Abstract
Terminal deoxynucleotidyl transferase (TdT) is an unusual DNA polymerase that adds untemplated dNTPs to 3′-ends of DNA. If a target protein is expressed as a TdT fusion and incubated with a DNA-encoded library (DEL) in the presence of dATP, the binders of the target induce proximity between TdT and the DNA, promoting the synthesis of a poly-adenine (polyA) tail. The polyA tail length is proportional to the binding affinity, effectively serving as a stable molecular record of binding events. The polyA tail is also a convenient handle to enrich binders with magnetic poly(dT)25 beads before sequencing. In a benchmarking system, we show that ligands spanning nanomolar to double-digit micromolar binding can be cleanly identified by TdT extension, whereas only the tightest binding ligands are identified by classical affinity selection. The method is simple to implement and can function on any DEL that bears a free 3′-end.
Introduction
Screening compound libraries for binding or inhibitory activity against target proteins is an essential part of early drug discovery. The industry-standard high-throughput screening (HTS) facility, however, comes with a sizeable infrastructural burden. DNA-encoded libraries (DELs) offer a radically different approach to initial hit identification.1−4 DEL technology uses DNA tags to track the synthetic history of individual members in a split-and-pool combinatorial synthesis scheme. Since each compound’s identity is encoded in its covalently connected DNA, the compounds can be pooled before performing an affinity selection against targets. After selection, the retained molecules are washed from beads, and their DNA tags are sequenced with next-generation sequencing (NGS). Sometimes including a selection condition with a known ligand can allow specific localization of the binding site.5 DNA sequencing is the final data readout, and the hope is that NGS read counts give a somewhat faithful representation of the binding affinity. While this concept works well for potent binders, screens that only yield moderate binders are more difficult to interpret due to noise. Here, we introduce a new solution-phase approach to DEL selection, which uses terminal deoxynucleotidyl transferase6 (TdT) to directly record the binding information into DEL DNA (Figure 1).
Figure 1.
Conceptual design for a molecular recorder of encoded library affinity data. Binding events are recorded by TdT (A), providing a handle to separate binders from non-binders (B). After NGS, the counts can be compared to a selection without the POI, revealing binders (C).
DEL technology has expanded rapidly since its conceptual birth7 and first practical demonstration,8−15 with much recent effort focused on DEL-compatible synthetic methods.1,16,17 Whether in solution18−27 or on bead (i.e., one-bead–one-compound DELs),28,29 examining different types of selections remains an active area of research with enormous potential for development.30,31 Particularly exciting are the selection methods that convert transient protein–ligand interactions into permanent covalent linkages,18,30 trigger a functional reaction upon binding,32,33 or allow the evolution of the library.24 More recently, the Krusemark lab presented the first example of a DEL selection using proximity-induced biotinylation.34 While these methods are strong contributions to DEL selection, innovations are sorely needed. For example, a common problem is that many new methods are not backward compatible with the most common types of DELs already available. Another problem in most methods is that single binding events must be individually captured to deliver a signal. Here, we introduce DEL selections using TdT polyadenylation recording (DELSTAR). DELSTAR is a solution-phase method that will work with any DEL bearing an extendable 3′-end. It is unique in that it directly records information about binding events onto the pendant DNA of the DEL. An additional feature of the method is that it uses more than 100-fold less protein than a typical affinity selection, meaning that small-scale protein production can support a selection campaign.
Figure 1 shows the conceptual design of DELSTAR. Target proteins of interest (POI) would be expressed as a fusion with TdT and then incubated with a DEL in the presence of dATP. The binders will induce proximity of the pendant DNA to the POI–TdT fusion, causing untemplated extension of the 3′-end to create a poly-A tail (step A, Figure 1). Commercial poly(dT)25 beads (typically used for mRNA purification) would then be used to separate tailed DNAs (i.e., binders) from untailed (i.e., nonbinders) (step B, Figure 1); alternatively by separating based on size, binders of differing affinity (different tail length) might be resolved (step C, Figure 1). Finally, the enriched libraries would be submitted for NGS and analyzed (step C, Figure 1).
Results
Selecting the Right TdT Variant
Many emerging applications of DNA (e.g., synthetic biology, DNA nanotechnology, and DNA information storage)35−38 require long de novo synthesis of sequences that are beyond the reach of chemistry and have no natural template.39 As such, enzymatic DNA synthesis with TdT has been intensely studied as a possible solution to long, template-free DNA synthesis.40−42 Examining this corpus led us to settle on TdTevo for our studies (hereafter referred to as TdT), as it had been optimized to have high activity and thermostability.43 Expression and testing of TdT and its fusion variants can be found in the Supporting Information.
Induced Proximity Can Control TdT Activity and Selectivity
The DELSTAR design relies on the possibility that induced proximity could control the activity and selectivity of TdT, which has never been shown. To study this, we designed a model system where DNA duplex formation (instead of protein/ligand interactions) would induce proximity between TdT and a DNA 3′-end; the advantage of DNA duplex formation is that binding affinity can be systematically controlled. We expressed TdT as an N-terminal fusion with SNAP-tag44 (hereafter SNAP–TdT) and coupled it to an oligonucleotide with a 5′-benzylguanine (Figure 2A) to create the DNA/protein chimaera SNAPDNA–TdT. Notably, the 3′-end of this oligonucleotide contains a 3′-deoxy base to prevent intramolecular TdT extension. We then examined the polyA-tailing of fluorescently labeled oligos that either matched (FAM) or mismatched (A590) to SNAPDNA–TdT. While control reactions with SNAP–TdT were extended with equal efficiency in both FAM and A590 (lanes 4–6), the matched reaction in a mixture of FAM and A590 showed a strong preference for extending the matched FAM oligo (lane 3, Figure 2A). Figure 2B demonstrates that this activation through induced proximity depends on the strength of the binding interaction. Specifically, we modified the FAM oligo to have varying affinities to the SNAPDNA–TdT, and indeed, we observe a correlation between binding strength and polyA tail length as we proceed from subnanomolar to micromolar affinity (compare lanes 2–6, Figure 2B).
Figure 2.
Induced proximity can control the activity and selectivity of TdT (A). TdT fused with a SNAP-tag (SNAP–TdT) is coupled to a short DNA with a 5′-benzylguanine and a 3′-deoxy end (see Supporting Information for complete structure of DNA) to create SNAPDNA–TdT; the proficiency of this chimeric fusion in creating 3′-extensions is then tested with fluorescently labeled oligonucleotides which are either matched (FAM) or mismatched (A590); lane 3 is the test condition and shows that the matched DNA is extended by TdT more efficiently than the unmatched. (B) Comparison of background activity of SNAP–TdT and SNAPDNA–TdT: lanes 4–6 in (A) show that SNAP–TdT has a substantial background extension; this is further confirmed by lane 3 in (B). A version of SNAPDNA–TdT, which is mismatched to the FAM oligonucleotide (SNAPmmDNA–TdT) shows a dramatically reduced background (lane 4). (C) The binding affinity of the FAM oligonucleotide is systematically varied through base-pairing rules and mismatches (calculated by the nearest neighbor method) to test whether affinity correlates to 3′-extension activity; comparing lanes 2–6 shows that as binding strength increases, 3′-extension also increases.
In the control lanes of Figure 2A (lanes 4–6) and Figure S3 (lane 7), we were troubled by the relatively high levels of untemplated extension with SNAP–TdT, particularly since it did not seem to jibe with the high discrimination we saw in the direct match versus mismatch experiment (lane 3, Figure 2A). We hypothesized that the covalently linked DNA in SNAPDNA–TdT might be saturating the native DNA-binding capacity of TdT, acting as a competitive inhibitor and accentuating the importance of induced proximity. To test this, we synthesized a version of the TdT where the attached DNA was mismatched with FAM (SNAPmmDNA–TdT) and compared the polyA-tailing ability of all three enzymes (Figure 2C). As predicted, the untemplated background activity of SNAPmmDNA–TdT was strongly attenuated, suggesting that in real DEL selections, we would need to saturate native DNA binding of TdT. In a typical DEL selection, a large amount of sheared salmon sperm DNA (sssDNA) is included to saturate any inherent DNA binding of target proteins—in our case, this should simultaneously serve to saturate DNA-binding of TdT. As seen in Figure 2C, this is indeed the case (lane 7), and the extension length increases with the binding affinity (lane 1–6). The study outlined in Figure 2 establishes for the first time that induced proximity can control the activity of TdT.
PolyA-Tailing Facilitates Highly Efficient Enrichment
PolyA-tailing leaves a molecular record of binding, and the next step was to enrich based on this tail. Selective PCR with a partially T-bearing primer sometimes showed enrichment but was unreliable. In contrast, poly(dT)25 purification showed highly specific discrimination between the extended and unextended DNAs. Specifically, if we take a mixture of FAM (matched to SNAPDNA–TdT) and A590 (mismatched to SNAPDNA–TdT) oligonucleotides and then incubate with the enzyme and dATP for 30 min, we see that FAM is selectively extended (lane 3, Figure 3A). Affinity enrichment with poly(dT)25 beads then delivers A590 in the flow-through and highly enriched FAM in the elution (compare lanes 4 and 5, Figure 3A). As our intention in DEL selections was to test both single- and double-stranded DELs, we performed an additional study to examine the impact of single-stranded versus blunt ends in the extension and enrichment (Figure 3B). Both single-stranded DNA (ssDNA) and double-stranded DNA (dsDNA) give pure extended product after enrichment (lanes 2 and 5, Figure 3B). However, the amount of recovered DNA is higher in the ssDNA case, consistent with the preference of TdT for unpaired 3′-ends.6 Despite the low absolute yield, qPCR measurements (Figure 3B) demonstrate that enrichment of the matched oligonucleotide is clearly observed in both ssDNA and dsDNA formats.
Figure 3.
(A) PolyA tailing with TdT is selective and can be purified with Poly(dT)25 beads. (B) The polyA tailing occurs on both ssDNA and dsDNA but is more efficient on ssDNA (compare lanes 2 and 5); qPCR measurement of DNA after enrichment in the templated (red bars) versus untemplated (blue bars) reactions show similar enrichment values for ssDNA and dsDNA. The error bars represent the standard error of mean across triplicates.
Sensitivity of PolyA-Tailing to Detect Small Molecule Binding in Mixtures
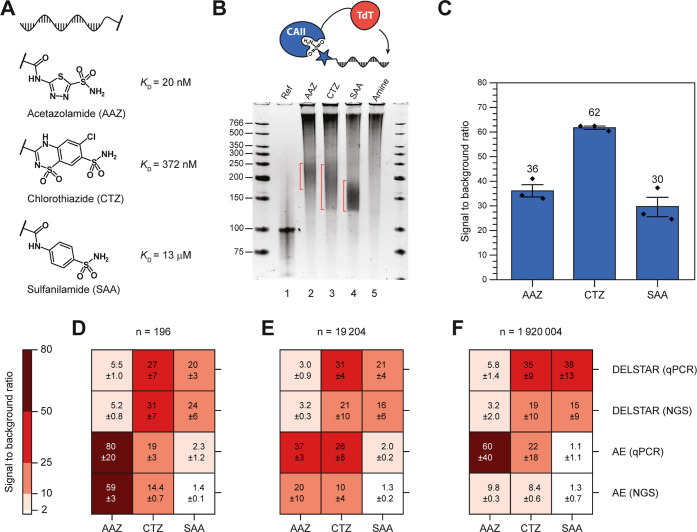
The concentration of each individual library member in a DEL decreases in an inverse relationship with library size. To understand the detection sensitivity of DELSTAR to libraries of different sizes, we looked at different copy numbers in a simulated library. We chose carbonic anhydrase II (CAII) as a model protein because binders45,46 spanning several orders of magnitude in binding efficiency are available, which we could use for benchmarking the TdT system. Hence, we synthesized DNA-linked versions of a series of three CAII binders [acetazolamide (AAZ), chlorothiazide (CTZ), and sulfanilamide (SAA)] spanning dissociation constants of double-digit nanomolar to double-digit micromolar (Figure 4A).
Figure 4.
Enrichments in a model library and the effect of library size. (A) Structures of CAII binders as DNA conjugates and the binding affinities of their parent molecules. (B) PolyA tailing using a CAII–TdT fusion gives a positive correlation between binding strength and tail length, while the non-binding control shows no tailing. Smear in all lanes comes from the background elongation of sssDNA. (C) qPCR data show strong enrichment of binders relative to non-binding control. The error bars represent the standard error of mean across triplicates. (D) NGS and qPCR data for a simulated library size of 196 compounds with known binders AAZ, CTZ, and SAA compared with AE or DELSTAR. Uncertainties are given as the 90% confidence interval from triplicates. (E) Same as (D), except the simulated library size is 19,204 compounds. (F) Same as (D), except simulated library size is 1,920,004 compounds.
Consistent with our earlier DNA models, exposing these molecules to a CAII–TdT fusion led to polyadenylation, where the tail length correlated to the measured binding affinity of the small molecules (Figure 4A). In this experiment, we used an excess of sssDNA to prevent background polyadenylation. While this excess DNA leads to smearing in each of the test lanes (lanes 2–5, Figure 4A), it succeeds in completely suppressing the background polyadenylation of a control DNA that has no CAII binder attached (lane 5). Magnetic (dT)25 bead purification and qPCR quantification showed that each binder has strong enrichment relative to the non-binding control (30–60-fold enrichment). We next took these binders and created a mock DEL of various sizes (102, 104, and 106) by diluting them into single-stranded DNA. We then performed selections using classical affinity enrichment (AE) and DELSTAR and measured the outcomes with both qPCR and NGS. As the heat maps in Figure 4 show, AE is good at identifying the tightest binders (AAZ & CTZ) in both NGS and qPCR when the simulated library sizes are small (Figure 4B,C). The micromolar binder SAA is, however, indistinguishable from noise with AE at any simulated library size in both qPCR and NGS. In contrast, DELSTAR can robustly identify all binders at any simulated library size tested. A strange observation with the DELSTAR technique in these experiments is that while AAZ is the tightest binder and gives the longest polyA tails (Figure 4B), it gives the weakest signal among the bona fide binders in the simulated library (Figure 4C–F). We suspected that the long polyA tail might be interfering with the elution, but changing the elution conditions by adding polyA DNA during the elution (to saturate the beads) or polyT-DNA (to saturate the tail) did not lead to any change (Figure S5A), nor did running the qPCR directly with the beads. Furthermore, reducing the concentration of dATP to shorten the overall tail lengths also did not improve the performance of AAZ (Figure S5B). Irrespective of the reason, since we saw no loss in enrichment signal across all simulated library sizes, we saw no cause for concern. These experiments convinced us that DELSTAR was robust enough to proceed with testing in a proper DEL.
Synthesis and Selection of a DEL in Both ssDNA and dsDNA Formats
We next tested DELSTAR in a DEL selection using both ss- and ds-DELs. Testing both types in otherwise identical libraries was important to assess the best DNA format for DELSTAR. Trichlorotriazine (a.k.a cyanuric chloride, TCT) is a common DEL hub scaffold, allowing selective consecutive substitution of each of the three chlorides with increasingly harsh reaction conditions. In a pioneering study, TCT substitution enabled the creation of huge three-cycle DEL libraries based on nucleophilic substitution (SNAr).47 Chemically, however, TCT can be diversified in other ways, including cross-coupling48,49 or azide–alkyne cycloaddition. Hence prior to library construction, we vetted some common DEL reactions on the TCT scaffold. Our scouting experiments suggested Suzuki–Miyaura cross-coupling (70 elements), CuAAC (666 elements), and nucleophilic substitution (282 elements) as excellent reactions; these also had the advantage of having diverse building block collections available.
We started library construction with pre-encoded amine-modified DNA to save biochemical steps. We modified each individual DNA with TCT and then ran the SNAr at optimal conditions for each nucleophile type: phenols and secondary amines were attached at 4 °C, primary amines at 20 °C, and anilines at 40 °C. After purification and pooling, we split the resulting library, DEL_1, into three aliquots, each aliquot destined for a different second-cycle chemistry—SNAr, Suzuki–Miyaura, and CuAAC. The SNAr sublibrary DEL_2c was first encoded (to prevent impaired ligation efficiency from residual reagents) and then diversified with a selection of phenols, amines, and anilines (282 in total). For the Suzuki–Miyaura sublibrary DEL_2a, encoding was also performed first, followed by diversification with 70 boronates. The CuAAC sublibrary DEL_2b proved to be more challenging, primarily because direct introduction of the azide failed. In the end, a workaround was found where we converted the library first to a quaternary ammonium salt with DABCO before substituting this activated system with sodium azide. The final cycloaddition with alkynes was possible using our standard conditions.50 As click reactions are known to induce DEL damage,51,52 only in this diversification did we encode after the chemical reaction. After pooling and encoding each sub-library, we then pooled the entire collection to yield ssDEL and dsDEL (Figure 5), each comprising 195,456 members. Importantly, each sublibrary contained several known ligands for CAII (see Figure 5b), allowing for benchmarking of the DEL selections.
Figure 5.

Construction of a chemically identical ss- and dsDEL. (a) SNAr of 5′-amine DNA with TCT followed by a second SNAr to install diversity element 1. (b) Diversification of dp2 by Suzuki–Miyaura cross-coupling. (c) 1. SNAr of dp2 with DABCO and NaN3, 2. CuAAC. (d) Diversification of dp2 by SNAr; (b–d) diversity elements were encoded by splint ligation. Before pooling of the three sublibraries, it was encoded for the type of reaction performed in the dp2 diversification either by splint ligation (e) ssDEL or by Klenow extension (f) dsDEL.
Using ssDEL and dsDEL synthesized in Figure 5, we performed both DELSTAR and classical AE against CAII. The 2D plots shown in Figure 6A examine the enrichment values relative to TdT selection alone. Some immediate conclusions can be drawn from the plot: first, it seems that DELSTAR with ssDEL is superior to all others in identifying the known binders in the library. This conclusion can be drawn from the quality (signal-to-noise) and completeness (all binders from nM to μM affinity identified) of the features in the top left plot (Figure 6A). While AE is excellent at identifying the strongest binder in both ssDEL and dsDEL, many expected features are missing or faint. In contrast, although the noise in DELSTAR with dsDEL is higher than that in ssDEL, many expected binders are seen (Figure 6A, lower panel). We performed a second series of selections and sequencing where we varied protein amounts and incubation time (Figure 6C–E), comparing the results by looking at histograms of enrichment numbers for different chemotypes. TdT selection with ssDEL still delivers similar enrichment whether 1 pmol (top panel Figure 6D) or 0.5 pmol (top panel Figure 6E) of CAII–TdT fusion is employed. The only noticeable change when less protein is used is that acetazolamide represents a greater overall percentage of the enriched hits. Another noteworthy finding is that increasing the incubation time from 30 to 60 min with dsDEL significantly increases the counts of known binders of CAII (compare lower panels of Figure 6D,E). Two new unexpected features also appeared in DELSTAR, dp2b-470 and dp1-101 (Figure 6B). Although the hydroxymethyl sulfonamide (dp2b-470, Figure 6B) was unknown to us as a CAII binder, a literature search revealed that this chemotype has already been biochemically and structurally characterized as a CAII inhibitor.53,54 The furan was unknown as a CAII binder, so we resynthesized off-DNA and found no binding—a puzzling observation as this feature was robustly observed across independent selections using DELSTAR (although not seen with AE). The normalization condition was selection against TdT alone, and this should exclude unselective protein binders; we nevertheless performed an additional selection with the completely unrelated fusion SNAP-TdT, which also led to no enrichment of this feature, ruling out unselective protein binding (see Figure S1, panel G and H), unless it is a feature unique to the CAII–TdT fusion. These experiments suggest a false positive.
Figure 6.
Selection results of ssDEL and dsDEL. (A) In the ssDEL (top panels), DELSTAR reveals a more complete binding picture than that of AE. DELSTAR performs similar to AE in the dsDEL (bottom), although acetazolamide is less prominent. The shaded area differentiates the three sublibraries. (B) Expected binders in the DEL (blue and red) and putative binders identified by DELSTAR (black). (C) Log-fold distributions in affinity enrichment for ssDEL (top) and dsDEL (bottom) of all retrieved library members [phenylsulfonamides (red), acetazolamides (purple), or all others (gray)]. The shaded area contains binders below 3 times the standard deviation and were not used for the scatter plot in panel (A). (D) DELSTAR performs better with ssDEL than with dsDEL at an equivalent incubation time (30 min). (E) Lowering the amount of protein (top panel) has little effect on DELSTAR results, while increasing incubation time to 60 min with dsDEL (bottom panel) improves the enrichment values of known binders [cf. with bottom panel in (D)]. (F) Counting the unique molecular identifier (UMI) shows that the DELSTAR signal is derived from 10-fold more individual binders than AE (top panel); this is also seen when counting the number of individual molecules with >10 copies (bottom panel). The shaded area in the top panel and the error bars in the bottom panel indicate the standard error of mean across three selections.
Aside from analyzing binders, other aspects of the NGS data merit mentioning. It is common practice in DELs (or any NGS experiment) to include a random nucleotide region in the DNA known as a unique molecular identifier (UMI) to differentiate individual molecules from copies generated during the PCR. We compared the UMIs between affinity enrichment and DELSTAR to see which assay led to the identification of more individual molecules. Comparing the fraction of reads of a specific DEL member that come from repetitive reads (we arbitrarily compare at >10 reads/UMI for visualization in Figure 6F) of a single UMI reveals a striking difference: AE comprises many more repetitive copies of UMIs than DELSTAR. This suggests that in AE, fewer individual molecules are retained and contribute to the overall signal as for DELSTAR. This has the consequences of a faster drop-off in signal for moderate binders and a greater probability of spurious effects for affinity enrichment.
Discussion
The affinity selection step is one of the most frustrating and variable steps in a DEL screen. Protein must be produced in large quantities and immobilized, protein loading on beads must be quantified, and the activity of the protein on the bead must be validated. Beads can be hydrophilic or hydrophobic, and there are no rules to determine which is best for a given protein. The immobilization strategy (for example, avi-tag or his-tag or unselective biotinylation) can have an impact on selection results, also in ways that are unpredictable. We have described a system that is solution-phase, uses tiny amounts of protein and DEL compared to those in conventional screens, and is backward compatible with most DELs (provided a 3′-extension can be integrated into blunt-ended DELs). Importantly, DELSTAR leaves a stable molecular record of binding events that can be used for selection. As such, DELSTAR reports on occupancy of the target protein to the DEL, rather than slow kinetic off-rates (the sole selection criterion for normal affinity selections). DELSTAR still involves a magnetic purification step with beads, but the beads are always the same irrespective of the target protein, which should facilitate standardization and automation of the selection conditions.
The low levels of noise, coupled with information on moderate affinity binders, opens new doors for using selection data to understand the chemistry of library construction. For example, looking at the plot in the top left of Figure 6A, we see that dp1-43 is strongly represented in the Suzuki–Miyaura (region a in the plots of Figure 6A) and SNAr sublibraries (region c in Figure 6A plots) but only poorly seen in the CuAAC sublibrary (region b in Figure 6A plots). This was striking to us as it perfectly reflected some observations we made during the reaction rehearsal phase prior to full DEL synthesis: we noticed that azide substitution in preparation for CuAAC caused some displacement of electron-deficient phenol-linked library members, i.e., double-azide substitutions were sometimes observed (Figure S6). Excluding those dp1 building blocks mitigated this in the testing phase, but the sequencing data suggests partial double-substitution likely still happened with other phenols during azide substitution (dp1-43 might be particularly susceptible since it too is electron-deficient). The related library member dp1-83, which bears an aniline at dp1, cannot be substituted by azide and, as a result, is represented strongly in the selection data across all three sublibraries. By looking at the AE data on the right side of Figure 6A, it is clear that such detailed comparative analyses of library construction chemistry would be impossible.
We observed an affinity-dependent polyA tail length in our model systems (see Figures 2B and 4B), but in the DEL selection presented, we have not yet capitalized on this. There are many ways DELSTAR could be expanded going forward, for example, a size selection prior to sequencing [rather than (dT)25 beads] could allow separate analysis of binders within different affinity regimes.
The field of encoded libraries has enjoyed an incredible expansion over the past decade, but low hit rates, substantial false positives, and unreliable SAR data demand further improvement in the technique. Some of the difficulties in DEL selection can be attributed to the fact that it is often employed as a last resort when other approaches have failed. However, the reality is that for the technique to excel in early drug discovery, it must perform well in the untilled soil of intractable targets. A large contributor to selection noise is the affinity selection protocol itself, which is different for every target and highly sensitive to experimental conditions. Another consideration is that affinity selection is a kinetic selection technique (selecting for slow koff under the wash conditions), while equilibrium binding information would give a more comprehensive picture of binding events. DELSTAR has the potential to overcome many of the open challenges in DEL selection. Here, we have introduced the concept and demonstrated the proof of principle on a known target with a purpose-built DEL. The next step will be to perform standardized side-by-side comparisons across many targets with different libraries. Since DELSTAR is cross- and backward-compatible with most types of DEL collections, it should be relatively simple for owners of DELs to test and compare DELSTAR against their own preferred selection conditions.
Methods
DELSTAR
In a typical selection with DELSTAR, a DEL (1 μL, 1 μM) is prepared in a solution with sssDNA (2 μL, 1 mg/mL), 10× assay buffer (20 μL, containing 500 mM KOAc, 200 mM tris acetate, 200 mM MgOAc2, pH 7.9), dATP (2 μL, 20 mM), and water (174 μL). In the end, protein (1 μL, 1 μM) is added to give a final volume of 200 μL. The reaction is incubated for 30 min (ssDEL) or 60 min (dsDEL) at 37 °C and inactivated for 10 min at 98 °C. Per 1 pmol of library, 5 μL of dT25 magnetic beads (NEB, S1419) are washed with a pull-down buffer (20 μL, 20 mM tris, 1 mM EDTA, 0.01 vol % Tween 20, pH 7.5) and subsequently added to the selection volume followed by Tween 20 (2 μL, 1 vol %). The sample was incubated for 30 min at room temperature, and the beads were washed with the pull-down buffer using a magnetic rack. For elution, the beads were resuspended in 20 μL of the pull-down buffer and heated to 98 °C for 10 min and quickly put on a magnetic rack to get the supernatant. The supernatant was subsequently used in PCR and sequenced (Illumina).
Acknowledgments
We would like to thank Timothy Sharpe for his help in measuring protein stabilities. Differential scanning fluorimetry measurements were performed at the Biophysics Facility at the Biozentrum at University of Basel. Calculations were performed at sciCORE (http://scicore.unibas.ch/) scientific computing center at the University of Basel. Funding from the Swiss National Science Foundation (SNF grants: 200020_185089 & 200020_215758) is gratefully acknowledged.
Data Availability Statement
Raw sequencing data is available on request. The code for enumerating the library and counting the codons is available on GitHub (https://github.com/Gillingham-Lab/DECL-Gen).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c05961.
Methods, characterization data, synthesis procedures of compounds, description of the data analysis pipeline, and complete list of all building blocks and DNA strands used for the DNA-encoded libraries (PDF)
The authors declare the following competing financial interest(s): A patent application has been filed to cover uses associated with the technology described in this manuscript.
Supplementary Material
References
- Gironda-Martínez A.; Donckele E. J.; Samain F.; Neri D. DNA-Encoded Chemical Libraries: A Comprehensive Review with Successful Stories and Future Challenges. ACS Pharmacol. Transl. Sci. 2021, 4, 1265–1279. 10.1021/acsptsci.1c00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favalli N.; Bassi G.; Scheuermann J.; Neri D. DNA-encoded chemical libraries – achievements and remaining challenges. FEBS Lett. 2018, 592, 2168–2180. 10.1002/1873-3468.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri D.; Lerner R. A. DNA-Encoded Chemical Libraries: A Selection System Based on Endowing Organic Compounds with Amplifiable Information. Annu. Rev. Biochem. 2018, 87, 479–502. 10.1146/annurev-biochem-062917-012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G.; Neri D. DNA-encoded chemical libraries: foundations and applications in lead discovery. Drug Discovery Today 2016, 21, 1828–1834. 10.1016/j.drudis.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daguer J. P.; Zambaldo C.; Ciobanu M.; Morieux P.; Barluenga S.; Winssinger N. DNA display of fragment pairs as a tool for the discovery of novel biologically active small molecules. Chem. Sci. 2015, 6, 739–744. 10.1039/c4sc01654h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J. D.; Suo Z. Biochemical, Structural, and Physiological Characterization of Terminal Deoxynucleotidyl Transferase. Chem. Rev. 2006, 106, 2092–2110. 10.1021/cr040445w. [DOI] [PubMed] [Google Scholar]
- Brenner S.; Lerner R. A. Encoded combinatorial chemistry. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 5381–5383. 10.1073/pnas.89.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannocci L.; Zhang Y.; Scheuermann J.; Leimbacher M.; De Bellis G.; Rizzi E.; Dumelin C.; Melkko S.; Neri D. High-throughput sequencing allows the identification of binding molecules isolated from DNA-encoded chemical libraries. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 17670–17675. 10.1073/pnas.0805130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkko S.; Scheuermann J.; Dumelin C. E.; Neri D. Encoded self-assembling chemical libraries. Nat. Biotechnol. 2004, 22, 568–574. 10.1038/nbt961. [DOI] [PubMed] [Google Scholar]
- Halpin D. R.; Harbury P. B. DNA Display I. Sequence-Encoded Routing of DNA Populations. PLoS Biol. 2004, 2, e173 10.1371/journal.pbio.0020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner Z. J.; Tse B. N.; Grubina R.; Doyon J. B.; Snyder T. M.; Liu D. R. DNA-Templated Organic Synthesis and Selection of a Library of Macrocycles. Science 2004, 305, 1601–1605. 10.1126/science.1102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Liu D. R. DNA-templated organic synthesis: Nature’s strategy for controlling chemical reactivity applied to synthetic molecules. Angew. Chem., Int. Ed. 2004, 43, 4848–4870. 10.1002/anie.200400656. [DOI] [PubMed] [Google Scholar]
- Gartner Z. J.; Liu D. R. The Generality of DNA-Templated Synthesis as a Basis for Evolving Non-Natural Small Molecules. J. Am. Chem. Soc. 2001, 123, 6961–6963. 10.1021/ja015873n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaene F.; Mejias L.; Harris J. L.; Winssinger N. Synthesis of a PNA-encoded cysteine protease inhibitor library. Tetrahedron 2004, 60, 8677–8690. 10.1016/j.tet.2004.05.107. [DOI] [Google Scholar]
- Harris J.; Mason D. E.; Li J.; Burdick K. W.; Backes B. J.; Chen T.; Shipway A.; Van Heeke G.; Gough L.; Ghaemmaghami A.; et al. Activity Profile of Dust Mite Allergen Extract Using Substrate Libraries and Functional Proteomic Microarrays. Chem. Biol. 2004, 11, 1361–1372. 10.1016/j.chembiol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Satz A. L.; Cai J.; Chen Y.; Goodnow R.; Gruber F.; Kowalczyk A.; Petersen A.; Naderi-Oboodi G.; Orzechowski L.; Strebel Q. DNA Compatible Multistep Synthesis and Applications to DNA Encoded Libraries. Bioconjugate Chem. 2015, 26, 1623–1632. 10.1021/acs.bioconjchem.5b00239. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Wu Y.-r.; Yu J.-q.; Zhang W.-n.; Zhuang C.-l. DNA-encoded libraries (DELs): a review of on-DNA chemistries and their output. RSC Adv. 2021, 11, 2359–2376. 10.1039/d0ra09889b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.; Li X. Evolution of the Selection Methods of DNA-Encoded Chemical Libraries. Acc. Chem. Res. 2021, 54, 3491–3503. 10.1021/acs.accounts.1c00375. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Meng L.; Nie Q.; Zhou Y.; Chen L.; Yang S.; Fung Y. M. E.; Li X.; Huang C.; Cao Y.; et al. Selection of DNA-encoded chemical libraries against endogenous membrane proteins on live cells. Nat. Chem. 2021, 13, 77–88. 10.1038/s41557-020-00605-x. [DOI] [PubMed] [Google Scholar]
- Oehler S.; Catalano M.; Scapozza I.; Bigatti M.; Bassi G.; Favalli N.; Mortensen M. R.; Samain F.; Scheuermann J.; Neri D. Affinity Selections of DNA-Encoded Chemical Libraries on Carbonic Anhydrase IX-Expressing Tumor Cells Reveal a Dependence on Ligand Valence. Chem.—Eur. J. 2021, 27, 8985–8993. 10.1002/chem.202100816. [DOI] [PubMed] [Google Scholar]
- Bassi G.; Favalli N.; Oehler S.; Martinelli A.; Catalano M.; Scheuermann J.; Neri D. Comparative evaluation of DNA-encoded chemical selections performed using DNA in single-stranded or double-stranded format. Biochem. Biophys. Res. Commun. 2020, 533, 223–229. 10.1016/j.bbrc.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino A.; Gironda-Martínez A.; Gorre É. M. D.; Prati L.; Piazzi J.; Scheuermann J.; Neri D.; Donckele E. J.; Samain F. Critical Evaluation of Photo-cross-linking Parameters for the Implementation of Efficient DNA-Encoded Chemical Library Selections. ACS Comb. Sci. 2020, 22, 204–212. 10.1021/acscombsci.0c00023. [DOI] [PubMed] [Google Scholar]
- Wichert M.; Krall N.; Decurtins W.; Franzini R. M.; Pretto F.; Schneider P.; Neri D.; Scheuermann J. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat. Chem. 2015, 7, 241–249. 10.1038/nchem.2158. [DOI] [PubMed] [Google Scholar]
- Vummidi B. R.; Farrera-Soler L.; Daguer J. P.; Dockerill M.; Barluenga S.; Winssinger N. A mating mechanism to generate diversity for the Darwinian selection of DNA-encoded synthetic molecules. Nat. Chem. 2022, 14, 141–152. 10.1038/s41557-021-00829-5. [DOI] [PubMed] [Google Scholar]
- Cai B.; Kim D.; Akhand S.; Sun Y.; Cassell R. J.; Alpsoy A.; Dykhuizen E. C.; Van Rijn R. M.; Wendt M. K.; Krusemark C. J. Selection of DNA-Encoded Libraries to Protein Targets within and on Living Cells. J. Am. Chem. Soc. 2019, 141, 17057–17061. 10.1021/jacs.9b08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L. K.; Christensen A. B.; Andersen J.; Folkesson C. G.; Kristensen O.; Andersen C.; Alzu A.; Sløk F. A.; Blakskjær P.; Madsen D.; et al. Screening of DNA-encoded small molecule libraries inside a living cell. J. Am. Chem. Soc. 2021, 143, 2751–2756. 10.1021/jacs.0c09213. [DOI] [PubMed] [Google Scholar]
- Cai B.; Krusemark C. J. Multiplexed Small-Molecule-Ligand Binding Assays by Affinity Labeling and DNA Sequence Analysis. Angew. Chem., Int. Ed. 2022, 61, e202113515 10.1002/anie.202113515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane W. G.; Malone M. L.; Dang V. Q.; Cavett V.; Satz A. L.; Paegel B. M. Activity-Based DNA-Encoded Library Screening. ACS Comb. Sci. 2019, 21, 425–435. 10.1021/acscombsci.9b00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes K. R.; Malone M. L.; Ndungu J. M.; Suponitsky-Kroyter I.; Cavett V. J.; McEnaney P. J.; MacConnell A. B.; Doran T. M.; Ronacher K.; Stanley K.; et al. High-throughput Identification of DNA-Encoded IgG Ligands that Distinguish Active and Latent Mycobacterium tuberculosis Infections. ACS Chem. Biol. 2017, 12, 234–243. 10.1021/acschembio.6b00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Li Y.; Li X. Strategies for developing DNA-encoded libraries beyond binding assays. Nat. Chem. 2022, 14, 129–140. 10.1038/s41557-021-00877-x. [DOI] [PubMed] [Google Scholar]
- Kodadek T.; Paciaroni N. G.; Balzarini M.; Dickson P. Beyond protein binding: recent advances in screening DNA-encoded libraries. Chem. Commun. 2019, 55, 13330–13341. 10.1039/c9cc06256d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. I.; McGregor L. M.; Jain T.; Liu D. R. Discovery of a Covalent Kinase Inhibitor from a DNA-Encoded Small-Molecule Library × Protein Library Selection. J. Am. Chem. Soc. 2017, 139, 10192–10195. 10.1021/jacs.7b04880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor L. M.; Jain T.; Liu D. R. Identification of Ligand–Target Pairs from Combined Libraries of Small Molecules and Unpurified Protein Targets in Cell Lysates. J. Am. Chem. Soc. 2014, 136, 3264–3270. 10.1021/ja412934t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B.; Mhetre A. B.; Krusemark C. J. Selection methods for proximity-dependent enrichment of ligands from DNA-encoded libraries using enzymatic fusion proteins. Chem. Sci. 2023, 14, 245–250. 10.1039/d2sc05495g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuri S.; Church G. M. Large-scale de novo DNA synthesis: technologies and applications. Nat. Methods 2014, 11, 499–507. 10.1038/nmeth.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N.; Bertone P.; Chen S.; Dessimoz C.; LeProust E. M.; Sipos B.; Birney E. Towards practical, high-capacity, low-maintenance information storage in synthesized DNA. Nature 2013, 494, 77–80. 10.1038/nature11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M.; Gao Y.; Kosuri S. Next-Generation Digital Information Storage in DNA. Science 2012, 337, 1628. 10.1126/science.1226355. [DOI] [PubMed] [Google Scholar]
- Jensen M. A.; Davis R. W. Template-Independent Enzymatic Oligonucleotide Synthesis (TiEOS): Its History, Prospects, and Challenges. Biochemistry 2018, 57, 1821–1832. 10.1021/acs.biochem.7b00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M. J.; Winkler S.; Hughes S. J.; Whitworth C.; Galant M.; Farnaby W.; Rumpel K.; Ciulli A. SPR-Measured Dissociation Kinetics of PROTAC Ternary Complexes Influence Target Degradation Rate. ACS Chem. Biol. 2019, 14, 361–368. 10.1021/acschembio.9b00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palluk S.; Arlow D. H.; de Rond T.; Barthel S.; Kang J. S.; Bector R.; Baghdassarian H. M.; Truong A. N.; Kim P. W.; Singh A. K.; et al. De novo DNA synthesis using polymerase-nucleotide conjugates. Nat. Biotechnol. 2018, 36, 645–650. 10.1038/nbt.4173. [DOI] [PubMed] [Google Scholar]
- Lu X.; Li J.; Li C.; Lou Q.; Peng K.; Cai B.; Liu Y.; Yao Y.; Lu L.; Tian Z.; et al. Enzymatic DNA Synthesis by Engineering Terminal Deoxynucleotidyl Transferase. ACS Catal. 2022, 12, 2988–2997. 10.1021/acscatal.1c04879. [DOI] [Google Scholar]
- Chua J. P. S.; Go M. K.; Osothprarop T.; Mcdonald S.; Karabadzhak A. G.; Yew W. S.; Peisajovich S.; Nirantar S. Evolving a Thermostable Terminal Deoxynucleotidyl Transferase. ACS Synth. Biol. 2020, 9, 1725–1735. 10.1021/acssynbio.0c00078. [DOI] [PubMed] [Google Scholar]
- Barthel S.; Palluk S.; Hillson N. J.; Keasling J. D.; Arlow D. H. Enhancing Terminal Deoxynucleotidyl Transferase Activity on Substrates with 3′ Terminal Structures for Enzymatic De Novo DNA Synthesis. Genes 2020, 11, 102. 10.3390/genes11010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier A.; Juillerat A.; Heinis C.; Corrêa I. R.; Kindermann M.; Beaufils F.; Johnsson K. An Engineered Protein Tag for Multiprotein Labeling in Living Cells. Chem. Biol. 2008, 15, 128–136. 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Iyer R.; Barrese III A. A.; Parakh S.; Parker C. N.; Tripp B. C. Inhibition Profiling of Human Carbonic Anhydrase II by High-Throughput Screening of Structurally Diverse, Biologically Active Compounds. SLAS Discovery 2006, 11, 782–791. 10.1177/1087057106289403. [DOI] [PubMed] [Google Scholar]
- Taylor P. W.; King R. W.; Burgen A. S. V. Kinetics of complex formation between human carbonic anhydrases and aromatic sulfonamides. Biochemistry 1970, 9, 2638–2645. 10.1021/bi00815a012. [DOI] [PubMed] [Google Scholar]
- Clark M. A.; Acharya R. A.; Arico-Muendel C. C.; Belyanskaya S. L.; Benjamin D. R.; Carlson N. R.; Centrella P. A.; Chiu C. H.; Creaser S. P.; Cuozzo J. W.; et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol. 2009, 5, 647–654. 10.1038/nchembio.211. [DOI] [PubMed] [Google Scholar]
- Xu H.; Ma F.; Wang N.; Hou W.; Xiong H.; Lu F.; Li J.; Wang S.; Ma P.; Yang G.; et al. DNA-Encoded Libraries: Aryl Fluorosulfonates as Versatile Electrophiles Enabling Facile On-DNA Suzuki, Sonogashira, and Buchwald Reactions. Advanced Science 2019, 6, 1901551. 10.1002/advs.201901551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favalli N.; Bassi G.; Zanetti T.; Scheuermann J.; Neri D. Screening of Three Transition Metal-Mediated Reactions Compatible with DNA-Encoded Chemical Libraries. Helv. Chim. Acta 2019, 102, e1900033 10.1002/hlca.201900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stress C. J.; Sauter B.; Schneider L. A.; Sharpe T.; Gillingham D. A DNA-Encoded Chemical Library Incorporating Elements of Natural Macrocycles. Angew. Chem., Int. Ed. 2019, 58, 9570–9574. 10.1002/anie.201902513. [DOI] [PubMed] [Google Scholar]
- Abel G. R.; Calabrese Z. A.; Ayco J.; Hein J. E.; Ye T. Measuring and Suppressing the Oxidative Damage to DNA During Cu(I)-Catalyzed Azide–Alkyne Cycloaddition. Bioconjugate Chem. 2016, 27, 698–704. 10.1021/acs.bioconjchem.5b00665. [DOI] [PubMed] [Google Scholar]
- Sauter B.; Schneider L.; Stress C.; Gillingham D. An assessment of the mutational load caused by various reactions used in DNA encoded libraries. Bioorg. Med. Chem. 2021, 52, 116508. 10.1016/j.bmc.2021.116508. [DOI] [PubMed] [Google Scholar]
- Di Fiore A.; Maresca A.; Alterio V.; Supuran C. T.; De Simone G. Carbonic anhydrase inhibitors: X-ray crystallographic studies for the binding of N-substituted benzenesulfonamides to human isoform II. Chem. Commun. 2011, 47, 11636–11638. 10.1039/c1cc14575d. [DOI] [PubMed] [Google Scholar]
- Briganti F.; Pierattelli R.; Scozzafava A.; Supuran C. T. Carbonic anhydrase inhibitors. Part 37. Novel classes of isozyme I and II inhibitors and their mechanism of action. Kinetic and spectroscopic investigations on native and cobalt-substituted enzymes. Eur. J. Med. Chem. 1996, 31, 1001–1010. 10.1016/s0223-5234(97)86179-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data is available on request. The code for enumerating the library and counting the codons is available on GitHub (https://github.com/Gillingham-Lab/DECL-Gen).