ABSTRACT.
This study aimed to isolate and characterize phages as an alternative treatment of multidrug- or pan-drug-resistant Pseudomonas aeruginosa. Phage titers and bacterial densities correlated, with the phages disappearing after bacteria were eliminated. We isolated phages in filtered sewage water by a double-layered agar spot test. Fifty-eight P. aeruginosa strains were used to screen the host spectrum of the 14 phages isolated. Random amplification of polymorphic DNA-typing polymerase chain reaction was used to analyze the genomic homologies of the 58 host bacteria strains and four phages with a broad host spectrum. Transmission electron microscopy was used to observe the morphology of the four phages with a broad host spectrum. Mice with intraabdominal P. aeruginosa infection were used as an in vivo animal model to investigate the therapeutic effect of the selected phage. Four virulent phages with a broad host spectrum specific to P. aeruginosa strains were isolated. They were all double-stranded DNA viruses and belonged to four different genotypes. The test curve showed that phage I had the highest adsorption rate, the shortest latent period, and the largest burst size. The infected mouse model indicated that small doses of phage I could prevent the death of infected mice. Phage titers and bacterial densities correlated, with phages disappearing after bacteria were eliminated. Phage I was the most effective and promising treatment of drug-resistant P. aeruginosa.
BACKGROUND
Pseudomonas aeruginosa is a common opportunistic pathogen belonging to the class of nonfermentative bacteria and is widely distributed in air, water, and soil. The bacteria are also present on the skin and in the airway and intestinal tract of healthy people. They can cause deadly infections in patients with cystic fibrosis, burns, AIDS, and other immunosuppressed conditions.1 Pseudomonas aeruginosa is the main gram-negative bacterial species in nosocomial infections, and is the most commonly isolated bacterial pathogen from patients in intensive care units.2–4 Recently, the detection rate of multidrug- or pan-drug-resistant P. aeruginosa has increased because of the incorrect use of antibiotics and the bacteria’s ability to acquire drug resistance. Consequently, the treatment of drug-resistant P. aeruginosa with currently available antibiotics has proven to be very challenging.5 Therefore, an investigation of novel therapeutic methods for treating multidrug-resistant pathogens is needed. As obligate bacterial viruses, phages are very host specific; they self-multiply and lyse bacteria. Bacteriophages (“phages”) that can specifically lyse their host bacteria are considered of promising potential for development as therapeutics.6,7 Although the application of phages as therapeutic agents to control pathogens has already been studied since the discovery of phages by Frederick Twort in 1915 and Félix d’Hérelle in 1917, there is renewed interest in phage therapy, especially when there is a limited range of antibiotics available for the treatment of a drug-resistant bacterial infection. During phage therapy, one problem is the evolution of phage-resistant bacteria, but this problem can be overcome by finding new phages, as Schooley et al. have reported.8 Therefore, the isolation and identification of novel, well-characterized phages with a broad antibacterial spectrum and strong lytic activity are vitally important for phage therapy. Hospitals have been considered as environments that harbor a variety of pathogens,9 and the sewage from hospitals usually contains a diversity of phages. In this study, we isolated four phages from hospital sewage with a broad host spectrum specific to P. aeruginosa. Further study regarding their characteristics and potential therapeutic value was carried out in a mouse model.
MATERIALS AND METHODS
Bacteria strains and preparations.
Eleven P. aeruginosa strains were selected to screen the P. aeruginosa-specific phages. Fifty-eight P. aeruginosa strains were used to identify phages with a broad host spectrum. All P. aeruginosa strains were the previous storage strains in our laboratory and were stored in skim milk at −20°C. The P. aeruginosa strains are numbered by our laboratory in chronological order (Supplemental Table 1). The Phoenix 100 (Becton, Dickinson, Franklin Lakes, NJ) was used to identify P. aeruginosa using a gram-negative bacterial identification card and antimicrobial susceptibility test card. The bacteria were revived on Luria-Bertani (LB) agar and then cultivated at 37°C overnight. The bacterial suspension was cultivated in LB broth at 37°C for 4.5 hours on a shaker at 200 rpm. Bacterial growth was monitored by testing their turbidity using the optical density at 600 nm (OD600); 1 OD corresponds to 3 × 108 cells per milliliter.
Isolation and enrichment of phages.
Sewage water from 16 hospitals in Beijing, China was collected and centrifuged (5,000 × g, 5 minutes at 4°C). The supernatants were filtered through a sterile filter membrane with a pore size of 0.45 μm. The filtrate was then tested for the presence of phages by a double-layered agar spot test (DLAST) with 11 P. aeruginosa strains. The test was carried out by dropping 5 μL of the filtrate onto double-layered agar made by topping 0.8% semisolid LB broth with the host bacteria onto the solid LB agar plate. The clear phage plaque formed on the top layer was picked and suspended in LB broth and then cultivated together with the host bacteria at 37°C on a shaker at 200 rpm. The host bacteria were all lysed and the phages were enriched with three repeated centrifugations at 10,000 rpm for 4 minutes and filtered using a membrane with a pore size of 0.45 μm to remove cell debris. The phage titer was tested by double-layered agar with a series of 2-fold dilutions. The enriched phage suspension was diluted in multiple proportions with LB broth. Then, 10 μL of the dilution was added to 200 μL of the host bacterial suspension (1 × 108 colony-forming units [CFUs] per milliliter) and mixed for 5 minutes. The mixture was added to 8 mL of 45°C 0.8% semisolid LB broth to produce the top agar and then was layered on the solid LB agar plate and incubated overnight at 37°C. The phage plaque-forming units (PFUs) were counted; phage titer (PFUs per milliliter) = the count of the PFUs × the dilution ratio × 100.
Host range test and covering spectrum test using clinically isolated P. aeruginosa strains.
The broad-host-spectrum phages were screened by DLAST using 58 P. aeruginosa strains. Briefly, 5 μL of phage suspension was spotted on the bacterial lawn as described above. The genomic homologies of the 58 host bacteria strains were analyzed by random amplification of polymorphic DNA-typing polymerase chain reaction (RAPD-PCR).10 The bacterial genome was extracted by boiling. The primer used in RAPD-PCR was primer 272 (5′-AGCGGGCCAA-3′).11 The amplification volume was 20 μL, which included 2 μL primers (100 μmol/L), 10 μL 2× PowerTaq PCR Master Mix, 1.5 μL template, and 6.5 μL double-distilled H2O. The parameters of RAPD-PCR were as follows: 4 cycles of 5 minutes at 94°C, 5 minutes at 36°C, and 5 minutes at 72°C; 30 cycles of 1 minute at 94°C, 1 minute at 36°C, and 2 minutes at 72°C; and a final extension at 72°C for 10 minutes. The products were analyzed on 0.8% agarose gels. The relatedness of the RAPD-PCR patterns was determined using the Dice coefficient/unweighted paired-group method with arithmetic mean analysis with 1% position tolerance. Pseudomonas aeruginosa strains with a similarity over 85% were considered to be the same clonal type.
Genome extraction and RAPD typing of phages.
The digested genomic profiles and RAPD typing were used for confirming the features of the phage genome and grouping the screened phages. Phage particles were concentrated with 10% polyethylene glycol (PEG) 6000 (11,000 × g, 10 minutes at 4°C), and the precipitate was resuspended in TM buffer (0.5M Tris-HCl (pH 7.5)/80mM MgSO4). The phage genome was obtained by the protease K/SDS (sodium dodecyl sulfate) method.12 Ethylenediaminetetraacetate, protease K, and SDS were added into the phage suspension at final concentrations of 20 mmol/L, 50 μg/mL, and 5 g/L, respectively. The mixture was incubated at 56°C for 1 hour and then centrifuged (10,000 rpm, 5 minute) to collect the water layer. Phenol:chloroform:isoamyl alcohol (25:24:1) was used to extract the phage genome from the water layer. DNase I, RNase, and restriction enzyme digestions were performed according to the manufacturer’s instructions (Sangon, Shanghai, China). Primer 106 (5′-AGTCAGCCAC-3′)13 was used for RAPD typing of the phages with a broad host spectrum as previously described.14 RAPD-PCR products were observed using 0.8% agarose gels stained with ethidium bromide. Phages with the same bands in the gels were placed in the same category.
Morphology of phage virions by electron microscopy.
Phage particles were prepared using PEG 6000 precipitation. Chloroform was used to remove residual PEG 6000. After three washes with 0.1 mol/L ammonium acetate solution (pH 7.0), the retained solution with the phage particles was used directly for negative staining by 2% uranyl acetate and examined by an H-7500 transmission electron microscope operated at 80 kV (Hitachi, Tokyo, Japan).
Multiplicity of infection, adsorption efficiency, and one-step growth curve experiment with phages.
Multiplicity of infection (MOI) = phage number/host bacteria number. The optimal MOI test is described briefly as follows. Tenfold serial dilutions (MOI 0.0001, 0.001, 0.01, 0.1, 1, 10) of phage solution were mixed with P. aeruginosa at the exponential growth phase (1 × 108 CFUs per milliliter) and the mixture was incubated at 37°C for 4 hours on a shaker at 200 rpm. Then the mixture was centrifuged at 10,000 × g for 10 minutes, and the supernatants containing phages were taken for titer determination to test the counts of the phages. Each MOI test was repeated three times and the phages with the optimal MOI were chosen for subsequent experiments.
An adsorption efficiency test of phages was performed using P. aeruginosa and phages at the optimal MOI, and then the mixture was incubated at room temperature on a shaker at 200 rpm. The adsorption efficiency of the phages was calculated by counting the nonadsorbed phage particles at the optimal MOI using the following equation: adsorption efficiency = 1 − (nonadsorbed phage particles/total phage particles). The incubated samples were taken out at time points 0, 2, 5, 10, 15, 20, and 30 minutes and centrifuged at 12,000 rpm for 5 minutes. The supernatants containing the phages that failed to adsorb onto the bacterial cells were counted by PFU test.
A one-step growth curve experiment was carried out to determine the latent period and phage burst size. Cells of P. aeruginosa incubated to mid-exponential phase were harvested by centrifugation and resuspended in fresh LB broth (108 CFUs per milliliter), and phages at the optimal MOI were added for adsorption for 5 minutes. The mixture was diluted 10,000-fold with LB broth and incubated at 37°C on a shaker at 200 rpm. Samples of mixed culture were taken out at intervals of 5–10 minutes until 100 minutes, and the phages in the mixture were counted by PFU test.
Abdominal cavity infection by P. aeruginosa and the determination of the minimum lethal dose in a mouse model.
Pseudomonas aeruginosa (strain number 1950) with multiple drug resistance, freshly identified from an in-patient case, was chosen to infect mice. Six- to 8-week-old BALB/c female mice were used to establish an animal model with intraperitoneal P. aeruginosa infection.15,16 Seven groups (each group with five mice and three independent trials) were set to test the minimum lethal dose (MLD). The bacterial suspension was grown in 15 mL LB broth at 37°C until the mid-log phase (OD600 0.5); the turbidity of the suspension was measured by a turbidity instrument, with 0.5 Mc unit equal to 1.5 × 108 CFUs per milliliter. The suspension was then centrifuged at 10,000 rpm for 5 minutes. The bacterial precipitate was washed and resuspended in 5 mL normal saline. With an appropriate dilution, the turbidity was measured to determine bacterial cell numbers; 1-mL serial dilutions of bacteria were injected intraperitoneally into mice to determine the MLD and the mice were observed for at least 5 days. Seven injection doses of the bacteria (P. aeruginosa strain 1950) were tested with bacterial concentrations at 3.0 × 108, 1.5 × 108, 7.5 × 107, 5.0 × 107, 3.0 × 107, 1.5 × 107, and 7.5 × 106 CFUs per milliliter. All experiments and surgical procedures were approved by the Laboratory Animal Care and Use Committee at Capital Medical University, which abides by the NIH Guide for the Care and Use of Laboratory Animals17 and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.18
Phage therapeutic effect in the mouse model.
Phage I, with the broadest host spectrum among the four screened phages, was chosen for the therapeutic test using the mouse model. Eight groups (including one control group) of mice (five mice in each group) were infected by intraperitoneal injection of P. aeruginosa strain 1950 at the MLD. Immediately, mice in the seven treatment groups were injected intraperitoneally with 1 mL of phage I, with MOIs at 0.0001, 0.001, 0.01, 0.1, 1, 10, and 100. The control group was treated with 1 mL of LB broth. The health condition of the mice was observed for at least 7 days.
Seven paired groups of mice (five mice in each group) were treated by intraperitoneal injection of P. aeruginosa strain 1950 at the MLD for delayed phage treatment. The injection of a single dose of phage I, 1 mL at an MOI of 1, was initiated at 0, 15, 30, 45, 60, 180, and 360 minutes after bacterial injection. The paired control group was simultaneously injected with the equivalent volume of LB broth. The health condition of the mice was observed for at least 7 days.
The phage mixture was also tested to treat the mouse model. Briefly, phages I, II, III, and IV with a broad host spectrum were mixed to produce a phage cocktail.19,20 Four P. aeruginosa strains (1748, 1768, 1958, and 1833) with multidrug resistance were selected to infect four paired groups of mice (five mice in each group, one strain per paired group) at the MLD (5.0 × 107 CFUs per milliliter). Pseudomonas aeruginosa strain 1833 could not be lysed by the four selected phages. For the four treatment groups, 1 mL of the phage mixture (MOI 1) was administered, whereas the four paired control groups were injected with the equivalent volume of LB broth. The survival rates of the mice were monitored for at least 7 days.
Distribution of phages and bacteria in vivo.
Three groups of mice (five mice in each) were intraperitoneally injected: one group with 1 mL P. aeruginosa strain 1950 at 5 × 107 CFUs per milliliter, the second group with phage I at 5 × 108 PFUs per milliliter, and the third group with both bacteria and phage I at the dose above. After the injection, at approximately 2, 4, 6, 24, 48, and 72 hours, 0.1 mL of blood was taken into a heparin anticoagulant tube by puncturing the inner canthus vein of the mice with a capillary tube. Then, the heparinized blood was diluted with normal saline, and the CFUs of the bacteria were tested to quantify bacterial cells on LB medium plates. PFUs of phage I were tested to count the phages. Blood samples were also collected from untreated mouse groups to ensure that the mice in the experiments were not contaminated by phages or bacteria.
Statistical analysis.
Descriptive statistical tests were used for the evaluation of adsorption efficiency, one-step growth curves of the phages, and results of the experiments using the mouse model. The χ2 test was used for frequency (in the phage therapy part, the survival rates of the mice treated with phages compared with those of the control group). In all analyses, a two-sided significance level of P < 0.05 was considered statistically significant.
RESULTS
Isolated phages and their spectra.
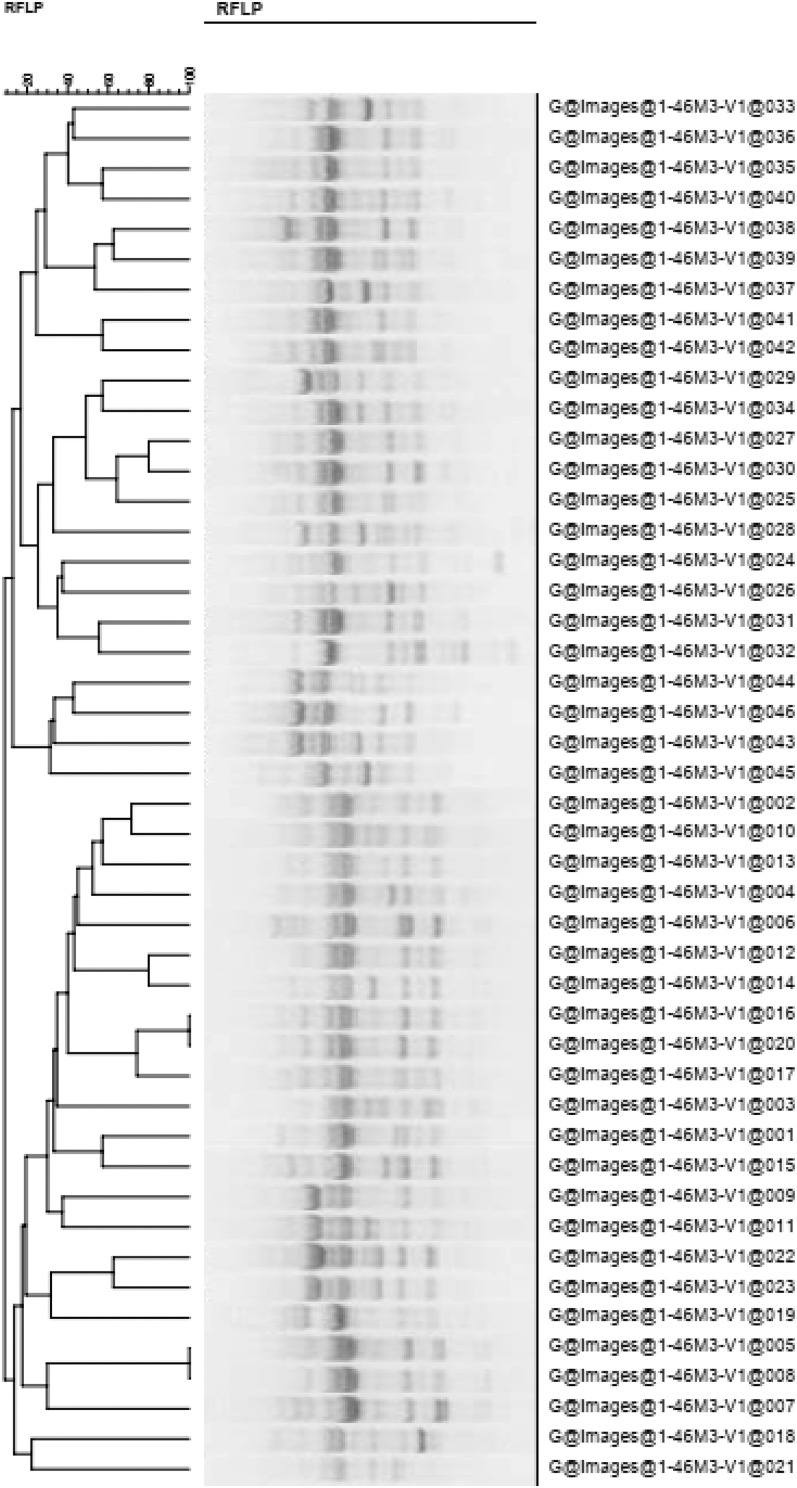
Fourteen phages were isolated from the sewage water of 16 hospitals in Beijing, China. The lysis spectrum of a combination of four phages covered 46 of the 58 P. aeruginosa strains tested. Each of the four phages could lyse more than 25 P. aeruginosa strains with different genotypes, and they were named as broad-host-spectrum phages I, II, III, and IV. Phages I, II, III, and IV could lyse 42, 35, 25, and 28 P. aeruginosa host strains with different genotypes, respectively (Supplemental Figure 1 and Figure 1).
Figure 1.
Homologous cluster analysis of the 46 Pseudomonas aeruginosa strains that could be lysed by the phages. This indicates that bacteria numbers 5, 8, 16, and 20 belonged to the same genotype, whereas the other host bacterial genotypes were different. RFLP = restriction fragment length polymorphism.
Genomic typing and electron microscopy of phages.
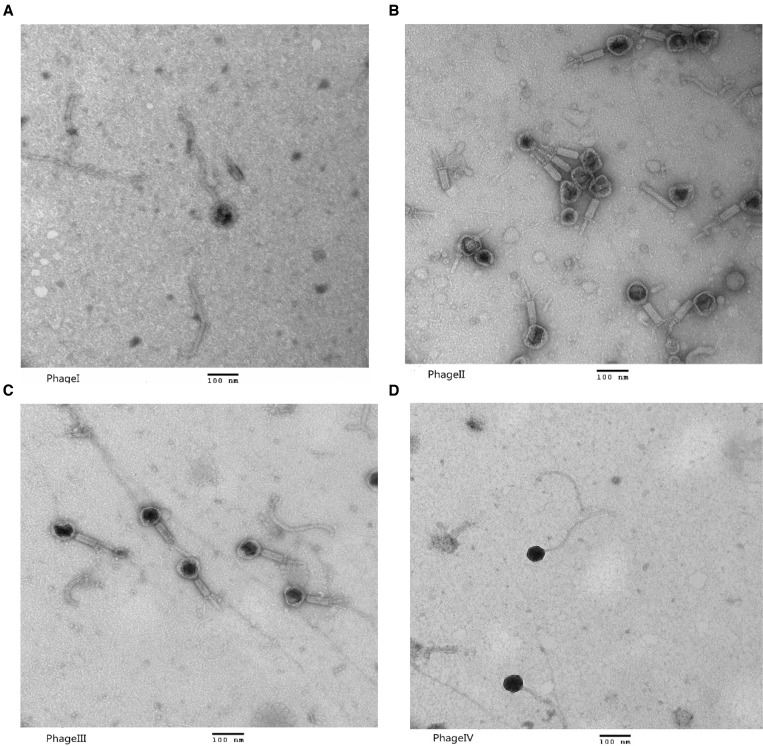
The four phages with a broad host spectrum were all DNA phages and their genomes could be digested by DNase I, but they belonged to different genotypes. The electron microscopy findings of these four phages are shown in Figure 2. Phages I and IV were novel phages of the Siphoviridae family, whereas phages II and III belonged to the Myoviridae family.
Figure 2.
Morphology of the four phages with a broad host spectrum. (A) Phage I belongs to the Siphoviridae family; the size of its head was approximately 100 nm and the length of its tail was approximately 405 nm. (B) Phage II belongs to the Myoviridae family; the size of its head was approximately 80 nm and the length of its tail was approximately 100 nm. (C) Phage III belongs to the Myoviridae family; the size of its head was approximately 78 nm and the length of its tail was approximately 100 nm. (D) Phage IV belongs to the Siphoviridae family; the size of its head was approximately 400 nm and the length of its tail was approximately 450 nm. Magnification, 150,000×. Scale bars, 100 nm.
The MOI of four broad-spectrum phages, their adsorption efficiency, and one-step growth curves.
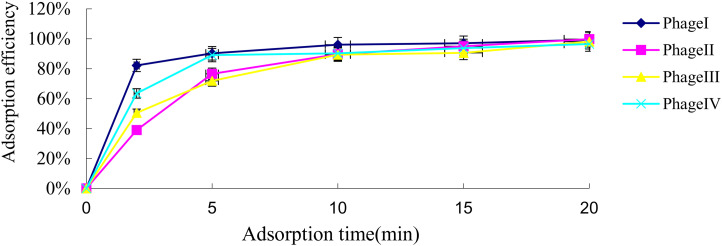
After three repeated experiments, the optimal MOIs of the four phages were all greater than 0.1 (the results were all similar with MOI 0.1, 1, and 10). The adsorption efficiencies of the four phages within 5 minutes were calculated (Figure 3), with the adsorption efficiency of phage I being the highest (P = 0.001).
Figure 3.
Adsorption efficiency of the four phages. The adsorption efficiency of the four phages was compared using the χ2 test (P = 0.001). Data are mean ± SD (error bars) from triplicate experiments.
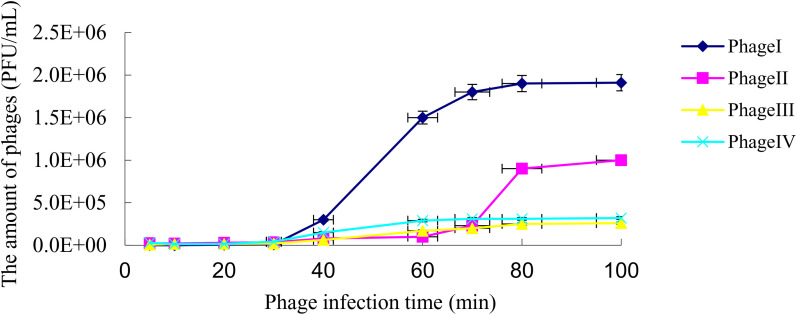
The one-step growth curve of a phage reflected the latent period, lysis period, and burst size. The curve of phage I showed that the latent phase was approximately 20 minutes, the rise phase was approximately 40 minutes, and the burst size was about 270 PFUs per cell. The latent phase, rise phase, and burst size of phage II were approximately 45 minutes, 20 minutes, and 220 PFUs per cell, respectively. The latent phase of phage III was approximately 30 minutes, the rise phase was approximately 20 minutes, and the burst size was approximately 60 PFUs per cell. For phage IV, the latent phase, rise phase, and burst size were approximately 25 minutes, 30 minutes, and 70 PFUs per cell. Phage I had the shortest incubation phase and the largest burst size (Figure 4).
Figure 4.
One-step growth curve of the four phages. Data are mean ± SD (error bars) from triplicate experiments. PFU = plaque-forming unit.
Therapeutic effect of phages in mice with abdominal cavity P. aeruginosa infection.
All mice were killed when the P. aeruginosa 1950 concentration reached 5.0 × 107 to ∼3.0 × 108 CFUs per milliliter. Therefore, 5.0 × 107 CFUs per milliliter was determined as the MLD. All mice infected with the MLD died in approximately 10 hours.
In the phage treatment test, all the mice in the control group died within 12 hours, whereas the mice in the treatment groups were all saved from death when phage I was given at MOI ≥ 0.01. The delayed treatment test indicated that even though phage I was injected 6 hours after the mice were infected with P. aeruginosa, it could still effectively protect the mice from death.
The phage cocktail consisting of the four broad-spectrum phages was able to effectively protect mice from death when they were infected with three P. aeruginosa strains sensitive to the four phages. However, this phage cocktail failed to protect the mice infected by P. aeruginosa strain 1833, which was outside the host spectrum.
Quantity distribution of phage I and P. aeruginosa in the mouse model.
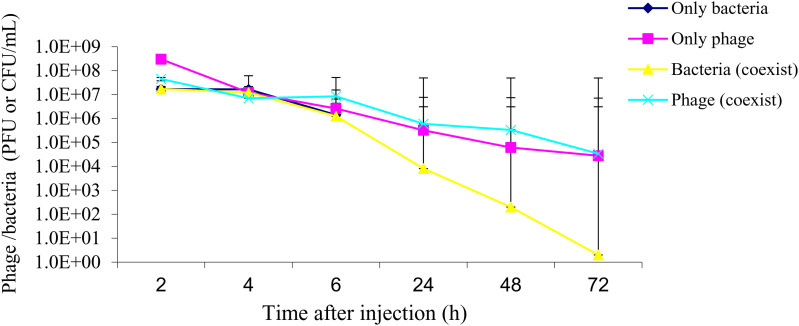
The results showed that phages could rapidly enter and spread in the bloodstream and could be maintained at high titers. The mice injected only with P. aeruginosa had a heavy bacterial load, indicated by the CFU counts, and all died within 12 hours. However, the bacterial load in mice injected with both P. aeruginosa and phage I was at a relatively low level and gradually decreased until it disappeared at 72 hours. In the meantime, the phage counts also dropped gradually, synchronously (Figure 5). The results indicated that application of the phages to P. aeruginosa-infected mice could not only cure the infection but also had few side effects in vivo, because the phage count decreased with the decreasing number of bacteria until the bacteria disappeared.
Figure 5.
Phage particles and Pseudomonas aeruginosa distribution in vivo. “Only bacteria” indicates mice injected with P. aeruginosa only. “Only phage” indicates mice injected with the phage only. “Bacteria (coexist)” indicates in vivo bacterial distribution in mice injected with both the bacteria and the phage. “Phage (coexist)” indicates in vivo distribution of the phages in mice injected with both the bacteria and the phage. The line for the “only bacteria” group stops at 6 hours because no treatment was given and all mice in this group died before the 24-hour time point. Data are mean ± SD (error bars) from three independent experiments with five mice per group. CFU = colony-forming unit; PFU = plaque-forming unit.
DISCUSSION
Pseudomonas aeruginosa is a common opportunistic pathogen among the top three gram-negative bacteria isolated from hospitals in China.21,22 Pseudomonas aeruginosa can easily form a biological biofilm, which refers to organized bacterial populations that are attached to the surface of living or inanimate objects, coated by bacterial extracellular macromolecules, and may develop a multidrug resistance mechanism,23 which makes its eradication difficult. With the incorrect use of antibiotics, P. aeruginosa isolates continuously acquire multidrug or pan-drug resistance,24,25 which makes clinical treatment even more difficult. Multidrug- or pan-drug-resistant P. aeruginosa can be a lethal hazard when it infects patients with serious diseases and immunodeficiency, such as patients in the intensive care unit.
In this study, we screened 14 strains of phages targeting P. aeruginosa from the sewage water of more than a dozen hospitals, of which four proved to have a broad host spectrum. RAPD typing indicated that these four broad-spectrum phages’ genetic materials were DNA and had different genotypes. Among the four phages, phage I was found to have the most extensive host spectrum, the highest adsorption efficiency, a short incubation phase, a long rise phase, and a large burst size. Compared with the findings of a previous report,26 phage I had a shorter incubation time and a larger burst size, and was a highly efficient lytic phage. Thereafter, it was chosen for experimental phage therapy using a mouse model with abdominal cavity P. aeruginosa infection. Upon treatment of infected mice with purified phage I, all the mice were protected from death and eventually recovered fully, but all the mice in the infected control group without phage administration died. Phage I displayed very good protection with MOI ≥ 0.01, even when administered after a 6-hour delay. The results indicated that phages have a good therapeutic effect on drug-resistant P. aeruginosa infection, and that more evaluation of phage I is needed because it has the potential for clinical use.
We found that when administered as a phage cocktail, the phages could treat P. aeruginosa infection within their lysis spectra but failed to protect mice infected by a P. aeruginosa strain outside their combined host range. These results indicated that phage therapy has a lysis spectrum limit, but this limitation can be compensated by adding new phage strains that can lyse the corresponding bacteria into the cocktail. Therefore, it is necessary to screen phages and construct phage libraries with different host spectra to treat the corresponding bacterial infection.
By detecting the distribution of the phages and P. aeruginosa in vivo during phage therapy, we found that the phages were well distributed in the bloodstream and that they could be maintained at considerably high titers as Figure 5 in the Results section shows. The quantity of phages and bacteria in vivo showed a covariant trend, indicating that phages are very host dependent. They only existed at the bacterial infection site and disappeared along with the clearance of the host bacteria, with no residual phage in the body.27 In our study, the infected mice treated with phages were cured without any toxic side effects but all the mice in the control groups died of the infection, suggesting that phage therapy would be effective and safe, as described in previous studies.28 In this study, we did not monitor the immunity status of the mice by detecting the inflammatory cytokine or immune cell transition. Therefore, further study regarding the immune response during phage therapy is needed. Compared with antibiotics, phages are highly host specific, which suggests that they may cause fewer side effects than antibiotics.29
Screening new phages only takes several weeks or months, whereas developing a new antibiotic usually needs years or even decades. This makes phages an excellent candidate for the treatment of bacterial infections, especially for multidrug- or pan-drug-resistant bacterial infections. Host range is an important parameter for the initial screening of new effective phages against target bacteria. In our study, phage I had the broadest host spectrum among the phages tested and showed strong bacteriolytic activity in vitro and therapeutic efficacy in vivo against the multidrug-resistant P. aeruginosa strains.
This work suggests that the new phages are safe and effective, and could be used for treating patients with target bacterial infection with multidrug resistance. However, phage therapy is a biotherapy, and additional research is needed before applying it in a clinical setting.30,31 Phage therapy alone or in combination with antibiotics is a promising alternative strategy for the treatment of drug-resistant bacterial infections and is worthy of further study.
CONCLUSION
In conclusion, we isolated four phages with a broad host spectrum specific to P. aeruginosa; of them, phage I was found to be the most effective and promising treatment of drug-resistant P. aeruginosa. These experimental results will be beneficial for further studies on the isolation and application of phages to clinical use.
Financial Disclosure
This study was supported by the China Primary Health Care Foundation-Youan Foundation of Liver Disease and AIDS and the Key Medical Professional Development Plan of the Beijing Hospital Authority (Grant no. ZYLX202124) and the Special Foundation for National Science and Technology Basic Research Program of China (Grant no. 2019FY101200).
Supplemental Materials
ACKNOWLEDGMENT
We appreciate the excellent technical assistance provided by Liguang Chen’s group at Tzu Chi Hospital in Taiwan.
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1. Wilson R, Aksamit T, Aliberti S, De Soyza A, Elborn JS, Goeminne P, Hill AT, Menendez R, Polverino E, 2016. Challenges in managing Pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respir Med 117: 179–189. [DOI] [PubMed] [Google Scholar]
- 2. Chen MJ, Wang H, 2003. Continuous surveillance of antimicrobial resistance among nosocomial gram-negative bacilli from intensive care units in China. Zhonghua Yi Xue Za Zhi 83: 375–381. [PubMed] [Google Scholar]
- 3. Sanford E, Farnaes L, Batalov S, Bainbridge M, Laubach S, Worthen HM, Tokita M, Kingsmore SF, Bradley J, 2018. Concomitant diagnosis of immune deficiency and Pseudomonas sepsis in a 19 month old with ecthyma gangrenosum by host whole-genome sequencing. Cold Spring Harb Mol Case Stud 4: a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jean SS, Harnod D, Hsueh PR, 2022. Global threat of carbapenem-resistant gram-negative bacteria. Front Cell Infect Microbiol 12: 823684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong DJ, Bae IK, Jang IH, Jeong SH, Kang HK, Lee K, 2015. Epidemiology and characteristics of metallo-β-lactamase-producing Pseudomonas aeruginosa . Infect Chemother 47: 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merril CR, Scholl D, Adhya SL, 2003. The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discov 2: 489–497. [DOI] [PubMed] [Google Scholar]
- 7. Sulakvelidze A, 2005. Phage therapy: an attractive option for dealing with antibiotic-resistant bacterial infections. Drug Discov Today 10: 807–809. [DOI] [PubMed] [Google Scholar]
- 8. Schooley RT. et al. , 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61: e00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahenthiralingam E, Campbell ME, Foster J, Lam JS, Speert DP, 1996. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J Clin Microbiol 34: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ceyssens PJ. et al. , 2009. Survey of Pseudomonas aeruginosa and its phages: de novo peptide sequencing as a novel tool to assess the diversity of worldwide collected viruses. Environ Microbiol 11: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 11. Premamalini T, Rajyoganandh V, Vijayakumar R, Veena H, Kindo AJ, Marak RS, 2021. Strain typing of Trichosporon asahii clinical isolates by random amplification of polymorphic DNA (RAPD) analysis. J Lab Physicians 13: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qamar W, Khan MR, Arafah A, 2017. Optimization of conditions to extract high quality DNA for PCR analysis from whole blood using SDS-proteinase K method. Saudi J Biol Sci 24: 1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li L, Yang H, Lin S, Jia S, 2010. Classification of 17 newly isolated virulent bacteriophages of Pseudomonas aeruginosa . Can J Microbiol 56: 925–933. [DOI] [PubMed] [Google Scholar]
- 14. Biswas B, Adhya S, Washart P, Paul B, Trostel AN, Powell B, Carlton R, Merril CR, 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium . Infect Immun 70: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang J. et al. , 2006. Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa . Int J Mol Med 17: 309–317. [PubMed] [Google Scholar]
- 16. Shivshetty N, Hosamani R, Ahmed L, Oli AK, Sannauallah S, Sharanbassappa S, Patil SA, Kelmani CR, 2014. Experimental protection of diabetic mice against lethal P. aeruginosa infection by bacteriophage. BioMed Res Int 2014: 793242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Institutes of Health , 2011. NIH Guide for the Care and Use of Laboratory Animals. Available at: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf. Accessed March 27, 2023.
- 18. Kilkenny C, Brown W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group, 2010. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Available at: 10.1111/j.1476-5381.2010.00872.x. Accessed March 27, 2023. [DOI]
- 19. Carter CD, Parks A, Abuladze T, Li M, Woolston J, Magnone J, Senecal A, Kropinski AM, Sulakvelidze A, 2012. Bacteriophage cocktail significantly reduces Escherichia coli O157:H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage 2: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaiswal A, Koley H, Ghosh A, Palit A, Sarkar B, 2013. Efficacy of cocktail phage therapy in treating Vibrio cholerae infection in rabbit model. Microbes Infect 15: 152–156. [DOI] [PubMed] [Google Scholar]
- 21. Ziyong S, Li L, Xuhui Z, Yue M, Jingyun L, Zhengyi S, Shaohong J, 2006. Analysis on antimicrobial resistance of clinical bacteria isolated from county hospitals and a teaching hospital. J Huazhong Univ Sci Technol (Med Sci) 26: 386–388. [DOI] [PubMed] [Google Scholar]
- 22. China Antimicrobial Resistance Surveillance System , 2018. National Bacterial Resistance Monitoring Report 2018 (Brief Edition). Available at: http://www.carss.cn/Report/Details?aId=648. Accessed March 27, 2023.
- 23. Betts JW, Hornsey M, Higgins PG, Lucassen K, Wille J, Salguero FJ, Seifert H, La Ragione RM, 2019. Restoring the activity of the antibiotic aztreonam using the polyphenol epigallocatechin gallate (EGCG) against multidrug-resistant clinical isolates of Pseudomonas aeruginosa . J Med Microbiol 68: 1552–1559. [DOI] [PubMed] [Google Scholar]
- 24. Yusuf E, Van Herendael B, Verbrugghe W, Ieven M, Goovaerts E, Bergs K, Wouters K, Jorens PG, Goossens H, 2017. Emergence of antimicrobial resistance to Pseudomonas aeruginosa in the intensive care unit: association with the duration of antibiotic exposure and mode of administration. Ann Intensive Care 7: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pachori P, Gothalwal R, Gandhi P, 2019. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis 6: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang C, Deng C, Zhang Y, Xiao C, Wang J, Rao X, Hu F, Lu S, 2018. Characterization and genomic analyses of Pseudomonas aeruginosa podovirus TC6: establishment of genus Pa11virus . Front Microbiol 9: 2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Melo ACC, da Mata Gomes A, Melo FL, Ardisson-Araújo DMP, de Vargas APC, Ely VL, Kitajima EW, Ribeiro BM, Wolff JLC, 2019. Characterization of a bacteriophage with broad host range against strains of Pseudomonas aeruginosa isolated from domestic animals. BMC Microbiol 19: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeon J, Yong D, 2019. Two novel bacteriophages improve survival in Galleria mellonella infection and mouse acute pneumonia models infected with extensively drug-resistant Pseudomonas aeruginosa . Appl Environ Microbiol 85: e02900–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cisek AA, Dabrowska I, Gregorczyk KP, Wyzewski Z, 2017. Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol 74: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El Haddad L, Harb CP, Gebara MA, Stibich MA, Chemaly RF, 2019. A systematic and critical review of bacteriophage therapy against multidrug-resistant ESKAPE organisms in humans. Nephrol Dial Transplant 69: 167–178. [DOI] [PubMed] [Google Scholar]
- 31. Wright RCT, Friman VP, Smith MCM, Brockhurst MA, 2019. Resistance evolution against phage combinations depends on the timing and order of exposure. mBio 10: e01652–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.