Abstract
This study aimed to summarise the findings of the studies assessing the effectiveness of ultraviolet C (UV-C) room disinfection in reducing the incidence rate of healthcare-associated multi-drug-resistant organism (MDRO) infections. A systematic screening was conducted using PubMed, EMBASE, and Scopus for randomised controlled trials (RCTs), quasi-experimental studies, and before–after studies, which assessed the efficacy of the UV-C disinfectant system in reducing the incidence of MDRO infections. A random-effects model was used for the analysis. Effect sizes were described as incidence rate ratio (IRR) with 95% confidence intervals (CI). Nine studies were included, all of which were conducted in the USA. No statistically significant reduction in Clostridioides difficile (CD) (IRR: 0.90, 95% CI; 0.62–1.32) and vancomycin-resistant enterococcal (VRE) infection rates (IRR 0.72, 95% CI; 0.38–1.37) was observed with the use of UV-C, but the risk of Gram-negative rod infection was reduced (IRR 0.82, 95% CI; 0.68–0.99).
Keywords: healthcare-associated infection, HCAI, meta-analysis, multi-drug-resistant organisms’, systematic review, ultraviolet C
Introduction
Nosocomial infections, also called healthcare-associated infections (HCAIs), are reported to account for approximately 7% of all infections in developed countries and 10% in developing countries [1, 2]. Recent evidence suggests that nosocomial infections affect nearly 15% of all hospitalised patients [3] and are associated with prolonged hospital stay, significant disability, and economic burden. Studies conducted in high-income settings in the USA and Europe showed that the incidence density of such infections is around 13–20 episodes per 1000 patient days [4] and is associated with a high financial burden [5]. This burden is expected to be much higher with the increasing emergence of multi-drug-resistant organisms (MDROs) [5]. Recent studies have shown that >70% of the bacteria implicated in HCAI are usually resistant to one or more of the antimicrobials used for the initial treatment of patients [6], and the attributable cost increase in treating resistant organisms ranges from 4000 to 4500 USD per infection per patient [7, 8].
Multi-drug-resistant bacteria can survive in the hospital environment for long periods [9], and all surfaces, porous or non-porous, in patient’s rooms are highly susceptible to contamination [10]. Consequently, effective infection prevention programmes have environmental hygiene as an integral component. A wide range of chemical disinfectants are commonly used in healthcare settings and include surface disinfectants such as quaternary ammonium compounds, sodium hypochlorite, peracetic acid, and liquid hydrogen peroxide [10]. No-touch technologies in addition to conventional cleaning measures are commonly used in hospital settings and include exposure to ultraviolet light or hydrogen peroxide vapour or mist [11–13]. Ultraviolet light sources are broadly categorised as UV-C devices and those that utilise pulsed xenon–UV light (PX-UVL). The former consist of mercury bulbs that emit continuous radiation of wavelength ranging from 200 to 270 nm [14, 15], while a PX-UVL system is characterised by short high-intensity bursts of radiation of UV wavelengths (100–280 nm) and visible (380–700 nm) spectra [14, 15].
A meta-analysis by Dong et al. [16] has shown that PX-UV may be useful in reducing the incidence rate of infections with Clostridium difficile (CD) and methicillin-resistant Staphylococcus aureus (MRSA) but was not effective in reducing the rates of vancomycin-resistant enterococcal (VRE) infection. Likewise, Marra et al. [17] pooled data on both types of UV technologies and found a statistically significant decrease in both CDI and VRE infection rates, but rates of MRSA and Gram-negative MDROs were unaffected. However, the latter analysis included only two studies utilising UV-C.
Recently, several studies have examined the effect of UV-C disinfection system on the rates of MDRO infections in hospitals. The current review aimed at synthesising the findings of all studies assessing the impact of UV-C room disinfection on reducing HCAI infection rates. In particular, the outcomes were related to the effect of UV-C disinfection on the risk of CDI, VRE, and Gram-negative multi-drug-resistant pathogens. The decision to focus only on UV-C disinfection systems (and exclude PX-UVL studies) was driven by the specific research question and objectives of the meta-analysis. The primary focus of our analysis was on disinfection systems that utilise continuous-wave UV-C light. PX-UVL systems differ from the latter in terms of the light source, technology, and application protocols. Nevertheless, the methods aim to achieve disinfection by damaging the genetic material of microorganisms. We considered that these differences made it challenging to directly compare the outcomes of PX-UVL evaluations with conventional UV-C systems.
Methods
Selection of studies
The review protocol was registered at PROSPERO (registration number CRD42023405885). Given its nature, oversight by an institutional board was not required before registration. The search strategy is presented in Supplementary Table S1. Three databases, that is PubMed, EMBASE, and Scopus, were screened for English language studies published up to 15 February 2023, in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [18]. The inclusion criteria were as follows: studies that had assessed the efficacy of an ultraviolet C (UV-C) disinfectant system for reduction in the incidence of MDRO infections; were conducted in all healthcare settings; of all sample sizes and types of patient population; and randomised controlled trials (RCTs), quasi-experimental studies, and before–after interventions. The exclusion criteria were as follows: those assessing the effect of PX-UVL; evaluations of the efficacy of UV-C in combination with other infection control measures; and reviews, case reports, and case series. After the removal of duplicates, the titles and abstracts of studies were independently screened by two of the study investigators for potential inclusion. The full texts of the remaining studies were then read, and final decisions for inclusion were made. A senior author was consulted in case of any discrepancies.
Data extraction and statistical analysis
Data were extracted using a pretested electronic template that consisted of variables related to study identifiers (author’s name, year of publication, study design, and country of study), type of health facility where the study was conducted, duration, and findings of relevance. Statistical analysis was conducted using STATA 16 software (Texas, USA). The pooled effect sizes were reported as an incidence rate ratio (IRR) with 95% confidence intervals (CI). We decided, a priori, to use a random-effects model for all analyses to account for potential variability such as characteristics of the hospitals studied, study design, and method of data collection. It was assumed that these differences would have led to substantial heterogeneity in the reported findings. A revised Cochrane risk-of-bias tool for randomised trials (RoB 2) was used [19]. For ‘before–after’ studies, the quality assessment tool developed by the National Heart Lung and Blood Institute [20] was used, and Egger’s test and funnel plots were used for the assessment of publication bias [21]. P < 0.05 was statistically significant. A subgroup analysis was conducted based on the study design (i.e. RCT/quasi-experimental and before–after design), for the risk of CDI as an outcome.
Results
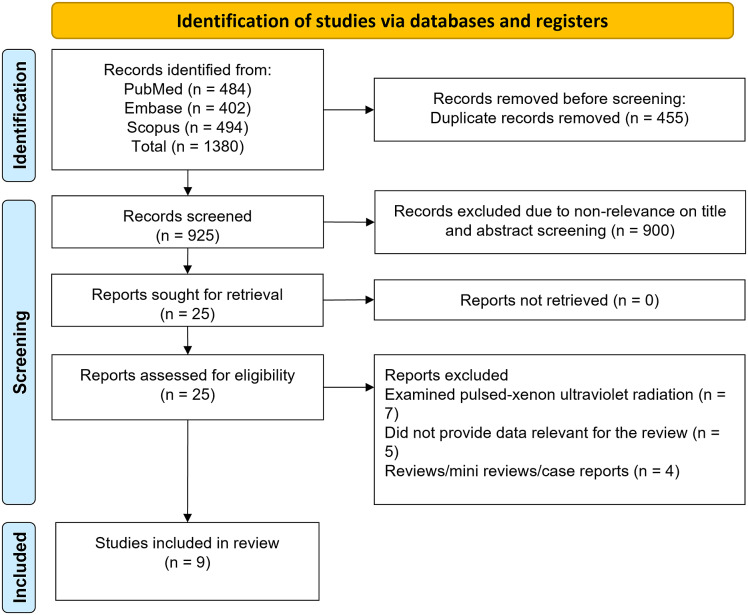
A systematic search across three databases identified 1380 studies. After the removal of the 455 duplicates, 925 unique studies remained. Screening based on their title and abstract led to the further exclusion of 900 studies. The full texts of the resulting 25 studies were screened, and an additional 16 were excluded (Figure 1) leaving nine studies [22–30] for this meta-analysis (summarised in Table 1). All studies were conducted in the USA and five were of a ‘before–after’ design, two were classed as quasi-experimental, and two were of a cluster-randomised crossover design. The included studies examined mainly CD, VRE, Gram-negative rod infection, and MRSA. Two studies specifically studied Gram-negative MDROs including Klebsiella, Acinetobacter, Pseudomonas, and E. coli. Schaffzin et al. [30] defined a Gram-negative as an isolate that was non-susceptible (intermediate or resistant) to at least one agent in at least three of nine antibiotic classes (anti-pseudomonal penicillin, third- or fourth-generation cephalosporin, carbapenem, fluoroquinolone, aminoglycoside, penicillin and beta-lactamase inhibitor, monobactam, polymyxin, and folate inhibitor). Five of the studies were conducted in academic medical facility or tertiary care hospitals, two in community hospitals, and one each in Veteran’s Health Administration (VHA) hospitals, and in a tertiary or community or VHA setting. The quality assessments for the cluster-randomised and quasi-experimental studies and for ‘before–after’ design studies are presented in Supplementary Figure S1 and Supplementary Table S2, respectively. It was concluded that most studies had a moderate risk of bias.
Figure 1.
Selection process of studies included in the review.
Table 1.
Characteristics of the studies included in the meta-analysis
| References | Study design and country | Hospital type (No. of beds) | Study period and comparisons | Outcomes |
|---|---|---|---|---|
| Rock [22] | Cluster-randomised crossover trial USA |
Academic medical facility (1059) Cancer and solid organ transplant in-patient units |
Phase 1: 12 months and 15 days Washout: 5 weeks Phase 2: 12 months and 15 days UV-C + standard environmental cleaning versus standard environmental cleaning |
Vancomycin-resistant enterococcal infection (VRE): IRR 0.98 (95% CI; 0.78, 1.22) Clostridioides difficile (CD) infection: IRR 1.43 (95% CI; 0.93, 2.21) |
| McMullen [23] | Before–after USA |
Acute care community hospital (472) | Pre-intervention: 12 months Post-intervention: 21 months Pre-intervention: Standard daily manual disinfection protocol Intervention: manual disinfection protocol along with UV-C-based disinfection |
CD infection: IRR 1.34 (95% CI; 0.84, 2.15) |
| Anderson [24] | Cluster-randomised crossover trial USA |
Nine hospitals representing multiple types (tertiary, community, Veterans Affairs) |
Each strategy used at every study hospital for four consecutive 7-month study periods. Each study period consisted of a 1-month wash-in period followed by a 6-month period of data collection Three strategies for enhanced terminal disinfection tested against the standard terminal disinfection Comparison of interest for this review: UV-C + standard quaternary ammonium disinfectant (bleach for C. difficile) versus standard quaternary ammonium disinfectant (bleach for C. difficile) |
VRE infection: IRR 0.46 (95% CI; 0.26, 0.82) CD infection: IRR 0.96 (95% CI; 0.61, 1.52) Methicillin-resistant staphylococcal infection (MRSA): IRR 0.73 (95% CI; 0.51, 1.04) |
| Abosi [25] | Before–after USA |
Academic medical centre (811) | 9 months UV-C + sporicidal agent (bleach wipes) versus sporicidal agent (bleach wipes) |
CD infection: IRR 1.05 (95% CI; 0.70, 1.58) |
| Steele [26] | Before–after USA |
Academic children hospital (364) Paediatric haematology–oncology unit |
Pre-intervention: 42 months Post-intervention: 18 months Pre-intervention: manual disinfection protocol along with UV-C-based disinfection. Post-intervention: standard daily manual disinfection protocol |
CD infection: IRR 0.38 (95% CI; 0.24, 0.61) |
| Napolitano [27] | Before–after USA |
Community hospital (420) All patient rooms, hospital-wide |
6 months Continuously monitored and frequently UV-C treatment; incidence of infection before and after the intervention period compared |
CD infection: IRR 0.54 (95% CI; 0.03, 10.58) VRE infection: IRR 0.88 (95% CI; 0.05, 15.36) |
| Pegues [28] | Quasi-experimental with interrupted time series USA |
Academic tertiary care hospital (789) Haematology–oncology units |
Pre-intervention: 12 months Post-intervention: 12 months Incidence rates of C. difficile infection compared between the baseline and intervention period |
CD infection: IRR 0.75 (95% CI; 0.43, 1.30) |
| Goto [29] | Quasi-experimental USA |
Veterans’ Health Administration (VHA) hospitals | Pre-intervention: standard disinfection protocol Intervention: enhanced terminal room cleaning with ultraviolet C (UV-C) |
Hospital-onset Gram-negative rod infection: IRR 0.81 (95% CI: 0.66, 0.97) |
| Schaffzin [30] | Before–after USA |
Large paediatric referral facility (449) | Pre-intervention: standard disinfection protocol (hydrogen peroxide or bleach) Intervention: enhanced cleaning with ultraviolet C (UV-C) |
Hospital-onset Gram-negative rod infection: IRR 2.12 (95% CI: 0.32, 14.2) |
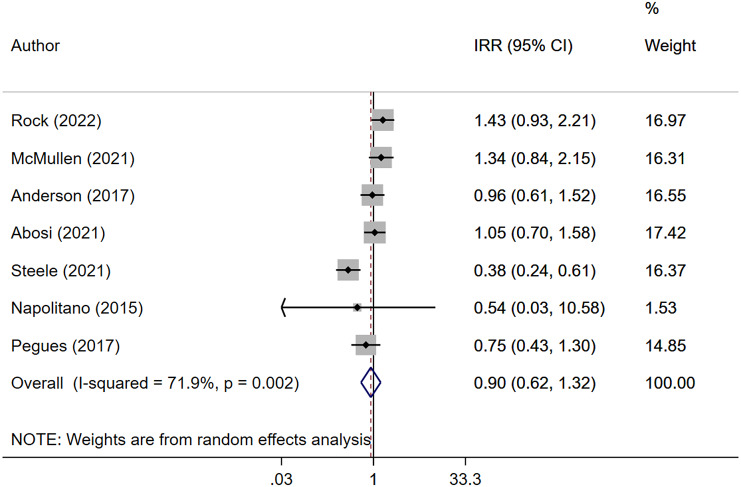
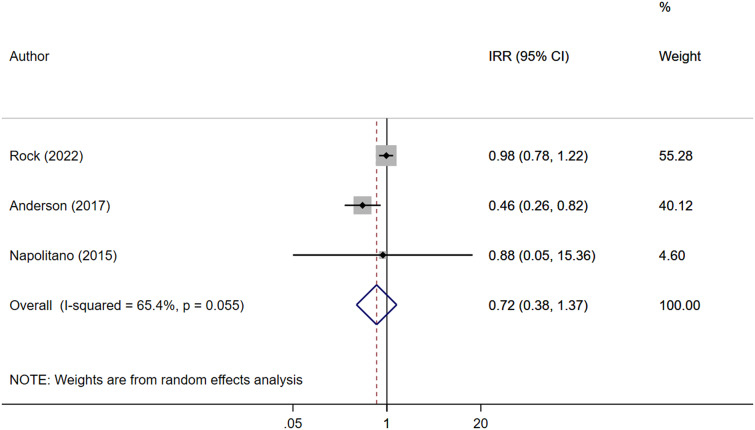
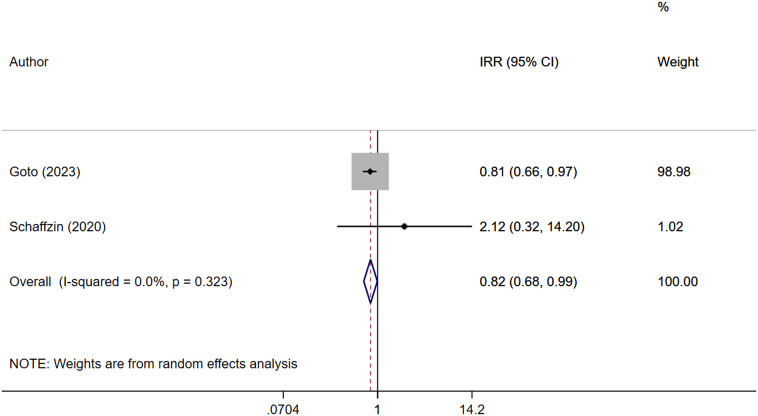
Pooled analysis indicated no statistically significant reduction in CD infection rates with the use of ultraviolet C disinfection systems (IRR: 0.90, 95% CI; 0.62–1.32, I2 = 71.9%, N = 7) (Figure 2). Subgroup analysis based on the study design also showed no effect of this system on risk of CD infection for both RCT/quasi-experimental studies (IRR: 1.04, 95% CI; 0.72–1.50, I2 = 43.2%, N = 3) and ‘before–after’ design (IRR: 0.80, 95% CI; 0.40–1.59, I2 = 81.4%, N = 4) (Supplementary Figure S2). No publication bias was shown by Egger’s test (P = 0.728) and funnel plots (Supplementary Figure S3). Similarly, no significant reduction in VRE infection was observed (IRR 0.72, 95% CI; 0.38–1.37, I2 = 65.4%, N = 3) (Figure 3), and no evidence of publication bias was found (Supplementary Figure S4). UV-C systems appeared to reduce the risk of Gram-negative rod infection (IRR 0.82, 95% CI; 0.68–0.99, I2 = 0.0%, N = 2), but the number of studies reporting this outcome was small (Figure 4). No evidence of publication bias was found (Supplementary Figure S5).
Figure 2.
Forest plot of IRRs of Clostridioides difficile (CD) infection for UV-C versus control.
Figure 3.
Forest plot of IRRs of vancomycin-resistant enterococcal infection (VRE) for UV-C versus control.
Figure 4.
Forest plot of IRRs of Gram-negative rod infection for UV-C versus control.
Discussion
Our study did not find any evidence of the benefit of using UV-C-based disinfectant systems in healthcare facilities to reduce the incidence of nosocomial infections, particularly MDROs such as CD and VRE. Nevertheless, some studies showed that UV-C (wavelength 200–270 nm) is effective in reducing the risk of Gram-negative rod infections by inducing DNA and RNA damage, through dimerisation of pyrimidine molecules, thereby reducing the replication of microorganisms [31, 32]. UV-C at the wavelengths of 250–270 nm appears to be the most efficient due to its maximal absorption by microbial nucleic acids [33]. However, one of the disadvantages of UV-C is that its penetration is affected by the presence of organic matter [34]. Additionally, there is an issue of costs and the requirement for the training of the personnel. We found no advantages to the use of UV-C in healthcare settings as an adjunct to conventional infection prevention modalities to reduce the incidence of MDRO.
The aspect of cost-effectiveness and staff training should also be considered further. Two previous studies that have conducted cost-effectiveness evaluation of ultraviolet disinfection systems after terminal cleaning [28, 35] have shown the cost to be around 200,000 to 300,000 USD per year. However, on average, cases of CDI and VRE can lead to a cost of c. 14,000 USD/case [36, 37]. Nursing professionals are essential for implementing disinfection protocols in healthcare facilities, as they are directly involved in the cleaning and disinfection of shared medical monitoring devices. It is therefore critical that any advancement in technology be known to them. In this regard, the current review further emphasises the importance of ‘no-touch’ disinfection systems in health facilities.
With the assumption that, in the included studies, the dosing and duration of exposure were appropriate, there may be some possible explanations for the lack of effectiveness of UV-C disinfectant systems. One reason could be the use of disinfectant as part of the standard protocol offered in both study groups. High compliance with disinfectants such as bleach or standard quaternary ammonium compounds may have led to relatively few residual spores for the UV-C device to eliminate. Second, UV-C disinfection relies on direct line-of-sight exposure to effectively kill microorganisms, and inadequately exposed surfaces to UV-C light could result in incomplete disinfection. Recent findings suggest that the role of the environment in the transmission of C. difficile may not be as significant as previously believed. Eyre et al. [38] examined 1250 isolates from cases of symptomatic CDI over four years using whole-genome sequencing. Surprisingly, possible environmental contamination was found to be responsible for linking only 2% of patients with genetically related C. difficile isolates. Furthermore, differences in study design, protocols, equipment, or the specific UV-C system most likely contribute to variations in effectiveness, as well as room size and layout, UV-C system placement, and operator training, which can influence the overall performance and outcomes of studies. On the contrary, Steele et al. [26] showed that UV-C irradiation reduced the risk of CDI in a paediatric haematology–oncology unit and suggested that this might depend on the pre-intervention CDI burden and that such high-risk units could have maximum benefit from use of such systems.
There are some limitations to our study. First, all reviewed studies were conducted in the USA, that is a high-income setting, and therefore, the generalisability of the findings is limited. Second, most of the included studies had a ‘before–after’ design, compared current data with a historical control group, and therefore did not consider changes in hospital practices that occur with time. Third, the included studies varied in terms of intervention sites and standard methods of disinfection used. For instance, some studies were conducted in cancer and solid organ transplant in-patient units where patients would have lower immunity and thus be at an increased risk of HCAI. Finally, our approach of limiting the study scope to English language studies could have introduced a degree of bias through missing relevant studies, as well as geographic, cultural, and publication bias.
Conclusion
The use of UV-C had no measurable impact on the incidence of CD and VRE infections but might be of some advantage in reducing the risk of Gram-negative rod infection, albeit with low confidence. Further studies to support or refute the outcome of this meta-analysis are needed.
Supporting information
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/S0950268823001371.
click here to view supplementary material
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author contribution
Conceptualization: Q.W., Y.S., Q.W.; Funding acquisition: Q.W.; Validation: Q.W., J.L., Y.S., Q.W.; Visualization: Q.W., Y.S., Q.W.; Data curation: J.L., Y.S., Q.W.; Formal analysis: J.L.; Methodology: J.L., Y.S., Q.W.; Project administration: J.L., Q.W.; Writing – original draft: J.L., Y.S., Q.W.; Writing – review & editing: J.L., Q.W.; Investigation: Q.W.; Resources: Q.W.
Competing interest
The authors declare none.
References
- [1].Danasekaran R, Mani G and Annadurai K (2014) Prevention of healthcare-associated infections: Protecting patients, saving lives. International Journal of Community Medicine and Public Health 1, 67–68. [Google Scholar]
- [2].Haque M, Sartelli M, McKimm J and Abu Bakar MB (2018) Health care-associated infections – An overview. Infection and Drug Resistance 11, 2321–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Raoofi S, Pashazadeh Kan F, Rafiei S, Hosseinipalangi Z, Noorani Mejareh Z, Khani S, Abdollahi B, Seyghalani Talab F, Sanaei M, Zarabi F, Dolati Y, Ahmadi N, Raoofi N, Sarhadi Y, Masoumi M, sadat Hosseini B, Vali N, Gholamali N, Asadi S, Ahmadi S, Ahmadi B, Beiramy Chomalu Z, Asadollahi, Rajabi M, Gharagozloo D, Nejatifar Z, Soheylirad R, Jalali S, Aghajani F, Navidriahy M, Deylami S, Nasiri M, Zareei M, Golmohammadi Z, Shabani H, Torabi F, Shabaninejad H, Nemati A, Amerzadeh M, Aryankhesal A and Ghashghaee A (2023) Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS One 18, e0274248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khan HA, Baig FK and Mehboob R (2017) Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pacific Journal of Tropical Biomedicine 7, 478–482. [Google Scholar]
- [5].Gidey K, Gidey MT, Hailu BY, Gebreamlak ZB and Niriayo YL (2023) Clinical and economic burden of healthcare-associated infections: A prospective cohort study. PLoS One 18, e0282141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marschall J, Agniel D, Fraser VJ, Doherty J and Warren DK (2008) Gram-negative bacteraemia in non-ICU patients: Factors associated with inadequate antibiotic therapy and impact on outcomes. Journal of Antimicrobial Chemotherapy 61, 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jia H, Li W, Hou T, Ma H, Yang Y, Wu A, Liu Y, Wen J, Yang H, Luo X, Xing Y, Zhang W, Wu Y, Ding L, Liu W, Lin L, Li Y, Chen M and Li L (2019) The attributable direct medical cost of healthcare associated infection caused by multidrug resistance organisms in 68 hospitals of China. BioMed Research International 2019, 7634528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Johnston KJ, Thorpe KE, Jacob JT and Murphy DJ (2019) The incremental cost of infections associated with multidrug-resistant organisms in the inpatient hospital setting-A national estimate. Health Services Research 54, 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Simmons BP and Larson EL (2015) Multiple drug resistant organisms in healthcare: The failure of contact precautions. Journal of Infection Prevention 16, 178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leas BF, Sullivan N, Han JH, Pegues DA, Kaczmarek JL and Umscheid CA. (2015) Environmental Cleaning for the Prevention of Healthcare-Associated Infections. Rockville, MD: Agency for Healthcare Research and Quality (US). Report No.: 15-EHC020-EF. PMID: 26290935. [PubMed] [Google Scholar]
- [11].Otter JA, Yezli S, Perl TM, Barbut F and French GL (2014) A guide to no-touch automated room disinfection (NTD) systems. In Walker JT (ed.) Decontamination in Hospitals and Healthcare. Cambridge: Woodhead Publishing Ltd, pp. 413–460. [Google Scholar]
- [12].Jinadatha C, Quezada R, Huber TW, Williams JB, Zeber JE and Copeland LA (2014) Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infectious Diseases 14, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Passaretti CL, Otter JA, Reich NG, Myers J, Shepard J, Ross T, Carroll KC, Lipsett P and Perl TM (2013) An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clinical Infectious Diseases 56, 27–35. [DOI] [PubMed] [Google Scholar]
- [14].Weber DJ, Anderson D and Rutala WA (2013) The role of the surface environment in healthcare-associated infections. Current Opinion in Infectious Diseases 26, 338–344. [DOI] [PubMed] [Google Scholar]
- [15].Nerandzic MM, Thota P, Sankar C. T, Jencson A, Cadnum JL, Ray AJ, Salata RA, Watkins RR and Donskey CJ (2015) Evaluation of a pulsed xenon ultraviolet disinfection system for reduction of healthcare-associated pathogens in hospital rooms. Infection Control and Hospital Epidemiology 36, 192–197. [DOI] [PubMed] [Google Scholar]
- [16].Dong Z, Zhou N, Liu G and Zhao L (2020) Role of pulsed-xenon ultraviolet light in reducing healthcare-associated infections: A systematic review and meta-analysis. Epidemiology and Infection 148, e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marra AR, Schweizer ML and Edmond MB. (2018) No-touch disinfection methods to decrease multidrug-resistant organism infections: A systematic review and meta-analysis. Infection Control and Hospital Epidemiology 39, 20–31. [DOI] [PubMed] [Google Scholar]
- [18].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P and Moher D (2021) The PRISMA 2020 statement: anupdated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF and Higgins JPT (2019) Rob 2: A revised tool for assessing risk of bias in randomised trials. British Medical Journal (Clinical Research Edition) 366, l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- [20].National Heart Lung and Blood Institute (2014) Study Quality Assessment Tools | NHLBI, NIH. Available at https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed 20 July 2023).
- [21].Egger M, Smith GD, Schneider M and Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. British Medical Journal (Clinical Research Edition) 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rock C, Hsu YJ, Curless MS, Carroll KC, Ross Howard T, Carson KA, Cummings S, Anderson M, Milstone AM and Maragakis LL (2022) Ultraviolet-C light evaluation as adjunct disinfection to remove multidrug-resistant organisms. Clinical Infectious Diseases 75, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McMullen K, Guth RM, Wood H, Mueller C, Dunn G, Wade R, Siddiqui A, Dubberke ER, Woeltje KF and Warren DK (2021) Impact of no-touch ultraviolet light room disinfection systems on Clostridioides difficile infections. American Journal of Infection Control 49, 646–648. [DOI] [PubMed] [Google Scholar]
- [24].Anderson DJ, Chen LF, Weber DJ, Moehring RW, Lewis SS, Triplett PF, Blocker M, Becherer P, Schwab JC, Knelson LP, Lokhnygina Y, Rutala WA, Kanamori H, Gergen MF, Sexton DJ and CDC Prevention Epicenters Program (2017) Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the benefits of enhanced terminal room disinfection study): A cluster-randomised, multicentre, crossover study. Lancet 389, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abosi OJ, Kobayashi T, Holley S, Kukla ME, Dains A, Alsuhaibani M, Marra AR, Jenn KE, Meacham H, Sheeler LL, Etienne W, Trannel A, Garringer J, Millard W, Diekema DJ, Edmond MB, Wellington M and Salinas JL (2021) Stable Clostridioides difficile infection rates after the discontinuation of ultraviolet light for terminal disinfection at a tertiary care center, Iowa 2019-2020. American Journal of Infection Control 49, 1567–1568. [DOI] [PubMed] [Google Scholar]
- [26].Steele M, Hurtado RR, Rychlik K, Bonebrake A, Bovee MC, O’Donnell A, Perryman J and Kociolek LK (2021) Impact of an automated multiple emitter whole-room ultraviolet-C disinfection system on hospital acquired infections: A quasi-experimental study. American Journal of Infection Control 49, 1200–1203. [DOI] [PubMed] [Google Scholar]
- [27].Napolitano NA, Mahapatra T and Tang W (2015) The effectiveness of UV-C radiation for facility-wide environmental disinfection to reduce health care-acquired infections. American Journal of Infection Control 43, 1342–1346. [DOI] [PubMed] [Google Scholar]
- [28].Pegues DA, Han J, Gilmar C, McDonnell B and Gaynes S (2017) Impact of ultraviolet germicidal irradiation for no-touch terminal room disinfection on Clostridium difficile infection incidence among hematology-oncology patients. Infection Control and Hospital Epidemiology 38, 39–44. [DOI] [PubMed] [Google Scholar]
- [29].Goto M, Hasegawa S, Balkenende EC, Clore GS, Safdar N, Perencevich EN, VA-CDC Practice-Based Research Network, Bradley SF, Morgan D, Gupta K, Hostler C, Evans C, Goetz M, Reisinger H, Safdar N, Lira GJB, DeVries A, Harris B, Bittner M, Pfeiffer C, Rubin M, Cadena-Zuluaga J and Suda K (2023) Effectiveness of ultraviolet-C disinfection on hospital-onset gram-negative rod bloodstream infection: A nationwide stepped-wedge time-series analysis. Clinical Infectious Diseases 76, 291–298. [DOI] [PubMed] [Google Scholar]
- [30].Schaffzin JK, Wilhite AW, Li Z, Finney D, Ankrum AL and Moore R (2020) Maximizing efficiency in a high occupancy setting to utilize ultraviolet disinfection for isolation rooms. American Journal of Infection Control 48, 903–909. [DOI] [PubMed] [Google Scholar]
- [31].Dai T, Vrahas MS, Murray CK and Hamblin MR (2012) Ultraviolet C irradiation: An alternative antimicrobial approach to localized infections? Expert Review of Anti-Infective Therapy 10, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chang JC, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG and Johnson JD (1985) UV inactivation of pathogenic and indicator microorganisms. Applied and Environmental Microbiology 49, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yin R, Dai T, Avci P, Jorge AES, de Melo WCMA, Vecchio D, Huang YY, Gupta A and Hamblin MR (2013) Light based anti-infectives: Ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Current Opinion in Pharmacology 13, 731–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Otaki M, Okuda A, Tajima K, Iwasaki T, Kinoshita S and Ohgaki S (2003) Inactivation differences of microorganisms by low pressure UV and pulsed xenon lamps. Water Science and Technology 47, 185–190. [PubMed] [Google Scholar]
- [35].Vianna PG, Dale CR Jr, Simmons S, Stibich M and Licitra CM (2016) Impact of pulsed xenon ultraviolet light on hospital-acquired infection rates in a community hospital. American Journal of Infection Control 44, 299–303. [DOI] [PubMed] [Google Scholar]
- [36].Dubberke ER and Olsen MA (2012) Burden of Clostridium difficile on the healthcare system. Clinical Infectious Diseases 55(Suppl 2), S88–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cosgrove SE (2006) The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clinical Infectious Diseases 42(Suppl 2), S82–S89. [DOI] [PubMed] [Google Scholar]
- [38].Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O’Connor L, Ip CLC, Golubchik T, Batty EM, Finney JM, Wyllie DH, Didelot X, Piazza P, Bowden R, Dingle KE, Harding RM, Crook DW, Wilcox MH, Peto TEA and Walker AS (2013) Diverse sources of C. difficile infection identified on whole-genome sequencing. New England Journal of Medicine 369, 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
Sun et al. supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/S0950268823001371.
click here to view supplementary material
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.