Abstract
Bacterial antimicrobial resistance (AMR) is among the leading global health challenges of the century. Animals and their products are known contributors to the human AMR burden, but the extent of this contribution is not clear. This systematic literature review aimed to identify studies investigating the direct impact of animal sources, defined as livestock, aquaculture, pets, and animal-based food, on human AMR. We searched four scientific databases and identified 31 relevant publications, including 12 risk assessments, 16 source attribution studies, and three other studies. Most studies were published between 2012 and 2022, and most came from Europe and North America, but we also identified five articles from South and South-East Asia. The studies differed in their methodologies, conceptual approaches (bottom-up, top-down, and complex), definitions of the AMR hazard and outcome, the number and type of sources they addressed, and the outcome measures they reported. The most frequently addressed animal source was chicken, followed by cattle and pigs. Most studies investigated bacteria–resistance combinations. Overall, studies on the direct contribution of animal sources of AMR are rare but increasing. More recent publications tailor their methodologies increasingly towards the AMR hazard as a whole, providing grounds for future research to build on.
Keywords: antibiotic resistance, antimicrobial resistance, infectious disease, human(s), risk assessment, source attribution
Introduction
Modern medicine and animal husbandry heavily depend on the effectiveness of antimicrobial drugs to combat infectious diseases. The unprecedented rise in resistance to antimicrobial substances throughout the past decades, believed to be linked to their extensive usage in medicine, agriculture, and aquaculture, poses a substantial threat to public health. Indeed, bacterial antimicrobial resistance (AMR) is currently a leading cause of global deaths and is predicted to become one of the greatest public health challenges of the 21st century [1].
AMR can be transferred between bacteria species via mobile genetic elements, which leads to crossovers between pathogens and commensals in humans, animals, and the environment [2], thus making AMR a prime example of a global ‘One Health’ issue [3]. While antimicrobial usage (AMU) and AMR in animals are known drivers of human AMR [4, 5], the extent to which animals are responsible for AMR in humans, as well as which specific animal sources are most relevant for AMR transmission to humans, is not clear.
Source attribution studies and risk assessments are well suited to investigate the importance of different sources of human AMR [6]. The main source attribution methods are microbial subtyping, comparative exposure assessments, and epidemiological approaches as have been described in detail elsewhere [7]. Briefly, microbial subtyping studies typically attribute human infections based either on the frequency of source-specific bacteria subtypes in human samples or on the genetic relatedness between human and source strains [7]. More recently, machine-learning methods, such as random forests, have been applied to, for example, process whole genome sequencing data [8]. Comparative exposure assessments determine the relative importance of different sources of human exposure. Epidemiological approaches include investigations of outbreaks, which summarise source information for multiple outbreaks, and meta-analyses of case–control studies of sporadic cases [6, 7].
Risk assessments are part of risk analysis and can be qualitative, quantitative, or semi-quantitative. For microbial risks, they traditionally follow ‘farm-to-fork’ approaches, which usually combine a hazard description, an assessment of the relationship between hazard and outcome, and an appraisal of the likelihood and magnitude of exposure to the hazard during different phases along the farm-to-fork continuum into a human health risk estimate [9]. There are also less data-demanding risk assessment approaches, which, for example, estimate the contribution of a specific source to the total number of human cases, starting at human surveillance data (e.g. [10, 11]).
Objective of the review
A better understanding of the relative contribution of animal sources to human AMR is crucial for planning effective AMR mitigation and control strategies in animals. It is also necessary for an accurate estimation of the economic and public health burden posed by animal diseases, which the Global Burden of Animal Disease study (GBADs), in the context of which this review is undertaken, seeks to produce.
This systematic review aims to summarise the current evidence on the relative contribution of animals or animal products to human AMR, focusing on the methodologies applied to address this question, the investigated antimicrobial hazards, and the included animal sources.
Methods
The completed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist [12], as well as additional information about the search strategy, protocol, study selection, data extraction, and compilation of study results, can be found in Supplementary material S1.
Definitions
Antimicrobial resistance describes the ability of microorganisms to survive and uphold pathogenic properties when treated with substances previously effective in eliminating them. It includes resistance to antibiotics, antivirals, antifungals, and antiparasitic agents [13]. This article addresses only the most extensively studied form of AMR, that is, bacterial resistance, and this abbreviation is henceforth used synonymously.
The term ‘animals’ is used to refer to domesticated animals, either for food production (livestock or aquaculture) or companionship (pets). Wildlife is excluded as it is typically studied as a marker for resistance in the environment [14]. We define ‘animal products’ as food items including meat, poultry, fish, shellfish, other aquatic animals, dairy, and eggs [15].
Data sources and search strategy
We searched four bibliographic databases (PubMed, Scopus, Embase, and Web of Science) with terms relating to the following key areas: 1) animals and animal products, 2) AMR, 3) relevant study types, and 4) humans. No publication date or language restrictions were applied, but all searches were conducted in English.
Eligibility criteria
We included studies on AMR transmitted from animals or animal products to humans. Studies focusing exclusively on non-human populations, exposure via wild animals or the environment, or other hazards, such as antibiotic agents (i.e., AMU in humans or animals or environmental antibiotic residues) or other types of resistance, were excluded.
Publications were included if they either 1) estimated the number of antibiotic-resistant human infections directly attributable to an animal source, or 2) assessed the relative contribution of at least one animal source to the burden of AMR in humans, either qualitatively or quantitatively. Eligible study types entailed source attribution studies, risk assessments, and any other meeting these criteria. Investigations of outbreaks were included only if they described five or more outbreaks. Those excluded were non-comparative exposure assessments, unrelated studies accidentally picked up by the search string, case studies, case series, single outbreak reports, conference abstracts, studies without new information (reviews, duplicates, or letters to the editors), and studies for which no full text was available. If two or more publications used the same data to illustrate different methodologies, all were included.
Data screening, selection, and extraction
Data screening was conducted in two steps: first, the titles and abstracts were examined based on the inclusion and exclusion criteria, and second, the full texts of the articles included during the first step were retrieved for the final selection. Additionally, we screened the reference lists of the included articles for studies fitting our research objectives and added them to the full-text screening (snowballing).
We defined a data extraction template in Excel and piloted it with a subset of included studies. The final data extraction table (Supplementary material S2) included variables relating to the publication, that is, the study setting, outcome, hazard, methods, and the conceptual approach. The software Sysrev [16] was used to track the study selection process, and citations were managed in Zotero [17]. Duplicate entries were removed in Rayyan.ai [18], as Sysrev currently does not offer this function.
Compiling the results of the studies
Due to the broad spectrum of methodologies, study designs, and hazards included, we did not assess the risk of bias or quality of the studies, and it was not possible jointly to analyse the results of the studies. However, to illustrate the relative importance of the animal sources in relation to each other, we assigned ranks according to the results reported by studies that included different animal sources, as described in Supplementary material S1. We also report the most important source for studies on animal and non-animal sources.
The figures were created using Microsoft Office.
Results
Literature review
As shown in Figure 1, 16,955 records were initially identified through database searches, 10,150 of which were non-duplicates and screened for eligibility. Of the 596 records selected for full-text screening, 30 articles were included; an additional relevant publication was retrieved via snowballing, thus totalling 31 included studies (Table 1).
Figure 1.
Flow chart of included studies.
Table 1.
Characteristics of the included studies
| Study | Location | Level | Description of study aims (relevant to this review) | Methods | Relevant outcomes |
|---|---|---|---|---|---|
| Microbial subtyping studies | |||||
| Hald, 2007 [33] | Denmark | National | Attribute resistant salmonellosis cases to different animal reservoirs, using resistance profiles |
Typing: serotyping & antibiotyping (phenotypic); phage typing (for susceptible isolates) Analysis: based on Hald model [49] |
Attribution of all infections; % of resistant cases due to each source |
| Vieira, 2016 [32] | United States | National | Evaluate the use of Salmonella Hadar retail contamination data and antibiotyping to attribute human illnesses to food sources, comparing four models |
Typing: PFGE (comparison) & antibiotyping (PT) Analysis: used Dutch model [50] |
Attribution (%) |
| Mughini-Gras, 2019 [31] | Netherlands | National | Attribute ESBL- and pAmpC-producing E. coli carriage in the community to different sources based on gene, prevalence, and human exposure data |
Typing: ESBL & pAmpC gene occurrence Analysis: based on modified Hald model [51] |
Attribution (%) |
| Parisi, 2020 [47] | Vietnam | Sub-national | Attribute invasive and non-invasive human non-typhoidal salmonellosis in Southern Vietnam to animal sources using serotyping and/or antibiotyping |
Typing: molecular serotyping (MLST) & antibiotyping (phenotypic) Analysis: own, Bayesian multinomial mixture model |
Attribution (% and total cases) |
| Duarte, 2021 [19] | Europe | Regional | Demonstrate the use of metagenomics for the attribution of the human resistome to different animal reservoirs with three models differing on whether they include a human/unknown source and on whether they were country dependent or independent |
Typing: metagenomics Analysis: own, random forests & dissimilarity analysis (SIMPER) |
Different plots illustrating attribution |
| Mitchell, 2021 [46] | India | Sub-national | Estimate the contribution of animals and water sources to resistant E. coli infections in children in rural India | Typing: antibiotyping (phenotypic) Analysis: SourceR [52] |
Attribution (% and total cases) |
| Perestrelo, 2022 [30] | Germany | National | Attribute human ESBL-producing E. coli colonisation to animal sources and nosocomial infections, using different combinations of three typing methods |
Typing: ESBL genotyping, phylogenetic grouping (PCR) & antibiotyping (phenotypic) Analysis: based on Hald model [49] |
Attribution (% and total cases) |
| Comparative exposure assessments | |||||
| Carmo, 2014 [24] | Denmark | National | Assess the relative contribution of different meat types to the consumer exposure to ESBL-/AmpC-producing E. coli |
Quantitative
Guideline: not specified |
Exposure attribution (%) |
| Evers, 2017 [23] | Netherlands | National | Quantify ESBL- & pAmpC-producing E. coli exposure in humans via meat consumption from pre-retail to exposure |
Quantitative
Guideline: based on swift QMRA [53] |
Exposure/ portion; exposure attribution (total and %) |
| Lechner, 202 [22] | Switzerland | National | Identify the most relevant AMR transmission pathways from animals to humans based on Swiss expert opinions |
Qualitative
Guideline: OIE framework for AMR [9] |
Person days at risk; bubble chart relating exposure, release & person days at risk |
| Investigation of outbreaks | |||||
| Holmberg, 1984 [29] | United States | National | Describe animal sources of antibiotic-resistant Salmonella outbreaks between 1971 and 1983 | Linked outbreak reports with resistance information | Number of resistant outbreaks caused by each source |
| Sahin, 2012 [28] | United States | National | Report of several outbreaks caused by Campylobacter jejuni clone SA as part of their investigation of its presence in human isolates | Identified relevant outbreaks via the PulseNet database for Campylobacter | Description of all clone SA outbreaks & their source |
| Brown, 2017 [27] | United States | National | Compare foods associated with antibiotic-resistant Salmonella outbreaks from 2003 to 2012 | Linked outbreak info to antibiotic susceptibility data | Numbers of resistant (& multidrug-resistant) outbreaks caused by each source |
| Folster, 2017 [26] | United States | National | Report the number of outbreaks caused by ceftriaxone-resistant Salmonella by source between 2011 and 2012 as part of a genetic analysis of the outbreak strains | Tested outbreak samples for ceftriaxone-resistance & linked positive samples to source information | Number of resistant outbreaks caused by each source |
| Waltenburg, 2021 [25] | United States | National | Describe all salmonellosis outbreaks caused by reptiles or amphibians between 2009 and 2018, including a description of the sources by resistance profile | Tested outbreak samples for resistance | Number of resistant outbreaks caused by each pet species |
| Other source attribution studies | |||||
| de Freitas Costa, 2022 [34] | Netherlands | National | Develop a dynamic risk model that accounts for the multi-directional spread of ESBL-producing E. coli between populations over time and may be used for exploring the effects of different food chain interventions |
Source attribution
Analysis: discrete-time model |
Attribution at equilibrium (%) |
| Risk assessments | |||||
| Vose, 2000 [11] | United States | National | Develop a model to assess the human health impact of fluoroquinolone-resistant Campylobacter attributed to chicken consumption that also allows for modelling future changes in the system |
Quantitative
Framework: own (FDA-CVM) |
% and ‘1 in x’ of being affected for all citizens, cases, cases seeking care & care-seeking cases who are prescribed antibiotics |
| Alban, 2022 [44] | Denmark | National | Assess whether dry-cured sausages produced with pork with Salmonella Typhimurium DT104 are a risk for consumers |
Quantitative
Framework: Codex [54] |
Maximal observed number of diarrhoea cases per year within 100 years |
| Presi, 2009 [43] | Switzerland | National | Compare the health risk for consumers arising from their exposure to resistant bacteria from meat of four different types |
Semi-Quantitative (Risk scoring) Framework: own model |
Ranking of different meat products according to high human health risk |
| Cox, 2014 [42] | United States | National | Estimate the excess number of human MRSA infections attributable to MRSA ST398 from pigs and pork |
Quantitative
Framework: own model |
Excess cases per year |
| Otto, 2014 [45] | Canada | Sub-national | Estimate number of ceftiofur-resistant Salmonella enterica Heidelberg cases in humans in Québec and Ontario attributable to chicken consumption |
Quantitative
Framework: based on FDA-CVM [11] |
Annual mean incidence due to chicken consumption |
| Doménech, 2015 [48] | Spain | Sub-national | Characterise the human health risk due to different resistances in Salmonella from pork, beef, and poultry meat |
Qualitative
Framework: Codex AMR [55] |
Level of risk for humans due to different resistances from different meats |
| Chereau, 2017 [20] | South East Asia | Regional | Characterise the level of risk of the emergence and spread of AMR in the WHO Southeast Asian region |
Qualitative
Framework: WHO rapid risk assessment guideline [56] |
High, medium, low & negligible risk transmission routes |
| Collineau, 2018 [41] | Switzerland | National | Develop a framework to rank the human health importance of combinations of pathogens, resistance to antimicrobials, and different meat types |
Semi-Quantitative (Risk ranking) Framework: Codex AMR & MCDA, identified via EFSA risk ranking review [55, 57] |
List of meat-pathogen-resistance combinations with the highest human health risk |
| Collineau, 2020 [40] | Canada | National | Define the baseline (2013) risk of human ceftiofur-resistant Salmonella Heidelberg infection due to chicken and compare it to alternative scenarios |
Qualitative
Framework: Based on Codex AMR & FAO/WHO model [55, 58] |
% of illness per serving; number of cases per year |
| Costard, 2020 [39] | United States | National | Estimate the risk for resistant non-typhoidal salmonellosis per beef meal using the yearly cases of resistant infections and number of meals made with beef and evaluate the change over time |
Quantitative
Framework: Based on [33] and USDA framework [10] |
Annual incidence attributable to beef, cases per 1 million beef meals |
| Schoen, 2020 [38] | United States | National | Assess the risk for MRSA colonisation from preparing contaminated pork meat |
Quantitative
Framework: not specified |
Risk per preparation event |
| Opatowski, 2021 [21] | South or South East Asia | National | Develop a model to combine annual ESBL-producing E. coli colonisation incidence due to five One Health transmission routes. Illustrate its application in hypothetical high- and low-income settings |
Quantitative
Framework: complementary to [20] |
Incidence due to animal-based food and animal contact per 100 persons per year |
| Other studies | |||||
| Bosch, 2016 [37] | Netherlands | National | Describe changing characteristics of livestock-associated MRSA, including the percentage of cases reporting livestock contact | / | % of cases who were in contact with livestock |
| Larsen, 2017 [36] | Denmark | National | Describe the emergence of livestock-associated MRSA CC398 in invasive human cases, including a summary of cases not reporting livestock contact | / | % of cases who were in contact with livestock |
| Booton, 2021 [35] | Thailand | National | Develop a One Health model to predict the maximum impact of reducing different AMR drivers in Thailand on the human AMR burden between 2020 and 2040 |
Prediction model, One Health
Analysis: compartmental model of ordinary differential equations |
Maximum human AMR reduction via elimination of animal-to-human transmission (%) |
Abbreviations: Codex, Codex Alimentarius; EFSA, The European Food Safety Authority; ESBL, extended-spectrum β-lactamase; FAO, Food and Agriculture Organization; FDA-VCM, Food and Drug Administration Center for Veterinary Medicine; MRSA, methicillin-resistant S. aureus; OIE, World Organisation for Animal Health (now WOAH); (p)AmpC, (plasmid)-mediated AmpC β-lactamase; QMRA, quantitative microbial risk assessment; USDA, United States Department of Agriculture; WHO, World Health Organization.
Country of origin, publication year, and study type
Of the 31 articles, two assessed the impact of different sources on human AMR at a regional level: one for Europe [19] and the other for South-East Asia [20]. One article provided a framework for any high- or low-income country in South or South-East Asia (SSEA) [21]. Twenty-four studies were designed in the context of specific countries [22–44], and four were sub-national [45–48]. Most national and sub-national studies were conducted in North American and European countries, but three studies originated from SSEA countries [35, 46, 47]. The highest number (10) of national study publications were from the United States [11, 25–29, 32, 38, 39, 42].
The articles were published over a range of nearly 40 years, mainly as a consequence of a 1984 investigation of resistant salmonellosis outbreaks in the United States [29]. However, most studies (87%) were published between 2012 and 2022, and 12 have been published since 2020 [19, 21, 22, 25, 30, 34, 35, 38–40, 46, 47].
Based on the authors’ descriptions and reported methods, 16 articles were categorised as source attribution studies, including seven microbial subtyping studies [19, 30–33, 46, 47], five investigations of outbreaks [25–29], three comparative exposure assessments [22–24], and one multi-directional dynamic risk model [34]. Twelve were classified as risk assessments [11, 20, 21, 38–45, 48], and the remaining three were of a different study type [35–37].
Methodological frameworks
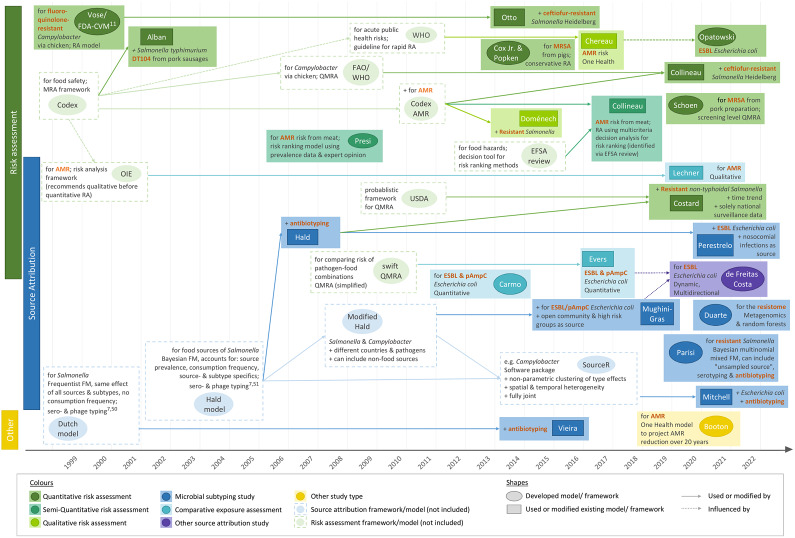
Table 1 summarises the countries of origin, relevant aims, methods, and outcome measures of the articles by study type. Figure 2 shows their timeline and the methods and guidelines they were based on to illustrate how they developed over time.
Figure 2.
Timeline of the studies and links between their methodologies (excluding investigations of outbreaks). Words printed in red describe how the study addressed AMR. AMR, bacterial antimicrobial resistance; EFSA, The European Food Safety Authority; ESBL, extended-spectrum ß-lactamase producing; FAO, Food and Agriculture Organization; FDA-VCM, Food and Drug Administration Center for Veterinary Medicine; FM, frequency matched model; MRSA, methicillin-resistant Staphylococcus aureus; (p)AmpC, (plasmid)-mediated AmpC ß-lactamase producing; RA, risk assessment; (Q)MRA, (quantitative) microbial RA; OIE, World Organisation for Animal Health (now WOAH); WHO, World Health Organization.
Microbial subtyping
Of the seven microbial subtyping studies, one used metagenomics and random forests to attribute the entirety of resistance genes, and the human resistome, to different sources [19]. The rest followed frequency-matched approaches. A study on resistant Salmonella Hadar infections used a well-known frequentist model [32], also called the ‘Dutch model’ [50]. One study on Salmonella spp. [33] and three on different Escherichia coli strains [30, 31, 46] were based on a Bayesian subtyping model, the ‘Hald model’ [49], its modification [51], or SourceR for quantitative source attribution [52]. Finally, Parisi and colleagues developed a Bayesian multinomial mixed model for their study on non-typhoidal salmonellosis [47]. More details about the models and adaptations made by the individual studies can be found in Figure 2.
Three microbial subtyping studies compared the results of different combinations of subtyping methods [30, 32, 47], and two assessed the impact of including ‘human-to-human transmission’ or an ‘unspecified’ source [19, 30]. Duarte and colleagues further defined country-dependent and country-independent models for the data they collected from several European countries [19]. The studies generally found that the more subtyping approaches were combined, the less likely that cases were attributed to specific animal sources and the more relevant unknown or human sources became.
Epidemiological approaches
Five studies from the United States reported on animal sources of outbreaks caused by resistant infections. For two of them, both on salmonellosis, the assessment of the relative contributions of the different sources was the main study aim [27, 29]. Two studies included the sources of resistant outbreaks as part of answering different research questions: one concerned Campylobacter jejuni clone SA, a tetracycline-resistant strain [28], and the other ceftriaxone-resistant Salmonella [26]. The fifth study reported the relative importance of different reptilian or amphibian pet species in causing antibiotic-resistant salmonellosis outbreaks [25]. We did not identify any meta-analyses on sporadic resistant infections in humans.
Comparative exposure assessments
Two of the three comparative exposure assessments identified were quantitative and focused on the relative contribution of different meat types to human exposure by antibiotic-resistant E. coli strains [23, 24]. The third study qualitatively assessed the relative importance of AMR exposure via different animal sources [22]. The frameworks of the studies are described in Figure 2.
Lastly, a study was identified which attributed extended-spectrum β-lactamase (ESBL)-producing E. coli infections in the community to different sources using a dynamic risk model that allows for multi-directional pathogen transmission between human and animal populations over time [34].
Risk assessments
Of the 12 risk assessments, seven were quantitative [11, 21, 38, 39, 41, 42, 45], two were semi-quantitative [41, 43], two were qualitative [20, 48], and one combined qualitative with quantitative parts [44]. Seven studies either followed the Codex Alimentarius guidelines for microbial food safety hazards risk assessments (Codex) [54, 55], or frameworks based on, or influenced by, them [20, 21, 39–41, 44, 48]. Another study did not specify any guidelines, but was also in line with the overall Codex framework [38]. Three risk assessments developed their own models [11, 42, 43]. One of them, the Food and Drug Administration Center for Veterinary Medicine (FDA-CVM) guideline [11], was applied by a separate Canadian study [45]; see Figure 2.
Other studies
Three studies did not fit into the above-described categories, namely: a One Health prediction model of the maximum future human health impact of eliminating different AMR transmission routes in Thailand [35] and two studies describing the proportion of cases of livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) in the Netherlands [37] and Denmark [36] among individuals who had livestock contact previous to their infection. While the latter two studies technically met the criteria for inclusion, they did not utilise any specific methodology to address our research objective and, therefore, will not be addressed further.
Conceptual approaches
The articles differed in their conceptual approaches for modelling the link between animal sources and human AMR. All comparative exposure assessments [22–24] and six risk assessments [38, 40, 41, 43, 44, 48] followed a bottom-up approach, starting at the hazard at one point of the food production chain and factoring in the effects of subsequent steps to estimate the human health impact at exposure (Figure 3a). In addition to food-related exposure, Lechner and colleagues included direct animal contact as an exposure route [22].
Figure 3.
Conceptual approaches of the identified studies. (a) Bottom-up approaches: start at one point along the farm-to-fork continuum and adjust for different factors to arrive at an estimate of the health outcome. (b) Top-down approaches: start at the outcome and attribute it to one or multiple sources, either at the level of exposure (i.e., consumption or contact) or at the animal reservoir. (c) Complex approaches: integrate all One Health domains or account for multi-directional hazard transmission. Arrows indicate the directionality of transmission. AMR, bacterial antimicrobial resistance; AMU, antimicrobial usage.
Sixteen studies started at the outcome and either estimated the contribution of one specific source to it [11, 39, 42, 45] or attributed it to different sources [19, 25–33, 46, 47]. All microbial subtyping studies [19, 30–33, 46, 47], all investigations of outbreaks [25–29], and four risk assessments [11, 39, 42, 45] adopted such ‘top-down’ concepts (Figure 3a). Eleven studies attributed infections at the point of consumption or contact (exposure), and the remaining five partitioned the outcome to animal reservoirs. The latter included one quantitative risk assessment [39] and all microbial subtyping studies, except Mughini-Gras et al. [31] and Hald et al. [33], both of which integrated information on the exposure frequency to attribute infections at the point of exposure.
Four studies employed more complex concepts that did not fit into the top-down or bottom-up approaches (Figure 3c). Two of these followed a One Health strategy [20, 21], another allowed for multi-directional AMR transfer between animals and humans [34], and the last had both a One Health concept and addressed multi-directional spread between populations [35].
Hazard and outcome definitions
Most studies investigated infections due to specific bacteria–resistance combinations. Three articles reported on bacterial subtypes known to be associated with specific resistances [28, 42, 44], and five focused on general resistance in a specific pathogen [25, 27, 29, 39, 48] (Figure 4a). Four microbial subtyping studies included all infections with the pathogen and accounted for resistance by using antibiotic profiles to subtype strain populations [32, 33, 46, 47]. Opatowski and colleagues built a generic quantitative model applicable for any resistant pathogen [21], whereas four other articles considered multiple bacteria–resistance combinations relevant to human health [41, 43] or qualitatively addressed AMR in general [20, 22]. The only quantitative assessment that focused on the resistome as a whole was that by Duarte and colleagues [19].
Of the articles on specific pathogens, 12 concerned Salmonella species. Seven investigated E. coli, mainly strains producing ESBL and/or (plasmid)-mediated AmpC β-lactamase. Two studies each were about Campylobacter and MRSA.
Sixteen studies defined the outcome as human illness due to resistant pathogens, six investigated colonisation with resistant bacteria, and all four qualitative and semi-quantitative risk assessments defined it as a health risk caused by AMR. The key outcome of the three exposure assessments was exposure to AMR (Figure 4b).
Figure 4.
Hazard definition (a), outcome measure (b), investigated animal-related (c) and non-animal-related sources (d), as well as the most important source found (e) by the included studies. Colourised fields indicate that the respective source(s) and mode(s) of transmission were addressed by the study.
*Outcomes: overall human health risk due to hazard (HH), human colonisation with hazard (H), human illness due to hazard (I), or exposure to hazard (E).
†Letters indicate whether food preparation and animal contact were occupational (O), non-occupational (N) or both (B). If no letter, it was not specified.
¶The numbers are ranks of importance (1 = highest importance). They are only given for studies reporting the relative contribution of different animal sources to human AMR in relation to each other. Studies examining risk due to only one specific animal source are shown with a T (total risk estimate).
§Only given for studies with both animal and non-animal sources. If the source is printed in bold, the study found it to be responsible for over 50% of the outcome. AMR, bacterial antimicrobial resistance; AMU, antimicrobial usage; CEA, comparative exposure assessment; DT, definite/phage type; ESBL, extended-spectrum ß-lactamase; FQ, fluoroquinolone; MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; OUT, investigation of outbreaks; (p)AmpC, (plasmid)-mediated AmpC ß-lactamase; RA, risk assessment; S-Q, semi-quantitative; Q, quantitative; Ql, qualitative.
Animal sources
Five studies investigated animal reservoirs [19, 30, 32, 46, 47], 15 focused on food consumption [11, 20, 23, 24, 26–28, 33, 39–41, 43–45, 48], one focused on animal contact [25], one focused on food preparation [38], and seven included different manners of acquisition [21, 22, 29, 31, 34, 35, 42] (Figure 4c).
Chicken was the most investigated animal source with 20 studies, followed by cattle (including veal calves) with 19 studies and pigs with 16 studies. Other animal sources included turkeys (n = 5), sheep or goat (n = 2), fish or seafood (n = 2), eggs (n = 2), raw milk (n = 1), and duck (n = 1) (Figure 4c). Four articles explored the role of pets as sources of AMR, one of which examined specifically the relative importance of pet reptiles or amphibians in causing resistant outbreaks [25].
Of the risk assessments estimating the human risk due to a single animal source of AMR, three concerned chicken products [11, 41, 45], three pig products [38, 42, 44], and one beef [39]. Fourteen studies also included non-animal-related sources, such as human-to-human exposure or environmental sources (Figure 4d).
Resulting estimates
The studies reported their results in one or more of the following ways: 1) as the relative contribution of different animal-related and non-animal sources to the human AMR burden (percentage, total numbers, or qualitatively), 2) as the relative importance of animal sources in relation to each other, or 3) as the total number of human cases due to a specific animal source (per population or per meal). Descriptions of the results reported by each study can be found in Table 1.
Figure 4e lists the most important contributors to the human AMR burden found by studies with outcome type 1. The estimates differed with the pathogen under investigation; while none of the five studies on resistant E. coli found animals to be the most important contributor, most studies on resistant Salmonella attributed the highest proportion of illness to animal sources.
In Figure 4c, the results of studies reporting outcomes of the second type are displayed in the form of ranks. Chicken was most frequently assigned the top rank (six times), followed by cattle which was most relevant according to four studies. One study found turkeys to be the major contributor [32] while in another study pets were more relevant than individual livestock species – but not when compared to all livestock species combined [31].
Discussion
We aimed to describe the current state of evidence on the relative direct contribution of animal sources to human AMR. Literature searches were kept broad to capture as many relevant publications as possible and screened over 10,000 records; however, we only found 31 studies that addressed our specific research objective. This illustrates that while there is a large and growing body of evidence on the topic of AMR, there is still a paucity of studies compiling this evidence to assess the concrete contribution of animals to human AMR.
Our work complements a 2018 review by Pires and colleagues, which describes the utility of risk assessments and source attribution studies for determining the relative importance of different sources of human AMR [6]. We systematically searched for publications following such or similar strategies, albeit focusing on animal sources of AMR. Our results support their observation of these study types still being in their infancy in the context of AMR [6]. However, there has been a marked recent increase of relevant publications as almost 40% of studies were published in the past three years alone. This rise coincided with the publication of the Global Action Plan on Antimicrobial Resistance (GAP) by WHO in 2015, which may have contributed to the momentum of AMR research [59].
Most microbial subtyping studies were published in Europe, which was unsurprising, given that this study type originated in Denmark and the Netherlands [49, 50]. North America contributed a third of the studies, including all five investigations of outbreaks, which were all from the United States. The availability of nationwide surveillance systems such as the National Antimicrobial Resistance Monitoring System (NARMS) [60], the CDC’s Foodborne Disease Active Surveillance Network (FoodNet) [61], and the National Outbreak Reporting System (NORS) [62] are likely among the reasons for why we identified multiple US studies compiling source information for resistant outbreaks. However, similar systems also exist in other countries, for example the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) [63] and FoodNet Canada [64]. The lack of comparable outbreak studies from other countries may, therefore, not reflect a true absence of evidence, but could also be influenced by the limitations of our search scope.
We did not find any studies from South America, Africa, or Oceania, which may indicate either that study types relevant to this review are not published in scientific journals in these regions or that no such studies are conducted there, possibly due to data scarcity or lack of awareness or interest in the issue.
Given the nature of the AMR hazard, capturing the complete burden it causes in humans via animal sources requires approaches accounting for horizontal gene transfer. However, most studies focused on specific pathogens, which reflects the way infectious disease data are currently collected. Only one study quantitatively investigated sources of the human resistome [19]. Increasing usage of (meta-)genomics for resistance characterisation in food safety in recent years, combined with decreasing sequencing costs [65], gives hope that the availability of data necessary to conduct similar studies will increase over the coming years.
A commonly stated limitation of the source attribution studies was their inability to account for bidirectional AMR transmission between animals and humans. However, only two studies identified explicitly integrated directionality into their models: one developing a multi-directional dynamic risk model [34] and the other specifying a human-to-animal transmission parameter in their prediction model [35].
While our focus was on the direct impact of animal sources on human AMR, we acknowledge that AMR is an issue spanning across animals, humans, and the environment and that more holistic approaches are necessary to describe fully all direct and indirect connections between them. Indeed, we found three studies that followed such One Health concepts [20, 21, 35].
None of the studies included subgroup analyses for vulnerable populations. Gender or socioeconomic factors were also not investigated. Such factors may likely have a considerable impact on the probability of AMR acquisition [66, 67] and should be kept in mind for future research.
Fish and seafood were only addressed by two studies. Given the rapid growth of the aquaculture sector and the accompanying use of antimicrobials, especially in regions without adequate regulations, pathogens of aquatic origins have high resistance proportions [68]. Aquaculture, therefore, presents a potential threat to human health and merits further research.
We compiled some results of the studies to give a broad overview, but when interpreting them, it should be kept in mind that no account was made for study design, quality, or uncertainty and the results were merely ranked for each study individually. The findings suggest differing degrees of importance of the animal sources depending on the type of hazard, which is in line with other studies observing different risks of exposure via the same type of meat source depending on the bacteria–resistance combination [69, 70].
By aiming at identifying studies that quantified the relative contribution of animal sources to human AMR, we implicitly posed the underlying assumption that there is a link between animal and human AMR. The question of whether transmission of resistance occurs between animals and humans has been addressed by previous systematic reviews. For example, such a review from 2018 found that 33 out of 45 eligible studies supported the view that transmission of resistant E. coli occurs between food-producing animals and humans [71]. Likewise, another systematic review summarised information on food-producing animals as potential origins for extra-intestinal E. coli resistant to cephalosporins and found overall supporting evidence, especially for poultry [5].
Given our focus on the direct contribution of animals to human AMR, we did not include AMU in our search strategy and excluded studies attributing cases of AMR in humans solely to AMU in animals. However, even when omitting AMU-specific search terms, during the screening, we observed several studies, especially risk assessments, relating AMU in animals to human AMR, indicating that there is considerable evidence available on this topic that could be explored by future research.
We did not search grey literature databases and, therefore, likely missed relevant publications, such as government reports. Furthermore, while articles were not excluded based on language, our searches were conducted in English, thereby likely missing relevant publications in other languages.
In conclusion, the body of evidence on the direct contribution of animal sources to AMR in humans is growing but still relatively small. Existing studies utilise a broad range of methodologies to address this question. Recent years have seen promising developments, such as using human resistome data for source attribution, that will aid in tailoring studies to the specific characteristics of the AMR hazard.
Supporting information
Fastl et al. supplementary material
Fastl et al. supplementary material
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268823001309.
Data availability statement
The search strategies and all extracted data can be found in the Supplementary Materials.
Author contribution
Conceptualization: S.M.P., L.M., B.D., C.D.B., J.S.A., S.B.M., N.V., D.P., C.F.; Writing – review & editing: S.M.P., L.M., B.H., B.D., C.D.B., H.C.D.C.F., J.R., J.S.A., S.B.M., N.V., D.P., C.F.; Funding acquisition: B.H., B.D., J.R., D.P.; Methodology: B.D., C.D.B., H.C.D.C.F., J.S.A., S.B.M., N.V., D.P.; Project administration: B.D., D.P.; Supervision: B.D.; Investigation: H.C.D.C.F., C.F.; Validation: H.C.D.C.F., C.F.; Data curation: C.F.; Visualization: C.F.; Writing – original draft: C.F.
Financial support
This research was performed in the framework of the Global Burden of Animal Diseases (GBADs) programme, which is led by the University of Liverpool and the World Organization for Animal Health (WOAH) (https://animalhealthmetrics.org/). This research was supported through the Grant Agreement Investment ID INV-005366 with the Bill & Melinda Gates Foundation and the UK Foreign, Commonwealth and Development Office (FCDO). GBADs case studies received additional funding from the following: European Commission, Australian Centre for International Agricultural Research (ACIAR), Brooke Foundation and the Food and Agriculture Organization of the United Nations (FAO). A full list of the GBADs collaborators can be accessed here: https://animalhealthmetrics.org/acknowledgements. This work was also supported by a Subgrant Agreement (award number 20220419) with Fleming Fund and WOAH.
Competing interest
The authors declare none.
References
- [1].Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC, Browne AJ, Chipeta MG, Fell F, Hackett S, Haines-Woodhouse G, Hamadani BHK, Kumaran EAP, McManigal B, Achalapong S, Agarwal R, Akech S, Albertson S, Amuasi J, Andrews J, Aravkin A, Ashley E, Babin F-X, Bailey F, Baker S, Basnyat B, Bekker A, Bender R, Berkley JA, Bethou A, Bielicki J, Boonkasidecha S, Bukosia J, Carvalheiro C, Castañeda-Orjuela C, Chansamouth V, Chaurasia S, Chiurchiù S, Chowdhury F, Donatien RC, Cook AJ, Cooper B, Cressey TR, Criollo-Mora E, Cunningham M, Darboe S, Day NPJ, Luca MD, Dokova K, Dramowski A, Dunachie SJ, Bich TD, Eckmanns T, Eibach D, Emami A, Feasey N, Fisher-Pearson N, Forrest K, Garcia C, Garrett D, Gastmeier P, Giref AZ, Greer RC, Gupta V, Haller S, Haselbeck A, Hay SI, Holm M, Hopkins S, Hsia Y, Iregbu KC, Jacobs J, Jarovsky D, Javanmardi F, Jenney AWJ, Khorana M, Khusuwan S, Kissoon N, Kobeissi E, Kostyanev T, Krapp F, Krumkamp R, Kumar A, Kyu HH, Lim C, Lim K, Limmathurotsakul D, Loftus MJ, Lunn M, Ma J, Manoharan A, Marks F, May J, Mayxay M, Mturi N, Munera-Huertas T, Musicha P, Musila LA, Mussi-Pinhata MM, Naidu RN, Nakamura T, Nanavati R, Nangia S, Newton P, Ngoun C, Novotney A, Nwakanma D, Obiero CW, Ochoa TJ, Olivas-Martinez A, Olliaro P, Ooko E, Ortiz-Brizuela E, Ounchanum P, Pak GD, Paredes JL, Peleg AY, Perrone C, Phe T, Phommasone K, Plakkal N, Ponce-de-Leon A, Raad M, Ramdin T, Rattanavong S, Riddell A, Roberts T, Robotham JV, Roca A, Rosenthal VD, Rudd KE, Russell N, Sader HS, Saengchan W, Schnall J, Scott JAG, Seekaew S, Sharland M, Shivamallappa M, Sifuentes-Osornio J, Simpson AJ, Steenkeste N, Stewardson AJ, Stoeva T, Tasak N, Thaiprakong A, Thwaites G, Tigoi C, Turner C, Turner P, Doorn HR van Velaphi S, Vongpradith A, Vongsouvath M, Vu H, Walsh T, Walson JL, Waner S, Wangrangsimakul T, Wannapinij P, Wozniak T, Sharma TEMWY, Yu KC, Zheng P, Sartorius B, Lopez AD, Stergachis A, Moore C, Dolecek C, Naghavi M (2022) Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. The Lancet 399, 629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].von Wintersdorff CJH, Penders J, van Niekerk JM, Mills ND, Majumder S, van Alphen LB, Savelkoul PHM, Wolffs PFG (2016) Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Frontiers in Microbiology 7, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Robinson TP, Bu DP, Carrique-Mas J, Fèvre EM, Gilbert M, Grace D, Hay SI, Jiwakanon J, Kakkar M, Kariuki S, Laxminarayan R, Lubroth J, Magnusson U, Thi Ngoc P, Van Boeckel TP, Woolhouse MEJ (2016) Antibiotic resistance is the quintessential one health issue. Transactions of the Royal Society of Tropical Medicine and Hygiene 110, 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].European Food Safety Authority, European Centre for Disease Prevention and Control, European Medicines Agency. (2021) Antimicrobial consumption and resistance in bacteria from humans and animals: Third joint inter-agency report on integrated analysis of antimicrobial agent consumption and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA: JIACRA III 2016–2018 [Internet]. LU: Publications Office; Available at https://data.europa.eu/doi/10.2900/056892. (accessed 6 July 2023)
- [5].Lazarus B, Paterson DL, Mollinger JL, Rogers BA (2015) Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review Clinical Infectious Diseases. 60, 439–452. [DOI] [PubMed] [Google Scholar]
- [6].Pires SM, Duarte AS, Hald T (2018) Source attribution and risk assessment of antimicrobial resistance. Microbiology Spectrum 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mughini-Gras L, Kooh P, Fravalo P, Augustin J-C, Guillier L, David J, Thébault A, Carlin F, Leclercq A, Jourdan-Da-Silva N, Pavio N, Villena I, Sanaa M, Watier L (2019) Critical orientation in the jungle of currently available methods and types of data for source attribution of foodborne diseases. Frontiers in Microbiology 10, 2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Munck N, Njage PMK, Leekitcharoenphon P, Litrup E, Hald T (2020) Application of whole-genome sequences and machine learning in source attribution of Salmonella Typhimurium. Risk Analysis 40, 1693–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vose DJ, Acar J, Anthony F, Franklin A, Gupta R, Nicholls TJ, Tamura Y, Thompson S, Threlfall EJ, Van Vuuren M, White DG, Wegener HC, Costarrica ML (2001) Antimicrobial resistance: Risk analysis methodology for the potential impact on public health of antimicrobial resistant bacteria of animal origin. Revue Scientifique et Technique 20, 811–827. [DOI] [PubMed] [Google Scholar]
- [10].Williams MS, Ebel ED and Vose D (2011) Framework for microbial food-safety risk assessments amenable to Bayesian modeling. Risk Analysis 31, 548–565. [DOI] [PubMed] [Google Scholar]
- [11].Vose D, Hollinger K, Bartholomew M. Human Health Impact of Fluoroquinolone Resistant Campylobacter Attributed to the Consumption of Chicken. Washington, DC: US Food and Drug Administration Center for Veterinary Medicine; 2000. Available at https://www.fda.gov/media/76429/download. [Google Scholar]
- [12].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLOS Medicine 18, e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Antimicrobial resistance [Internet] (Nov 17th 2021). [cited 2023 Mar 21]. Available at https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. (accessed 6 July 2023)
- [14].Plaza-Rodríguez C, Alt K, Grobbel M, Hammerl JA, Irrgang A, Szabo I, Stingl K, Schuh E, Wiehle L, Pfefferkorn B, Naumann S, Kaesbohrer A, Tenhagen B-A (2021) Wildlife as sentinels of antimicrobial resistance in Germany? Frontiers in Veterinary Science 7, 627821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Richardson LC, Bazaco MC, Parker CC, Dewey-Mattia D, Golden N, Jones K, Klontz K, Travis C, Kufel JZ, Cole D (2017) An updated scheme for categorizing foods implicated in foodborne disease outbreaks: A tri-Agency collaboration. Foodborne Pathogens and Disease 14, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bozada T, Borden J, Workman J, Del Cid M, Malinowski J, Luechtefeld T (2021) Sysrev: A FAIR platform for data curation and systematic evidence review. Frontiers in Artificial Intelligence 4, 685298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Roy Rosenzweig Center for History and New Media. (2006) Zotero [Internet]. [cited 2023 Jun 29]. Available at https://www.zotero.org/. (accessed 6 July 2023)
- [18].Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—A web and mobile app for systematic reviews. Systematic Reviews 5, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duarte ASR, Röder T, Van Gompel L, Petersen TN, Hansen RB, Hansen IM, Bossers A, Aarestrup FM, Wagenaar JA, Hald T (2021) Metagenomics-based approach to source-attribution of antimicrobial resistance determinants – Identification of reservoir resistome signatures. Frontiers in Microbiology 11, 601407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chereau F, Opatowski L, Tourdjman M, Vong S (2017) Risk assessment for antibiotic resistance in South East Asia. British Medical Journal 358, j3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Opatowski L, Opatowski M, Vong S, Temime L (2021) A one‐health quantitative model to assess the risk of antibiotic resistance acquisition in Asian populations: Impact of exposure through food, water, livestock and humans. Risk Analysis 41, 1427–1446. [DOI] [PubMed] [Google Scholar]
- [22].Lechner I, Freivogel C, Stärk KDC, Visschers VHM (2020) Exposure pathways to antimicrobial resistance at the human-animal interface—A qualitative comparison of Swiss expert and consumer opinions. Frontiers in Public Health 8, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Evers EG, Pielaat A, Smid JH, van Duijkeren E, Vennemann FBC, Wijnands LM, Chardon JE (2017) Comparative exposure assessment of ESBL-producing Escherichia coli through meat consumption. PLoS One 12, e0169589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Carmo LP, Nielsen LR, da Costa PM, Alban L (2014) Exposure assessment of extended-spectrum beta-lactamases/AmpC beta-lactamases-producing Escherichia coli in meat in Denmark. Infection Ecology & Epidemiology 4, 22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Waltenburg MA, Perez A, Salah Z, Karp BE, Whichard J, Tolar B, Gollarza L, Koski L, Blackstock A, Basler C, Nichols M (2022) Multistate reptile- and amphibian-associated salmonellosis outbreaks in humans, United States, 2009–2018. Zoonoses and Public Health 69, 925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Folster JP, Grass JE, Bicknese A, Taylor J, Friedman CR, Whichard JM (2017) Characterization of resistance genes and plasmids from outbreaks and illness clusters caused by Salmonella resistant to ceftriaxone in the United States, 2011–2012. Microbial Drug Resistance 23, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brown AC, Grass JE, Richardson LC, Nisler AL, Bicknese AS, Gould LH (2017) Antimicrobial resistance in Salmonella that caused foodborne disease outbreaks: United States, 2003–2012. Epidemiology and Infection 145, 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sahin O, Fitzgerald C, Stroika S, Zhao S, Sippy RJ, Kwan P, Plummer PJ, Han J, Yaeger MJ, Zhang Q (2012) Molecular evidence for zoonotic transmission of an emergent, highly pathogenic Campylobacter jejuni clone in the United States. Journal of Clinical Microbiology 50, 680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Holmberg SD, Wells JG and Cohen ML (1984) Animal-to-man transmission of antimicrobial-resistant salmonella: Investigations of U.S. outbreaks, 1971-1983. Science 225(4664), 833–835. [DOI] [PubMed] [Google Scholar]
- [30].Perestrelo S, Correia Carreira G, Valentin L, Fischer J, Pfeifer Y, Werner G, Schmiedel J, Falgenhauer L, Imirzalioglu C, Chakraborty T, Käsbohrer A (2022) Comparison of approaches for source attribution of ESBL-producing Escherichia coli in Germany. PLoS One 17, e0271317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mughini-Gras L, Dorado-García A, van Duijkeren E, van den Bunt G, Dierikx CM, Bonten MJM, Bootsma MCJ, Schmitt H, Hald T, Evers EG, de Koeijer A, van Pelt W, Franz E, Mevius DJ, Heederik DJJ (2019) Attributable sources of community-acquired carriage of Escherichia coli containing β-lactam antibiotic resistance genes: A population-based modelling study. Lancet Planetary Health 3, e357–e369. [DOI] [PubMed] [Google Scholar]
- [32].Vieira AR, Grass J, Fedorka-Cray PJ, Plumblee JR, Tate H, Cole DJ (2016) Attribution of Salmonella enterica serotype Hadar infections using antimicrobial resistance data from two points in the food supply system. Epidemiology and Infection 144, 1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hald T, Lo Fo Wong DMA and Aarestrup FM (2007) The attribution of human infections with antimicrobial resistant Salmonella bacteria in Denmark to sources of animal origin. Foodborne Pathogens and Disease 4, 313–326. [DOI] [PubMed] [Google Scholar]
- [34].de Freitas Costa E, Hagenaars TJ, Dame-Korevaar A, Brouwer MSM, de Vos CJ (2022) Multidirectional dynamic model for the spread of extended-spectrum-β-lactamase-producing Escherichia coli in the Netherlands. Microbial Risk Analysis 22, 100230. [Google Scholar]
- [35].Booton RD, Meeyai A, Alhusein N, Buller H, Feil E, Lambert H, Mongkolsuk S, Pitchforth E, Reyher KK, Sakcamduang W, Satayavivad J, Singer AC, Sringernyuang L, Thamlikitkul V, Vass L, Avison MB, Alhusein N, Booton RD, Buller H, Chantong B, Charoenlap N, Couto N, Dulyayangkul P, Feil E, Gibbon MJ, Gould VC, Lambert H, Meeyai A, Mongkolsuk S, Montrivade V, Pitchforth E, Phoonsawad K, Rangkadilok N, Ratanakorn P, Reyher KK, Sakcamduang W, Satayavivad J, Singer AC, Sirikanchana K, Sringernyuang L, Suriyo T, Suwanpakdee S, Thamlikitkul V, Turner KME, Vass L, Wichuwaranan K, Wiratsudakul A, Avison MB, Turner KME (2021) One health drivers of antibacterial resistance: Quantifying the relative impacts of human, animal and environmental use and transmission. One Health 12, 100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Larsen J, Petersen A, Larsen AR, Sieber RN, Stegger M, Koch A, Aarestrup FM, Price LB, Skov RL, for the Danish MRSA Study Group Johansen HK, Westh H, Pedersen M, Jensen US, Jensen MLS, Chen M, Strøbæk S, Østergaard C, Lomborg S, Ellermann-Eriksen S, Ripadal P (2017) Emergence of livestock-associated methicillin-resistant Staphylococcus aureus bloodstream infections in Denmark. Clinical Infectious Diseases 65, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bosch T, van Luit M, Pluister GN, Frentz D, Haenen A, Landman F, Witteveen S, van Marm-Wattimena N, van der Heide HG, Schouls LM (2016) Changing characteristics of livestock-associated meticillin-resistant Staphylococcus aureus isolated from humans – Emergence of a subclade transmitted without livestock exposure, the Netherlands, 2003 to 2014. Eurosurveillance 21, 30236. [DOI] [PubMed] [Google Scholar]
- [38].Schoen ME, Peckham TK, Shirai JH, Kissel JC, Thapaliya D, Smith TC, Meschke JS (2020) Risk of nasal colonization of methicillin-resistant Staphylococcus aureus during preparation of contaminated retail pork meat. Microbial Risk Analysis 16, 100136. [Google Scholar]
- [39].Costard S, Pouzou JG, Belk KE, Morley PS, Schmidt JW, Wheeler TL, Arthur TM, Zagmutt FJ (2020) No change in risk for antibiotic-resistant salmonellosis from beef, United States, 2002–2010. Emerging Infectious Diseases 26, 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Collineau L, et al. (2020) A farm-to-fork quantitative risk assessment model for Salmonella Heidelberg resistant to third-generation cephalosporins in broiler chickens in Canada. International Journal of Food Microbiology 330, 108559. [DOI] [PubMed] [Google Scholar]
- [41].Collineau L, et al. (2018) Risk ranking of antimicrobial-resistant hazards found in meat in Switzerland. Risk Analysis 38, 1070–1084. [DOI] [PubMed] [Google Scholar]
- [42].Cox LA and Popken DA (2014) Quantitative assessment of human MRSA risks from swine. Risk Analysis 34, 1639–1650. [DOI] [PubMed] [Google Scholar]
- [43].Presi P, et al. (2009) Risk scoring for setting priorities in a monitoring of antimicrobial resistance in meat and meat products. International Journal of Food Microbiology 130, 94–100. [DOI] [PubMed] [Google Scholar]
- [44].Alban L, Olsen A.-M, Nielsen B, Sørensen R, & Jessen B (2002) Qualitative and quantitative risk assessment for human salmonellosis due to multi-resistant Salmonella typhimurium DT104 from consumption of Danish dry-cured pork sausages. Preventive Veterinary Medicine 52, 251–265. [DOI] [PubMed] [Google Scholar]
- [45].Otto SJG, Carson CA, Finley RL, Thomas MK, Reid-Smith RJ, & McEwen SA (2014) Estimating the number of human cases of Ceftiofur-resistant Salmonella enterica Serovar Heidelberg in Quebec and Ontario, Canada. Clinical Infectious Diseases 59, 1281–1290. [DOI] [PubMed] [Google Scholar]
- [46].Mitchell J, Purohit M, Jewell CP, Read JM, Marrone G, Diwan V, & Stålsby Lundborg C (2021) Trends, relationships and case attribution of antibiotic resistance between children and environmental sources in rural India. Scientific Reports 11, 22599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Parisi A, Phuong TLT, Mather AE, Jombart T, Tuyen HT, Lan NPH, Trang NHT, Carrique-Mas J, Campbell JI, Trung NV, Glass K, Kirk MD, & Baker S (2020) The role of animals as a source of antimicrobial resistant nontyphoidal Salmonella causing invasive and non-invasive human disease in Vietnam. Infection, Genetics and Evolution 85, 104534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Doménech E, Jiménez-Belenguer A, Pérez R, Ferrús MA, & Escriche I (2015) Risk characterization of antimicrobial resistance of Salmonella in meat products. Food Control 57, 18–23. [Google Scholar]
- [49].Hald T, Vose D, Wegener HC, & Koupeev T (2004) A Bayesian approach to quantify the contribution of animal‐food sources to human Salmonellosis. Risk Analysis 24, 255–269. [DOI] [PubMed] [Google Scholar]
- [50].van Pelt W, Giessen AW and Leeuwen WJ (1999) Oorsprong, omvang en kosten van humane salmonellose. Deel 1. Oorsprong van humane salmonellose met betrekking tot varken, rund, kip, ei en overige bronnen. Infectieziekten Bulletin 10, 240–243. [Google Scholar]
- [51].Mullner P, Jones G, Noble A, Spencer SEF, Hathaway S, & French NP (2009) Source attribution of food-borne zoonoses in New Zealand: A modified Hald model. Risk Analysis 29, 970–984. [DOI] [PubMed] [Google Scholar]
- [52].Miller P, Marshall J, French N, & Jewell C (2017) sourceR: Classification and source attribution of infectious agents among heterogeneous populations. PLoS Computational Biology 13, e1005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Evers EG and Chardon JE (2010) A swift quantitative microbiological risk assessment (sQMRA) tool. Food Control 21, 319–330. [Google Scholar]
- [54].Codex Alimentarius Commission (1999) Principles and guidelines for the conduct of microbiological risk assessment. CAC/GL-30. Available at: https://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/en/. (accessed 6 July 2023)
- [55].Codex Alimentarius Commission (2011) Guidelines for risk analysis of foodborne antimicrobial resistance. CAC/GL-77. Available at https://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/en/. (accessed 6 July 2023)
- [56].World Health Organization (2012) Rapid risk assessment of acute public health events (WHO/HSE/GAR/ARO/2012.1). Available at https://apps.who.int/iris/handle/10665/70810. (accessed 6 July 2023)
- [57].van der Fels-Klerx Hj, van Asselt Ed, Raley M, Poulsen M, Korsgaard H, Bredsdorff L, Nauta M, Flari V, d’Agostino M, Coles D, & Frewer L (2015) Critical review of methodology and application of risk ranking for prioritisation of food and feed related issues, on the basis of the size of anticipated health impact. EFSA Supporting Publications 12, 710E. [Google Scholar]
- [58].World Health Organization (2009) Risk assessment of Campylobacter spp. in broiler chickens: Technical report. Available at https://apps.who.int/iris/handle/10665/43731. (accessed 6 July 2023)
- [59].World Health Organization (2015) Global action plan on antimicrobial resistance. Available at https://apps.who.int/iris/handle/10665/193736. (accessed 6 July 2023)
- [60].Centers for Disease Control and Prevention (2023) National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS). Available at https://www.cdc.gov/narms/index.html. (accessed 6 July 2023)
- [61].Centers for Disease Control and Prevention (2022) Foodborne Diseases Active Surveillance Network (FoodNet). Available at https://www.cdc.gov/foodnet/index.html. (accessed 6 July 2023)
- [62].Centers for Disease Control and Prevention (2023) National Outbreak Reporting System (NORS). Available at https://www.cdc.gov/nors/index.html. (accessed 6 July 2023)
- [63].Public Health Agency of Canada (2007) Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS). Available at https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillance-cipars.html. (accessed 6 July 2023)
- [64].Public Health Agency of Canada (2006) About FoodNet Canada (formerly C-EnterNet). Available at https://www.canada.ca/en/public-health/services/surveillance/foodnet-canada.html. (accessed 6 July 2023)
- [65].Pennone V, Cobo-Díaz JF, Prieto M, & Alvarez-Ordóñez A (2022) Application of genomics and metagenomics to improve food safety based on an enhanced characterisation of antimicrobial resistance. Current Opinion in Food Science 43, 183–188. [Google Scholar]
- [66].Forrester JD, Cao S, Schaps D, Liou R, Patil A, Stave C, Sokolow SH, & Leo GD (2022) Influence of socioeconomic and environmental determinants of health on human infection and colonization with antibiotic-resistant and antibiotic-associated pathogens: A scoping review. Surgical Infections 23, 209–225. [DOI] [PubMed] [Google Scholar]
- [67].Jones N, Mitchell J, Cooke P, Baral S, Arjyal A, Shrestha A, & King R (2022) Gender and antimicrobial resistance: What can we learn from applying a gendered lens to data analysis using a participatory arts case study? Frontiers in Global Women’s Health 3, 745862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Watts JEM, Schreier HJ, Lanska L, & Hale MS (2017) The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Marine Drugs 15, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang Y, Schmidt JW, Arthur TM, Wheeler TL, & Wang B (2021) A comparative quantitative assessment of human exposure to various antimicrobial-resistant bacteria among U.S. ground beef consumers. Journal of Food Protection 84, 736–759. [DOI] [PubMed] [Google Scholar]
- [70].Plaza-Rodríguez C, Mesa-Varona O, Alt K, Grobbel M, Tenhagen B.-A, & Kaesbohrer A (2021) Comparative analysis of consumer exposure to resistant bacteria through chicken meat consumption in Germany. Microorganisms 9, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Muloi D, Ward MJ, Pedersen AB, Fèvre EM, Woolhouse MEJ, & van Bunnik BAD (2018) Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A Systematic Review Foodborne Pathogens and Disease 15, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fastl et al. supplementary material
Fastl et al. supplementary material
Data Availability Statement
The search strategies and all extracted data can be found in the Supplementary Materials.