Abstract
Background
Ascaridia galli is the largest gut‐dwelling helminth of chickens, which confers adverse effects on meat and egg production; thus, on the animal protein supply and the economy. Both adult and immature parasites affect gut health, but larval stages play a major role in pathology.
Aims
Here, we present immunology and pathology of A. galli in chickens.
Materials and Methods
Literatures were surveyed through online platforms such as PubMed, Google Scholar and Researchgate.
Results
The larvae cause excessive mucus production, damage to the intestinal gland, hemorrhage, anemia, diarrhea, and malnutrition. The adult worms can cause death by intestinal obstruction and intussusception. Although both cellular and humoral immunity are involved in fighting against ascariasis, the role of naturally acquired immunity is poorly defined. In cellular immunity, Th‐2 cytokines (IL‐4, IL‐5, IL‐9, and IL‐13), goblet cells (mucin), gut‐associated lymphoid tissues, CD8α+ intraepithelial cells, TCRγδ + T cells, and TGF‐β4 form a protective band. Type 2 immunity provides protection by forming a network of endogenous damage‐associated molecular patterns, chitin, and parasitic antigens. Among antibodies, IgY is the most prominent in chickens and provides temporary humoral protection. During parasitic infection, infiltration of various immune cells is evident, especially in the intestinal epithelium, lamina propria, and crypts of the duodenum and jejunum. In chickens older than 12 weeks, gradual reduction of worm burden is more successful than the younger birds. Female chickens exert a short‐lived but higher level of protection by passing IgY to chicks in the form of egg yolk antibodies. In laying conditions, immunity differs between breeds. This review provides an overview of the silent but inevitable pathological changes induced by A. galli and the interaction of host immunity with the parasite.
Keywords: age, sex and breed specific immunity; Immunology; maternal immunity; pathology; Ascaridia galli
Ascaridia galli is the most common helminth in chickens. It is more common in young chickens. The helminth induces huge economic losses in poultry.

1. INTRODUCTION
The poultry sector, which has an extremely important place in terms of food safety and nutrition, is the fastest‐growing agricultural subsector, especially in developing countries. Poultry is raised by approximately 80% of rural households in developing countries. According to FAO estimates, the global poultry population has produced 83 million tons of eggs and 33 million tons of meat in 2021. 1 Indigenous chickens are popularly reared in backyard or semi‐intensive systems, which is very attractive to the resource‐deprived segment of the global population, particularly by women because of minimum requirement of investment and lower food supplement. Backyard system contributes 8% of total egg production and 2% of total meat production. 2 , 3 The scavenging nature of the chickens facilitate easy fecal contamination of the premises, and the global warming/hot humid climatic condition provides suitable condition for parasite egg development, including the soil‐transmitted helminths. Ascaridia galli (Schrank, 1788), the largest nematode of chickens, is the most frequently encountered problem (up to 90%) of indigenous chickens. 4 Ascaridiasis in chickens is highly prevalent in many countries around the globe, like Germany (88%), Sweden (77.1%), Bangladesh (61%), India (32.97%), and Tanzania (32.3%), where birds were reared in free range system compared to Ghana (30%) and Serbia (15.6%–24%), where intensive rearing system was applied. 5 , 6 , 7 , 8 , 9 , 10 , 11
Ascaridia galli affects the small intestine, especially the duodenum of chickens, pigeons, and wild birds. 12 , 13 , 14 The parasite can be associated with anemia, emaciation, and reduction in production efficiency. 15 , 16 Direct losses are caused by obstruction and damage to the intestinal tract, resulting in malabsorption and malnutrition, alteration of beneficial gut microflora, immunosuppression, and increased susceptibility to concurrent infections. 17 In addition, A. galli can act as a vector for other pathogens such as bacteria (e.g., Salmonella enterica) 18 and can impair the humoral immune responses (HIRs) following vaccination against other pathogens (e.g., Newcastle disease virus). 19 Concurrent infection with A. galli and Escherichia coli 20 or Pasteurella multocida 21 has been shown to have significant effects on weight gain and egg production. Because the life cycle of A. galli is direct, transmission of the parasite is very rapid, especially in deep litter systems. In addition, earthworms act as transport hosts and may play a critical role in the transmission cycle in rural scavenging and semi‐scavenging settings. 22 , 23
The intestinal epithelium acts as a communication network for this gut‐dwelling nematode as it transmits signals to the immune systems (innate and acquired) in the underlying mucosa. 24 Macroscopically, A. galli infection includes a thickened intestinal wall with hemorrhagic spots along with edema and infiltration of lymphoid cells mixed with eosinophils. 25 Occasionally, ulcerative proventriculitis can also be detected in ascaridiasis. 26 The immunological consequences are the alteration in the number and activity of Th2 lymphocytes, Toll‐like receptor expression, the release of the endogenous damage‐associated molecular pattern (eDAMP) or alarmins, and immunomodulatory proteins. 27 , 28 Since an anthelmintic vaccine against A. galli is yet to be developed and commercialized, an anthelmintic‐based control program is the main tool against this helminth. 29 Piperazine and levamisole have been used against A. galli for decades. 17 Although anthelmintic resistance (AR) against A. galli is yet to be reported, however, AR to other helminths is well documented. 30 , 31 In addition, recently, we found that piperazine has very limited activity against several gastro‐intestinal tract dwelling helminths, including A. galli (unpublished data). While the concept of AR is one of the burning issues around the world, the use of plant materials with anthelmintic activity like papaya seed, papaya latex, neem seed, and leaf extract of pine apple could be an easier and cheaper escape route. 32 , 33 Therefore, a thorough, up‐to‐date understanding of the biology, pathology, and immunology of A. galli is needed to develop a sustainable control strategy to mitigate the problem and make poultry production economically viable. This review focuses on the pathobiology and immunity induced against A. galli infection in chickens.
2. TRANSMISSION AND PATHOBIOLOGY
Ascridia galli leads a direct life cycle (Figure 1) involving a single host. The sexually mature adult worms live in the small intestine, lay eggs, and are expelled into the external environment. The oval eggs are enveloped with three layers, namely, the vitelline membrane (the inner permeable layer), a thick, resistant covering, and a thin albuminous layer, 34 which make them resistant to desiccation and allow long survival in the external environment. Eggs do not hatch in the environment; rather develop larvae within the egg, molt continuously, and eventually become the third larval stage (L3). An egg with L3 is the infective stage. During development, eggs begin to divide into the two‐cell stage within 24 h and continue to develop into the three‐cell stage within 48 h and the four‐cell stage within 72 h. This fourth stage develops within eggs, known as morula with blastomeres. A fully mature infectious L3 stage is formed after 11–12 days. 35 Transmission occurs through ingestion of contaminated food/water or mechanically by earthworms. 13 , 34 Earthworms act as paratenic hosts that ingest the infectious stage and become infectious to the definitive hosts. Infection by ingestion of earthworms is easier than picking up eggs in nature. However, the earthworm must be consumed within 96 h to result in effective transmission of A. galli; otherwise, the infective stage may be excreted with the gut contents of the annelids, or the eggs can be hatched in the earthworm intestine. 23 The stimulating factors such as temperature, pH, and carbon dioxide levels initiate egg hatching and release L3 within 24 h of infection, 36 in the anterior portion of the jejunum, the first predilection site of newly hatched larvae. 37 The life cycle of A. galli includes both free‐living larvae and parasitic stages (infective eggs to adult worms). Both stages are responsible for pathological sequelae 38 , 39 and adopt sequentially through the mucosal and histotrophic stages. 40 In necropsy, bundles of parasites are usually seen, indicating a massive dose of infection.
Figure 1.
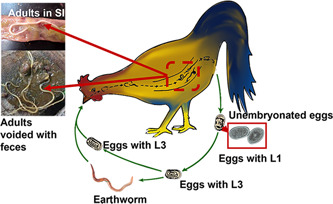
Schematic presentation of the life cycle of Ascaridia galli. L, larva; SI, small intestine.
During the first week of infection, most larvae settle in the anterior half of the jejunoileal section but migrate posteriorly as infection progresses. Thus, one subpopulation of larvae, which inhabit mainly in the lumen, grows over time, while another subpopulation remains dwarfed and attached with the mucosa. Later, both subpopulations migrate to a posterior localization in the gastro‐intestinal tract. 41
The next larval stage is histotrophic, which can last 3–54 days before the larvae move to a final stage in the lumen. Most larvae (63%) are found in the lumen in contact with the epithelium in the crypts of Lieberkühn after 3 days of infection, and 37% of larvae are found in the tunica mucosa. 40 This study also suggested that the highest number of larvae remained in the crypts (51%), followed by the transitional zone (31%) and the villous zone (18%). 40 The duration of this phase is usually dose‐dependent. When the histotrophic phase ends, the larvae return to the lumen of the intestine and become adults. After maturation, adult female worms produce a large number of eggs. The prepatent period is about 4–8 weeks, 42 depending on the age of the host. During the so‐called mucosal or histotrophic phase, when large numbers of larvae invade the duodenal mucosa, they can cause hemorrhagic enteritis by destroying the intestinal epithelium, leading to malabsorption and eventually malnutrition. 43 Ascaridiasis is often associated with anemia, decreased sugar levels, and necrosis of the mucosa, resulting in diarrhea, anorexia, weakness, ruffled feathers, and a dirty vent. 26 , 44 The pathogenesis of A. galli in chickens is mostly associated with hypertrophy of the intestinal villi infiltration of inflammatory cells, especially eosinophils, lymphocytes, and macrophages. Necrosis of the crypts of Lieberkühn is associated with the histotrophic phase of larvae. 24 Infection can result in poor growth followed by poor performance. 45 Infection with large numbers of adult worms results in obstruction of the small intestinal lumen and also intussusception of the intestine due to hypermotility leading to death. 45 Sometimes, A. galli toxins are released with the secretory and excretory (E/S) products of the worms, which impair the enzymatic process of the intestine and further impede the absorption of nutrients through the intestinal wall. 46
3. IMMUNITY
The defense mechanism of the body depends on both cellular and humoral components. In poultry, blood parameters (e.g., acute phase proteins) and intestinal lymphocyte subpopulations such as innate lymphoid cells are greatly modulated by helminth infections. 47 Blood parameters like mannose‐binding lectin and alpha 1‐acid glycoprotein can even be used as indicators for early disease diagnosis, 48 which can be very useful for monitoring the health status of poultry. Worm expulsion was closely related to the developmental stage of the worms, with the elimination of juvenile stages being particularly high. A very small percentage of the worms are nevertheless able to survive and reach sexual maturity. 41
4. CELLULAR IMMUNITY AGAINST A. galli
The endogenous immunity of chickens counters both intracellular (e.g., bacteria) and extracellular (e.g., nematodes) invaders mainly through Th1‐ and Th2‐type immune responses, respectively. 17 , 49 The collagen‐based cuticle with carbohydrate‐rich surface coatings of the parasites, as well as their ability to change antigenic surfaces through multiple molts during the development cycle, 50 play a critical role in how the parasitic worms evade the host's innate immune system. 51 , 52 There are a few studies describing naturally acquired immunity to A. galli. 45 Although specific information on A. galli is not known, it is generally assumed for other gastrointestinal nematodes that the Type 2 response includes several biological processes that serve to disrupt the parasite niche in the gut by strengthening the physical barrier and promoting tissue repair. 53 These mechanisms are highly coordinated and involve several different cell types and effector molecules that have been implicated at various stages of response. 30 , 54 Given this, intestinal nematodes tend to cause more tissue destruction than other pathogens due to their body size and invasiveness. It is perhaps more plausible that Type 2 immunity results from the combined recognition of eDAMP or alarmins and worm‐derived molecules (including chitin) that become available for uptake after larval molting. In addition, parasite‐derived antigens that are continuously secreted during infection also elicit an immune response. 54 Pattern recognition receptors are constitutively expressed at key ports (e.g., skin, lungs, and intestinal epithelia) of pathogens and have been shown to interact with pathogen‐associated molecular patterns (PAMPs) or eDAMP during the progression of various parasitic infections, including helminths and others. 55 , 56
CD4+ Th2‐mediated immunity is characterized by secretion of Type 2 signature cytokines (IL‐4, IL‐5, IL‐9, and IL‐13), activation of alternatively activated macrophages, eosinophils, basophils, and mast cells. 56 , 57 Such a response to enteric nematodes, including A. galli, is associated with hyperplasia of goblet cells, increased numbers and degranulation of mast cells, 58 and an increase in heterophils. 40 Goblet cells are the cells of the innate immune system that are the main source of mucins. Mucins are the main macromolecules of the intestinal mucus barrier. 57 The enlargement of goblet cells leads to increased secretion of mucins. The mucus restricts the movement of the worms by covering their cuticle; thus, it hampers the attachment of worms to the intestinal mucosa, and the worms are eventually removed from the intestinal lumen with the feces. 59 , 60 Granulocytes, especially eosinophils, release toxic substances that are considered effective against extracellular organisms like nematodes. 61 In addition, the gut mucosal immune system is a compartmentalized part of the immune system that provides local immunity because it possesses secondary lymphoid tissues (T, B, and dendritic cells). Once mucosal immune cells are stimulated by luminal antigens, they infiltrate into the diffuse areas of mucosal tissue (e.g., the lamina propria of intestinal villi) and exhibit immune effector functions. 62 Gut‐associated lymphoid tissues (GALTs) are well‐developed in birds. It consists of lymphoid cells located in the epithelial lining and the lamina propria, as well as specialized lymphoid structures such as Peyer's patches and cecal tonsils. 63
The follicle‐associated epithelium covering the GALTs has specialized microfold cells that actively internalize luminal antigens to trigger antigen‐specific immunoglobulins, such as IgA production. 64 , 65 Chickens exposed to nematodes induce polarization of Th1/Th2 immune responses. 17 , 66 Immunity to A. galli is highly gut‐specific. 40 , 44 , 49 Coordinated and measured involvement of different subpopulations of immune cells in the different layers of the intestinal wall, particularly in the epithelium, lamina propria, and crypts of duodenum and jejunum, was elegantly demonstrated. 44 It was also described that the population of CD8α+ intraepithelial lymphocytes (CD8α+ IEL) in the crypts of the jejunum decreased significantly 2–3 weeks after infection. Another study reported that the number of CD4+ IEL in the duodenum and jejunum infected with A. galli increased twofold to threefold 1‐week postinfection (pi). However, the relative number of CD8α+ IEL decreased in the duodenum and jejunum 2‐week pi. 67 TCRγδ+ T cells were also shown to increase (∼45%) in A. galli infections within 1‐week pi in the duodenum. However, no changes were observed in IgA + B lymphocytes in the jejunum (2‐week pi) and duodenum (1‐ and 2‐week pi) (Table 1).
Table 1.
Reports on cellular and humoral immunity against Ascaridia galli infection in chickens.
| Types of immunity | Source |
|---|---|
| A. galli infection develops both cellular (T helper type 2/Th2 immune response) and humoral (IgY antibody‐mediated) immunity. However, elevated IgY level does not confer permanent protection against A. galli. | [17, 66, 67, 68, 69, 70] |
| Immunity to A. galli is highly gut‐specific. | [49, 69] |
| Immune cells are predominantly found in duodenum and jejunum. | [44] |
| In CD4+ Th2‐mediated immunity, IL‐4, IL‐5, IL‐9, and IL‐13 are involved. | [57, 71, 72] |
| A. galli induced goblet cell hyperplasia, increased number of heterophils and mast cells. | [73] |
| CD4+ intraepithelial lymphocytes are increased two to three times in infected duodenum and jejunum at 1‐week postinfection. | [44] |
| IFN‐γ, IL‐1β, IL‐2β, and IL‐18 from splenic cells increased at 6‐week postinfection. | [66] |
| DEFβ1 (Beta‐defensin 1) significantly declined at 2 weeks postinfection but increased after 6 and 8 weeks. | [74] |
| TCRγδ+ T cells in the duodenum are increased within 1‐week postinfection. Worm burden is associated with the influx of both αβ, including CD4+ cells and γδ T cells in the jejunal mucosa. | [44] |
| Mannose‐binding protein (MBP) significantly increased in splenic tissues. | [74] |
| Jejunal mast cells result in the degradation of the A. galli cuticle. | [74] |
| Humoral immune response can only impair larval growth. | [42, 70] |
| IL‐13 activates the STAT6 pathway in A. galli infection, resulting expulsion of worms. | [19, 44, 75] |
| Th2 cytokines associated with short histotrophic phase of A. galli. | [49, 76] |
| A. galli larvae control the antibody production level in both plasma and egg yolk. | [77] |
Upon infection with A. galli, Th2 cytokines, interleukin IL‐4, and IL‐13 are upregulated in the spleen and ileal tissue. 71 In addition, splenocytes from chickens infected with A. galli showed increased expression of IFN‐ƴ, IL‐1β, IL‐12β, and IL‐18 at Week 6 pi, but not at Week 2 or Week 9 pi, except for IL‐8. 66 Expression of IL‐8 was upregulated at both Week 2 and Week 6 pi. On the other hand, DEFβ1 (beta‐defensin 1) expression significantly decreased at 2‐week pi and increased at Weeks 6 and 8 pi. Acute phase proteins, such as mannose‐binding protein, are significantly increased in the spleen tissue of chickens infected with A. galli. 66 Increased expression of TGF‐β4 was also observed; however, IL‐10 was not increased but rather decreased. 71 The Th2 signature cytokine IL‐13 was upregulated in the spleens of chickens infected with A. galli at 2 weeks pi but not at later stages of infection 66 (Table 1). It remains to be determined whether the proinflammatory responses are caused by A. galli‐specific PAMPs, host‐specific DAMPs released by tissue damage, or the DAMP homologues secreted by the parasites or opportunistic secondary microbial pathogens. In the jejunal mucosa, there is an influx of both αβ, including CD4+ cells and ƴδ T cells associated with worm burden, and the highest worm burden results in the highest mRNA expression. 44
5. HUMORAL IMMUNITY AGAINST A. galli
Chickens infected with A. galli showed upregulation of mRNA of the cytokines IL‐4 and IL‐13 in spleen and ileal tissue 14 , 44 , 71 and developed a systemic HIR. 68 , 69 Other research has shown that birds infected with A. galli develop both cellular (T2‐type) and humoral (IgY antibodies, referred to as IgG) immune responses. 70 Chicken sera contain three different types of antibodies, such as IgA, IgM, and IgY. Considering the structure, molecular weight, and immunoelectrophoretic mobility, chicken IgA and IgM are similar to mammalian IgA and IgM, 78 and IgY is the most dominant antibody in birds, reptiles, amphibians, and lungfish. 79 Elevated levels of IgY level have been found in A. galli infection, but this does not provide any permanent protection. 67 , 69 , 70 In fact, the HIR does not provide adequate protection against this nematode and, therefore, cannot prevent reinfection. It can only affect larval growth rather than a complete elimination from the host. 42 , 70 The onset of HIR occurs within 2 weeks of infection with A. galli, but serum titers do not correlate with worm burden or fecundity of the worm. 37 , 70 However, the number of A. galli larvae count correlates significantly with IgY levels at 2 weeks pi. 49 The delayed trend of HIR and the lower IgY level might be related to the age of the bird. 49
Both humoral and local immune responses are associated with worm expulsion, with the latter likely to have strong effects. 49 There are three distinct phases of A. galli expulsion: (1) The first phase, which depends on larval hatchability and the transit time of the host's digesta, allows effective larval establishment on the first day of infection. (2) The second phase, which is the most efficient. It is partially species‐specific and acts on both tissue‐associated larvae and juvenile stages located in the lumen by activating humoral and cell‐mediated immunity (Table 1). Targeted expulsion of the first‐generation worms, slow‐growing larvae, and posterior localization are more evident in this phase. (3) In the third phase, only a few A. galli are expelled. 49 An immunological orchestra elicited against A. galli infection is depicted in Figure 2.
Figure 2.
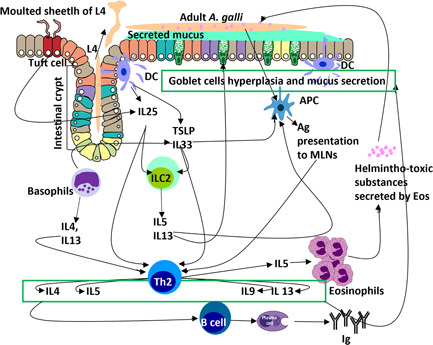
Schematic presentation of immunological orchestra elicited against Ascaridia galli infection. Ag, antigen; APC, antigen‐presenting cells; DC, dendritic cells; Eos, eosinophils; ILC, innate lymphoid cells; IL, interleukin; MLNs, mesenteric lymph nodes; TSLP, thymic stromal lymphopoietin.
The short‐term upregulation of Th2 cytokines may be related to the short histotrophic phase of A. galli, which is an obligatory component of the early infection stage. 49 This is consistent with larval‐dependent antibody production. 77 In fact, the number of larvae, rather than the number of mature worms, influences levels of antibodies in both plasma and egg yolk. The antibody titers might be influenced by several factors, such as the antigen type and dose, the used adjuvant, the route of application, the inoculation frequency, age, and stage of development in birds. 80
6. AGE‐RELATED IMMUNITY AGAINST A. galli
A plethora of literatures suggest that there is age‐dependent immunity. 81 , 82 , 83 , 84 Older chickens are more able to resist infection with A. galli than younger birds. 83 In birds aged >3 months, the histotrophic phase is much longer than in younger birds (≤3 months); therefore, worms mature more rapidly in younger birds and affect the prepatent period. 13 In the Lohmann LSL layer, age does not count as a significant determinant of resistance against A. galli, but a bird's hormonal and immune status does. 84 In another study, high levels of growth inhibitory factors in older birds were found to prevent the development of A. galli infection. 85 Interestingly, age does not ensure protection in layers (Table 2). Laying hens are more susceptible to this nematode infection due to hormonal changes and lower antibody levels, making them immunocompromised. 84
Table 2.
Reports on age, sex‐, and breed‐related immunity against Ascaridia galli infection in chickens.
| Source | |
|---|---|
| Age‐related immunity | |
| Adult chickens (>3 months) can prevent A. galli infection more strongly than younger birds. | [12, 86] |
| In young birds, the prepatent period is short because of the shorter histotrophic phase | [86] |
| Laying hens are more susceptible to A. galli infection due to alteration of hormone level | [85] |
| High levels of growth‐inhibiting factors in older birds resist the infection. | [86] |
| Sex‐related immunity | |
| Female chicks have higher levels of antibody than male due to A. galli infection, but it is not protective enough. | [87, 88] |
| Alteration in protective immune responses (humoral and cellular) greatly hampers vaccine‐induced immunity in laying hens. | [19] |
| Breed‐related immunity | |
| Susceptibility to A. galli infection differs genetically in different layer‐lines like Danish Landrace and Lohman Brown. | [89] |
7. SEX‐ AND BREED‐RELATED IMMUNITY AGAINST A. galli
A. galli‐specific serum antibodies develop to a greater extent in female chicks than in males, independent of dam/offspring infection levels. As chickens become adults, the population of T lymphocytes decreases. 52 , 87 , 88 On the other hand, infection with A. galli can alter protective immune responses (humoral and cellular), thereby severely compromising vaccine‐induced immunity in laying hens. 19 The major histocompatibility complex or B‐complex in chickens is associated with resistance or susceptibility to disease at the individual level. 90 , 91 Susceptibility to infection with A. galli differs genetically in different lines of layers. The Danish Landrace (DL) was found to have a higher worm burden and egg count than the Lohmann Brown (LB) (Table 2) breed of chickens. Both DL and LB showed a self‐cure mechanism, that is, elimination of adult parasites at the time of infection with infectious stages, which is a well‐recognized phenomenon in sheep indicated against Haemonchus contortus. 91
8. MATERNAL IMMUNITY AGAINST A. galli
Maternal immunity, a natural passive immunity, is conferred by the administration of preformed immunoglobulins (Igs) and provides immediate but short‐lived protection against any infection. 92 , 93 Studies suggest that maternal antibodies are the main escape route for very young chicks. 94 , 95 IgG (IgY) transferred from the hen can ensure an effective immune response in the young chicks. IgY accumulates predominantly in the egg yolk (5–15 mg/mL), while IgA (0.3–0.5 mg/mL) and IgM (1–3 mg/mL) are found in egg whites. 96 Egg yolk, ovarian follicles, yolk sac membranes, and oviductal secretions are the main repositories for transferring maternal IgY to hatchlings. 68 , 97 The yolk sac membrane has specific receptors for IgY, 98 and the amount of IgY transferred to the yolk is directly proportional to serum antibodies. 94 Studies have confirmed that there is a strong correlation between plasma and egg yolk IgY levels in hens infected with A. galli. 52 , 94 The production of A. galli‐specific Ig in plasma and egg yolk is dependent on the infective dose and the duration of infection. Plasma antibody (PAB) is induced much earlier than egg yolk antibody (EAB); therefore, PAB is better for providing evidence of early infection. 77 However, both PAB and EAB only indicate the infection dynamics of the parasite, not protection against the worm (Table 3). The number of antibodies present in the egg yolk correlates strongly with the antibody levels of the hens. Nevertheless, maternal antibodies can provide some protection to the chicks, but they do not ensure permanent protective immunity against infection with A. galli. 52
Table 3.
Reports on maternal immunity against Ascaridia galli infection in chickens.
| Maternal immunity | Source |
|---|---|
| Egg yolk, ovarian follicles, yolk sac membranes, and oviduct are the major sites to transfer maternal IgY to hatchlings. | [68, 97, 99] |
| IgY is predominantly found in egg yolk (5–15 mg/mL). | [96, 100] |
| Ascaridia galli‐specific antibodies production in plasma and egg yolk is dependent on the infective dose and duration of infection. | [77] |
| Plasma and egg yolk antibodies indicate only infection but do not give any protection against the worm. | [52] |
| Plasma antibody is more suitable to get hints regarding early infection. | [77] |
| Maternal immunity exerts immediate but short‐lived protection. | [92] |
9. CONCLUSIONS
Ascariasis is a highly prevalent helminth infection in chickens reared in scavenging and semi‐scavenging settings. It mainly affects young birds but is not infrequent in adults as well. Until recently, there is no effective vaccine, and anthelmintics are the main means of tackling the problem. As the prepatent period of A. galli is 4–8 weeks, birds can be treated with an effective anthelmintic (e.g., levamisole) at 4‐ to 6‐week intervals. Humoral immunity does not provide protection; therefore, fostering research activities targeting cell‐mediated immunity is essential. The development or detection of resistant breeds or strains and their dissemination among farmers will help to alleviate the problem. The identification of gene(s) associated with the helminth resistance phenomenon and a controlled breeding policy to develop resistant poultry strain(s) would be another way to reduce the losses associated with A. galli in the poultry industry. Besides, proper nutrition of poultry and the use of botanicals with anthelmintic activity could be revolutionary approaches for sustainable control of A. galli.
AUTHOR CONTRIBUTIONS
Methodology, writing original draft, revision: Nusrat Nowrin Shohana, Sharmin Aqter Rony, and Md. Haydar Ali. Methodology, writing original draft, revision, and editing: Md. Shahadat Hossain, Sharmin Shahid Labony, Anita Rani Dey, and Thahsin Farjana. Conceptualization, writing review, editing, and revision: Anisuzzaman, Md. Abdul Alim, and Mohammad Zahangir Alam. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the generous help of Md. Rayhan Uddin Fakir, Lecturer, Department of Fine Arts, Islamic University, Kushtia.
Shohana NN, Rony SA, Ali MH, et al. Ascaridia galli infection in chicken: Pathobiology and immunological orchestra. Immun Inflamm Dis. 2023;11:e1001. 10.1002/iid3.1001
REFERENCES
- 1. FAOSTAT . Food and Agriculture Organization of the United Nations Statistical database, Rome, Italy. 2019. Accessed November 30, 2022. https://www.fao.org/faostat/en/#data/QCL
- 2. Mottet A, Tempio G. Global poultry production: current state and future outlook and challenges. Worlds Poult Sci J. 2017;73:245‐256. 10.1017/S0043933917000071 [DOI] [Google Scholar]
- 3. Sonaiya EB. Family poultry, food security and the impact of HPAI. Worlds Poult Sci J. 2007;63:132‐138. 10.1017/S0043933907001353 [DOI] [Google Scholar]
- 4. Shifaw A, Feyera T, Walkden‐Brown SW, Sharpe B, Elliott T, Ruhnke I. Global and regional prevalence of helminth infection in chickens over time: a systematic review and meta‐analysis. Poult Sci. 2021;100:101082. 10.1016/j.psj.2021.101082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashika T, Islam A, Anisuzzaman A, Amin N, Begum N, Khan M. Epidemiology of gastro‐intestinal nematode infections in indigenous chickens of Bangladesh. J Bangladesh Agric Univ. 2021;19(4):500‐504. 10.5455/JBAU.73556 [DOI] [Google Scholar]
- 6. Tamara I, Novica Đ, Katarina N, Danica B, Jelena A, Sanda D. Importance of parasitological screening in extensive poultry farming based on organic production. Acta Parasitol. 2019;64:336‐346. 10.2478/s11686-019-00042-y [DOI] [PubMed] [Google Scholar]
- 7. Anane A, Dufailu OA, Addy F. Ascaridia galli and Heterakis gallinarum prevalence and genetic variance of A. galli in rural chicken from the Northern Region, Ghana. Vet Parasitol Reg Stud Reports. 2022;29:100692. 10.1016/j.vprsr.2022.100692 [DOI] [PubMed] [Google Scholar]
- 8. Ara I, Khan H, Syed T, Bhat B. Prevalence and seasonal dynamics of gastrointestinal nematodes of domestic fowls (Gallus gallus domesticus) in Kashmir, India. J Adv Vet Anim Res. 2021;8:448‐453. 10.5455/javar.2021.h533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Permin A, Magwisha H, Kassuku AA, et al. A cross‐sectional study of helminths in rural scavenging poultry in Tanzania in relation to season and climate. J Helminthol. 1997;71:233‐240. 10.1017/S0022149X00015972 [DOI] [PubMed] [Google Scholar]
- 10. Kaufmann F, Daş G, Sohnrey B, Gauly M. Helminth infections in laying hens kept in organic free range systems in Germany. Livest Sci. 2011;141:182‐187. 10.1016/j.livsci.2011.05.015 [DOI] [Google Scholar]
- 11. Jansson DS, Nyman A, Vågsholm I, et al. Ascarid infections in laying hens kept in different housing systems. Avian Pathol. 2010;39:525‐532. 10.1080/03079457.2010.527923 [DOI] [PubMed] [Google Scholar]
- 12. Daş G, Kaufmann F, Abel H, Gauly M. Effect of extra dietary lysine in Ascaridia galli‐infected grower layers. Vet Parasitol. 2010;170:238‐243. 10.1016/j.vetpar.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 13. Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th ed. Baillière Tindall; 1982. [Google Scholar]
- 14. Alkharigy FA, El Naas AS, El Maghrbi AA. Survey of parasites in domestic pigeons (Columba livia) in Tripoli, Libya. Open Vet J. 2018;8:360‐366. 10.4314/OVJ.V8I4.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dänicke S, Moors E, Beineke A, Gauly M. Ascaridia galli infection of pullets and intestinal viscosity: consequences for nutrient retention and gut morphology. Br Poult Sci. 2009;50:512‐520. 10.1080/00071660903124530 [DOI] [PubMed] [Google Scholar]
- 16. Phiri IK, Phiri AM, Ziela M, Chota A, Masuku M, Monrad J. Prevalence and distribution of gastrointestinal helminths and their effects on weight gain in free‐range chickens in Central Zambia. Trop Anim Health Prod. 2007;39:309‐315. 10.1007/S11250-007-9021-5 [DOI] [PubMed] [Google Scholar]
- 17. Sharma N, Hunt PW, Hine BC, Ruhnke I. The impacts of Ascaridia galli on performance, health, and immune responses of laying hens: new insights into an old problem. Poult Sci. 2019;98:6517‐6526. 10.3382/ps/pez422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eigaard NM, Schou TW, Permin A, et al. Infection and excretion of Salmonella Enteritidis in two different chicken lines with concurrent Ascaridia galli infection. Avian Pathol. 2006;35:487‐493. 10.1080/03079450601071696 [DOI] [PubMed] [Google Scholar]
- 19. Pleidrup J, Dalgaard TS, Norup LR, et al. Ascaridia galli infection influences the development of both humoral and cell‐mediated immunity after Newcastle Disease vaccination in chickens. Vaccine. 2014;32:383‐392. 10.1016/j.vaccine.2013.11.034 [DOI] [PubMed] [Google Scholar]
- 20. Permin A, Christensen JP, Bisgaard M. Consequences of concurrent Ascaridia galli and Escherichia coli infections in chickens. Acta Vet Scand. 2006;47:43. 10.1186/1751-0147-47-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dahl C, Permin A, Christensen JP, et al. The effect of concurrent infections with Pasteurella multocida and Ascaridia galli on free range chickens. Vet Microbiol. 2002;86:313‐324. 10.1016/S0378-1135(02)00015-9 [DOI] [PubMed] [Google Scholar]
- 22. Anderson RC. Order Ascaridida. Nematode Parasites of Vertebrates: Their Development and Transmission. CABI Publishing; 1999:265. 10.1079/9780851994215.0245 [DOI] [Google Scholar]
- 23. Augustine PC, Lund EE. The fate of eggs and larvae of Ascaridia galli in earthworms. Avian Dis. 1974;18:394‐398. 10.2307/1589106 [DOI] [PubMed] [Google Scholar]
- 24. Malatji DP, van Marle‐Koster E, Muchadeyi FC. Gene expression profiles of the small intestine of village chickens from an Ascaridia galli infested environment. Vet Parasitol. 2019;276:100012. 10.1016/j.vpoa.2019.100012 [DOI] [PubMed] [Google Scholar]
- 25. Levkut M, Levkutová M, Grešáková Ľ, et al. Production of intestinal mucins, sIgA, and metallothionein after administration of zinc and infection of Ascaridia galli in chickens: preliminary data. Life. 2022;13:67. 10.3390/life13010067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brar RS, Kumar R, Leishangthem GD, Banga HS, Singh ND, Singh H. Ascaridia galli induced ulcerative proventriculitis in a poultry bird. J Parasit Dis. 2016;40:562‐564. 10.1007/s12639-014-0509-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol. 2006;28:515‐523. 10.1111/J.1365-3024.2006.00879.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10:3‐12. 10.1007/s11882-009-0085-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tarbiat B, Jansson DS, Tydén E, Höglund J. Comparison between anthelmintic treatment strategies against Ascaridia galli in commercial laying hens. Vet Parasitol. 2016;226:109‐115. 10.1016/j.vetpar.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 30. Anisuzzaman □, Tsuji N. Schistosomiasis and hookworm infection in humans: disease burden, pathobiology and anthelmintic vaccines. Parasitol Int. 2020;75:102051. 10.1016/j.parint.2020.102051 [DOI] [PubMed] [Google Scholar]
- 31. Dey AR, Begum N, Anisuzzaman □, Alim MA, Alam MZ. Multiple anthelmintic resistance in gastrointestinal nematodes of small ruminants in Bangladesh. Parasitol Int. 2020;77:102105. 10.1016/J.PARINT.2020.102105 [DOI] [PubMed] [Google Scholar]
- 32. Feroza S. Effect of papaya and neem seeds on Ascaridia galli infection in broiler chicken. Pak J Nematol. 2017;35:105‐111. 10.18681/pjn.v35.i01.p105-111 [DOI] [Google Scholar]
- 33. Patra G, Lyngdoh WM, Ali MA, et al. Comparative anthelmintic efficacy of pineapple and neem leaves in broiler chickens experimentally infected with Ascaridia galli . Int J Poult Sci. 2010;9:1120‐1124. 10.3923/ijps.2010.1120.1124 [DOI] [Google Scholar]
- 34. Perry RN, Wharton DA. Molecular and Physiological Basis of Nematode Survival. CAB International; 2011. 10.1079/9781845936877.0000 [DOI] [Google Scholar]
- 35. Tarbiat B, Rahimian S, Jansson DS, Halvarsson P, Höglund J. Developmental capacity of Ascaridia galli eggs is preserved after anaerobic storage in faeces. Vet Parasitol. 2018;255:38‐42. 10.1016/j.vetpar.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 36. Tarbiat B, Jansson DS, Höglund J. Environmental tolerance of free‐living stages of the poultry roundworm Ascaridia galli . Vet Parasitol. 2015;209:101‐107. 10.1016/j.vetpar.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 37. Ferdushy T, Nejsum P, Roepstorff A, Thamsborg SM, Kyvsgaard NC. Ascaridia galli in chickens: intestinal localization and comparison of methods to isolate the larvae within the first week of infection. Parasitol Res. 2012;111:2273‐2279. 10.1007/s00436-012-3079-3 [DOI] [PubMed] [Google Scholar]
- 38. Daş G, Abel H, Humburg J, et al. The effects of dietary non‐starch polysaccharides on Ascaridia galli infection in grower layers. Parasitology. 2012;139:110‐119. 10.1017/S0031182011001636 [DOI] [PubMed] [Google Scholar]
- 39. Luna‐Olivares LA, Kyvsgaard NC, Ferdushy T, et al. The jejunal cellular responses in chickens infected with a single dose of Ascaridia galli eggs. Parasitol Res. 2015;114:2507‐2515. 10.1007/s00436-015-4450-y [DOI] [PubMed] [Google Scholar]
- 40. Luna‐Olivares LA, Ferdushy T, Kyvsgaard NC, et al. Localization of Ascaridia galli larvae in the jejunum of chickens 3 days post infection. Vet Parasitol. 2012;185:186‐193. 10.1016/j.vetpar.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 41. Ferdushy T, Luna‐Olivares LA, Nejsum P, Roepstorff AK, Thamsborg SM, Kyvsgaard NC. Population dynamics of Ascaridia galli following single infection in young chickens. Parasitology. 2013;140:1078‐1084. 10.1017/S0031182013000401 [DOI] [PubMed] [Google Scholar]
- 42. Andersen JP, Norup LR, Dalgaard TS, et al. No protection in chickens immunized by the oral or intra‐muscular immunization route with Ascaridia galli soluble antigen. Avian Pathol. 2013;42:276‐282. 10.1080/03079457.2013.783199 [DOI] [PubMed] [Google Scholar]
- 43. Hinrichsen LK, Labouriau R, Engberg RM, Knierim U, Sørensen JT. Helminth infection is associated with hen mortality in Danish organic egg production. Vet Rec. 2016;179:196. 10.1136/vr.103614 [DOI] [PubMed] [Google Scholar]
- 44. Schwarz A, Gauly M, Abel H, et al. Immunopathogenesis of Ascaridia galli infection in layer chicken. Dev Comp Immunol. 2011;35:774‐784. 10.1016/j.dci.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 45. Daş G, Gauly M. Response to Ascaridia galli infection in growing chickens in relation to their body weight. Parasitol Res. 2014;113:1985‐1988. 10.1007/s00436-014-3832-x [DOI] [PubMed] [Google Scholar]
- 46. Vassilev I, Ossikovski E, Bozhkov S, Kambourov P, Bankov I, Roupova L. On the pathogenesis of ascaridiosis in turkeys. Bull Cent Helminthol Lab. 1973;16:43‐58. [Google Scholar]
- 47. Murata H, Shimada N, Yoshioka M. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J. 2004;168:28‐40. 10.1016/S1090-0233(03)00119-9 [DOI] [PubMed] [Google Scholar]
- 48. Nakamura K, Mitarai Y, Yoshioka M, Koizumi N, Shibahara T, Nakajima Y. Serum levels of interleukin‐6, alpha1‐acid glycoprotein, and corticosterone in two‐week‐old chickens inoculated with Escherichia coli lipopolysaccharide. Poult Sci. 1998;77:908‐911. 10.1093/PS/77.6.908 [DOI] [PubMed] [Google Scholar]
- 49. Stehr M, Sciascia Q, Metges CC, Gauly M, Daş G. Co‐expulsion of Ascaridia galli and Heterakis gallinarum by chickens. Int J Parasitol. 2018;48:1003‐1016. 10.1016/j.ijpara.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 50. Blaxter ML, Page AP, Rudin W, Maizels RM. Nematode surface coats: actively evading immunity. Parasitol Today. 1992;8:243‐247. 10.1016/0169-4758(92)90126-M [DOI] [PubMed] [Google Scholar]
- 51. De Veer MJ, Kemp JM, Meeusen ENT. The innate host defence against nematode parasites. Parasite Immunol. 2007;29:1‐9. 10.1111/j.1365-3024.2006.00910.x [DOI] [PubMed] [Google Scholar]
- 52. Rahimian S, Daş G, Gauly M. Maternal protection against Ascaridia galli? Vet Parasitol. 2017;233:43‐47. 10.1016/j.vetpar.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 53. Grencis RK, Humphreys NE, Bancroft AJ. Immunity to gastrointestinal nematodes: mechanisms and myths. Immunol Rev. 2014;260:183‐205. 10.1111/imr.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sorobetea D, Svensson‐Frej M, Grencis R. Immunity to gastrointestinal nematode infections. Mucosal Immunol. 2018;11:304‐315. 10.1038/mi.2017.113 [DOI] [PubMed] [Google Scholar]
- 55. Tsubokawa D, Kikuchi T, Lee JM, Kusakabe T, Yamamoto Y, Maruyama H. Venestatin from parasitic helminths interferes with receptor for advanced glycation end products (RAGE)‐mediated immune responses to promote larval migration. PLoS Pathog. 2021;17:e1009649. 10.1371/journal.ppat.1009649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anisuzzaman □, Hatta T, Miyoshi T, et al. Longistatin in tick saliva blocks advanced glycation end‐product receptor activation. J Clin Invest. 2014;124:4429‐4444. 10.1172/JCI74917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sharpe C, Thornton DJ, Grencis RK. A sticky end for gastrointestinal helminths; the role of the mucus barrier. Parasite Immunol. 2018;40:e12517. 10.1111/pim.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Knight PA, Brown JK, Pemberton AD. Innate immune response mechanisms in the intestinal epithelium: potential roles for mast cells and goblet cells in the expulsion of adult Trichinella spiralis . Parasitology. 2008;135:655‐670. 10.1017/S0031182008004319 [DOI] [PubMed] [Google Scholar]
- 59. Levy DA, Frondoza C. Immunity to intestinal parasites: role of mast cells and goblet cells. Fed Proc. 1983;42:1750‐1755. [PubMed] [Google Scholar]
- 60. Ishikawa N, Horii Y, Oinuma T, Suganuma T, Nawa Y. Goblet cell mucins as the selective barrier for the intestinal helminths: t‐cell‐independent alteration of goblet cell mucins by immunologically 'damaged' Nippostrongylus brasiliensis worms and its significance on the challenge infection with homologous and heterologous parasites. Immunology. 1994;81:480‐486. [PMC free article] [PubMed] [Google Scholar]
- 61. Sorci G, Faivre B. Inflammation and oxidative stress in vertebrate host‐parasite systems. Philos Trans R Soc B. 2009;364:71‐83. 10.1098/rstb.2008.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nochi T, Jansen CA, Toyomizu M, Eden W. The well‐developed mucosal immune systems of birds and mammals allow for similar approaches of mucosal vaccination in both types of animals. Front Nutr. 2018;5:60. 10.3389/fnut.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lamichhane A, Azegami T, Kiyono H. The mucosal immune system for vaccine development. Vaccine. 2014;32:6711‐6723. 10.1016/j.vaccine.2014.08.089 [DOI] [PubMed] [Google Scholar]
- 64. Takahashi D, Kimura S, Hase K. Intestinal immunity: to be, or not to be, induced? That is the question. Int Immunol. 2021;33:755‐759. 10.1093/intimm/dxab051 [DOI] [PubMed] [Google Scholar]
- 65. Nakamura K, Kassem S, Cleynen A, et al. Dysregulated IL‐18 is a key driver of immunosuppression and a possible therapeutic target in the multiple myeloma microenvironment. Cancer Cell. 2018;33:634‐648. 10.1016/j.ccell.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 66. Dalgaard TS, Skovgaard K, Norup LR, et al. Immune gene expression in the spleen of chickens experimentally infected with Ascaridia galli . Vet Immunol Immunopathol. 2015;164:79‐86. 10.1016/j.vetimm.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 67. Ruhnke I, Andronicos NM, Swick RA, et al. Immune responses following experimental infection with Ascaridia galli and necrotic enteritis in broiler chickens. Avian Pathol. 2017;46:602‐609. 10.1080/03079457.2017.1330536 [DOI] [PubMed] [Google Scholar]
- 68. Marcos‐Atxutegi C, Gandolfi B, Arangüena T, Sepúlveda R, Arévalo M, Simón F. Antibody and inflammatory responses in laying hens with experimental primary infections of Ascaridia galli . Vet Parasitol. 2009;161:69‐75. 10.1016/j.vetpar.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 69. Ferdushy T, Schou TW, Norup LR, et al. Acquisition of resistance after continuous infection with Ascaridia galli in chickens. Parasitology. 2014;141:1603‐1610. 10.1017/S0031182014000742 [DOI] [PubMed] [Google Scholar]
- 70. Norup LR, Dalgaard TS, Pleidrup J, et al. Comparison of parasite‐specific immunoglobulin levels in two chicken lines during sustained infection with Ascaridia galli . Vet Parasitol. 2013;191:187‐190. 10.1016/j.vetpar.2012.07.031 [DOI] [PubMed] [Google Scholar]
- 71. Degen WGJ, Daal N, Rothwell L, Kaiser P, Schijns VEJC. Th1/Th2 polarization by viral and helminth infection in birds. Vet Microbiol. 2005;105:163‐167. 10.1016/j.vetmic.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 72. Kaiser P. The avian immune genome—a glass half‐full or half‐empty? Cytogenet Genome Res. 2007;117:221‐230. 10.1159/000103183 [DOI] [PubMed] [Google Scholar]
- 73. Darmawi D, Balqis U, Hambal M, Tiuria R, Frengki F, Priosoeryanto BP. Mucosal mast cells response in the jejunum of Ascaridia galli‐infected laying hens. Media Peternakan. 2013;36:113‐119. 10.5398/medpet.2013.36.2.113 [DOI] [Google Scholar]
- 74. Guo X, Wang L, Cui D, Ruan W, Liu F, Li H. Differential expression of the toll‐like receptor pathway and related genes of chicken bursa after experimental infection with infectious bursa disease virus. Arch Virol. 2012;157:2189‐2199. 10.1007/s00705-012-1403-y [DOI] [PubMed] [Google Scholar]
- 75. Oeser K, Schwartz C, Voehringer D. Conditional IL‐4/IL‐13‐deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol. 2015;8:672‐682. 10.1038/mi.2014.101 [DOI] [PubMed] [Google Scholar]
- 76. Harris N, Gause WC. To B or not to B: B cells and the Th2‐type immune response to helminths. Trends Immunol. 2011;32:80‐88. 10.1016/j.it.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Daş G, Hennies M, Tuchscherer A, Gauly M. Time‐ and dose‐dependent development of humoral immune responses to Ascaridia galli in experimentally and naturally infected chickens. Vet Parasitol. 2018;255:10‐19. 10.1016/j.vetpar.2018.03.021 [DOI] [PubMed] [Google Scholar]
- 78. Leslie GA, Clem LW. Phylogeny of immunoglobulin structure and function. J Exp Med. 1969;130:1337‐1352. 10.1084/jem.130.6.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunol Today. 1995;16:392‐398. 10.1016/0167-5699(95)80008-5 [DOI] [PubMed] [Google Scholar]
- 80. Schade R, Calzado EG, Sarmiento R, Chacana PA, Porankiewicz‐Asplund J, Terzolo HR. Chicken egg yolk antibodies (IgY‐technology): a review of progress in production and use in research and human and veterinary medicine. ATLA: Altern Lab Anim. 2005;33:129‐154. 10.1177/026119290503300208 [DOI] [PubMed] [Google Scholar]
- 81. Gauly M, Duss C, Erhardt G. Influence of Ascaridia galli infections and anthelmintic treatments on the behaviour and social ranks of laying hens (Gallus gallus domesticus). Vet Parasitol. 2007;146:271‐280. 10.1016/j.vetpar.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 82. Egerton JR, Hansen MF. Immunity and tolerance of chickens to the roundworm, Ascaridia galli (Schrank). Exp Parasitol. 1955;4:335‐350. 10.1016/0014-4894(55)90011-4 [DOI] [PubMed] [Google Scholar]
- 83. Idi A, Permin A, Murrell KD. Host age only partially affects resistance to primary and secondary infections with Ascaridia galli (Schrank, 1788) in chickens. Vet Parasitol. 2004;122:221‐231. 10.1016/j.vetpar.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 84. Gauly M, Homann T, Erhardt G. Age‐related differences of Ascaridia galli egg output and worm burden in chickens following a single dose infection. Vet Parasitol. 2005;128:141‐148. 10.1016/j.vetpar.2004.11.023 [DOI] [PubMed] [Google Scholar]
- 85. Silva AP, Gallardo RA. The chicken MHC: insights into genetic resistance, immunity, and inflammation following infectious bronchitis virus infections. Vaccines. 2020;8:637. 10.3390/VACCINES8040637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nalule AS, Mbaria JM, Olila D, Kimenju JW. Ethnopharmacological practices in management of livestock helminthes by pastoral communities in the drylands of Uganda. Livest Res Rural Dev. 2011;23:36. [Google Scholar]
- 87. Bettridge JM, Lynch SE, Brena MC, et al. Infection‐interactions in Ethiopian village chickens. Prev Vet Med. 2014;117:358‐366. 10.1016/j.prevetmed.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Johnston CE, Hartley C, Salisbury A‐M, Wigley P. Immunological changes at Point‐of‐Lay increase susceptibility to Salmonella enterica serovar enteritidis infection in vaccinated chickens. PLoS One. 2012;7:e48195. 10.1371/journal.pone.0048195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Permin A, Ranvig H. Genetic resistance to Ascaridia galli infections in chickens. Vet Parasitol. 2001;102:101‐111. 10.1016/S0304-4017(01)00525-8 [DOI] [PubMed] [Google Scholar]
- 90. Schou T, Permin A, Roepstorff A, Sorensen P, Kjær J. Comparative genetic resistance to Ascaridia galli infections of 4 different commercial layer‐lines. Br Poult Sci. 2003;44:182‐185. 10.1080/0007166031000088335 [DOI] [PubMed] [Google Scholar]
- 91. Schou TW, Permin A, Juul‐Madsen HR, et al. Gastrointestinal helminths in indigenous and exotic chickens in Vietnam: association of the intensity of infection with the major histocompatibility complex. Parasitology. 2007;134:561‐573. 10.1017/S0031182006002046 [DOI] [PubMed] [Google Scholar]
- 92. Kovacs‐Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annu Rev Food Sci Technol. 2012;3:163‐182. 10.1146/annurev-food-022811-101137 [DOI] [PubMed] [Google Scholar]
- 93. Marcotte H, Hammarström L. Passive immunization: toward magic bullets. Mucosal Immunol Fourth Ed. 2015;2–2:1403‐1434. 10.1016/B978-0-12-415847-4.00071-9 [DOI] [Google Scholar]
- 94. Hamal KR, Burgess SC, Pevzner IY, Erf GF. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult Sci. 2006;85:1364‐1372. 10.1093/ps/85.8.1364 [DOI] [PubMed] [Google Scholar]
- 95. Gharaibeh S, Mahmoud K, Al‐Natour M. Field evaluation of maternal antibody transfer to a group of pathogens in meat‐type chickens. Poult Sci. 2008;87:1550‐1555. 10.3382/ps.2008-00119 [DOI] [PubMed] [Google Scholar]
- 96. Härtle S, Magor KE, Göbel TW, Davison F, Kaspers B. Structure and evolution of avian immunoglobulins. In: Schat KA, Kaspers B, Kaiser P, eds. Avian Immunol. 2nd ed. Academic Press; 2014:103‐120. 10.1016/B978-0-12-396965-1.00006-6 [DOI] [Google Scholar]
- 97. West AP, Herr AB, Bjorkman PJ. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC‐related Fc receptor, is a phospholipase A2 receptor homolog. Immunity. 2004;20:601‐610. 10.1016/S1074-7613(04)00113-X [DOI] [PubMed] [Google Scholar]
- 98. Morrison SL, Mohammed MS, Wims LA, Trinh R, Etches R. Sequences in antibody molecules important for receptor‐mediated transport into the chicken egg yolk. Mol Immunol. 2002;38:619‐625. 10.1016/S0161-5890(01)00095-5 [DOI] [PubMed] [Google Scholar]
- 99. Grindstaff JL, Brodie ED, Ketterson ED. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc R Soc Lond. 2003;270:2309‐2319. 10.1098/rspb.2003.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rose ME, Orlans E. Immunoglobulins in the egg, embryo and young chick. Dev Comp Immunol. 1981;5:15‐20. 10.1016/S0145-305X(81)80003-1 [DOI] [PubMed] [Google Scholar]


