This randomized clinical trial examines whether lapatinib combined with a cisplatin-based combination radiation regimen is effective for the treatment of patients with locally advanced head and neck cancer with poor prognosis.
Key Points
Question
Does the addition of lapatinib, a dual epidermal growth factor receptor and ERBB2 inhibitor, increase the effectiveness of chemoradiotherapy for definitive therapy of stage III to IV non–human papillomavirus head and neck cancer?
Findings
In this placebo-controlled randomized clinical trial of 127 patients, lapatinib plus cisplatin-based chemoradiotherapy did not improve progression-free or overall survival compared with chemoradiation alone.
Meaning
Despite preclinical activity of dual epidermal growth factor receptor and ERBB2 inhibition in head and neck squamous cell carcinoma, lapatinib is insufficiently effective to warrant further study in the definitive setting with chemoradiotherapy.
Abstract
Importance
Patients with locally advanced non–human papillomavirus (HPV) head and neck cancer (HNC) carry an unfavorable prognosis. Chemoradiotherapy (CRT) with cisplatin or anti–epidermal growth factor receptor (EGFR) antibody improves overall survival (OS) of patients with stage III to IV HNC, and preclinical data suggest that a small-molecule tyrosine kinase inhibitor dual EGFR and ERBB2 (formerly HER2 or HER2/neu) inhibitor may be more effective than anti-EGFR antibody therapy in HNC.
Objective
To examine whether adding lapatinib, a dual EGFR and HER2 inhibitor, to radiation plus cisplatin for frontline therapy of stage III to IV non-HPV HNC improves progression-free survival (PFS).
Design, Setting, and Participants
This multicenter, phase 2, double-blind, placebo-controlled randomized clinical trial enrolled 142 patients with stage III to IV carcinoma of the oropharynx (p16 negative), larynx, and hypopharynx with a Zubrod performance status of 0 to 1 who met predefined blood chemistry criteria from October 18, 2012, to April 18, 2017 (median follow-up, 4.1 years). Data analysis was performed from December 1, 2020, to December 4, 2020.
Intervention
Patients were randomized (1:1) to 70 Gy (6 weeks) plus 2 cycles of cisplatin (every 3 weeks) plus either 1500 mg per day of lapatinib (CRT plus lapatinib) or placebo (CRT plus placebo).
Main Outcomes and Measures
The primary end point was PFS, with 69 events required. Progression-free survival rates between arms for all randomized patients were compared by 1-sided log-rank test. Secondary end points included OS.
Results
Of the 142 patients enrolled, 127 (median [IQR] age, 58 [53-63] years; 98 [77.2%] male) were randomized; 63 to CRT plus lapatinib and 64 to CRT plus placebo. Final analysis did not suggest improvement in PFS (hazard ratio, 0.91; 95% CI, 0.56-1.46; P = .34) or OS (hazard ratio, 1.06; 95% CI, 0.61-1.86; P = .58) with the addition of lapatinib. There were no significant differences in grade 3 to 4 acute adverse event rates (83.3% [95% CI, 73.9%-92.8%] with CRT plus lapatinib vs 79.7% [95% CI, 69.4%-89.9%] with CRT plus placebo; P = .64) or late adverse event rates (44.4% [95% CI, 30.2%-57.8%] with CRT plus lapatinib vs 40.8% [95% CI, 27.1%-54.6%] with CRT plus placebo; P = .84).
Conclusion and Relevance
In this randomized clinical trial, dual EGFR-ERBB2 inhibition with lapatinib did not appear to enhance the benefit of CRT. Although the results of this trial indicate that accrual to a non-HPV HNC-specific trial is feasible, new strategies must be investigated to improve the outcome for this population with a poor prognosis.
Trial Registration
ClinicalTrials.gov Identifier: NCT01711658
Introduction
Although human papillomavirus (HPV)–associated oropharynx cancer is the most prevalent subtype of head and neck squamous cell carcinomas (HNC), non-HPV HNCs represent a significant and problematic population of HNC. Development of non-HPV HNC is associated with tobacco use and carries a worse prognosis compared with its viral-associated counterpart. Worldwide, particularly in less developed countries, non-HPV is the predominant form of HNC.1 For these reasons, new and more aggressive combinatory studies have been sought for the management of non-HPV HNC, in contrast to the direction of clinical trials for low-risk HPV-associated HNC in which dose deescalation of therapy is the focus of many clinical research trials. Cisplatin-based concurrent therapy has emerged as the standard of care for locally advanced HNC treated with radiotherapy.2,3,4,5 Therefore, intensification of cisplatin-based chemoradiotherapy (CRT) regimens has been a focus of investigation.
Epidermal growth factor receptor (EGFR), a member of the ErbB (formerly HER) family of receptor tyrosine kinase, has proven to be an effective target for many diseases. Targeting EGFR with monoclonal antibodies has been successful in combination with chemotherapy for recurrent and metastatic HNC as well as in combination with radiation for treatment of locally advanced HNC.6,7 However, the addition of EGFR inhibition with cetuximab to cisplatin-based CRT did not increase efficacy compared with CRT alone for locally advanced HNC.4 Alternatively, small-molecule tyrosine kinase inhibitors (TKIs), which bind to the adenosine triphosphate binding site of the tyrosine kinase domain, inhibit signal transduction downstream of EGFR. A number of these TKIs have demonstrated varying degrees of efficacy in HNC.8 In addition, heterodimerization and cross-talk between the EGFR and other HER family members stimulate signal transduction pathways.9 Lapatinib is a small-molecule TKI and US Food and Drug Administration–approved oncology drug and has dual EGFR and ERBB2 (formerly HER2 or HER2/neu) effects. An escape mechanism conferring EGFR resistance is thought to occur through autocrine events leading to activation of other ErbB family receptors.10 Lapatinib may therefore carry an advantage over anti-EGFR antibodies by circumventing acquired EGFR resistance. Resistance to cetuximab is associated with a mechanism of increased activation of ERBB2 and ERBB3.11 On the basis of its dual mechanism of action, lapatinib may be more effective than monoclonal antibody inhibitors of the EGFR pathway and, therefore, an attractive agent for investigation in HNC. Analysis of a subset of HPV-negative patients from a phase 2 randomized clinical trial of CRT with or without lapatinib for locally advanced HNC demonstrated a signal for improved progression-free survival (PFS) favoring lapatinib in this group with a poor prognosis.12 These observations set the stage for the TRYHARD (Radiation Therapy Plus Cisplatin With or Without Lapatinib in Treating Patients With Head and Neck Cancer) study. We hypothesized that lapatinib would produce potent anticancer effects when combined with a cisplatin-based combination radiation regimen, in particular for patients with locally advanced HNC with poor prognosis.
Methods
Trial Design and Participants
In this randomized clinical trial, eligible patients, not enriched by EGFR expression or other features, had untreated stage III to IV carcinoma of the oropharynx (p16 negative), larynx, and hypopharynx; a Zubrod performance status of 0 to 1; age of 18 years or older; any tobacco status; and adequate bone marrow, hepatic, and kidney functions. Patients with a history of congestive heart failure or significant cardiac disease were excluded. Patients were enrolled from October 18, 2012, to April 18, 2017 (median follow-up, 4.1 years). Self-reported race and ethnicity data were collected to assess the diversity of the population. Patients were stratified by age (≤65 vs >65 years), T stage (T1-3 vs T4), and nodal stage (N0-N2a vs N2b-N3) and were randomly assigned (1:1) using permuted blocks to chemoradiation plus lapatinib arm (CRT plus lapatinib) or chemoradiation plus placebo arm (CRT plus placebo). The TRYHARD study (Radiation Therapy Oncology Group [RTOG] 3501) was registered with the National Institutes of Health and approved by the American College of Radiology Institutional Review Board and institutional review boards at 21 participating centers. All patients provided written informed consent to participate. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol is given in Supplement 1.
Procedures
Patients received 70 Gy (6 weeks) plus 2 cycles of cisplatin (every 3 weeks) plus either lapatinib, 1500 mg per day (CRT plus lapatinib arm) or placebo (CRT plus placebo arm) starting 1 week before radiotherapy (RT) and continuing concurrently with RT and for 3 months after RT. Accelerated radiotherapy involved 70 Gy in 35 fractions (2 Gy per fraction) delivered for 6 weeks, with twice per day dosing once weekly for 5 weeks, with mandatory intensity-modulated radiation therapy and image-guided radiation therapy when using less than 5-mm planning treatment volume expansions. Investigators at each institution registered patients using an electronic system. Treatment assignment was centrally generated at the RTOG Foundation Statistics and Data Management Center and provided to the institution when the patient was registered. All investigators, patients, and funders of the study were masked to group allocation. The active drug and matching placebo were identical in packaging, labeling, appearance, and schedule of administration to preserve the masking.
The cisplatin dose was 100 mg/m2 on days 1 and 22 of RT based on the findings of NRG/RTOG 0129, which showed similar survival with accelerated fractionation plus 2 cycles of cisplatin and standard fractionation and 3 cycles of cisplatin.3 In a double-blinded fashion, lapatinib (1500 mg) or placebo once daily by mouth was started 7 days before RT and continued after completion of RT for 3 months of maintenance therapy. Adverse events (AEs) were evaluated weekly during RT and then during follow-up using Common Terminology Criteria for Adverse Events, version 4. Follow-up was every 3 months for years 1 to 2, every 6 months for years 3 to 5, then annually. Imaging was performed at 3, 6, and 12 months after completion of RT and then annually for 4 years to assess tumor status.
Outcomes
The primary end point was PFS in all randomized patients, which was defined as the time from randomization to locoregional failure (LRF), distant metastasis (DM), or death due to any cause. The primary hypothesis was that the addition of lapatinib to CRT sufficiently improves PFS to warrant a definitive clinical trial. Secondary end points were overall survival (OS), LRF, DM, acute and late treatment-related (definitely, probably, or possibly related) AEs, adherence with protocol-defined treatment delivery by central review, and quality of life. Acute and late AEs were defined as those that occurred 180 or less and more than 180 days, respectively, from end of RT. Locoregional failure was defined as local or regional progression, salvage surgery of the primary tumor with the tumor present or the outcome unknown, salvage neck dissection with the tumor present or the outcome unknown at more than 20 weeks after the end of RT, death because of the study cancer without documented progression, or death because of any unknown cause without documented progression. Distant metastasis and death from other causes were considered competing risks. Locoregional failure and death were considered competing risks for the DM end point. Quality of life and correlative studies will be reported separately.
Statistical Analysis
RTOG 3501 was initially designed with 69 PFS events from 176 patients to detect a 35% reduction in the PFS hazard (hazard ratio [HR], 0.65) with 80% power, a 1-sided α = .20, and 1 interim efficacy and futility analysis at 50% information. The Lan and Demets spending function boundary for efficacy and ρ family spending function with a parameter of 1.5 for futility were used to derive a nonbinding rule. In 2016, a protocol amendment was approved to reduce the sample size to 142 patients (128 analyzable) because of a lower-than-expected accrual rate. The significance level and targeted HR remained unchanged. The PFS rates between arms for all randomized patients were compared using a log-rank test (1-sided α = .18) after accounting for an interim efficacy analysis at 50% information. Despite slower-than-projected accrual and longer-than-projected follow-up, it was estimated that at least 1 to 2 more years of follow-up would have been needed to reach 69 PFS events. Therefore, the RTOG Foundation Data Monitoring Committee recommended reporting the results with 67 PFS events, which had a minor impact on statistical power (<1%) for the primary end point analysis.
The PFS and OS probabilities were estimated using the Kaplan-Meier method, and the LRF and DM probabilities were estimated using the cumulative incidence method.13 Progression-free survival and OS were compared using 1-sided log-rank tests, and LRF and DM were compared using 1-sided cause-specific log-rank tests. Adverse event and treatment adherence (per protocol or acceptable variation) rates were compared using Fisher exact tests (2-sided α = .05). Rates and 95% CIs based on normal approximation for a binomial outcome for individual grade 3 to 4 AE terms occurring in at least 5% of patients in either arm and for feeding tubes (post hoc) were also reported. Treatment effect HRs with 95% CIs were estimated using Cox proportional (cause-specific) hazards regression models14 with or without adjustment for stratification factors. Cox proportional hazards regression model assumptions were assessed using diagnostics based on scaled Schoenfeld residuals and cumulative martingale residuals. Sensitivity analysis for the primary end point was performed in the eligible patient population (see Figure 1 for exclusions). Two-year rates and 95% CIs for all clinical outcomes based on the same analytic methods used for the between-arm comparison were reported. Median PFS by group using the Brookmeyer and Crowley method with 95% CIs were also estimated.
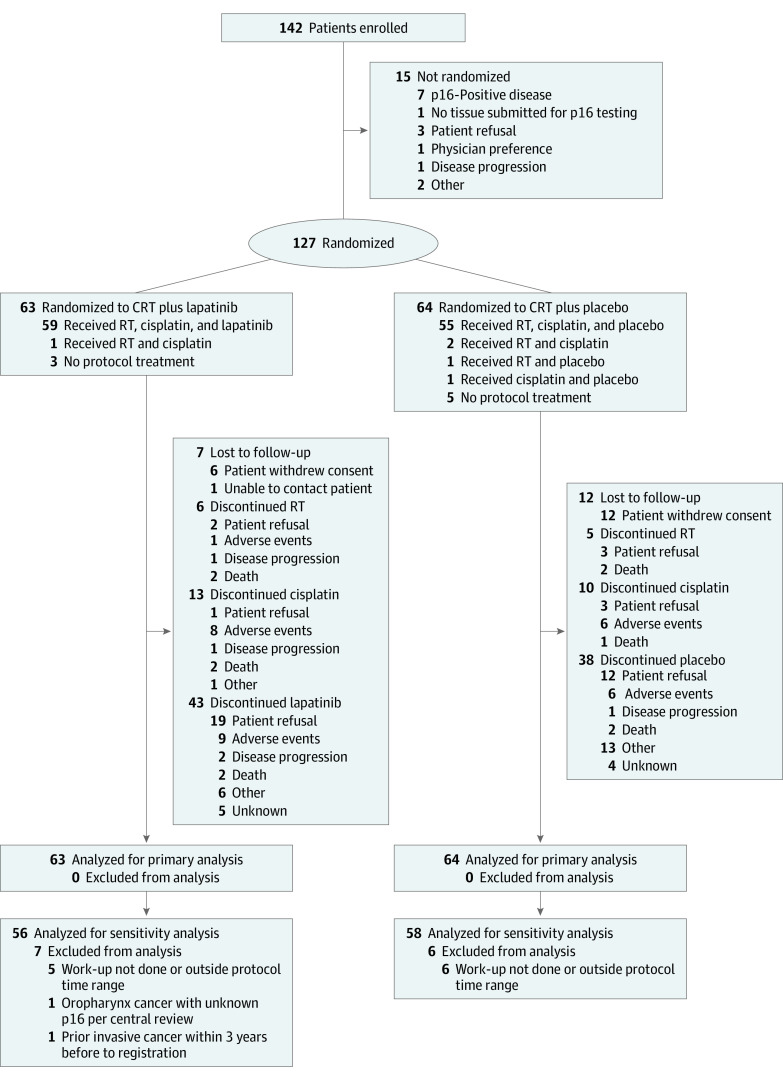
Figure 1. CONSORT Diagram.
CRT indicates chemoradiotherapy; RT, radiotherapy.
All time-to-event end points were measured from the date of randomization to that of the event. Patients alive at the time of analysis were censored at the last follow-up (administrative censoring). All clinical end point analyses except AEs were based on an intent-to-treat approach (ie, all randomized patients). Analysis of AEs was limited to patients who received any protocol treatment.
Study design, implementation, data collection, analysis, interpretations, and article preparation were performed by the authors as representatives of the RTOG Foundation and the RTOG Foundation Statistics and Data Management Center. Data analysis was performed from December 1, 2020, to December 4, 2020. All analyses were performed in SAS software, version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
Of the 142 patients enrolled, 127 (median [IQR] age, 58 [53-63] years; 29 (22.8%) female; 98 [77.2%] male; 2 [1.6%] American Indian or Alaska Native, 2 [1.6%] Asian, 19 [15.0%] Black or African American, 2 [1.6%] Native Hawaiian or Other Pacific Islander, 99 [78.0%] White, 1 [0.8%] multiracial, and 2 [1.6%] unknown or not reported) were randomized and analyzed (Figure 1). Three patients receiving CRT plus lapatinib and 5 receiving CRT plus placebo received no protocol treatment; 6 patients receiving CRT plus lapatinib and 12 receiving CRT plus placebo withdrew consent. Table 1 lists the demographic and baseline characteristics of 127 randomized patients (63 in the CRT plus lapatinib arm and 64 in the CRT plus placebo arm). Sixty patients (47.2%) had a Zubrod performance status score of 0; sites of disease were larynx (72 [57.6%]), oropharynx (32 [25.2%]), and hypopharynx (23 [18.1%]).
Table 1. Demographic and Baseline Characteristicsa.
| Characteristic | CRT plus lapatinib (n = 63) | CRT plus placebo (n = 64) | Total (N = 127) |
|---|---|---|---|
| Age, yb | |||
| Median (IQR) [range] | 57 (52-63) [33-75] | 58 (53.5-63) [42-80] | 58 (53-63) [33-80] |
| ≤65 | 56 (88.9) | 54 (84.4) | 110 (86.6) |
| >65 | 7 (11.1) | 10 (15.6) | 17 (13.4) |
| Sex | |||
| Male | 47 (74.6) | 51 (79.7) | 98 (77.2) |
| Female | 16 (25.4) | 13 (20.3) | 29 (22.8) |
| Race | |||
| American Indian or Alaska Native | 1 (1.6) | 1 (1.6) | 2 (1.6) |
| Asian | 0 | 2 (3.1) | 2 (1.6) |
| Black or African American | 10 (15.9) | 9 (14.1) | 19 (15.0) |
| Native Hawaiian or Other Pacific Islander | 0 | 2 (3.1) | 2 (1.6) |
| White | 50 (79.4) | 49 (76.6) | 99 (78.0) |
| Multiracial | 1 (1.6) | 0 | 1 (0.8) |
| Unknown or not reported | 1 (1.6) | 1 (1.6) | 2 (1.6) |
| Ethnicity | |||
| Hispanic or Latino | 1 (1.6) | 3 (4.7) | 4 (3.1) |
| Not Hispanic or Latino | 61 (96.8) | 60 (93.8) | 121 (95.3) |
| Unknown | 1 (1.6) | 1 (1.6) | 2 (1.6) |
| Zubrod performance status | |||
| 0 | 27 (42.9) | 33 (51.6) | 60 (47.2) |
| 1 | 36 (57.1) | 31 (48.4) | 67 (52.8) |
| Smoking history, pack-years | (n = 62) | (n = 63) | (n = 125) |
| Median (IQR) [range] | 29.5 (10-42) [0-168] | 38 (12.5-58.5) [0-126] | 30 (12-48) [0-168] |
| ≤10 | 17 (27.4) | 13 (20.6) | 30 (24.0) |
| >10 | 45 (72.6) | 50 (79.4) | 95 (76.0) |
| Primary site | |||
| Oropharynx, p16-negative (central review) | 16 (25.4) | 15 (23.4) | 31 (24.4) |
| Oropharynx, unknown p16 (central review)c | 1 (1.6) | 0 | 1 (0.8) |
| Hypopharynx | 7 (11.1) | 16 (25.0) | 23 (18.1) |
| Larynx | 39 (61.9) | 33 (51.6) | 72 (56.7) |
| T stage (AJCC 7th edition)b | |||
| T1 | 0 | 2 (3.1) | 2 (1.6) |
| T2 | 7 (11.1) | 9 (14.1) | 16 (12.6) |
| T3 | 38 (60.3) | 35 (54.7) | 73 (57.5) |
| T4 | 18 (28.6) | 18 (28.1) | 36 (28.3) |
| N stage (AJCC 7th edition)b | |||
| N0 | 20 (31.7) | 18 (28.1) | 38 (29.9) |
| N1 | 8 (12.7) | 7 (10.9) | 15 (11.8) |
| N2a | 2 (3.2) | 3 (4.7) | 5 (3.9) |
| N2b | 19 (30.2) | 19 (29.7) | 38 (29.9) |
| N2c | 13 (20.6) | 15 (23.4) | 28 (22.0) |
| N3 | 1 (1.6) | 2 (3.1) | 3 (2.4) |
| Overall stage (AJCC 7th edition) | |||
| II | 0 | 1 (1.6) | 1 (0.8) |
| III | 20 (31.7) | 18 (28.1) | 38 (29.9) |
| IV | 43 (68.3) | 45 (70.3) | 88 (69.3) |
Abbreviations: AJCC, American Joint Committee on Cancer; CRT, chemoradiotherapy.
Data are presented as number (percentage) of patients unless otherwise indicated.
Stratification factor.
Randomized with a nonoropharynx primary site that was later revised to oropharynx. Per local testing, p16 status is negative.
Outcomes
An interim efficacy and futility analysis was conducted with 32 PFS events reported through March 22, 2017. With 32 events, if the 1-sided log-rank test P value was ≤.06, a recommendation for stopping due to efficacy (ie, futility) would have been made. The 1-sided log-rank test P value was .48. The CRT plus lapatinib/CRT plus placebo HR was 0.98 (95% CI, 0.49-1.96). The 1-sided P value did not cross either boundary. Therefore, the data monitoring committee recommended continuation of the trial per protocol.
At the time of analysis, 78 patients were alive, with a median (range) follow-up time of 4.1 (0.003-7.1) years. Median PFS was 2.2 years (95% CI, 1.3 years to not reached) in the CRT plus lapatinib group and 2.7 years (95% CI, 1.3-4.2 years) in the CRT plus placebo group. The primary analysis does not suggest improvement in PFS with adding lapatinib to CRT (CRT plus lapatinib/CRT plus placebo HR, 0.91; 95% CI, 0.56-1.46; 82% lower confidence bound = 0.72; log-rank test P = .34). Furthermore, the primary analysis was able (with 82% confidence) to rule out an effect of CRT plus lapatinib on PFS below an HR of 0.72. The 2-year PFS rates were 50.6% (95% CI, 37.5%-63.7%) for CRT plus lapatinib and 56.2% (95% CI, 43.0%-69.4%) for CRT plus placebo (Table 2). Multivariable analysis after adjusting for the stratifying factors (age, T stage, and nodal status) led to a treatment effect HR of 0.97 (95% CI, 0.59-1.57) (eTable 1 in Supplement 2). Unadjusted and adjusted sensitivity analysis, limited to eligible patients (see Figure 1 for exclusions), yielded similar results (unadjusted analysis HR, 0.89; 95% CI, 0.54-1.47; adjusted analysis HR, 0.96; 95% CI, 0.58-1.60; log-rank test P = .33).
Table 2. Progression-Free and Overall Survival Estimates by Assigned Treatment.
| Year | Estimate (95% CI), % | |||
|---|---|---|---|---|
| Progression-free survival | Overall survival | |||
| CRT plus lapatinib | CRT plus placebo | CRT plus lapatinib | CRT plus placebo | |
| 0 | 100 [Reference] | 100 [Reference] | 100 [Reference] | 100 [Reference] |
| 1 | 70.4 (58.6-82.2) | 71.1 (59.2-83.1) | 86.2 (77.3-95.1) | 93.1 (86.5-99.6) |
| 2 | 50.6 (37.5-63.7) | 56.2 (43.0-69.4) | 71.8 (60.1-83.5) | 76.0 (64.5-87.4) |
| 3 | 43.1 (30.1-56.1) | 48.4 (35.0-61.8) | 62.6 (49.9-75.3) | 72.1 (60.0-84.1) |
| 4 | 43.1 (30.1-56.1) | 37.1 (23.6-50.5) | 58.3 (45.2-71.5) | 58.0 (43.9-72.0) |
| 5 | 43.1 (30.1-56.1) | 34.6 (21.2-48.0) | 49.9 (35.5-64.3) | 55.1 (40.6-69.5) |
Abbreviation: CRT, chemoradiotherapy.
No significant differences were observed in OS (unadjusted HR, 1.06; 95% CI, 0.61-1.86; log-rank P = .58), LRF (HR, 1.15; 95% CI, 0.62-2.17; cause-specific log-rank test P = .67), or DM (HR, 0.64; 95% CI, 0.25-1.65; cause-specific log-rank test P = .17) (Figure 2). See eTables 2, 3, and 4 in Supplement 2 for univariate and multivariable Cox proportional hazards regression model results for OS, LRF, and DM, respectively. The 2-year OS rates were 71.8% (95% CI, 60.1%-83.5%) for CRT plus lapatinib and 76.0% (95% CI, 64.5%-87.4%) for CRT plus placebo (Table 2). The 2-year LRF rates were 30.2% (95% CI, 18.7%-42.5%) for CRT plus lapatinib and 29.3% (95% CI, 17.8%-41.8%) for CRT plus placebo. The 2-year DM rates were 12.4% (95% CI, 5.4%-22.5%) for CRT plus lapatinib and 9.2% (95% CI, 3.3%-18.8%) for CRT plus placebo. Fifteen of 26 patients (57.7%) receiving CRT plus lapatinib and 9 of 23 (39.1%) receiving CRT plus placebo were due to the study cancer (eTable 5 in Supplement 2).
Figure 2. Kaplan-Meier Estimates of Progression-Free Survival (PFS) and Overall Survival (OS) and Cumulative Incidence Estimates of Locoregional Failure (LRF) and Distant Metastasis (DM) by Assigned Treatment.
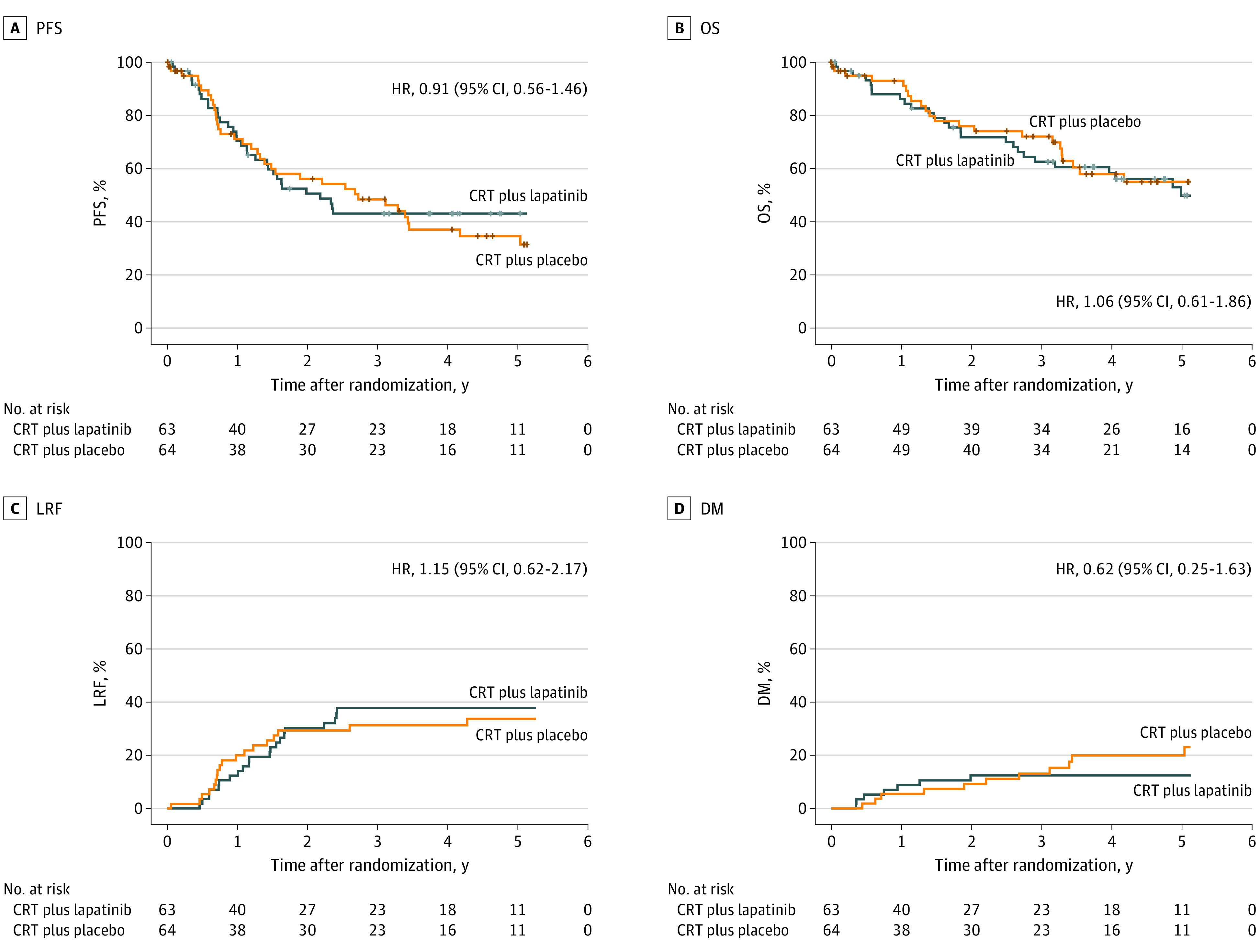
Plus sign indicates censored data. CRT indicates chemoradiotherapy; HR, hazard ratio.
Adverse Events
Table 3 lists the distribution of worst-grade acute and late AEs overall and limited to terms occurring in at least 5% of patients in either study arm. There were no significant differences in grade 3 to 4 acute AE rates (83.3% [95% CI, 73.9%-92.8%] in the CRT plus lapatinib arm vs 79.7% [95% CI, 69.4%-89.9%] in the CRT plus placebo arm; P = .64) or late AE rates (44.4% [95% CI, 30.2%-57.8%] in the CRT plus lapatinib vs 40.8% [95% CI, 27.1%-54.6%] in the CRT plus placebo arms; P = .84). Treatment-related grade 5 AEs were rare, with 0 of 60 AEs in the CRT plus lapatinib arm and 4 of 59 (6.8%) in the CRT plus placebo arm, with 2 treatment-related grade 5 events during both the acute (cardiac arrest and sudden death not otherwise specified) and late (tracheal hemorrhage and oral hemorrhage) periods. Acute grade 3 dysphagia occurred in 19 of 60 patients (31.7%) in the CRT plus lapatinib arm and 27 of 59 (45.8%) in the CRT plus placebo arm. Rates of acute grade 3 oral mucositis, grade 3 pain, grade 3 radiation dermatitis, grade 3 dehydration, and grade 3 weight loss were similar in both arms. Acute low-grade diarrhea (grades 1-2) was more common in the CRT plus lapatinib arm (23 of 60 [38.3%]) compared with the CRT plus placebo arm (3 of 59 [5.1%]), but grade 3 diarrhea (2 of 60 [3.3%] vs 0 of 59) was not. Late grade 3 dysphagia occurred in 13 of 50 patients (26.0%) in the CRT plus lapatinib arm and 12 of 49 (24.5%) in the CRT plus placebo arm. Feeding tube rates peaked at the end of RT and decreased over time, with no substantial differences between arms (eFigure in Supplement 2).
Table 3. Acute and Late Treatment-Related Adverse Events Occurring in at Least 5% of Patients in Either Arma.
| Adverse event | Estimate (95% CI), % | P value | |
|---|---|---|---|
| CRT plus lapatinib | CRT plus placebo | ||
| Acute events (60 lapatinib patients and 59 placebo patients) | |||
| Grade 5 overall | 0.0 (0.0-0.0) | 3.4 (0.0-8.0) | .64 |
| Grade 3-4 overall | 83.3 (73.9-92.8) | 79.7 (69.4-89.9) | |
| Grade 3 dysphagiab | 31.7 (19.9-43.4) | 45.8 (33.1-58.5) | |
| Grade 3-4 decreased lymphocyte count | 26.7 (15.5-37.9) | 18.6 (8.7-28.6) | |
| Grade 2-3 dry mouthc | 25.0 (14.0-36.0) | 32.2 (20.3-44.1) | |
| Grade 3-4 anorexia | 18.3 (8.5-28.1) | 16.9 (7.4-26.5) | |
| Grade 3 nauseac | 16.7 (7.2-26.1) | 16.9 (7.4-26.5) | |
| Grade 3 mucositis oralb | 16.7 (7.2-26.1) | 11.9 (3.6-20.1) | |
| Grade 3 vomitingb | 16.7 (7.2-26.1) | 10.2 (2.5-17.9) | |
| Grade 3 dehydrationb | 13.3 (4.7-21.9) | 16.9 (7.4-26.5) | |
| Grade 3-4 decreased neutrophil count | 13.3 (4.7-21.9) | 10.2 (2.5-17.9) | |
| Grade 3 acute kidney injuryb | 11.7 (3.5-19.8) | 8.5 (1.4-15.6) | |
| Grade 3 hyponatremiab | 10.0 (2.4-17.6) | 10.2 (2.5-17.9) | |
| Grade 3-4 decreased white blood cell | 8.3 (1.3-15.3) | 13.6 (4.8-22.3) | |
| Grade 3 weight lossc | 8.3 (1.3-15.3) | 6.8 (0.4-13.2) | |
| Grade 3-4 hypokalemia | 6.7 (0.4-13.0) | 5.1 (0.0-10.7) | |
| Grade 3 sore throatc | 5.0 (0.0-10.5) | 8.5 (1.4-15.6) | |
| Grade 3 anemiab | 5.0 (0.0-10.5) | 6.8 (0.4-13.2) | |
| Grade 3 radiation dermatitisb | 5.0 (0.0-10.5) | 6.8 (0.4-13.2) | |
| Grade 3 pharyngeal mucositisb | 5.0 (0.0-10.5) | 5.1 (0.0-10.7) | |
| Grade 3 painc | 5.0 (0.0-10.5) | 3.4 (0.0-8.0) | |
| Grade 3-4 increased creatinine | 3.3 (0.0-7.9) | 6.8 (0.4-13.2) | |
| Grade 3-4 laryngeal edema | 3.3 (0.0-7.9) | 5.1 (0.0-10.7) | |
| Grade 3 dyspneab | 1.7 (0.0-4.9) | 5.1 (0.0-10.7) | |
| Grade 3 hoarsenessc | 1.7 (0.0-4.9) | 5.1 (0.0-10.7) | |
| Grade 3 fatiguec | 0.0 (0.0-0.0) | 5.1 (0.0-10.7) | |
| Late events (n = 50 lapatinib patients and 49 placebo patients) | |||
| Grade 5 overall | 0.0 (0.0-0.0) | 4.1 (0.0-9.6) | .84 |
| Grade 3-4 overall | 44.0 (30.2-57.8) | 40.8 (27.1-54.6) | |
| Grade 2-3 dry mouthc | 36.0 (22.7-49.3) | 28.6 (15.9-41.2) | |
| Grade 3 dysphagiab | 26.0 (13.8-38.2) | 24.5 (12.4-36.5) | |
| Grade 3 weight lossc | 8.0 (0.5-15.5) | 12.2 (3.1-21.4) | |
| Grade 3-4 decreased lymphocyte count | 8.0 (0.5-15.5) | 4.1 (0.0-9.6) | |
| Grade 3 hyponatremiab | 8.0 (0.5-15.5) | 2.0 (0.0-6.0) | |
| Grade 3 hoarsenessc | 8.0 (0.5-15.5) | 0.0 (0.0-0.0) | |
| Grade 3 esophageal stenosisb | 6.0 (0.0-12.6) | 6.1 (0.0-12.8) | |
| Grade 3 impaired hearingb | 6.0 (0.0-12.6) | 2.0 (0.0-6.0) | |
| Grade 3 pharyngeal mucositisb | 2.0 (0.0-5.9) | 8.2 (0.5-15.8) | |
Abbreviation: CRT, chemoradiotherapy.
Adverse events were graded with Common Terminology Criteria for Adverse Events, version 4. Treatment related is defined as definitely, probably, or possibly related to treatment. Acute is defined as 180 days or less from the end of radiation therapy and late as more than 180 days from the end of radiation therapy. P values are from the Fisher exact test.
No grade 4 events were reported for this term.
Grade 4 does not exist for this term in Common Terminology Criteria for Adverse Events, version 4.
Treatment Adherence
Radiotherapy (≥70 Gy) was completed in 54 of 63 patients (85.7%) in the CRT plus lapatinib arm and 53 of 64 patients (82.8%) in the CRT plus placebo arm. Cisplatin (200 mg/m2 ± 5%) was completed in 41 of 63 patients in the CRT plus lapatinib arm (65.1%) and 45 of 64 patients (70.3%) in the CRT plus placebo arm. Seventy-five percent of patients received 2 doses of cisplatin (47 of 63 in the lapatinib arm and 48 of 64 patients in the placebo arm). The median (IQR) total doses were 87 000 (21 000-193 250) mg for lapatinib and 125 250 (34 500-205 750) mg for placebo. Cisplatin adherence per study chair review overall was high for both arms (≥88%) (eTable 6 in Supplement 2). Delivery of lapatinib and placebo, respectively, was scored per protocol or with acceptable variation in 87.3% (n = 55) and 82.8% (n = 53) for the pre–intensity-modulated radiotherapy period (P = .80), 84.1% (n = 53) and 79.7% (n = 51) for the concurrent period (P = .65), and 49.2% (n = 31) and 56.3% (n = 36) for the maintenance period (P = .48)
Discussion
Early deciphering of the ErbB family signaling network inferred significant potential clinical benefit of targeted therapy in many diseases, including HNC.9 Ample evidence has demonstrated that activity of anti-EGFR therapy (antibody as well as small molecule) in HNC is modest as a single agent and much more active when combined with other anticancer therapies.6,7,15 Lapatinib, as a small-molecule anti-EGFR agent that also interferes with heterodimerization and cross-talk between EGFR and other ErbB family members, has had a strong rationale as a combination agent in HNC. In contrast, newer evidence suggests that ERBB3 expression can contribute to resistance to dual or pan ErbB inhibitors.16 The findings of the TRYHARD study, however, showed no signal of enhanced efficacy for the combination of lapatinib plus cisplatin-based CRT compared with standard cisplatin-based CRT alone. Neither the primary end point (PFS) nor the secondary end points (OS, LRF, and DM) indicated improvement with the addition of lapatinib. Lapatinib was well tolerated as indicated by AE rates. The rates of RT and cisplatin completion were similar in each arm. Of note, 75% of patients in each arm completed the planned 2 cycles of cisplatin compared with 90% in the RTOG 0129 and 0522 clinical trials.3,4 Because TRYHARD enrolled solely patients without HPV, it is possible that this population is somewhat less robust in contrast to these other trials, which accrued a mixture of HPV-positive and HPV-negative patients. Hence, the overall findings of our study do not appear to be attributable to excess toxic effects because the addition of lapatinib to CRT was safe and tolerable. Insight as to the interpretation of the TRYHARD study can be further comprehended in the context of findings from other anti-EGFR CRT trials.
Other attempts to enhance the effectiveness of cisplatin-based CRT by addition of anti-EGFR therapy have not been successful. A phase 3 trial, NRG/RTOG 0522, examined concurrent cisplatin CRT with or without the anti-EGFR antibody agent cetuximab for stage III or IV HNC.4 The addition of cetuximab did not improve OS (primary end point), PFS, or DM. In the postoperative setting for resected high-risk (positive surgical margin or extranodal tumor extension) HNC, a phase 3 trial examined cisplatin-based CRT plus concurrent and maintenance lapatinib vs placebo.17 Again, this study did not show an improvement in PFS (primary end point) or OS. It is arguable that if EGFR blockade enhances the effectiveness of cisplatin-based CRT, the results are more likely observed in the definitive treatment setting rather than the postoperative setting. The results of the TRYHARD study indicate that lapatinib is insufficiently active and do not support further study of this agent in this setting. The role of anti-EGFR therapy in the management of locally advanced HNC is currently limited to cisplatin-ineligible patients for whom concurrent cetuximab plus radiation is a standard option. Intensification of cisplatin-based CRT with other novel agents remains a viable investigational strategy for the high-risk non-HPV population.
Limitations
This study has several limitations. Although we demonstrate that lapatinib does not enhance the efficacy of CRT, it cannot be concluded that further investigation of dual EGFR-ErbB inhibition in the CRT setting lacks scientific merit. More active small-molecule dual EGFR-ErbB inhibitors could yield greater clinical efficacy in combination with CRT. This study is also limited in that non–platin-containing CRT regimens, such as docetaxel, may have greater synergy with anti–EGFR-ERBB2 agents compared with platin-based regimens.
Conclusions
Although this randomized clinical trial was successful in completing accrual of a shrinking HNC subpopulation within a multicenter setting, an amendment was introduced to reduce the required number of enrolled patients because of an accrual rate that was lower than projected. This observation should help to encourage the conduct of future clinical trials focusing on this population, although feasibility discussions should be conducted according to specific trial conditions and site participation.
Trial Protocol
eTable 1. Cox Model Results for Progression-Free Survival (n = 127; 67 Events)
eTable 2. Cox Model Results for Overall Survival (n = 127; 49 Events)
eTable 3. Cause-Specific Cox Model Results for Locoregional Failure (n = 127; 39 Events)
eTable 4. Cause-Specific Cox Model Results for Distant Metastasis (n = 127; 18 Events)
eTable 5. Cause of Death
eTable 6. Treatment Compliance per Study Chair (Central) Modality Reviews
eFigure. Feeding Tube Rates Over Time (Time Points 3 Months Post-RT and Later Are ±6 Weeks)
Data Sharing Statement
References
- 1.Aupérin A. Epidemiology of head and neck cancers: an update. Curr Opin Oncol. 2020;32(3):178-186. doi: 10.1097/CCO.0000000000000629 [DOI] [PubMed] [Google Scholar]
- 2.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92-98. doi: 10.1200/JCO.2003.01.008 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32(34):3858-3866. doi: 10.1200/JCO.2014.55.3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940-2950. doi: 10.1200/JCO.2013.53.5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40-50. doi: 10.1016/S0140-6736(18)32779-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116-1127. doi: 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 7.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578. doi: 10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- 8.Xu MJ, Johnson DE, Grandis JR. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017;36(3):463-473. doi: 10.1007/s10555-017-9687-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127-137. doi: 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 10.Kong A, Calleja V, Leboucher P, Harris A, Parker PJ, Larijani B. HER2 oncogenic function escapes EGFR tyrosine kinase inhibitors via activation of alternative HER receptors in breast cancer cells. PLoS One. 2008;3(8):e2881. doi: 10.1371/journal.pone.0002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27(28):3944-3956. doi: 10.1038/onc.2008.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington K, Berrier A, Robinson M, et al. Randomised phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. Eur J Cancer. 2013;49(7):1609-1618. doi: 10.1016/j.ejca.2012.11.023 [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Non-parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Stat Soc A. 1972;34:187-202. doi: 10.1111/j.2517-6161.1972.tb00899.x [DOI] [Google Scholar]
- 15.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171-2177. doi: 10.1200/JCO.2006.06.7447 [DOI] [PubMed] [Google Scholar]
- 16.Cohen EEW, Licitra LF, Burtness B, et al. Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer. Ann Oncol. 2017;28(10):2526-2532. doi: 10.1093/annonc/mdx344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington K, Temam S, Mehanna H, et al. Postoperative adjuvant lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: a phase III, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2015;33(35):4202-4209. doi: 10.1200/JCO.2015.61.4370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Cox Model Results for Progression-Free Survival (n = 127; 67 Events)
eTable 2. Cox Model Results for Overall Survival (n = 127; 49 Events)
eTable 3. Cause-Specific Cox Model Results for Locoregional Failure (n = 127; 39 Events)
eTable 4. Cause-Specific Cox Model Results for Distant Metastasis (n = 127; 18 Events)
eTable 5. Cause of Death
eTable 6. Treatment Compliance per Study Chair (Central) Modality Reviews
eFigure. Feeding Tube Rates Over Time (Time Points 3 Months Post-RT and Later Are ±6 Weeks)
Data Sharing Statement