ABSTRACT.
At least a third of tuberculosis (TB) cases remain undiagnosed, disproportionately so in children and adolescents, which is hampering global elimination goals. Prolonged symptom duration presents a high-risk scenario for childhood TB in endemic areas, but the prolonged period of symptoms and its impact on educational attainment are rarely documented. Using a mixed method approach, we aimed to quantify the duration of respiratory symptoms and describe their impact on education among children from a rural area of Tanzania. We used data from a prospectively enrolled cohort of children and adolescents aged 4–17 years in rural Tanzania at the start of active TB treatment. We report on the cohort’s baseline characteristics and explore the correlation between duration of symptoms and other variables. In-depth qualitative interviews were designed on the basis of a grounded theory approach to explore the impact of TB on educational attainment among school-aged children. In this cohort, children and adolescents diagnosed with TB experienced symptoms for a median of 85 days (interquartile range: 30, 231 days) prior to treatment initiation. In addition, 56 participants (65%) had a TB exposure in the household. Of the 16 families with school-aged children who were interviewed, 15 (94%) reported a significant negative impact of TB on the schooling of their children. Children in this cohort experienced a long duration of TB symptoms; the extent of illness impacted absenteeism at school. Screening initiatives for households affected by TB may lead to a shortened duration of symptoms and may minimize the impact on school attendance.
INTRODUCTION
The United Nations has identified tuberculosis (TB) as a priority in its sustainable development goals, with the aim of ending the TB epidemic by 2030 by reducing TB incidence by 80% compared with 2015 levels.1 However, millions of people with TB continue to go uncounted, a challenge that has been exacerbated by the COVID-19 pandemic.2,3 Global public health advocacy groups emphasize the need for urgent and creative strategies to meet the ambitious TB elimination targets.4 National TB management guidelines in high-burden countries often under-prioritize TB prevention efforts among children and adolescents despite the disproportionate risk of morbidity experienced in these age groups.5,6 Focusing on prevention and early detection could avert both transmission and disease-related impacts on development; however, globally more than 75% of eligible household members under the age of 5 years did not access TB preventive therapy as recommended by the guidelines.2 This gap has led to delayed diagnosis of TB among children in affected households, which may have a significant downstream impact on their well-being and educational attainment.7
Tanzania is one country that experiences a high burden of TB. In response, Tanzania built a strong active case finding (ACF) program in the early 1980s that relied on outreach to patients and their households, as suggested by TB guidelines at the time.8 However, a surge in HIV-related TB over the past 2 decades has led to a diversion of resources from ACF toward TB and HIV treatment, which likely contributed to the growing detection gap.9 In 2019 and before the COVID-19 pandemic, Tanzania reported 12,244 cases of TB among children under the age of 15 years and TB preventive treatment coverage of 39% among children under the age of 5 years.10 In this report, we highlight the potential benefits of reigniting ACF efforts throughout Tanzania by examining the delay in TB diagnosis and its impact on schooling as experienced by children and adolescents with the disease.
MATERIALS AND METHODS
Study design and population.
This was a concurrent mixed method study performed at Haydom Lutheran Hospital (HLH), as part of a larger multisite study assessing TB pharmacokinetics and outcomes (ClinicalTrials.gov Identifier: NCT05283967). We aimed to identify the median symptom duration prior to active TB diagnosis in pediatric and adolescent individuals from a high TB–burden setting to identify opportunities for early detection of active TB and explore the downstream effects of a delayed diagnosis. Between January 2020 and March 2021, this study enrolled people aged 4–17 years at HLH, a rural district hospital in Haydom, Tanzania, with a large catchment area extending into the Manyara and Singida regions of northern Tanzania.11 Participants who were diagnosed with any form of TB and were started on active TB therapy were serially recruited from HLH’s inpatient wards and its attached outpatient TB clinic. Tuberculosis diagnosis and treatment were initiated by local TB clinicians following national TB guidelines in Tanzania.12 In addition, the study involved in-depth interviews with a subset of participants and their caregivers selected by convenience sampling to explore the experiences of children recently diagnosed with TB.
Study procedures.
All participants.
Participants were asked to estimate how long they had been ill and then were specifically asked for a main symptom. After that, participants were asked if they had one of the following symptoms: cough, swollen lymph nodes, fevers, sweats, chills, loss of appetite, weight loss, headache, diarrhea, abdominal pain, shortness of breath, chest pain, and lethargy. We also assessed the nutritional status of participants using body mass index (BMI) for age z-score. Body mass index measurements were obtained after participants had started TB treatment. In addition, demographic and clinical data were collected, including epidemiological contacts with people with TB and the distance from the primary research site at HLH to the home village of the participant.
Subset of participants for qualitative interview.
A subset of participants were selected for an in-depth interview via convenience sampling to represent a balanced distribution of ages and gender. In-depth interviews with children attending school who were enrolled at HLH were conducted by trained researchers using an interview guide in the presence of a parent/guardian, interview guide is detailed in the supplemental materials. During the interviews, participants were asked about their daily routine before they had TB and how it had changed as a result of illness. Specifically, school-aged children and their families were asked how TB had impacted the child’s schooling, the child’s relationships with friends, and their relationships with other children in the neighborhood and/or school. Questions regarding the effects of TB on schooling exclude impacts related to any TB isolation requirements. All interviews were conducted in Kiswahili and were audiotaped. Audio files were transcribed by authors S. L., D. A., P. M., R. B., and C. M. and were translated into English by authors S. L., D. A., P. M., and R. B.
Data analysis.
We hypothesized that a known exposure to TB in the household and shorter distance from the TB clinic at HLH would be associated with a shorter duration of symptoms prior to TB diagnosis. We also hypothesized that a shorter duration of symptoms prior to diagnosis would result in less adverse impacts on schooling in the subset tested by a qualitative interview. A two-sample Wilcoxon rank-sum test was used to compare the median duration of symptoms and nutritional status based on reported TB exposure and distance from HLH (i.e., the Haydom area, which was defined as the area within 15.5 km of HLH, was compared with the remainder of cohort). The analysis was performed using RStudio Version 1.4.1717 (R Foundation for Statistical Computing, Vienna, Austria) and Stata 16.1 (StataCorp, College Station, TX).
To analyze the impact on schooling, study team members developed a preliminary codebook following the principles of grounded theory until thematic saturation was reached.13 Themes from the finalized codebook were applied to all transcripts. We used Dedoose for coding and thematic analysis (version 9.0 [2021]; SocioCultural Research Consultants, LLC, Los Angeles, CA).14
Ethics statement.
All caregivers provided written informed consent to participate in this study, which was approved by the Tanzanian National Institute of Medical Research and the University of Virginia. In addition, all participants ≥ 7 years provided verbal assent.
RESULTS
Overall cohort.
Eighty-six children and adolescents with a mean age of 9.04 years were included, 60% of whom were female. Their average BMI for age z-score was −1.4 SD (interquartile range [IQR]: −2.34, −0.40), and 10% required hospitalization. Table 1 shows additional baseline characteristics.
Table 1.
Baseline characteristics of study participants
| Baseline characteristics | All participants | Participants who reported previous exposure to TB | Participants without previous reported exposure to TB | Participants with unknown exposure to TB |
|---|---|---|---|---|
| (N = 86) | (N = 56) | (N = 26) | (N = 4) | |
| Females, n (%) | 52 (60%) | 34 (61%) | 16 (64%) | 2 (50%) |
| Median age in years (IQR) | 9.04 (5.67, 12.92) | 9.08 (5.79, 12.92) | 8.58 (5.17, 13.42) | 10.83 (10.42, 11.25) |
| Living with HIV, n (%)* | 2 (2%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Median duration of symptoms prior to treatment initiation in days (IQR)† | 85 (30, 231) | 60 (30, 261.5) | 90 (30, 212) | 201 (72, 330) |
| Main symptoms‡ | ||||
| Cough, n (%) | 44 (51%) | 31 (55%) | 11 (42%) | 2 (50%) |
| Lymph node swelling, n (%) | 43 (50%) | 29 (52%) | 11 (42%) | 3 (75%) |
| Other, n (%)§ | 8 (9%) | 2 (4%) | 5 (20%) | 1 (25%) |
| BMI for age z-score in SD units† | −1.40 (−2.34, −0.40) | −1.01 (−1.98, −0.36) | −1.95 (−2.70, −0.79) | −1.08 (−1.85, 2.84) |
| Hospitalized, n (%) | 9 (10%) | 4 (7%) | 4 (15%) | 1 (25%) |
| Median distance in kilometers from HLH (IQR)† | 24.2 (13.1, 34.5) | 22.65 (11.05, 34.5) | 24.2 (15.5, 34.5) | 27.3 (24.2, 51.1) |
BMI = body mass index; HLH = Haydom Lutheran Hospital; IQR = interquartile range; TB = tuberculosis.
Information on HIV status was available for 65 participants (76%).
Difference was not statistically significant according to a two-sample Wilcoxon rank-sum test comparing the median duration of symptoms based on reported TB exposure and distance from HLH (P = 0.62, P = 0.14, and P = 0.16, respectively).
Does not add to 100% as some patients reported cough and lymph node swelling as the main symptoms at the same time.
Other symptoms include fevers, sweats, chills, loss of appetite, weight loss, headache, diarrhea, abdominal pain, shortness of breath, chest pain, and lethargy.
Close contact to someone with active TB within the prior year was reported by 56 participants (65%), of whom 40 (71%) lived with and 21 (38%) slept in the same room as the person with active TB. When participants were grouped by reported exposure to TB, the median duration of symptoms prior to treatment initiation was 60 days (IQR: 30, 261.5 days) among those previously exposed to TB in the household compared with 90 days (IQR: 30, 212 days) for those without a known TB exposure (P = 0.62). Of note, those with reported previous exposure to TB had a smaller proportion of hospitalized participants (7% versus 15%, P = 0.24) and had better nutritional status, with a BMI for age z-score of −1.01 SD (IQR: −1.98, −0.36) compared with those without reported previous exposure to TB (−1.95 SD [IQR: −2.70, −0.79]); the difference was not statistically significant (P = 0.14).
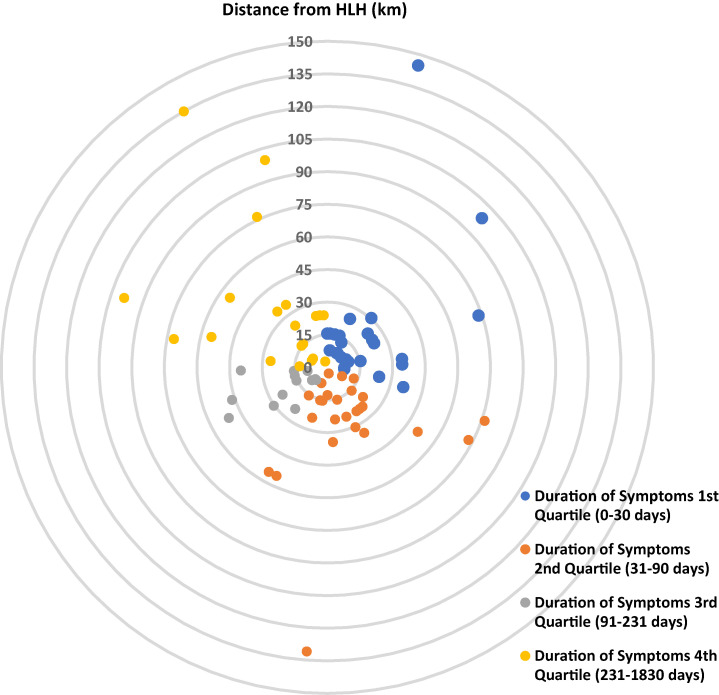
Figure 1 shows a schematic representation of distance from HLH per participant based on duration of symptoms; the median distance was 24.2 km (IQR: 13.1, 34.5 km). When duration of symptoms based on distance from HLH was compared, participants from the Haydom area had a median duration of symptoms of 75 days (IQR: 30, 210 days) compared with 85 days (IQR: 35, 345 days) in the remainder of the cohort (P = 0.17).
Figure 1.
A schematic representation showing the estimated distance for each participant from Haydom Lutheran Hospital (HLH, center) in kilometers, color-coded according to duration of symptoms quartiles. Blue: Duration of Symptoms 1st Quartile (0–30 days), orange: Duration of Symptoms 2nd Quartile (31–90 days), grey: Duration of Symptoms 3rd Quartile (91–231 days), and yellow: Duration of Symptoms 4th Quartile (> 231 days).
Impact on schooling (N = 16 from subset with qualitative interview).
Of the total cohort, 16 participants and their caregivers were recruited to complete in-depth interviews, and their characteristics are summarized in the Supplemental Table. The prolonged duration of symptoms in the total cohort resulted in a significant impact on schooling among school-aged children and adolescents and their caregivers interviewed: 15 of the 16 participants (94%) reported that TB negatively affected the child’s educational experience. Nine of these children (56%) missed at least 8 weeks of class instruction because of their illness and had TB symptoms for ≥ 30 days (range: 30–408 days) prior to diagnosis. Two children (13%) stopped going to school altogether, and two other children (13%) had to repeat grades as a result of prolonged absence. This hardship, which was experienced both during the prolonged diagnostic period and following treatment initiation, was highlighted in the words of the mother of a 12-year-old boy who experienced symptoms for 72 days:
“School attendance has decreased because most of the time, he is lethargic and the school is far from home. It is located in the hilly land, so climbing the hills makes him feel very sick. He has not attended school for a long time because he is taking medication and his health condition is not very good.”
A 13-year-old participant had symptoms for 210 days, and the impact of her prolonged illness was highlighted by her brother:
“She was active, she was participating in school activities, but for now, she is not doing all this. This child, when she was healthy, she had good school attendance, but after becoming sick, she does not attend school. She complains of chest, neck. When pains begin, she barely can turn around.”
The impact of the illness was not limited to the child with TB but also impacted other siblings who had to miss school to help the household with other tasks; this was highlighted by the mother of 12-year-old participant:
“I wake up early to ask for permission from the school for one child to stay at home for responsibilities so that I can leave with this one to come to clinic.”
Four children (25%) experienced a temporary disruption to their learning, between 1 and 3 weeks in duration. One family unenrolled their 10-year-old daughter from school entirely because they “were worried about the side effects of the medications. But it reached a point we discussed with the teachers and agreed that she continue with her studies.” Only one caregiver (7%) shared that TB had no impact on her 14-year-old daughter’s education.
DISCUSSION
Reports on the duration of illness prior to initiation of TB treatment among children are rare; one study in urban TB centers in India reported a median duration of symptoms prior to treatment initiation of 44 days in a group of 175 children aged 0–14 years,15 whereas another study from Malawi reported a median duration of symptoms of 8 weeks in 150 children hospitalized for TB.16 In contrast, a TB referral center in Portugal (low TB burden) that used an extensive and timely diagnostic workup reported a median of 2 weeks in a group of 46 children diagnosed with TB, of whom 65.2% reported a known exposure to TB.17 Reports of TB impacts on schooling are even less frequent; one review citing data from Peru and Ukraine highlighted the negative impact of TB symptoms and treatment on attending school and educational attainment in an urban setting.7 Another study highlighted the negative impact of prolonged isolation on school attendance in Peru.18 Our current study from a high TB–burden region in rural Tanzania found considerably longer durations of illness and a similar negative impact on the schooling experience for those with the disease as well as for siblings. Active case finding is a known strategy that can identify children at higher risk for TB early in the course of their illness, thus preventing additional downstream morbidity and mortality.19
In an effort to reduce the TB burden among children and adolescents and minimize the duration of symptoms and subsequent impacts on education, we advocate for expanding TB preventive efforts and reigniting ACF programs. Tanzanian guidelines have consistently recommended screening of all household members of those diagnosed with TB.12 Although these recommendations were once part of a concerted programmatic effort, they are now inconsistently applied.10 Furthermore, many households in Tanzania report barriers to accessing care for children despite exposure to household members with TB. A recent survey by Kilale et al.20 reported a median cost of 154.90 US dollars incurred by patients in Tanzania while seeking services along the TB care cascade. This survey identified nutritional supplements and transportation as the highest cost categories, but importantly did not assess the cost of screening additional household TB contacts or the downstream financial implications of lost learning.20
Reigniting strategies that move beyond the care of people with active TB to engage those with high-risk TB exposure in endemic areas can benefit from approaches used in other aspects of TB care. For instance, Wingfield et al.21 found that participants in Peru who received a conditional cash transfer were more likely to complete active TB treatment, and their contacts were more likely to initiate TB preventive therapy. Beyanga et al.22 reported that when home visits were used to screen and test household contacts of TB patients in Tanzania, 6.4% of those screened had active TB. Furthermore, approaches that incorporate enhanced communication with healthcare professionals through mobile technologies may alleviate the financial burden incurred by families as a result of accessing screening services, even among marginalized populations.23 These technologies can also improve access to screening services.
The effect of these approaches will need to be evaluated in relevant settings before implementation on a larger scale. Active case finding efforts are frequently described as expensive and difficult to sustain long-term.24,25 To best use the limited resources of ACF programs, we need additional studies that are powered to evaluate the correlation between delayed TB diagnosis and factors such as exposure to TB in the household, distance from TB centers, and symptom duration prior to pediatric and adolescent TB diagnosis. As suggested in this study, these factors may serve as important markers when measuring the implementation benefit of community detection efforts and may better target limited ACF resources. Consequently, we join the call for household contact tracing to be completed shortly after diagnosis as part of a comprehensive approach to TB prevention to reduce progression and duration of symptoms of the disease, especially in children.26
This study has several limitations. First, this was a secondary data analysis that was not powered to evaluate associations between the duration of symptoms and other baseline characteristics. Second, BMI was calculated after initiation of TB treatment, even though weight was used for TB medication dosing at initiation and height was not measured until study participation, which allowed enrollment up to 14 days after treatment initiation. This approach could have systematically underestimated the degree of malnutrition in the cohort. Lastly, the study relied on participants’ ability to report symptoms and their duration, which may be subject to recall bias; however, our research team minimized this possibility by using prompts to solicit more details regarding the timeline.
In conclusion, prolonged duration of TB symptoms and subsequent consequences may be preventable by earlier detection. Researchers and other stakeholders must explore novel and locally relevant approaches that improve pediatric and adolescent TB case detection in high-burden settings such as rural Tanzania.
Financial Disclosure
This work was funded by NIH training grant 5T32AI007046 and NIH R01AI137080 (to S. K. H.).
Supplemental Materials
ACKNOWLEDGMENTS
We acknowledge the participants and their families for their time and efforts to help complete this study.
Note: Supplemental table and interview guide appear at www.ajtmh.org.
REFERENCES
- 1. United Nations , 2022. Sustainable Development Goals. Goal 3: Ensure Healthy Lives and Promote Well-Being for All at All Ages. Available at: https://www.un.org/sustainabledevelopment/health/. Accessed April 29, 2022.
- 2. World Health Organization , 2021. Global Tuberculosis Report 2021. Geneva, Switzerland: WHO. [Google Scholar]
- 3. Migliori GB. et al. , 2021. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur Respir J 58: 2101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization , 2018. Roadmap Towards Ending TB in Children and Adolescents. Geneva, Switzerland: WHO. [Google Scholar]
- 5. Ilaiwy G, Heysell SK, Thomas TA, 2022. National approaches to TB care in adolescents. Int J Tuberc Lung Dis 26: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perez-Velez CM, Marais BJ, 2012. Tuberculosis in children. N Engl J Med 367: 348–361. [DOI] [PubMed] [Google Scholar]
- 7. Moscibrodzki P, Enane LA, Hoddinott G, Brooks MB, Byron V, Furin J, Seddon JA, Meyersohn L, Chiang SS, 2021. The impact of tuberculosis on the well-being of adolescents and young adults. Pathogens 10: 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joint Study Group on Tuberculosis Control of the World Health Organization and International Union against Tuberculosis , 1982. Tuberculosis Control: Report of a Joint IUAT/WHO Study Group [Meeting Held in Geneva from 14 to 18 September 1981]. Geneva, Switzerland: WHO. [Google Scholar]
- 9. Graf P, Chum HT, 1993. Challenge in Tanzania. World Health 46: 16–18. [Google Scholar]
- 10. National Tuberculosis and Leprosy Programme, The Ministry of Health of the United Republic of Tanzania , 2019. Annual Report for 2019. Dodoma, Tanzania. Available at: https://ntlp.go.tz/site/assets/files/1113/ntlp_annual_report_2019-1.pdf. Accessed April 14, 2023. [Google Scholar]
- 11. Mduma ER. et al. , 2014. The etiology, risk factors, and interactions of enteric infections and malnutrition and the consequences for child health and development study (MAL-ED): description of the Tanzanian site. Clin Infect Dis 59 (Suppl 4): S325–S330. [DOI] [PubMed] [Google Scholar]
- 12. National Tuberculosis and Leprosy Programme, The Ministry of Health of the United Republic of Tanzania , 2016. National Guidelines for the Management of Tuberculosis in Children. Available at: https://ntlp.go.tz/site/assets/files/1087/pdf_final_draft_ped_guideline_02_05_2017.pdf. Accessed April 14, 2023.
- 13. Glaser BG, Strauss AL, 2017. The Discovery of Grounded Theory: Strategies for Qualitative Research. Abingdon, United Kingdom: Routledge. [Google Scholar]
- 14. Deterding NM, Waters MC, 2021. Flexible coding of in-depth interviews: a twenty-first-century approach. Sociol Methods Res 50: 708–739. [Google Scholar]
- 15. Kalra A, 2017. Care seeking and treatment related delay among childhood tuberculosis patients in Delhi, India. Int J Tuberc Lung Dis 21: 645–650. [DOI] [PubMed] [Google Scholar]
- 16. Weismuller MM, Graham SM, Claessens NJ, Meijnen S, Salaniponi FM, Harries AD, 2002. Diagnosis of childhood tuberculosis in Malawi: an audit of hospital practice. Int J Tuberc Lung Dis 6: 432–438. [PubMed] [Google Scholar]
- 17. Silva JB, Santos JC, Barbosa L, Carvalho I, 2021. Tuberculosis in the paediatric age group: a reflection on transmission. An Pediatr (Engl Ed) 94: 403–411. [DOI] [PubMed] [Google Scholar]
- 18. Oliva Rapoport VE. et al. , 2022. Impact of prolonged isolation on adolescents with drug-susceptible tuberculosis in Lima, Peru: a qualitative study. BMJ Open 12: e063287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamid M, Brooks MB, Madhani F, Ali H, Naseer MJ, Becerra M, Amanullah F, 2019. Risk factors for unsuccessful tuberculosis treatment outcomes in children. PLoS One 14: e0222776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilale AM. et al. , 2022. Economic burden of tuberculosis in Tanzania: a national survey of costs faced by tuberculosis-affected households. BMC Public Health 22: 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wingfield T. et al. , 2017. A randomized controlled study of socioeconomic support to enhance tuberculosis prevention and treatment, Peru. Bull World Health Organ 95: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beyanga M, Kidenya BR, Gerwing-Adima L, Ochodo E, Mshana SE, Kasang C, 2018. Investigation of household contacts of pulmonary tuberculosis patients increases case detection in Mwanza City, Tanzania. BMC Infect Dis 18: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hodges J. et al. , 2022. Process evaluation for the adaptation, testing and dissemination of a mobile health platform to support people with HIV and tuberculosis in Irkutsk, Siberia. BMJ Open 12: e054867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burke RM. et al. , 2021. Community-based active case-finding interventions for tuberculosis: a systematic review. Lancet Public Health 6: e283–e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hinderaker SG, Rusen ID, Chiang CY, Yan L, Heldal E, Enarson DA, 2011. The FIDELIS initiative: innovative strategies for increased case finding. Int J Tuberc Lung Dis 15: 71–76. [PubMed] [Google Scholar]
- 26. Kasaie P, Andrews JR, Kelton WD, Dowdy DW, 2014. Timing of tuberculosis transmission and the impact of household contact tracing. An agent-based simulation model. Am J Respir Crit Care Med 189: 845–852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.