101024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC cancer staging manual. 7th ed. Springer; 2010. [Google Scholar]
- 19.Caudell JJ, Gillison ML, Maghami E, et al. NCCN guidelines insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022;20(3):224-234. doi: 10.6004/jnccn.2022.0016 [DOI] [PubMed] [Google Scholar]
- 20.Amin MB, Edge SB, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 21.Smits RWH, van Lanschot CGF, Aaboubout Y, et al. Intraoperative assessment of the resection specimen facilitates achievement of adequate margins in oral carcinoma. Front Oncol. 2020;10:614593. doi: 10.3389/fonc.2020.614593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwich P, MacKay C, Bullock M, et al. Specimen oriented intraoperative margin assessment in oral cavity and oropharyngeal squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2021;50(1):37. doi: 10.1186/s40463-021-00501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sridharan S, Thompson LDR, Purgina B, et al. Early squamous cell carcinoma of the oral tongue with histologically benign lymph nodes: a model predicting local control and vetting of the eighth edition of the American Joint Committee on Cancer pathologic T stage. Cancer. 2019;125(18):3198-3207. doi: 10.1002/cncr.32199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel RS, Goldstein DP, Guillemaud J, et al. Impact of positive frozen section microscopic tumor cut-through revised to negative on oral carcinoma control and survival rates. Head Neck. 2010;32(11):1444-1451. doi: 10.1002/hed.21334 [DOI] [PubMed] [Google Scholar]
- 25.Jäckel MC, Ambrosch P, Martin A, Steiner W. Impact of re-resection for inadequate margins on the prognosis of upper aerodigestive tract cancer treated by laser microsurgery. Laryngoscope. 2007;117(2):350-356. doi: 10.1097/01.mlg.0000251165.48830.89 [DOI] [PubMed] [Google Scholar]
- 26.Reece M, Saluja H, Hollington P, et al. The use of circulating tumor DNA to monitor and predict response to treatment in colorectal cancer. Front Genet. 2019;10:1118. doi: 10.3389/fgene.2019.01118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimbrough CW, St Hill CR, Martin RCG, McMasters KM, Scoggins CR. Tumor-positive resection margins reflect an aggressive tumor biology in pancreatic cancer. J Surg Oncol. 2013;107(6):602-607. doi: 10.1002/jso.23299 [DOI] [PubMed] [Google Scholar]
- 28.Stepan KO, Li MM, Kang SY, Puram SV. Molecular margins in head and neck cancer: current techniques and future directions. Oral Oncol. 2020;110:104893. doi: 10.1016/j.oraloncology.2020.104893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubik MW, Sridharan S, Varvares MA, et al. Intraoperative margin assessment in head and neck cancer: a case of misuse and abuse? Head Neck Pathol. 2020;14(2):291-302. doi: 10.1007/s12105-019-01121-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li MM, Puram SV, Silverman DA, Old MO, Rocco JW, Kang SY. Margin analysis in head and neck cancer: state of the art and future directions. Ann Surg Oncol. 2019;26(12):4070-4080. doi: 10.1245/s10434-019-07645-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. 2015;125(10):2298-2307. doi: 10.1002/lary.25397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Diagram of Patient Population Stratified by Localized vs Advanced Tumors
eTable 1. Competing Risk Regression for Recurrence Among Patients With Localized Oral Cavity Squamous Cell Carcinoma
eTable 2. Competing Risk Regression for Recurrence Among Patients With Advanced Oral Cavity Squamous Cell Carcinoma
Data Sharing Statement