Abstract
Background:
Factors that determine individual disease course of patients with primary sclerosing cholangitis (PSC) are poorly understood. Although an association between gut microbes and disease outcome has been suggested, little is known about the role of microbes in the biliary tract.
Methods:
We analyzed microbial cultures from bile specimens obtained during routine endoscopic retrograde cholangiopancreatography (ERCP) and intraoperatively before liver transplantation in 114 patients with PSC in our tertiary academic center. The presence of bacterial and fungal species was correlated with clinical characteristics and outcome data.
Results:
A total of 87 patients (76%) had positive bile culture results. The presence of concomitant inflammatory bowel disease (IBD) was associated with positive bile culture results in multivariate analysis (OR, 4.707; 95% CI, 1.688–13.128; p=0.003). Enterococcus spp. in the bile was associated with a more frequent occurrence of liver transplantation and/or death (OR, 2.778; 95% CI, 1.147–6.728; p=0.021) and recurrent (≥3) cholangitis episodes (OR, 2.839; 95% CI, 1.037–7.768; p=0.037). Biliary candidiasis was linked to a higher frequency of recurrent (≥3) cholangitis episodes (OR, 5.677; 95% CI, 1.940–16.616; p=0.001). Proton pump inhibitor intake conferred a clinical feature associated with biliary candidiasis in multivariate analysis (OR, 3.559; 95% CI, 1.275–9.937; p=0.016).
Conclusions:
Our data indicate that in patients with PSC, presence of Enterococcus spp. and Candida spp. in bile is associated with an adverse outcome. Concomitant IBD is linked to presence of microbes in bile, and proton pump inhibitor intake is a feature associated with biliary candidiasis in patients with PSC.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic inflammatory liver disease characterized by biliary inflammation, multifocal bile duct strictures, and cholestasis. PSC frequently progresses to end-stage liver disease and is associated with a significant risk of cancer in the bile ducts and colon. Up to 80% of patients with PSC suffer concomitantly from inflammatory bowel disease (IBD). Currently, no medical treatment with proven benefit on transplant-free survival is available, and liver transplantation is the only curative treatment option.1,2
Although the last decades have shown considerable advances in the molecular understanding of PSC, many aspects of its pathogenesis remain poorly understood. It is generally accepted that the interplay between genetic susceptibility and environmental factors, such as transmission of bacterial microbes from the gut due to increased permeability of the portal venous system, contributes to the pathogenesis and disease progression.3–5 Moreover, recent experimental data demonstrate that specific gut microbes such as Klebsiella pneumonia can disrupt the epithelial barrier to initiate bacterial translocation and ameliorate T helper 17 cell responses in the liver, increasing susceptibility to hepatobiliary injuries.6
Partly related to the difficulty in obtaining bile samples, data on the role of bacteria colonizing the biliary tract of patients with PSC are limited. Only very few clinical studies have focused on the role of specific, culturable microbes in the bile of patients with PSC. Analysis of biliary isolates from explanted livers of patients with PSC and primary biliary cholangitis revealed bacterial colonization of bile in a majority of patients with PSC but not in patients with primary biliary cholangitis.7 Of note, patients with PSC with a high load of bacteria had a shorter time to liver transplantation, which points toward a harmful effect of bacterial colonization on liver function.7
The aim of our study was to investigate the association of microbiological culture results of bile fluid from patients with PSC, obtained either during endoscopic retrograde cholangiopancreatography (ERCP) or intraoperatively before liver transplantation, with clinical outcome in a large PSC cohort from our tertiary academic liver center. Furthermore, we explored whether endogenous and external features are associated with the presence of pathogens in bile of patients with PSC.
METHODS
Study design and study population
Within our monocentric cohort study, we analyzed bile fluid culture results in a cohort of 114 patients with diagnosed PSC who were treated in our hepatology and gastroenterology unit at Charité - Universitätsmedizin Berlin from 2000 until August 2022. During the study period, a total of 402 patients with diagnosed PSC were treated at our tertiary center. Bile fluid samples were obtained routinely during ERCP in 103 patients and intraoperatively before liver transplantation in 11 patients. Only patients with at least 1 endoscopic intervention with ERCP and available bile culture tests for bacterial and fungal species were included in the analysis. Patients with prior liver transplantation were excluded from the analysis. We included a control cohort with 50 patients who received bile sampling before liver transplantation due to other reasons than PSC (HCC, alcohol-associated liver disease, and NASH). The 114 patients with PSC who fulfilled the selection criteria were followed until recently. ERCP reports of all patients were analyzed and evaluated. Medical data were extracted from electronic medical charts. One bile sample result per patient was reported.
Diagnosis of PSC was confirmed in accordance with the current American College of Gastroenterology guidelines, using a combination of clinical, biochemical, and cholangiographic (magnetic resonance cholangiopancreatography and/or ERCP) features.8–11 Acute cholangitis was defined according to the revised Tokyo Guidelines 2018, based on a combination of systemic inflammation (fever and/or shaking chills, increase of serum C-reactive protein levels, and abnormal white blood cell counts), cholestasis (jaundice/abnormal liver function tests), and imaging (biliary dilatation and evidence of the etiology on imaging, eg, stricture and stone).2,12 Only cholangitis episodes requiring i.v. antibiotic treatment and hospitalization were considered. For the diagnosis of an overlap syndrome with autoimmune hepatitis (AIH), simplified diagnostic criteria for AIH in clinical practice were used. Diagnosis of hepatobiliary malignancies was confirmed histologically. A relevant stenosis was defined as a high-grade biliary stricture in the common bile duct or hepatic ducts with signs or symptoms of obstructive disease and/or cholangitis according to current European Association for the Study of Liver (EASL) guidelines.2 The diagnosis of liver cirrhosis was based on measurement of liver stiffness (cutoff ≥14.4 kPa) or the presence of (morphological) signs of hepatic decompensation such as portal hypertension (ascites and esophageal varices) and/or hepatic encephalopathy.13,14 The model of end-stage liver disease (MELD) was calculated using published algorithms.15 The definition of IBD was based on endoscopic and histological findings according to accepted criteria.16 The observation period is defined as time period after bile sampling for patients who received ERCP; when samples were obtained in the context of liver transplantation, it was set to 0. Enterobacteriaceae include Escherichia coli, Klebsiella species (spp.) Enterobacter spp., and Citrobacter spp. Candida spp. include Candida albicans, Candida dubliniensis, Candida tropicalis, Candida krusei, Candida kefyr, and Candida glabrata. Enterococcus species include Enterococcus faecalis, Enterococcus faecium, Enterococcus gallinarum, and Enterococcus casseliflavus.
Bile sampling and bile cultivation
Bile cultures were collected from patients during ERCP and tested for the presence of bacterial and fungal species. All bile samples during ERCP were obtained after standardized cannulation of the common bile duct and before application of contrast media. Endoscopic interventions were performed by a group of 6 experienced endoscopists. Initial endoscopic interventions were performed according to the current European guidelines, in which endoscopic treatment is recommended for patients who (1) develop clinically relevant or worsening symptoms such as pruritus, jaundice, and cholangitis; (2) display a fast rise in cholestatic enzyme levels; or (3) present with a new dominant stricture or progression of existing dominant strictures identified at magnetic resonance cholangiopancreatography imaging.9,17 After initial intervention, follow-up ERCP procedures were performed in regular intervals for follow-up treatment of biliary strictures.
During the ERCP procedures, bile fluid was drawn into a sterile syringe and delivered to the microbiology laboratory within 2 hours. In patients undergoing liver transplantation, bile samples were taken intraoperatively from the common bile duct after hepatectomy. Bile specimens were processed in the clinical microbiology laboratory using routine methods. Basically, specimens were cultivated on conventional solid media and incubated in aerobic and anaerobic atmospheres at 37 °C. Aerobic microorganisms were routinely identified using the VITEK 2 System (bioMérieux, France) or matrix-associated laser desorption/ionization time-of-flight mass spectrometry (MALDI-MS; Bruker, MA, before 2011, or bioMérieux, France, since 2011), and anaerobic bacteria were identified by MALDI-MS.
Antibiotic treatment
All patients with PSC undergoing ERCP received peri-interventional antibiotics. The standard regimen in our institution was i.v. administration of a single dose (2 g) of ceftriaxone about 30 minutes before each procedure. If required, antibiotic regimen was adapted according to previous bile culture results. In case of known allergies, ciprofloxacin or ampicillin/sulbactam was used. Before liver transplantation, patients received antibiotic prophylaxis with ceftriaxone and metronidazole.
Statistical analysis
Categorical data were described as frequency and percentage. Continuous data were evaluated for normal distribution using histogram and Q-Q plot and are reported as median and range. Frequencies were compared using χ2 test or Fisher exact test, where appropriate. To identify factors that are associated with positive bile culture and biliary candidiasis results, we performed univariable and multivariable binary logistic regression analyses, respectively. We included the following factors: sex, presence of IBD, presence of AIH, age at initial diagnosis of PSC, proton pump inhibitor (PPI) intake, and number of ERCPs. Categorical variables were described as frequency and percentage. All statistical analyses were performed with SPSS (Version 26.0. Armonk, NY: IBM Corp). A p-value of < 0.05 was considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001).
Consent
The study protocol was reviewed and approved by the ethics committee of the Charité – Universitätsmedizin Berlin (ethical approval number EA1/142/21; EA1/416/20) and was done in accordance with the Declaration of Helsinki and Istanbul. The need for informed consent was waived for this observational study.
RESULTS
Patient cohort characteristics
Our cohort included 114 patients with confirmed PSC. A total of 103 patients received bile sampling during ERCP, and 11 patients received bile sampling intraoperatively before liver transplantation with testing for the presence of bacterial and fungal species. The median age of patients with PSC at the time of bile analysis was 42 years (18–74). Overall, 30% of the patients (n = 34) were female and 70% (n = 80) were male. A total of 83 patients (73%) suffered concomitantly from IBD; among these, 67 patients (81%) had ulcerative colitis and 14 patients (12%) had a diagnosis of Crohn disease, while 2 patients (2%) showed evidence of indeterminate colitis. A relevant biliary tract stenosis was present in 105 (92%) patients. The median number of performed ERCPs per patient was 5 (1–6). A total of 18 (16%) patients suffered from an acute cholangitis episode at the time of bile sampling. Overall, 5 patients (4%) already suffered from cholangiocarcinoma before bile sample collection. Further baseline patient and ERCP characteristics of the PSC cohort are shown in Table 1.
TABLE 1.
Patient characteristics
| PSC cohort n = 114 | |
|---|---|
| Sex, n (%) | |
| Female | 34 (30) |
| Male | 80 (70) |
| Median age at initial diagnosis (range) | 30 (10–67) |
| Median age at time of bile sampling (range) | 42 (18–74) |
| Overlap syndrome with AIH, n (%) | 14 (12) |
| Presence of IBD, n (%) | 83 (73) |
| Ulcerative colitis | 67 (81) |
| Crohn disease | 14 (12) |
| Colitis indeterminate | 2 (2) |
| Liver cirrhosis, n (%) | 48 (42) |
| Cholangiocarcinoma at the time of bile sampling, n (%) | 5 (4) |
| Relevant stenosis, n (%) | 105 (92) |
| Proton pump inhibitor intake at the time of bile sampling, n (%) | 29 (25) |
| Immunosuppressive treatment at the time of bile samplinga, n (%) | 31 (27) |
| Immunosuppressantsb | 22 (19) |
| Biologicalsc | 9 (8) |
| Cortisone (oral) | 9 (8) |
| Cholangitis episode at the time of sampling, n (%) | 18 (16) |
| Median baseline laboratory parameters at the time of sampling (range) | — |
| Median bilirubin [mg/dL] (range) | 1.2 (0.15–33) |
| Median ALT [U/L] (range) | 75 (14–954) |
| Median AST [U/L] (range) | 64 (12–1165) |
| Median ALP [U/L] (range) | 263 (15–1565) |
| Median GGT [U/L] range) | 224 (12–1908) |
| Median creatinine [mg/dL] (range) | 0.78 (0.44–2.12) |
| Median INR (range) | 1.1 (0.88–3.69) |
| Median MELD (range) | 8 (6–32) |
Note: Data are represented as n (%) of patients, unless indicated otherwise. The percentages were rounded and may not sum to 100%.
Laboratory reference values: bilirubin < 1.2 mg/dL, ALT < 31 U/L, AST 35 U/L, ALP 35–105 U/L, GGT 5–36 U/L, creatinine 0.5–0.96 mg/dL, and INR 0.9–1.25
Patients using at least 1 immunosuppressant/biological (with/without simultaneous cortisone intake) are included.
Immunosuppressants: azathioprine (n = 14), tacrolimus (n = 3), mycophenolate mofetil (n = 2), and tofacitinib (n = 4). One patient received azathioprine and mycophenolate mofetil.
Biologicals: adalimumab (n = 1), vedolizumab (n = 6), ustekinumab (n = 1), golimumab (n = 1).
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; AIH, autoimmune hepatitis; ERCP, endoscopic retrograde cholangiopancreatography; GGT, gamma-glutamyl transferase; IBD, inflammatory bowel disease; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease.
Distribution of detected pathogens in bile samples of patients with PSC
The collected bile samples were tested for the presence of bacterial and fungal species. Positive bile cultures were obtained from 87 patients (76%). The median number of isolated bacteria or fungi per patient was 1 (0–6). In 42 patients (37%), only 1 pathogen was isolated, while in 45 patients (40%), multiple pathogens were detected. Enterococcus spp. (n = 42; 37%) were the most prevalent bacteria, followed by Enterobacteriaceae spp. (n = 28; 25%). Streptococcus spp. and/or Staphylococcus spp. were isolated in 31 patients (27%). Candida spp. were identified in bile cultures of 22 (19%) patients (Table 2). In a next step, we analyzed bile samples of 50 patients who received bile sampling before liver transplantation for reasons other than PSC (NAFLD, alcohol-associated fatty liver disease, and HCC). Microbial analysis revealed positive bile culture results in only 12% (6/50) (Supplemental Table S1, http://links.lww.com/HC9/A288).
TABLE 2.
Distribution of detected pathogens in bile samples of patients with PSC
| PSC cohort n = 114, n (%) | |
|---|---|
| Median no. bacterial/fungal isolates per patients (range) | 1 (0–6) |
| No. patients with positive cultures | 87 (76) |
| Enterobacteriaceae a | 28 (25) |
| Enterococcus spp.b | 42 (37) |
| Candida spp.c | 22 (19) |
| Other Streptococcus spp. and/or Staphylococcus spp.)d | 31 (27) |
| Other Streptococcus spp. | 25 (22) |
| Staphylococcus spp. | 10 (9) |
| Only 1 pathogen isolated | 42 (37) |
| Multiple (≥2) pathogens isolated | 45 (40) |
Note: Data are n (%) of patients, unless indicated otherwise. Primary sclerosing cholangitis (PSC); species (spp.).
Enterobacteriaceae include Escherichia coli, Klebsiella spp, Enterobacter spp., and Citrobacter spp.
Enterococcus spp. include Enterococcus faecalis, Enterococcus faecium, Enterococcus gallinarum, and Enterococcus casseliflacus.
Candida spp. include Candida albicans, Candida dubliniensis, Candida tropicalis, Candida krusei, Candida kefyr, and Candida glabrata.
Other Streptococcus and/or Staphylococcus include Staphylococcus haemolyticus, Staphylococcus aureus, Staphylococcus lentus, Staphylococcus epidermidis, Streptococcus parasanguinis, Sreptococcus salivinarius, Streptococcus sanguinis, Streptococcus mitis, Streptococcus oralis, Streptococcus cristatus, and Streptococcus anginosus.
Outcome analysis
Positive bile cultures are linked to an adverse outcome
During the observation period, 22 patients (19%) received a liver transplant and 5 patients (4%) died from a liver-related cause. Recurrent cholangitis episodes (ie, ≥3 episodes with the necessity of hospitalization and i.v. antibiotic treatment) occurred in 19 patients (17%) (Figure 1). Of the patients with positive bile cultures, 28% (n = 24) received liver transplants and/or died from a liver-related cause, while the proportion of patients with negative culture was 11% (n = 3) (OR, 3.048; 95% CI, 0.840–11.061; p = 0.079 (Supplemental Figure S1A, http://links.lww.com/HC9/A288, Supplemental Table S2, http://links.lww.com/HC9/A288). Patients with positive bile culture results suffered significantly more often from recurrent (≥3) cholangitis compared with patients with negative bile cultures (OR, 6.783; 95% CI, 0.861–53.406; p = 0.039) (Supplemental Figure S1B, http://links.lww.com/HC9/A288, Supplemental Table S2, http://links.lww.com/HC9/A288). Overall, 56% (n = 49) of patients with positive bile culture results suffered from at least 1 episode with cholangitis, which was significantly more frequent as compared with patients with negative bile cultures (OR, 5.674; 95% CI, 1.967–16.367; p = 0.001) (Supplemental Figure S1C, http://links.lww.com/HC9/A288, Supplemental Table S2, http://links.lww.com/HC9/A288).
FIGURE 1.
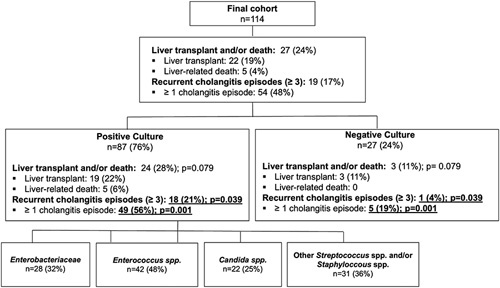
Flowchart with overview of outcome parameters of the total cohort and subcohorts. The p-values are based on the Pearson χ² test. Significant results (p < 0.05) are underlined and shown in bold type. Abbreviation: spp., species.
Presence of enterococcus spp. in bile is linked to adverse outcome
We next analyzed which specific microbes were linked to an unfavorable outcome with respect to liver transplantation and/or death as well as recurrent cholangitis episodes (Figure 2). Our data revealed that presence of Enterococcus spp. in bile was linked to a higher rate of liver transplantation and/or liver-related death as well as recurrent cholangitis episodes. A total of 27 patients (24%) received a liver transplant or died from liver-related causes. Of these, 15 patients revealed Enterococcus spp. in bile (OR, 2.778; 95% CI, 1.147–6.728; p=0.021) (Figure 3A, Supplemental Table S1, http://links.lww.com/HC9/A288). Interestingly, presence of Enterococcus spp. in bile was associated with occurrence of recurrent (≥3) cholangitis episodes (26% (n = 11); OR, 2.839; 95% CI, 1.037–7.768; p=0.037) (Figure 3B, Supplemental Table S1, http://links.lww.com/HC9/A288).
FIGURE 2.
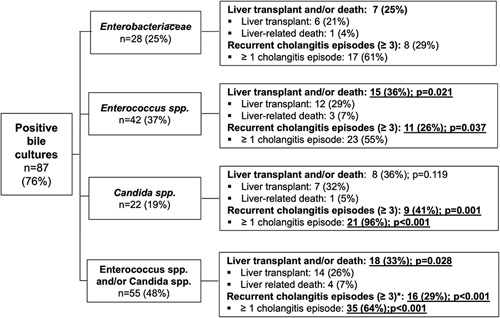
Flowchart with overview of outcome parameters of particular pathobionts. The p-values are based on the Pearson χ² test. Significant results (p < 0.05) are underlined and shown in bold type. Abbreviation: spp., species.
FIGURE 3.
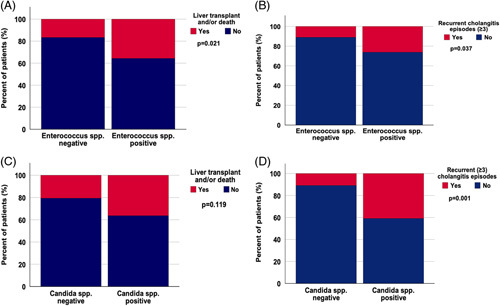
Presence of Enterococcus spp. is associated with poorer transplant-free survival and recurrent cholangitis episodes. (A) and (B) demonstrate the link between presence of Enterococcus spp. in bile and occurrence of liver transplant and/or liver-related death (OR, 2.778; 95% CI, 1.147–6.728; p = 0.021) (A) and recurrent cholangitis episodes (≥3) (OR, 2.839; 95% CI, 1.037–7.768; p = 0.037) (B). No significant correlation was found between biliary candidiasis and liver transplant and/or liver-related death (OR, 2.195; 95% CI, 0.804–5.996; p = 0.119) (C). There was a strong correlation between biliary candidiasis and recurrent cholangitis episodes (≥3) (OR, 5.677; 95% CI, 1.940–16.616; p < 0.001) (D). A p-value < 0.05 was considered significant. Abbreviation: spp., species.
Previous studies have reported an association between presence of Enterobacteriaceae spp. in bile with a poorer outcome.18 In contrast, we did not find an association with a shorter transplant-free survival, which might be related to the small number of patients in this subgroup. However, we found a strong (but not significant) association between Enterobacteriacae spp. in bile and recurrent cholangitis episodes (≥3) (OR, 2.727; 95% CI, 0.968–7.683; p=0.052) (Supplemental Table S1, http://links.lww.com/HC9/A288).
Patients with Candida spp. in bile were associated with recurrent cholangitis episodes
Analysis of patients with Candida spp. in bile revealed a higher frequency of cholangitis episodes in this subgroup. Remarkably, 96% of patients with biliary candidiasis suffered from at least 1 cholangitis episode (OR, 37.545; 95% CI, 4.829–291.891; p<0.001), and over 40% suffered from recurrent episodes (≥3) (OR, 5.677; 95% CI, 1.940–16.616; p=0.001). Although the proportion of patients who received a liver transplant and/or died from a liver-related cause was similarly high for Candida spp. as for Enterococcus spp., it did not reach statistical significance in patients with Candida spp., likely due to their overall low numbers (Figure 3C, D, Supplemental Table S1, http://links.lww.com/HC9/A288).
We thus combined patients positive for Enterococcus spp. and/or Candida spp. and confirmed a significantly higher occurrence of liver transplantation and/or liver-related death [18 (33%), OR, 2.703; 95% CI, 1.092–6.688; p=0.028]. Moreover, the presence of either Enterococcus spp. and/or Candida spp. in bile was linked to a higher frequency of developing recurrent cholangitis episodes (≥3) (OR, 7.658; 95% CI, 2.089–28.075; p=0.001). Overall, 64% of patients with Enterococcus spp. and/or Candida spp. developed at least 1 episode of cholangitis (OR, 3.684; 95% CI, 1.698–7.994; p=0.001) (Supplemental Figure S2A–C, http://links.lww.com/HC9/A288, Supplemental Table S1, http://links.lww.com/HC9/A288).
Identification of patient features that are linked to positive bile cultures
We next aimed to identify features associated with positive bile cultures and therefore analyzed various factors such as age at initial diagnosis, sex, liver cirrhosis, presence of concomitant diseases (IBD, AIH), presence of relevant stenosis, and medication intake (Table 3).
TABLE 3.
Clinical characteristics and OR for positive bile culture results
| Parameter | No. patients, n (%) | p | OR (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 27 (31) | 0.612 | 1.286 (0.486–3.403) |
| Male | 60 (69) | — | — |
| Age ≥30 y at initial diagnosis | 45 (52) | 0.835 | 1.098 (0.456–2.640) |
| Overlap syndrome with AIH | 10 (12) | 0.646 | 0.747 (0.214–2.605) |
| Immunosuppressant/biological intake | 24 (28) | 0.865 | 1.088 (0408–2.902) |
| Inflammatory bowel disease | 69 (79) | 0.005 | 3.560 (1.424–8.896) |
| Proton pump inhibitor intake | 23 (26) | 0.660 | 1.258 (0.451–3.505) |
| ≥2 ERCPs per patient | 81 (93) | 0.928 | 1.080 (0.205–5.691) |
| Relevant stenosis | 78 (90) | 0.082 | NA |
Note: Significant results (p < 0.05) are shown in bold type.
Abbreviations: AIH, Autoimmune hepatitis; ERCP, endoscopic retrograde cholangiopancreatography.
Concomitant IBD is a feature associated with positive bile cultures
Overall, 79% of patients with a positive bile culture result suffered concomitantly from IBD. Remarkably, we found a significant association between both factors (OR, 3.560; 95% CI, 1.424–8.896; p=0.005) (Table 2A). Multivariable logistic regression analysis including age at initial diagnosis, sex, presence of IBD, and number of performed ERCPs as potential confounders confirmed the presence of IBD as an independent factor that is linked to positive bile culture results (OR, 4.707; 95% CI, 1.688–13.128; p=0.003). Our data revealed no significant impact of age at initial diagnosis (OR, 1.018; 95% CI, 0.983–1.054; p = 0.323) or sex (OR, 1.394; 95% CI, 0.484–4.018; p=0.538) on the presence of pathogens in bile cultures of patients with PSC (Figure 4A, Supplemental Table S3, http://links.lww.com/HC9/A288).
FIGURE 4.
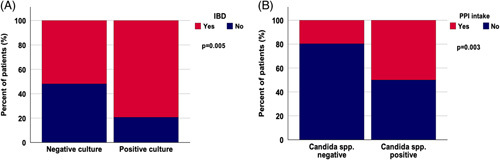
Presence of IBD is associated with positive bile culture results, and PPI intake is linked to biliary candidiasis. Presence of IBD is significantly associated with presence of microbes in bile (OR, 3.560; 95% CI, 1.424–8.896; p = 0.005) (A). PPI intake was significantly associated with biliary candidiasis (OR, 4.111; 95% CI, 1.540–10.973; p = 0.003) (B). A p-value < 0.05 was considered significant. Abbreviations: IBD, inflammatory bowel disease; PPI, Proton pump inhibitor; spp., species.
Identification of features associated with bacteriobilia and biliary candidiasis in patients with PSC
Features associated with bacteriobilia
Since different approaches are necessary to treat bacteriobilia and biliary candidiasis, we next subdivided our cohort of patients with positive bile cultures to identify confounders for bacteriobilia in bile and for biliary candidiasis, respectively.
In 71% of patients (n=81), different species of bacteria could be identified in bile. Contrary to previous reports, no association between age at initial diagnosis and bacteriobilia could be found (Table 3). However, subgroup analysis of patients with bacteriobilia revealed a significant association between the presence of Enterococcus spp. in bile and age at initial diagnosis (OR, 2.667; 95% CI, 1.201–5.919; p=0.015) (Supplemental Table S4, http://links.lww.com/HC9/A288).
Furthermore, no association between bacteriobilia and sex, presence of concomitant diseases such as AIH and IBD, PPI intake, presence of relevant stenosis, or a higher number of ERCPs could be detected (Supplemental Table S4, http://links.lww.com/HC9/A288).
Features associated with biliary candidiasis
PPI intake is associated with biliary candidiasis: As described above, presence of Candida spp. in bile was associated with a poorer outcome. Next, we performed further analysis to identify clinical features associated with biliary candidiasis. PPI intake was associated with the presence of biliary candidiasis (OR, 4.111; 95% CI, 1.540–10.972; p=0.003) (Table 4). Overall, 50% of patients with biliary candidiasis used PPIs at the time of bile sampling compared with 20% in the group without the presence of Candida spp. (Figure 4B). Multivariable binary logistic regression analysis validated this finding by demonstrating that PPI intake is a feature associated with biliary candidiasis with an HR of 3.559 (95% CI, 1.275–9.937; p=0.015) (Supplemental Table S5, http://links.lww.com/HC9/A288). We did not find a link between biliary candidiasis and sex, age at initial diagnosis, presence of concomitant diseases such as AIH and IBD, presence of relevant stenosis, and a higher number of ERCPs (Table 4).
TABLE 4.
Clinical characteristics and OR of patients with bacteriobilia and biliary candidiasis
| Bacteriobilia (n = 81) | Biliary candidiasis (n = 22) | |||||
|---|---|---|---|---|---|---|
| No. patients, n (%) | OR (95% CI) | p | No. patients, n (%) | OR (95% CI) | p | |
| Sex | ||||||
| Female | 26 (32) | 2.364 (0.263–21.271) | 0.430 | 8 (36) | 1.451 (0.544–3.865) | 0.455 |
| Male | 55 (68) | — | — | 14 (64) | — | — |
| Age at ID (≥30 y) | 44 (54) | 4.757 (0.509–44.436) | 0.136 | 11 (52) | 1.030 (0.398–2.663) | 0.952 |
| Overlap with AIH | 9 (11) | 0.625 (0.065–5.966) | 0.681 | 3 (14) | 1.163 (0.295–4.580) | 0.829 |
| Inflammatory bowel disease | 64 (79) | 0.753 (0.082–6.882) | 0.801 | 17 (77) | 1.339 (0.448–4.006) | 0.600 |
| Proton pump inhibitor use | 21 (26) | 0.700 (0.119–4.104) | 0.691 | 11 (50) | 4.111 (1.540–10.973) | 0.003 |
| Relevant stenosis | 72 (89) | NA | 0.389 | 19 (86) | 0.442 (0.101–1.926) | 0.266 |
| ≥2 ERCPS per patient | 75 (93) | NA | 0.490 | 22 (100) | NA | 0.151 |
Note: Significant results (p < 0.05) are shown in bold type.
Abbreviations: AIH, autoimmune hepatitis; ERCP, endoscopic retrograde cholangiopancreatography; ID, initial diagnosis.
DISCUSSION
Here, we investigated the association of bacterial or fungal occurrence in bile fluid of patients with PSC with the clinical characteristics and outcome. Our results indicate that bacteriobilia and fungobilia are associated with an unfavorable outcome. We identified the presence of concomitant IBD as a feature associated with the presence of pathogens in bile of patients with PSC and revealed that PPI intake is linked to biliary candidiasis.
Our data demonstrate that the presence of Enterococcus spp. in bile samples of patients with PSC is associated with a poorer outcome regarding recurrent cholangitis episodes and a higher frequency of liver transplantation and/or liver-related death. Notably, this finding is in line with recent PSC patient data from another high-volume tertiary liver center in Germany, in which bile samples obtained during routine ERCP were collected and analyzed.18 However, the authors used a different study design by excluding all patients with acute cholangitis episodes and recent antibiotic use. Moreover, contrary to our procedure guidelines, they applied antibiotic prophylaxis after obtaining bile samples.19 Despite the 2 different approaches, both studies identified a connection between Enterococcus spp. and disease progression. This is also reflected by emerging data from mechanistic studies, which demonstrated that bacterial microbes such as E. faecalis, the most common identified genus in bile culture studies, and E. gallinarum, induce a T helper cell type 17 immune response in patients with PSC.3,20,21 Because of its production of matrix metalloproteinases such as gelatinase, E. faecalis contributes to mucosal inflammation and an impaired intestinal barrier.20–23 Of note, microbial dysbiosis characterized by high Enterococcus abundance is linked to an increase in the potentially cancerogenic and proinflammatory bile acid taurolithocholic acid.20 These data along with our findings should prompt further research to investigate the pathogenic role of Enterococcus spp. For development and progression of PSC. Targeting Enterococcus spp. might represent a promising therapeutic strategy for PSC. In this context, studies including oral vancomycin treatment, which is highly effective against gram-positive bacteria such as Enterococcus spp., have already shown some promising effects such as biochemical improvement.24,25 However, another study could not confirm this beneficial effect.26 Whether oral vancomycin is indeed sufficient to efferently target biliary enterococci is not clear. Bacteriophages are another promising therapeutic agent to target particular pathobionts. Remarkably, combined phage-based treatment against Klebsiella pneumoniae was recently used in IBD models.27
In addition to bacterial infections common for PSC, fungal infections appear to be associated with a poor prognosis. We detected Candida spp. in 19% of all patients, which is in line with previous studies.18,28 These patients showed markedly increased rates of recurrent cholangitis episodes and higher rates of liver transplantation and/or death, although this parameter did not reach statistical significance, likely due to low patient numbers. Our data are in line with previous clinical studies, which have shown an influence of Candida spp. on outcome.18,28,29 In addition, some experimental studies indicate high Th17 cell response in patients with Candida albicans, although further mechanistic studies are required.3,30 It still needs to be explored if biliary candidiasis is the cause of poorer outcome or simply an epiphenomenon showing advanced PSC with recurrent complications. Therefore, at present, it is unclear whether and how patients with PSC and biliary candidiasis will benefit from antifungal treatment. Indeed, antifungal medication can aggravate liver damage, making it even more important to prevent biliary candidiasis in these patients. Up to now, studies investigating biliary candidiasis in liver disorders have not analyzed the effect of PPI intake.28,31 Thus, to our knowledge, our data are the first to reveal PPI intake as a feature associated with biliary candidiasis in patients with PSC. In line with our findings, a higher risk for various infections has already been reported in patients with prolonged PPI intake.32 This higher risk for infection has been explained by bacterial overgrowth in the intestine, resulting in increased bacterial translocation and migration of bacteria through the portal vein into the liver.33–35 In addition, acid-suppressive therapy is known to alter immune defense mechanisms through impaired production of the neutrophil extracellular and intracellular oxygen intermediates necessary for bactericidal effects and reduced expression of adhesion molecules, thereby attenuating endothelial-leukocyte interactions.36,37 Accordingly, several studies have detected Candida spp. overgrowth in duodenal aspirates, gastric juice, and jejunal fluid on PPI treatment.33,38–40 Moreover, PPI intake has been proposed as a risk factor for Candida esophagitis in immunocompetent individuals, and case-controlled studies have indicated that chronic PPI use might be a predisposing factor to Candida-associated intra-abdominal infections.41,42 PPIs are widely used drugs in clinical practice, and the rate of inappropriate prescriptions without indication is high. Our data implicate that particularly in patients with PSC, a critical evaluation of the indication for PPI is necessary to avoid biliary candidiasis and further disease progression.
We identified an association between the presence of concomitant IBD and positive bile culture results. A possible explanation for this finding could be the previously reported higher number of biliary strictures in patients with PSC-IBD.43,44 However, in our study, we only found a trend toward an association between the presence of relevant stenosis and positive bile cultures. Reasons for the absence of a significant association might be the overall high proportion of patients with relevant strictures in our cohort (90%). Another explanation for the association between IBD-PSC and positive bile culture might be the intake of immunosuppressants in patients with IBD, which makes these patients more susceptible for bacterial colonization. Since all patients were treated at a single center, confirming the results in a multicenter study would greatly strengthen the data. The low patient numbers included in different subgroup analyses might have masked existing differences between these groups. Moreover, culture-based techniques as opposed to 16S ribosomal RNA (rRNA) analysis have limitations for identifying bacterial and fungal composition of bile. However, our results are in line with findings from studies including 16S rRNA analysis.20 Furthermore, it should be noted that a substantial number of patients with PSC treated at our tertiary liver center do not fulfill the criteria for ERCP. Thus, our patient cohorts might include rather patients with higher disease activity compared with the general/average PSC population.
Collectively, the present study demonstrates that presence of Enterococcus spp. and/or Candida spp. in patients with PSC is associated with an adverse outcome. We identified the presence of concomitant IBD as a clinical feature associated with positive bile cultures in patients with PSC and revealed that PPI intake is linked to biliary candidiasis. The exact pathogenic role of specific bacteria and their virulence factors for the initiation and progression of PSC should be investigated in the future. Our data warrant the initiation of prospective trials to explore how the presence and the eradication of specific pathogens in bile will affect the course of disease in patients with PSC.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Rita Zietlow for editing the manuscript. Burcin Özdirik is a participant in the BIH Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité (BIH).
FUNDING INFORMATION
Michael Sigal has received funding from the German Research Foundation (DFG Si 1983/4-1). Frank Tacke is supported by the German Research Foundation CRC 1382 (project ID 403224013) and CRC/TR 296.
CONFLICTS OF INTEREST
Frank Tacke consults, advises, is on the speakers’ bureau, and received grants from Gilead. He consults, advises, and is on the speakers’ bureau for AbbVie. He consults, advises, and received grants from Allergan, Bristol-Myers Squibb, and Inventiva. He consults and advises Alnylam, Intercept, Pfizer, Novartis, Novo Nordisk, and Sanofi. He is on the speakers’ bureau for Falk, Merz, and Orphalan. The remaining authors have no conflicts to report.
DATA AVAILABILITY STATEMENT
Data are available on request from the Department of Hepatology and Gastroenterology of the Charité University Medicine Berlin for researchers who meet the criteria for access to confidential data (gastro-cvk@charite.de).
Footnotes
Abbreviations: AIH, autoimmune hepatitis; EASL, European Association for the study of Liver disease; ERCP, endoscopic retrograde cholangiography-pancreatography; IBD, inflammatory bowel disease; ID, initial diagnosis; INR, international normalized ratio; MALDI-MS, matrix-associated laser desorption/ionization time-of-flight mass spectrometry; MELD, model of end-stage liver disease; PSC, primary sclerosing cholangitis; PPI, proton pump inhibitor; spp., species.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
Burcin Özdirik, Email: burcin.oezdirik@charite.de.
Maria Scherf, Email: maria.scherf@charite.de.
Ana Brumercek, Email: Ana.brumercek@charite.de.
Jule M. Nicklaus, Email: jule-marie.nicklaus@charite.de.
Tassilo Kruis, Email: tassilo.kruis@laborberlin.de.
Philipp K. Haber, Email: Phillipp.haber@charite.de.
Johann Pratschke, Email: Johann.Pratschke@charite.de.
Frank Tacke, Email: frank.tacke@charite.de.
Michael Sigal, Email: michael.sigal@charite.de.
REFERENCES
- 1. Hov JR, Karlsen TH. The microbiota and the gut-liver axis in primary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol. 2022;20:135–54. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on Sclerosing Cholangitis. J Hepatol. 2022. doi: 10.1016/j.jhep.2022.05.011 [DOI] [PubMed] [Google Scholar]
- 3. Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E, et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology. 2013;58:1084–93. [DOI] [PubMed] [Google Scholar]
- 4. Lazaridis KN, Gores GJ. Primary sclerosing cholangitis and cholangiocarcinoma. Semin Liver Dis. 2006;26:42–51. [DOI] [PubMed] [Google Scholar]
- 5. Karlsen TH, Vesterhus M, Boberg KM. Review article: controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment Pharmacol Ther. 2014;39:282–301. [DOI] [PubMed] [Google Scholar]
- 6. Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T, et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol. 2019;4:492–503. [DOI] [PubMed] [Google Scholar]
- 7. Olsson R, Björnsson E, Bäckman L, Friman S, Höckerstedt K, Kaijser B, et al. Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. J Hepatol. 1998;28:426–32. [DOI] [PubMed] [Google Scholar]
- 8. Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. [DOI] [PubMed] [Google Scholar]
- 9. Lindor KD, Kowdley KV, Harrison ME. American College of Gastroenterology. ACG Clinical Guideline: primary sclerosing cholangitis. Am J Gastroenterol. 2015;110:646–59. [DOI] [PubMed] [Google Scholar]
- 10. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–67. [DOI] [PubMed] [Google Scholar]
- 11. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis—a comprehensive review. J Hepatol. 2017;67:1298–1323. [DOI] [PubMed] [Google Scholar]
- 12. Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17–30. [DOI] [PubMed] [Google Scholar]
- 13. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair: EASL Governing Board representative: Panel members: EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659–689. [DOI] [PubMed] [Google Scholar]
- 14. Corpechot C, Gaouar F, El Naggar A, Kemgang A, Wendum D, Poupon R, et al. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. 2014;146:970–979; quiz e915-976. [DOI] [PubMed] [Google Scholar]
- 15. Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: the model for end-stage liver disease—should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis ? Aliment Pharmacol Ther. 2005;22:1079–1089. [DOI] [PubMed] [Google Scholar]
- 16. Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. [DOI] [PubMed] [Google Scholar]
- 17.European Society of Gastrointestinal Endoscopy; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J Hepatol. 2017;66:1265–81. [DOI] [PubMed] [Google Scholar]
- 18. Zigmond E, Zecher BF, Bartels AL, Ziv-Baran T, Rösch T, Schachschal G, et al. Bile duct colonization with Enterococcus sp. associates with disease progression in Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2022;S1542-3565:00879–5. [DOI] [PubMed] [Google Scholar]
- 19. Zigmond E, Zecher BZ, Bartels A, Ziv T, Rösch T, Schachschal G, et al. Bacterial and fungal colonisation of bile ducts associates with the prognosis of primary sclerosing cholangitis In Proceedings of the International Liver Congress (ILC). 2021.
- 20. Liwinski T, Zenouzi R, John C, Ehlken H, Rühlemann MC, Bang C, et al. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 2020;69:665–72. [DOI] [PubMed] [Google Scholar]
- 21. Kwon W, Jang JY, Kim EC, Park JW, Han IW, Kang MJ, et al. Changing trend in bile microbiology and antibiotic susceptibilities: over 12 years of experience. Infection. 2013;41:93–102. [DOI] [PubMed] [Google Scholar]
- 22. Özdirik B, Müller T, Wree A, Tacke F, Sigal M. The role of microbiota in primary sclerosing cholangitis and related biliary malignancies. Int J Mol Sci. 2021;22:6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steck N, Hoffmann M, Sava IG, Kim SC, Hahne H, Tonkonogy SL, et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology. 2011;141:959–71. [DOI] [PubMed] [Google Scholar]
- 24. Ali AH, Damman J, Shah SB, Davies Y, Hurwitz M, Stephen M, et al. Open-label prospective therapeutic clinical trials: oral vancomycin in children and adults with primary sclerosing cholangitis. Scand J Gastroenterol. 2020;55:941–50. [DOI] [PubMed] [Google Scholar]
- 25. Tabibian JH, Weeding E, Jorgensen RA, Petz JL, Keach JC, Talwalkar JA, et al. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther. 2013;37:604–12. [DOI] [PubMed] [Google Scholar]
- 26. Deneau MR, Mack C, Mogul D, Perito ER, Valentino PL, Amir AZ, et al. Oral vancomycin, ursodeoxycholic acid, or no therapy for pediatric primary sclerosing cholangitis: a matched analysis. Hepatology. 2021;73:1061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Federici S, Kredo-Russo S, Valdés-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185:2879–98.e2824. [DOI] [PubMed] [Google Scholar]
- 28. Rupp C, Bode KA, Chahoud F, Wannhoff A, Friedrich K, Weiss KH, et al. Risk factors and outcome in patients with primary sclerosing cholangitis with persistent biliary candidiasis. BMC Infect Dis. 2014;14:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rudolph G, Gotthardt D, Klöters-Plachky P, Kulaksiz H, Rost D, Stiehl A. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J Hepatol. 2009;51:149–55. [DOI] [PubMed] [Google Scholar]
- 30. Bacher P, Hohnstein T, Beerbaum E, Röcker M, Blango MG, Kaufmann S, et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell. 2019;176:1340–55.e1315. [DOI] [PubMed] [Google Scholar]
- 31. Lenz P, Eckelskemper F, Erichsen T, Lankisch T, Dechêne A, Lubritz G, et al. Prospective observational multicenter study to define a diagnostic algorithm for biliary candidiasis. World J Gastroenterol. 2014;20:12260–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bettinger D, Martin D, Rieg S, Schultheiss M, Buettner N, Thimme R, et al. Treatment with proton pump inhibitors is associated with increased mortality in patients with pyogenic liver abscess. Aliment Pharmacol Ther. 2018;47:801–808. [DOI] [PubMed] [Google Scholar]
- 33. Fisher L, Fisher A. Acid-suppressive therapy and risk of infections: pros and cons. Clin Drug Investig. 2017;37:587–624. [DOI] [PubMed] [Google Scholar]
- 34. Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483–90. [DOI] [PubMed] [Google Scholar]
- 35. Lin HF, Liao KF, Chang CM, Lin CL, Lai SW. Correlation between proton pump inhibitors and risk of pyogenic liver abscess. Eur J Clin Pharmacol. 2017;73:1019–25. [DOI] [PubMed] [Google Scholar]
- 36. Zedtwitz-Liebenstein K, Wenisch C, Patruta S, Parschalk B, Daxböck F, Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit Care Med. 2002;30:1118–22. [DOI] [PubMed] [Google Scholar]
- 37. Yoshida N, Yoshikawa T, Tanaka Y, Fujita N, Kassai K, Naito Y, et al. A new mechanism for anti-inflammatory actions of proton pump inhibitors--inhibitory effects on neutrophil-endothelial cell interactions. Aliment Pharmacol Ther. 2000;14(suppl 1):74–81. [DOI] [PubMed] [Google Scholar]
- 38. Shindo K, Machida M, Fukumura M, Koide K, Yamazaki R. Omeprazole induces altered bile acid metabolism. Gut. 1998;42:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh S, Singh N, Kochhar R, Talwar P, Mehta SK. Cimetidine therapy and duodenal candidiasis: role in healing process. Indian J Gastroenterol. 1992;11:21–22. [PubMed] [Google Scholar]
- 40. Goenka MK, Kochhar R, Chakrabarti A, Kumar A, Gupta O, Talwar P, et al. Candida overgrowth after treatment of duodenal ulcer. A comparison of cimetidine, famotidine, and omeprazole. J Clin Gastroenterol. 1996;23:7–10. [DOI] [PubMed] [Google Scholar]
- 41. Nassar Y, Eljabbour T, Lee H, Batool A. Possible risk factors for Candida esophagitis in immunocompetent individuals. Gastroenterology Res. 2018;11:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cat TB, Charash W, Hebert J, Marden BT, Corbett SM, Ahern J, et al. Potential influence of antisecretory therapy on the development of Candida-associated intraabdominal infection. Ann Pharmacother. 2008;42:185–91. [DOI] [PubMed] [Google Scholar]
- 43. Rabinovitz M, Gavaler JS, Schade RR, Dindzans VJ, Chien MC, Van Thiel DH. Does primary sclerosing cholangitis occurring in association with inflammatory bowel disease differ from that occurring in the absence of inflammatory bowel disease? A study of sixty-six subjects. Hepatology. 1990;11:7–11. [DOI] [PubMed] [Google Scholar]
- 44. Navaneethan U, Venkatesh PG, Lashner BA, Shen B, Kiran RP. The impact of ulcerative colitis on the long-term outcome of patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012;35:1045–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the Department of Hepatology and Gastroenterology of the Charité University Medicine Berlin for researchers who meet the criteria for access to confidential data (gastro-cvk@charite.de).


