ABSTRACT.
Melioidosis is an infectious disease caused by the bacterium Burkholderia pseudomallei. Although this environmental organism is endemic in certain regions of Australia, it is not considered endemic in Southern Queensland, where the last case was reported 21 years ago. We report a climate change–associated outbreak of melioidosis occurring during two La Niña events in a region previously considered nonendemic for B. pseudomallei. During a 15-month period, 14 cases of locally acquired melioidosis were identified. Twelve patients were adults (> 50 years), with diabetes mellitus the most common risk factor in 6 of 12 patients (50%). Eleven patients (79%) had direct exposure to floodwaters or the flooded environment. This study suggests an association between climate change and an increased incidence of melioidosis. In addition, this is the first report of environmental sampling and whole-genome analysis to prove endemicity and local acquisition in this region.
Burkholderia pseudomallei is an environmental bacteria found predominantly in soil and surface water of tropical and subtropical regions.1 In Australia, B. pseudomallei is most commonly found north of lat. 20°S, and is endemic in the Northern Territory, Far North Queensland, and parts of Western Australia.2
The organism is an opportunistic pathogen and the causative agent of the emerging infectious disease melioidosis. This infection is most commonly acquired through inhalation or direct cutaneous inoculation of the organism into the host.3 The reported incidence of melioidosis in these regions is highest in the more heavily populated cities of Darwin, Cairns, and Townsville.4–6 Because of the environmental niche of B. pseudomallei and common modes of transmission, the greatest burden of infections occurs in the setting of severe weather events such as flooding or tropical cyclones.7,8 The most frequently identified focus of infection is pneumonia, which occurred in up to 69% of patients in Townsville,6 although the presence of bacteremia is the most common feature that occurred in up to 74% of patients in Cairns.5 In Australia the case fatality rate ranges from 12% to 23%, although it has improved in Darwin and Cairns to 6% and 9%, respectively.4,5
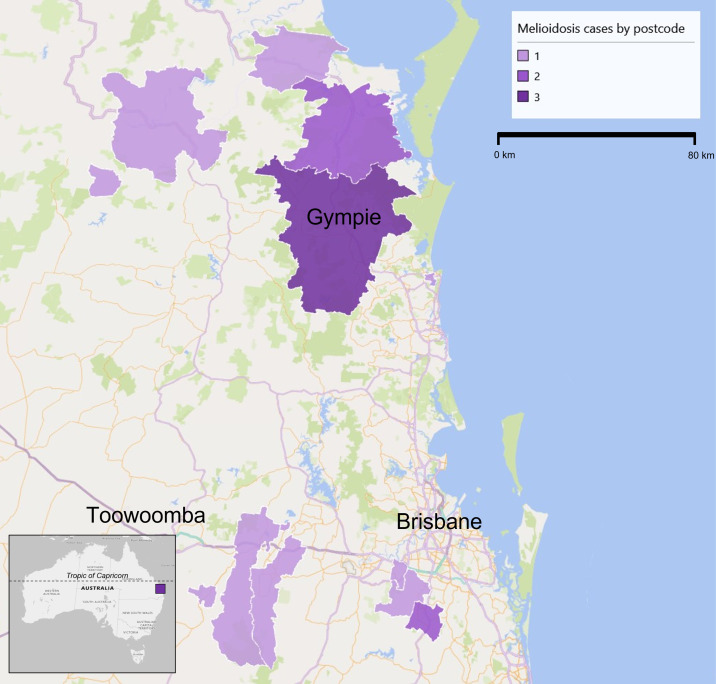
Melioidosis is a notifiable condition, and there were no documented cases of locally acquired melioidosis in Southern Queensland (below lat. 24°S) between 1999 and 2020. Prior to this, only four cases of locally acquired human melioidosis have been reported in Queensland below The Tropic of Capricorn (lat. 23°S), and all were associated with floodwater exposure.9–11 A case from 1974 is not included because no clinical details were available to assist in determining the location of acquisition.9 Three of these cases were thought to be acquired near the city of Toowoomba (Figure 1). Melioidosis has therefore not been an endemic disease in Southern Queensland.
Figure 1.
Map of South Queensland representing regions where locally-acquired melioidosis cases were identified.
We report an outbreak of 14 melioidosis cases in Southern Queensland between April 2021 and June 2022. These cases occurred in the context of climate change, with two successive La Niña years, resulting in above-average rainfall across the entire state of Queensland, and associated flood events in February and March of both years (Supplemental Figure 1).12,13
Fourteen patients (12 adult, 2 children) were diagnosed with culture-confirmed melioidosis (Table 1). Acute melioidosis (< 2 months of symptoms) accounted for the majority of presentations (13 of 14, 93%). Ten of 14 cases (71%) were bacteremic and 7 of 14 patients (50%) presented with pneumonia. Five patients (36%) presented with osteomyelitis or septic arthritis, and three (21%) with isolated skin and soft tissue infection. Solid organ abscess occurred in 4 of 14 patients (29%), with 2 of 12 men (17%) presenting with a prostate abscess. Last, one patient had a cerebral abscess and one had possible neuromelioidosis (a neurocognitive disorder that improved with antimicrobial treatment).
Table 1.
Summary of locally acquired South Queensland melioidosis cases
| Case | Diagnosis month/year | Age, years | Gender | Exposure | Risk factors | Infection site | Treatment | Outcome | Relapse |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4/2021 | 60 | M | Floodwater | Diabetes, alcohol excess, smoker | Blood, lung, brain | MEM, 56 days; SXT, 6 months | Alive | No |
| 2 | 5/2021 | 5 | M | Mud | None | Skin | CAZ, 14 days; SXT, 6 months | Alive | No |
| 3 | 6/2021 | 51 | F | Mud | Diabetes, smoker | Lung, bone (hallux), abscess (liver) | CAZ, 28 days; SXT, 6 months | Alive | No |
| 4 | 2/2022 | 77 | F | Floodwater | Pancreatic cancer (chemotherapy) | Blood | MEM, 18 days; AMC, 4 months | Dead | – |
| 5 | 2/2022 | 71 | M | Mud | Alcohol excess, ex-smoker, prostate cancer (radiotherapy) | Blood, lung, bone (femur), brain (possible) | MEM, 56 days; SXT, 4 months | Alive | No |
| 6 | 3/2022 | 83 | M | Unknown | Chronic kidney disease, lung disease, chronic liver disease, immunosuppression (azathioprine) | Blood, lung | MEM, 28 days; SXT, 3 months | Alive | No |
| 7 | 3/2022 | 59 | M | Floodwater | Lung disease, smoker | Blood | MEM, 14 days; SXT, 3 months | Alive | No |
| 8 | 3/2022 | 63 | M | Floodwater | Diabetes, smoker | Blood, joint (shoulder) | MEM, 28 days; SXT, 6 months | Alive | No |
| 9 | 3/2022 | 66 | M | Floodwater | Smoker | Blood, lung, abscess (spleen), bone (humerus), skin | MEM, 28 days; SXT, 1 month; AMC, 6 months | Alive | No |
| 10 | 3/2022 | 71 | M | Floodwater | Alcohol excess | Blood, lung | AMC, 1 day | Dead | – |
| 11 | 4/2022 | 11 | M | Unknown | None | Skin, bone (tibia) | MEM, 47 days; CAZ, 56 days; SXT, 8 months | Alive | No |
| 12* | 4/2022 | 74 | M | Unknown | Diabetes, alcohol excess | Blood, lung, abscess (liver, spleen, prostate), skin | MEM, 77 days; SXT, 6 months | Alive | No |
| 13 | 5/2022 | 60 | M | Floodwater | Diabetes, alcohol excess, smoker | Blood, abscess (prostate) | CAZ, 14 days; MEM, 14 days | Alive | No |
| 14 | 6/2022 | 82 | M | Floodwater | Diabetes | Skin | MEM, 14 days; SXT, 3 months | Alive | No |
AMC = amoxicillin–clavulanic acid; CAZ = ceftazidime; F = female; M = male; MEM = meropenem; SXT = trimethoprim–sulfamethoxazole.
Presented with chronic melioidosis (symptoms > 2 months).
All 12 adult patients were older than 50 years, and 6 of 12 (50%) had a history of diabetes mellitus. Only two patients had a diagnosis of chronic lung disease, but six were active smokers. Eleven patients (79%) had direct exposure to floodwater or the recently flooded environment. There was no history of exposure to construction sites. Only two patients reported a history of having traveled to a melioidosis-endemic region; both were during the dry season and occurred 1 and 3 years prior to symptom onset.
Two of the 14 patients died, although only one death (7%) was clearly attributable to the infection. There were no episodes of recurrence, which is defined as new signs of infection and a positive culture after response to therapy (range of follow-up, 6–18 months).
Because of resource constraints, environmental health officers only sampled water and soil at two properties south of Brisbane. A total of 21 soil and 12 water samples were collected per consensus guidelines14 and Baker et al.15 The location of soil to be sampled was identified using a thorough history of case exposure to floodwaters within semirural properties. Soil samples were taken from specific locations at a depth of 30 cm and at 2.5-m intervals.14 Water was sampled from two dams within one property as well as from indoor taps. All environmental samples were enriched in Ashdown broth and subcultured per protocol.14 Four isolates of B. pseudomallei were cultured from three soil samples collected at one property. In the case of the positive environmental sample, human presentation was of skin lesions in the leg that were exposed to the contaminated muddy waters.
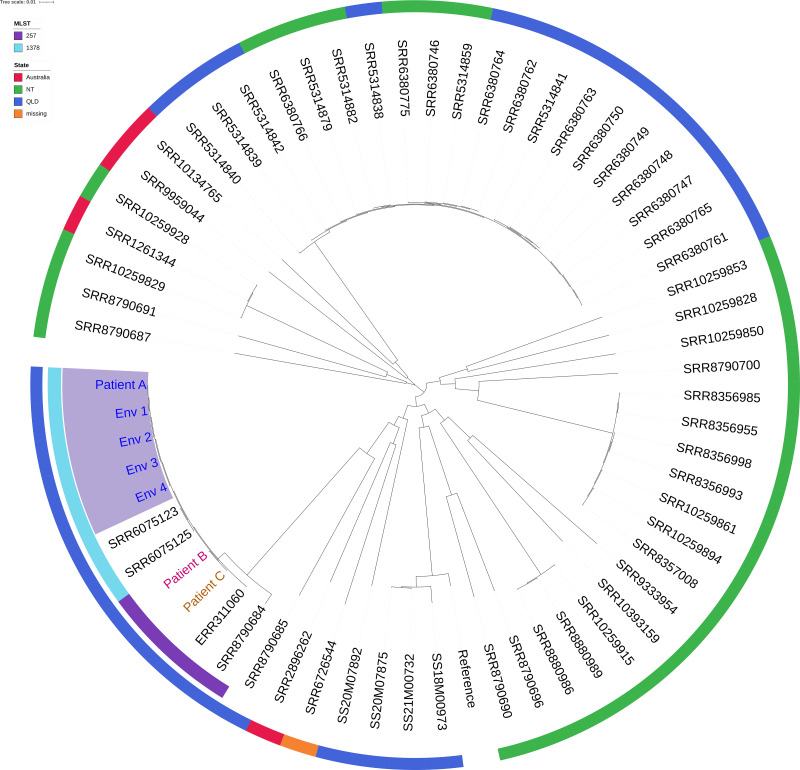
Whole-genome sequencing was performed on three clinical and four environmental samples using Illumina NextSeq500 (Illumina Inc., San Diego, CA) (Genbank project accession no. PRJEB56477). Genomic analysis using multilocus sequencing typing (MLST) and core genome MLST showed there was a high level of genomic similarity between the B. pseudomallei isolated from the soil at the patient’s property and the patient isolate (patient A, Figure 2). Analysis of these isolates in the context of publicly available B. pseudomallei sequences from Australia showed that the soil and patient isolates were more closely related to each other than to unrelated strains (Figure 2). Core genome MLST showed that these ST1378 isolates fall into a genetic clade with two previous ST257 cases sequenced from SEQ (patients B and C) as well as two publicly available ST257 sequences, all historical cases from the same region.
Figure 2.
Phylogenetic Maximum Likelihood Tree built using SNP differences between isolates. The inner ring represents the MLST ST and the outer ring indicates geographic location of isolation. The cluster containing Patient A and corresponding soil isolates is highlighted. The length of branches represents distance between groups as indicated by the scale bar.
We describe 14 cases of melioidosis diagnosed over a 15-month period in a geographic region that has not reported locally acquired infection in more than 21 years. Although reactivation of latent melioidosis occurs in approximately 4% of cases, this is unlikely to be significant in this cohort.3 The majority of patients presented with acute melioidosis in the setting of direct contact with floodwater or the flooded environment. Only one patient had traveled to a melioidosis-endemic region previously and did not have known contact with the flooded environment.
The incidence of melioidosis in B. pseudomallei–endemic regions has been linked to both environmental and climatic factors. Specific environmental factors such as soil temperature, moisture, salinity, pH, and clay-rich subsoil content all affect organism survival.16 In addition, anthropogenic manipulation of garden soil with irrigation and fertilizers can increase the B. pseudomallei bacterial load.16 Furthermore, recent evidence has emerged regarding an association between increased incidence and construction of a motorway in a melioidosis-endemic region.17 As many towns and cities across Queensland expand, the alteration of the natural habitat as well as the increase in population density in newly discovered melioidosis-endemic areas is likely to result in an increase in cases.
Previous publications have demonstrated clearly the association between rainfall intensity and incidence of melioidosis cases.7,18 As seen in one case series, a climatic factors analysis performed by Kaestli et al.7 demonstrated an association between increased incidence and La Niña events. Furthermore, genomic analysis has demonstrated that a particular genetic clone of B. pseudomallei is responsible for a number of melioidosis cases in the southeastern region of Queensland, and the detection of this same clone in soil samples suggests that it may be endemic in the environment.
A clear cause for this outbreak has not been identified. However, a recent report by the Climate Council of Australia13 demonstrated that, compared with the previous flood event in 2011, the 2022 weather event affecting Southern Queensland resulted in 50% more water accumulated in half the time. The report also provided evidence that climate change was “firmly embedded” in the flood emergency. It is unclear which additional factors contributed to this outbreak; therefore, further environmental analysis is warranted.
This case series demonstrates a substantial increase in B. pseudomallei infections throughout Southern Queensland. It appears that climate change has contributed to this increased incidence. In addition, this report is the first to demonstrate cultivation of B. pseudomallei from environmental sampling in this region combined with whole-genome sequencing analysis to prove local acquisition of infection.
Supplemental Materials
ACKNOWLEDGMENTS
We thank each patient for consenting to publication, and the staff in the Public Health Microbiology laboratory for culture and sequencing work.
Note: Supplemental figure appears at www.ajtmh.org.
REFERENCES
- 1. Limmathurotsakul D. et al. , 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1: 15008. [DOI] [PubMed] [Google Scholar]
- 2. Smith S, Hanson J, Currie BJ, 2018. Melioidosis: an Australian perspective. Trop Med Infect Dis 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gassiep I, Armstrong M, Norton R, 2020. Human melioidosis. Clin Microbiol Rev 33: e00006–e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Currie BJ. et al. , 2021. The Darwin Prospective Melioidosis Study: a 30-year prospective, observational investigation. Lancet Infect Dis 21: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 5. Stewart JD, Smith S, Binotto E, McBride WJ, Currie BJ, Hanson J, 2017. The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management. PLoS Negl Trop Dis 11: e0005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gassiep I, Ganeshalingam V, Chatfield MD, Harris PNA, Norton RE, 2021. The epidemiology of melioidosis in Townsville, Australia. Trans R Soc Trop Med Hyg 116: 328–335. [DOI] [PubMed] [Google Scholar]
- 7. Kaestli M, Grist EPM, Ward L, Hill A, Mayo M, Currie BJ, 2016. The association of melioidosis with climatic factors in Darwin, Australia: a 23-year time-series analysis. J Infect 72: 687–697. [DOI] [PubMed] [Google Scholar]
- 8. Cheng AC, Jacups SP, Gal D, Mayo M, Currie BJ, 2006. Extreme weather events and environmental contamination are associated with case-clusters of melioidosis in the Northern Territory of Australia. Int J Epidemiol 35: 323–329. [DOI] [PubMed] [Google Scholar]
- 9. Scott IA, Bell AM, Staines DR, 1997. Fatal human melioidosis in south-eastern Queensland. Med J Aust 166: 197–199. [DOI] [PubMed] [Google Scholar]
- 10. Munckhof WJ, Mayo MJ, Scott I, Currie BJ, 2001. Fatal human melioidosis acquired in a subtropical Australian city. Am J Trop Med Hyg 65: 325–328. [DOI] [PubMed] [Google Scholar]
- 11. Guard RW, Morero PJ, Yi W, Mackay MJ, 2009. Melioidosis in south-eastern Queensland. Med J Aust 191: 290. [DOI] [PubMed] [Google Scholar]
- 12. Australian Government Bureau of Meteorology , 2022. Monthly Rainfall—Brisbane. Available at: http://www.bom.gov.au/climate/data/. Accessed August 30, 2022.
- 13. Rice M, Hughes L, Steffen W, Bradshaw S, Bambrick H, Hutley N, Arndt D, Dean A, Morgan W, 2022. A Supercharged Climate: Rain Bombs, Flash Flooding and Destruction. NSW, Australia: Climate Council of Australia Limited. [Google Scholar]
- 14. Limmathurotsakul D. et al. , 2013. Systematic review and consensus guidelines for environmental sampling of Burkholderia pseudomallei . PLoS Negl Trop Dis 7: e2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker A, Tahani D, Gardiner C, Bristow KL, Greenhill AR, Warner J, 2011. Groundwater seeps facilitate exposure to Burkholderia pseudomallei . Appl Environ Microbiol 77: 7243–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaestli M, Harrington G, Mayo M, Chatfield MD, Harrington I, Hill A, Munksgaard N, Gibb K, Currie BJ, 2015. What drives the occurrence of the melioidosis bacterium Burkholderia pseudomallei in domestic gardens? PLoS Negl Trop Dis 9: e0003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith S, Horne P, Rubenach S, Gair R, Stewart J, Fairhead L, Hanson J, 2021. Increased incidence of melioidosis in Far North Queensland, Queensland, Australia, 1998–2019. Emerg Infect Dis 27: 3119–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Currie BJ, Jacups SP, 2003. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis 9: 1538–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.