ABSTRACT.
Encephalomyelitis is the most frequent manifestation of neuromelioidosis in Australia. It is hypothesized that Burkholderia pseudomallei causes encephalomyelitis after entering the brain directly, if complicating a scalp infection, or after traveling to the brain within peripheral or cranial nerves. A 76-year-old man presented with fever, dysphonia, and hiccups. Chest imaging demonstrated extensive bilateral pneumonia with mediastinal lymphadenopathy, blood cultures isolated B. pseudomallei, and nasendoscopy confirmed a left vocal cord palsy. Magnetic resonance imaging identified no intracranial abnormality but demonstrated an enlarged, enhancing left vagus nerve, consistent with neuritis. We hypothesize that B. pseudomallei invaded the vagus nerve in the thorax, was traveling proximally—involving the left recurrent laryngeal nerve and causing the left vocal cord palsy, but had not yet reached the brainstem. Given the frequency of pneumonia in cases of melioidosis, the vagus nerve may represent an alternative, and indeed common, route for B. pseudomallei to enter the brainstem in cases of melioidosis-related encephalomyelitis.
CASE REPORT
A 76-year-old Caucasian man presented to our hospital during the local monsoonal wet season with a 1-month history of fever, anorexia, odynophagia, dysphonia, and intermittent hiccups. He also reported pain and swelling of the left anterior neck and dyspnea on exertion. His history was notable for suboptimally controlled type 2 diabetes mellitus and prior cigarette smoking. He was an avid gardener and recalled a large amount of muddy water splashing into his mouth approximately 7 days before the onset of symptoms.
At presentation to the emergency department, he was febrile (40.1°C), his heart rate was 110 beats per minute, his blood pressure was 144/76 mm Hg, his respiratory rate was 26 breaths per minute, and his oxygen saturations on room air were 94% by pulse oximetry. There was no neck swelling or palpable lymphadenopathy on examination, but he had crepitations throughout the middle and upper zones of his right lung. He had persistent hiccups and a very hoarse voice, but a neurological examination—including assessment of his cranial nerves—was otherwise normal.
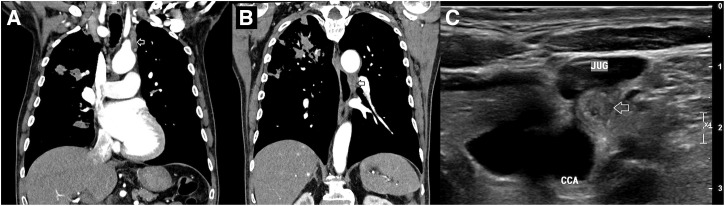
Investigations were notable for a neutrophilia (17.2 × 109/L [reference range (RR): 2.0–8.0 × 109/L], a C-reactive protein of 194 mg/L (RR < 5 mg/L), and a glycosylated hemoglobin of 10.9% (RR: 4.3–6.0%) (Table 1). A chest x-ray demonstrated extensive consolidation within the right lung, particularly the right upper zone. A computerized tomography (CT) scan of his chest confirmed this and identified a cavity in the right upper lobe and smaller nodular opacities in the left lingula and left lower lobe, consistent with extensive pneumonic consolidation. Lymphadenopathy was present in the hilar and mediastinal stations, and the vagus nerve was thickened in the superior mediastinum and below the aorto-pulmonary window (Figure 1). Ultrasound imaging of the neck also identified a grossly enlarged vagus nerve (Figure 1). He was commenced on intravenous meropenem, and on day 2, blood cultures that had been collected on admission isolated Burkholderia pseudomallei, consistent with a diagnosis of melioidosis.
Table 1.
Relevant laboratory values on admission
| Laboratory test | Reference range | Value on admission |
|---|---|---|
| Hemoglobin | 120–180 g/L | 129 g/L |
| White cell count | 3.5–11.0 × 109/L | 18.8 × 109/L |
| Platelet count | 140–400 × 109/L | 274 × 109/L |
| Neutrophil count | 2.0–8.0 × 109/L | 17.2 × 109/L |
| Serum creatinine | 60–110 μmol/L | 78 μmol/L |
| C-reactive protein | < 5 mg/L | 194 mg/L |
| Random blood glucose | 3.0–7.8 mmol/L | 10.7 mmol/L |
| Glycosylated hemoglobin (HbA1c) | 4.3–6.0% | 10.9% |
Figure 1.
Imaging of the chest and neck demonstrating extensive B. pseudomallei pneumonia and left vagus nerve enlargement. (A) Post-contrast CT of chest with reconstructed coronal views demonstrating a thickened left vagus nerve in the superior mediastinum (arrowed). (B) Post-contrast CT of chest with reconstructed coronal views demonstrating a thickened left vagus nerve below the aorto-pulmonary window (arrowed). (C) Ultrasound of left neck, transverse plan demonstrating the enlarged left vagus nerve (arrowed), adjacent to the left jugular vein (JUG) and the left common carotid artery (CCA).
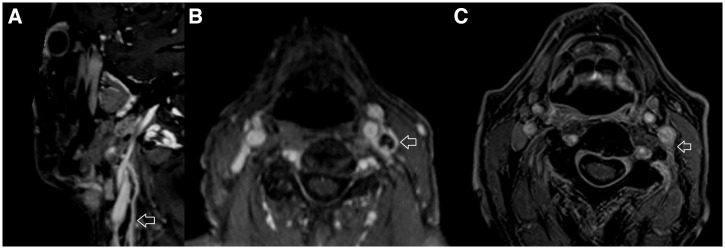
His hoarse voice suggested involvement of the recurrent laryngeal nerve—a branch of the vagus nerve (cranial nerve X), and flexible nasendoscopy confirmed a left vocal cord palsy (Supplemental video). A magnetic resonance imaging (MRI) scan of the brain and neck found no intracranial abnormality but identified marked tubular enhancement within the left carotid space extending from the skull base inferiorly, compatible with vagus nerve neuritis (Figure 2). The patient’s antibiotics were changed to intravenous (IV) ceftazidime plus adjunctive oral trimethoprim/sulfamethoxazole (TMP/SMX) with folic acid to facilitate outpatient therapy. His hoarseness slowly improved, and a follow-up MRI of the head and neck performed after 6 weeks of therapy showed resolution of the inflammatory changes of the vagus nerve (Figure 2). He was prescribed oral TMP/SMX with folic acid for a further 6 months. After 5 months of therapy, CT chest imaging revealed partial resolution of the right upper lobe changes. After 6 months of oral TMP/SMX with folic acid, he was systemically well and had returned to normal activities, and after intensification of his diabetes mellitus regimen, his glycosylated hemoglobin fell to 5.7%. However, his hoarse voice persisted, and the decision was made to continue the oral TMP/SMX for an additional 3 months because of concerns about the possibility of persisting vagal nerve infection with B. pseudomallei and the potential for evolution to life-threatening brainstem involvement.
Figure 2.
MRI imaging demonstrating vagus nerve neuritis; brain imaging was normal. (A) Fat saturated T1 weighted post-contrast sagittal MRI of neck demonstrating enlarged left vagus nerve with avid peripheral enhancement (arrowed). (B) Fat-saturated T1 weighted post-contrast axial MRI of neck demonstrating enlarged left vagus nerve with avid peripheral enhancement (arrowed). (C) Fat saturated T1 weighted post-contrast axial MRI of neck, performed 6 weeks later, demonstrating almost complete resolution of the left vagus nerve changes. The arrow points to residual enhancement in the left carotid space in the region of the left vagus nerve.
DISCUSSION
Burkholderia pseudomallei—the organism that causes melioidosis—is an opportunistic, environmental gram-negative bacterium that is endemic to northern Australia, southeast Asia, and other tropical countries.1 Melioidosis most commonly presents as pneumonia—with or without bacteremia, but almost any organ can be involved including the skin, liver, spleen, prostate, bone, and joints.2,3 The central nervous system (CNS) can also be affected, although this is seen in only about 3% of Australian cases.4,5 Central nervous system melioidosis has a broad range of clinical phenotypes that include encephalomyelitis, brain abscess, meningitis, and extra-meningeal disease. The mechanism that B. pseudomallei employs to enter the CNS is believed to play an important role in determining which of these clinical phenotypes is present.4,6
Although most organ involvement in melioidosis can be explained by either hematogenous dissemination (e.g., liver or splenic abscess in patients with bacteremia) or direct inoculation (e.g., pneumonia acquired through inhalation), the pathophysiology of neuromelioidosis is less well understood. Although brain abscesses are likely to follow bacteremia, encephalomyelitis has been hypothesized to develop after B. pseudomallei enters the CNS either directly from cutaneous scalp infections or by traveling within peripheral or cranial nerves.4,7,8 This hypothesis is supported by a mouse model that has demonstrated B. pseudomallei traversing the olfactory mucosa and cribriform plate to travel along the olfactory and trigeminal nerves and directly invading the brain.9,10 Encephalomyelitis—the most common manifestation of CNS melioidosis in Australia—has been documented after scalp infection in Australia, but head and neck involvement is less common in Australia than in other parts of the world.7,11,12 In contrast, pneumonia was present in more than 70% of cases in a recent Australian series and is frequently accompanied by mediastinal lymphadenopathy.13,14
The vagus nerve arises from the medulla, exits the skull through the jugular foramen, and descends within the carotid sheath. It then runs posterior to the lung hilum and down into the abdomen, forming the cardiac, pulmonary, esophageal, gastric, and celiac plexuses with the contralateral vagus nerve along this course. The vagus nerve has multiple branches in the lung, which mediate cough, changes in breathing pattern, and autonomic drive, all of which are important for normal lung homeostasis.15–17 The left recurrent laryngeal nerve branches from the vagus nerve at the level of the aortic arch and innervates the intrinsic laryngeal muscles, including the posterior cricoarytenoid muscle, the only muscle that abducts the vocal cord. The CT images in this case showed an enlarged vagal nerve inferior to the aorto-pulmonary window, suggesting that B. pseudomallei invaded the vagus nerve below this level, most likely from the extensive consolidation present in both lungs. We hypothesize that the infection then traveled cranially within the vagus nerve to involve the recurrent laryngeal nerve, causing the left vocal cord palsy. On this occasion, B. pseudomallei infection was diagnosed before the organism entered the brainstem.
Why some patients with melioidosis develop CNS disease is incompletely understood; however, a virulence gene that encodes the BimA autotransporter may be responsible. In an Australian study, patients infected with the Burkholderia mallei–like BimABm variant were more likely to have a primary CNS presentation and to have brainstem involvement and encephalomyelitis.4 Unfortunately, in this case, we were unable to perform genotyping of the patient’s isolate to determine if he was infected with the BimABm variant.
Neurological involvement in melioidosis is associated with a higher case-fatality rate and long-term disability; therefore, prompt identification is crucial.4 For CNS disease, current guidelines recommend a prolonged intensive phase of intravenous antibiotics followed by 6 months of oral eradication therapy. In our patient, an excellent outcome was achieved with 6 weeks of IV antibiotics followed by 9 months of oral antibiotics. His diabetes management was also optimized to reduce the risk of relapse and improve his long-term prognosis.18
CONCLUSION
The cranial nerves are implicated in the pathogenesis of CNS melioidosis; however, we are the first, to our knowledge, to describe an image-proven cranial neuropathy due to B. pseudomallei without intracranial involvement. This case supports the hypothesis that brainstem involvement may complicate pneumonia, with the organism traveling to the CNS in the vagus nerve. It is plausible that, given the relative frequency with which melioidosis involves the lung and mediastinum, the vagus nerve represents an alternative—and indeed common—route for B. pseudomallei to enter the brainstem in cases of encephalomyelitis.
Supplemental Materials
Note: Supplemental video appears at www.ajtmh.org.
REFERENCES
- 1. Gassiep I, Armstrong M, Norton R, 2020. Human melioidosis. Clin Microbiol Rev 33: e00006–e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Currie BJ. et al. , 2021. The Darwin Prospective Melioidosis Study: a 30-year prospective, observational investigation. Lancet Infect Dis 21: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 3. Stewart JD, Smith S, Binotto E, McBride WJ, Currie BJ, Hanson J, 2017. The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management. PLoS Negl Trop Dis 11: e0005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gora H, Hasan T, Smith S, Wilson I, Mayo M, Woerle C, Webb JR, Currie BJ, Hanson J, Meumann EM, 2022. Melioidosis of the central nervous system; impact of the bimABm allele on patient presentation and outcome. Clin Infect Dis (in press). doi: 10.1093/cid/ciac111. [DOI] [PubMed] [Google Scholar]
- 5. Smith S, Hanson J, Currie BJ, 2018. Melioidosis: an Australian perspective. Trop Med Infect Dis 3: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Currie BJ, Fisher DA, Howard DM, Burrow JN, 2000. Neurological melioidosis. Acta Trop 74: 145–151. [DOI] [PubMed] [Google Scholar]
- 7. McLeod C, Morris PS, Bauert PA, Kilburn CJ, Ward LM, Baird RW, Currie BJ, 2015. Clinical presentation and medical management of melioidosis in children: a 24-year prospective study in the Northern Territory of Australia and review of the literature. Clin Infect Dis 60: 21–26. [DOI] [PubMed] [Google Scholar]
- 8. Liu PJ, Chen YS, Lin HH, Ni WF, Hsieh TH, Chen HT, Chen YL, 2013. Induction of mouse melioidosis with meningitis by CD11b+ phagocytic cells harboring intracellular B. pseudomallei as a Trojan horse. PLoS Negl Trop Dis 7: e2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. St John JA. et al. , 2014. Burkholderia pseudomallei penetrates the brain via destruction of the olfactory and trigeminal nerves: implications for the pathogenesis of neurological melioidosis. MBio 5: e00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. St John JA, Walkden H, Nazareth L, Beagley KW, Ulett GC, Batzloff MR, Beacham IR, Ekberg JA, 2016. Burkholderia pseudomallei rapidly infects the brain stem and spinal cord via the trigeminal nerve after intranasal inoculation. Infect Immun 84: 2681–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim WK, Gurdeep GS, Norain K, 2001. Melioidosis of the head and neck. Med J Malaysia 56: 471–477. [PubMed] [Google Scholar]
- 12. Mohanty S, Sarkar S, Mishra B, 2020. Melioidosis of the head and neck: a case series from Eastern India. Infect Dis Rep 12: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gadsby BA, Oaten A, Davies P, Simpson G, Vincent S, 2022. Mediastinal melioidosis: a case series from far North Queensland. Respirol Case Rep 10: e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies P, Smith S, Wilcox R, Stewart JD, Davis TJ, McKenna K, Hanson J, 2022. Examination of the independent contribution of rheumatic heart disease and congestive cardiac failure to the development and outcome of melioidosis in Far North Queensland, tropical Australia. PLoS Negl Trop Dis 16: e0010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berthoud HR, Neuhuber WL, 2000. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17. [DOI] [PubMed] [Google Scholar]
- 16. Canning BJ, Spina D, 2009. Sensory nerves and airway irritability. Handb Exp Pharmacol 194: 139–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weijs TJ, Ruurda JP, Luyer MD, Nieuwenhuijzen GA, van Hillegersberg R, Bleys RL, 2015. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J Anat 227: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanson J, Smith S, 2019. High rates of premature and potentially preventable death among patients surviving melioidosis in tropical Australia. Am J Trop Med Hyg 101: 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.