Abstract
The pharmacokinetics, absolute bioavailability, accumulation, and tolerability over 8 days of an oral formulation of foscarnet (90 mg/kg of body weight once daily [QD] [n = 6], 90 mg/kg twice daily [BID] [n = 6], and 180 mg/kg QD [n = 3]) were investigated in 15 asymptomatic, human immunodeficiency virus-seropositive male patients free of active cytomegalovirus infection and with normal upper gastrointestinal function. Peak plasma drug concentrations were (mean ± standard deviation) 46.4 ± 10.8 μM (90 mg/kg QD), 45.7 ± 6.9 μM (90 mg/kg BID), and 64.9 ± 31.7 μM (180 mg/kg QD) on day 1 and rose to 86.2 ± 35.8, 78.7 ± 35.2, and 86.4 ± 25.0 μM, respectively, on day 8. The mean peak concentration in plasma following the intravenous administration of foscarnet (90 mg/kg) was 887.3 ± 102.7 μM (n = 13). The terminal half-life in plasma remained unchanged, averaging 5.5 ± 2.2 h on day 1 (n = 15) and 6.6 ± 1.9 h on day 8 (n = 13), whereas it was 5.7 ± 0.7 h following intravenous dosing. Oral bioavailabilities were 9.1% ± 2.2% (90 mg/kg QD), 9.5% ± 1.7% (90 mg/kg BID), and 7.6% ± 3.7% (180 mg/kg QD); the accumulation ratios on the 8th day of dosing were 2.1 ± 1.1, 1.8 ± 0.4, and 1.7 ± 0.7, respectively. The overall 24-h urinary excretion of oral foscarnet averaged 7.8% ± 2.6% (day 1) and 13.4% ± 6.0% (day 8), whereas it was 95.0% ± 4.9% after intravenous dosing. The glomerular filtration rate and creatinine clearance remained constant, and the mean 24-h renal clearances of foscarnet for the entire study group were 96 ± 18 ml/min (day 1), 88 ± 13 ml/min (day 8), and 103 ± 16 ml/min after intravenous dosing. Adverse effects were largely confined to gastrointestinal disturbances, with all subjects experiencing diarrhea that was dose dependent in its severity. The results suggest that the formulation studied would require significant improvement with respect to tolerability and bioavailability to gain clinical acceptance.
Foscarnet (trisodium phosphonoformate hexahydrate) inhibits viral DNA polymerase and is used in the treatment of cytomegalovirus (CMV) disease in immunodeficient patients (2, 12, 17, 19). Foscarnet is usually administered as an intermittent infusion at 8- to 12-h intervals, and with adequate prehydration any predisposition to nephrotoxicity is minimized (2, 12, 25). However, foscarnet infusion requires placement of a central venous catheter to facilitate acute and subsequent maintenance therapy. The availability of an oral formulation of the drug would be an obvious advantage and would improve the quality of life for patients. Other major drugs used in the management of CMV disease, ganciclovir (23, 24) and cidofovir (20), are both nucleoside analogs and are also poorly absorbed when administered orally (1, 6, 23). Despite its poor bioavailability, oral ganciclovir has been shown to have clinical efficacy, although the drug still retains its myelotoxic potential (24). Cidofovir has the distinct advantage of requiring administration at 1- to 3-week intervals (6), but its nephrotoxic potential remains a significant problem (20).
Attempts to administer intravenous preparations of foscarnet orally have resulted in both poor and erratic drug absorption, with absolute bioavailability, calculated from the urinary excretion of foscarnet, being between 12 and 22% of the administered dose (3, 22). In order to improve gastrointestinal absorption, an oral formulation containing 1% carboxymethyl cellulose and capric acid (0.1%) was developed. This has been used in the present study to determine the pharmacokinetics and absolute bioavailability following 8 consecutive days of oral treatment in a group of 15 human immunodeficiency virus (HIV)-seropositive subjects.
(Preliminary findings of part of this study were presented in abstract form at the 23rd Annual Meeting of the American College of Clinical Pharmacology, October 1994 [14a].)
MATERIALS AND METHODS
Patients.
Fifteen, asymptomatic, HIV-seropositive male subjects free of active CMV infection were recruited into the study, which was approved by Riverside Research Ethics Committee. The mean age of the subjects was 34 years (age range, 30 to 49 years), the mean weight was 69 kg (weight range, 59 to 83 kg), the mean height was 176 cm (height range, 160 to 186 cm), and the mean CD4+ count was 550 (CD4+ count range, 130 to 1,280). Subjects were either Caucasian (14 of 15) or of mixed race in origin (1 of 15). Informed consent was obtained and the patients were divided into three dosing groups: 90 mg/kg of body weight once daily (QD) (n = 6), 90 mg/kg twice daily (BID) (n = 6), and 180 mg/kg QD (n = 3) for 8 consecutive days. After a 7-day washout interval, all subjects received a standard intravenous dose of foscarnet (90 mg/kg coinfused with 500 ml of dextrose [5%] over 2-h period). Existing medication consisting of acyclovir (4 of 15 subjects), zidovudine (2 of 15), co-trimoxazole (2 of 15), and dapsone (2 of 15) was permitted during the study but was withheld on pharmacokinetic study days 1 and 8 and on the day of intravenous dosing (day 15).
d-Xylose excretion test.
Upper gastrointestinal (GI) function was assessed a few days before foscarnet administration by using a standard oral dose of d-ylose (5 g). Urine fractions were collected over 0 to 2 and 2 to 5 h, and d-xylose excretion was measured. A 5-h cumulative urinary excretion of d-xylose in excess of 21% of the administered dose was regarded as the cutoff for normal upper gastrointestinal absorptive function (10).
Study outline.
On the morning of the pharmacokinetic study days (days 1, 8, and 15), subjects were cannulated in each arm and were initially asked to drink 500 ml of water followed by 200 ml of water every hour for the next 10 h. The glomerular filtration rate (GFR) was measured by using 51Cr-EDTA. A priming dose of 51Cr-EDTA (25 μCi) was administered, followed by a sustained infusion at 7.5 μCi/h of 51Cr-EDTA (75 μCi dissolved in 1,000 ml of dextrose [4.5%] and saline [0.18%]) for approximately 10 h (i.e., 1.5 to 2 h predosing and 8 h postdosing). Subjects were asked to empty their bladder 45 to 60 min after the start of the 51Cr-EDTA infusion. Baseline plasma and urine samples were collected over the next hour before commencing the foscarnet infusion. Plasma samples were collected at regularly timed intervals on days 1 and 8 of oral dosing at −1 and 0 h (predosing) and at 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 16, and 24 h postdosing and on the intravenous dosing day (day 15) at −1 and 0 (predosing) and at 1, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 16, and 24 h postdosing. Timed urine fractions were collected at −1 to 0 (predosing), 0 to 2, 2 to 4, 4 to 8, 8 to 12, and 12 to 24 h postdosing on each of the study days.
Analytical methods.
Aliquots of plasma and urine were analyzed for creatinine by the Jaffe reaction (4) and for 51Cr-EDTA by using a gamma counter (Canberra Packard, Pangbourne, United Kingdom). GFR was calculated as renal clearance (CLR) of 51Cr-EDTA by a standard clearance formula: GFR = (U × V)/(P × T), where U is counts per minute in the urine sample, P is counts per minute in the plasma sample, V is the volume of urine (in milliliters), and T is the urine collection interval (in minutes). At each visit, GFR was determined in all fractions up to 8 h postdosing, and as an alternative but less accurate estimate of GFR, creatinine clearance was determined over the complete 24-h period (5, 8, 9).
Foscarnet concentrations in plasma and urine samples were measured by a modified high-pressure liquid chromatography (HPLC) method with electrochemical detection (15, 18). Standard curves were prepared for both plasma and urine (low range, 2 to 27 μM; high range, 27 to 360 μM). The assay method and associated modifications are briefly described below.
Analysis of foscarnet in plasma.
A total of 100 μl of plasma was mixed with 800 μl of 0.001 M pyrophosphoric acid (Fluka Chemicals, Poole, United Kingdom) and 100 μl of distilled water. When samples were analyzed by using the standard curve for the low concentration range, 25 mg of activated charcoal (Mallinckrodt, Paris, Ky.) was added at this stage and the mixture was vortexed for 30 s. The mixture was placed in ultrafiltration units (Centricon 30; Amicon Ltd., Stonehouse, United Kingdom) and centrifuged (2,000 × g for 45 min); the resulting ultrafiltrate was inactivated by heating at 100°C for 20 min. A total of 20 μl of the inactivated ultrafiltrate (or of the ultrafiltrate after appropriate dilution with 0.001 M pyrophosphoric acid) was injected onto an HPLC column (3 μm Spherisorb ODS-2 packed column; 100 by 4.6 mm [inner diameter]; Keystone Scientific, Bellefonte, Pa.). Methanol-phosphate buffer (0.043 M; 30:70 [vol/vol]) plus 0.0002 M pyrophosphoric acid and 0.001 M tetrahexylammonium hydrogen sulfate (Fluka Chemicals, Gillingham, United Kingdom) with a final pH of 5.8 was used as the mobile phase and was pumped at a flow rate of 1 ml/min. The foscarnet peak was eluted at 5.5 min and was detected with an ESA Coulochem detector (model 5100A; ESA, Bedford, Mass.) with a 5020 Guard cell (placed after the analytical column; +0.65 V) and a 5010 Analytical cell (cell 1 was at +0.70 V and the monitoring cell was at +0.95 V). The limit of quantification of foscarnet in plasma samples was 2 μM (taken as 10 times the absolute detection limit of 0.2 μM). Assay curves were linear, and the interassay coefficient of variation for standard curves for the low and high concentration ranges were less than 9 and 6%, respectively.
Analysis of foscarnet in urine.
A total of 200 μl of diluted urine was mixed with 600 μl of 0.01 M pyrophosphoric acid before the addition of 25 mg of activated charcoal. The mixture was vortexed for 30 s and was allowed to react with the charcoal for a further 5 min before being placed in ultrafiltration units (Centricon 30; Amicon Ltd.) and centrifuged (at 2,000 × g for 7 min). The 200 μl of the ultrafiltrate was then mixed with 200 μl of 0.02 M sodium hydroxide (Mallinckrodt) and 400 μl of absolute ethanol prior to heat inactivation (56°C for 30 min). After cooling, the contents were diluted 1 to 5 with 0.001 M pyrophosphoric acid (pH 5.8) before injecting 20 μl of this final mixture onto the same HPLC column as detailed above. The interassay coefficient of variation was less than 10%, and the limit of quantification was 2 μM foscarnet in urine.
Pharmacokinetic analysis.
Plasma concentration-time data were analyzed by noncompartmental methods. The maximum concentration in plasma (Cmax) was determined directly from the data. The terminal half-life (t1/2z) was calculated as a ratio of 0.693 and the slope (λz) of the terminal portion of the log-linear plasma concentration-time curve. In most cases data for the last five to six time points before the next dose were used to determine λz. The area under the plasma concentration-time curve from time zero to time t (AUC0–t) and the first moment of the area under the concentration-time curve from time zero to time t (AUMC0–t) were calculated by using the linear trapezoidal rule and were extrapolated to infinity [AUCt–∞ = Ct/λz; AUMCt–∞ = Ct/λz + t · Ct/(λz)2, where Ct is the concentration at time t]. Total body clearance (CL) was calculated as drug dose/AUC0–∞, and CLR was calculated from the total amount of foscarnet excreted by the kidney from time zero to time t/AUC0–t. Bioavailability was calculated as the ratio of oral AUC0–∞ to intravenous AUC0–∞. The accumulation ratio for foscarnet following 8 days of dosing was calculated as AUC0–t on day 8/AUC0–∞ after administration of the first dose on day 1. Because foscarnet is excreted unchanged by the kidney, the bioavailability of oral foscarnet was also estimated by using the comparative ratio of the amount of drug excreted following the administration of oral and intravenous doses. Two subjects from the 90-mg/kg BID group withdrew from the study on day 3 and day 5, respectively, and were not available for further investigation. For this reason, investigations for day 8 and day 15 were carried out with data for 13 of the 15 subjects.
Statistical analysis.
All data are presented as means ± standard deviations (SDs) or means with ranges. Data were analyzed by linear regression or the paired t test after log transformation (13), and the results were considered significant at a P value of < 0.05.
RESULTS
d-Xylose excretion.
Upper GI absorptive capacity, as assessed by the 5-h d-xylose excretion test, was found to be abnormal in one subject (15%) and borderline (21%) in two other subjects. The mean 5-h urinary excretion of d-xylose was 33% ± 9% (range, 15 to 48%) of the administered dose.
Pharmacokinetics of foscarnet in plasma.
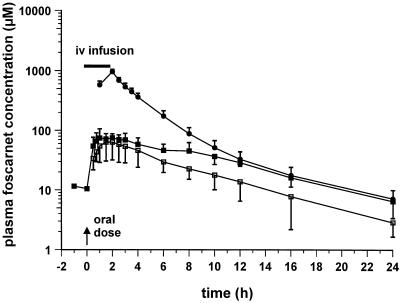
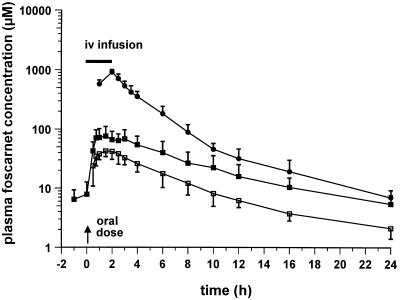
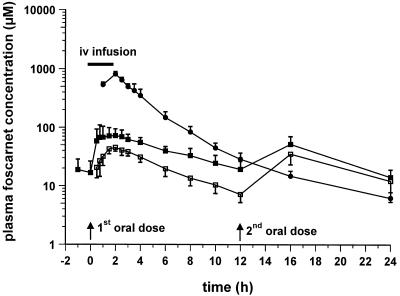
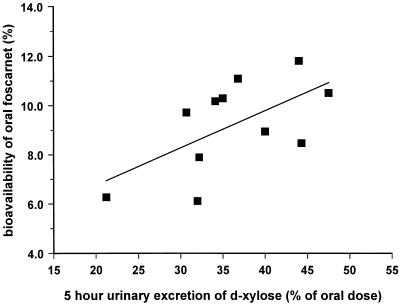
Semilogarithmic plots of the foscarnet profiles in plasma obtained after oral and intravenous dosing are illustrated in Fig. 1 to 3. Figure 1 shows the responses to the 90-mg/kg QD dose, with mean ± SD Cmaxs of 46.4 ± 10.8 μM (day 1; oral dose), 86.2 ± 35.8 μM (day 8; oral dose), and 909.0 ± 96.3 μM (day 15; intravenous dose). Figure 2 shows the responses to the 90-mg/kg BID dose, with Cmaxs of 45.7 ± 6.9 μM (day 1; oral dose), 78.7 ± 35.2 μM (day 8; oral dose), and 807.3 ± 83.8 μM (day 15; intravenous dose), while Fig. 3 shows the responses to the 180-mg/kg QD dose, with Cmaxs of 64.9 ± 31.7 μM (day 1; oral dose), 86.4 ± 25.0 μM (day 8; oral dose), and 950.7 ± 96.5 μM (day 15; intravenous dose). The results of pharmacokinetic parameters for all the dosing schedules are summarized in Table 1. Overall, the circulating drug concentrations after oral administration of foscarnet were higher on day 8 compared to those seen on day 1 (Fig. 1 to 3), yielding accumulation ratios of 2.1 ± 1.1, 1.8 ± 0.4, and 1.7 ± 0.7 for each of the respective oral dosing schedules, with the mean accumulation ratio for the whole group being 1.9 ± 0.8 (n = 13). The volume of distribution of foscarnet, derived from the pooled responses after intravenous dosing, averaged 24.0 ± 0.3 liters (n = 13), while CL was estimated to be 107 ± 16 ml/min (n = 13). Bioavailability was similar for each of the dosing schedules at 9.1% (90 mg/kg QD; range, 6.1 to 11.1%; n = 6), 9.5% (90 mg/kg BID; range, 7.9 to 11.8%; n = 4), and 7.6% (180 mg/kg QD; range, 3.4 to 10.5%; n = 3), with an overall mean oral bioavailability of 8.9% ± 2.4% (n = 13) for the entire study group. For 11 of the 13 subjects, estimates of bioavailability correlated closely with 5-h urinary d-xylose excretion (Fig. 4; r = 0.606; P < 0.05). The remaining two subjects were excluded because of diarrhea after foscarnet dosing.
FIG. 1.
Mean ± SD concentrations of foscarnet in plasma following oral administration (day 1 [□] and day 8 [▪] of daily dosing with 90 mg/kg). Intravenous (iv) foscarnet (90 mg/kg) was infused over 2 h into a group of six patients (——).
FIG. 3.
Mean ± SD concentrations of foscarnet in plasma following oral administration (day 1 [□] and day 8 [▪] of daily dosing with 180 mg/kg). Intravenous (iv) foscarnet (90 mg/kg) was infused over 2 h into a group of three patients (——).
FIG. 2.
Mean ± SD concentrations of foscarnet in plasma following oral administration (day 1 [n = 6; □] and day 8 [n = 4; ▪] of dosing with 90 mg/kg BID). Intravenous (iv) foscarnet (90 mg/kg) was infused over a 2-h period (——), [n = 4]). Two of the six patients in this group withdrew from the study and were unavailable for investigations on day 8 and for intravenous dosing.
TABLE 1.
Pharmacokinetic parameters for a group of HIV-seropositive receiving foscarnet orally and intravenouslya
| Group and day | Cmax (μM) | Tmax (h) | t1/2z (h) | AUC0–∞ (μM · h) | F (%) | Accumulation ratio | Urinary excretion of foscarnet over 24 h (% of dose) |
|---|---|---|---|---|---|---|---|
| 90 mg/kg QD | |||||||
| Day 1 (n = 6) | 46.4 ± 10.8 | 1.6 ± 0.7 | 6.7 ± 2.6 | 300 ± 60 | 9.1 ± 2.2 | 7.7 ± 3.0 | |
| Day 8 (n = 6) | 86.2 ± 35.8b | 1.4 ± 0.6 | 7.6 ± 1.2 | 646 ± 243c | 2.1 ± 1.1 | 16.6 ± 7.4b | |
| 90 mg/kg BIDd | |||||||
| Day 1 (n = 6) | 45.7 ± 6.9 | 2.0 ± 0.3 | 4.0 ± 1.3 | 298 ± 47 | 9.5 ± 1.7 | 8.7 ± 2.1 | |
| Day 8 (n = 4e) | 78.7 ± 35.2 | 1.8 ± 0.7 | 5.7 ± 2.7 | 688 ± 223b | 1.8 ± 0.4 | 11.3 ± 2.8 | |
| 180 mg/kg QD | |||||||
| Day 1 (n = 3) | 64.9 ± 31.7 | 1.7 ± 0.3 | 5.4 ± 0.4 | 494 ± 188 | 7.6 ± 3.7 | 6.0 ± 2.7 | |
| Day 8 (n = 3) | 86.4 ± 25.0 | 2.0 ± 1.0 | 5.6 ± 0.4 | 826 ± 195 | 1.7 ± 0.7 | 9.9 ± 2.5 | |
| 90 mg/kg intravenously (n = 13e) | 887.3 ± 102.7 | 2.0 ± 0.0 | 5.7 ± 0.2 | 3,308 ± 496 | 95.3 ± 4.9 |
Tmax, time to Cmax; F, bioavailability; the other abbreviations are defined in the text. Values are means ± standard deviations.
P < 0.05 versus day 1.
P < 0.01 versus day 1.
Data are for first dose of BID dosing schedule.
Excludes data for two patients who withdrew from the study.
FIG. 4.
Relationship between the overall GI absorptive function, as measured by the renal excretion of d-xylose over 0 to 5 h, and the oral bioavailability of foscarnet. Only data for 11 of the 13 subjects are shown (r = 0.606; P < 0.05) because the remaining 2 subjects were excluded following GI disturbances after the administration of foscarnet.
Urinary excretion and CLR of foscarnet.
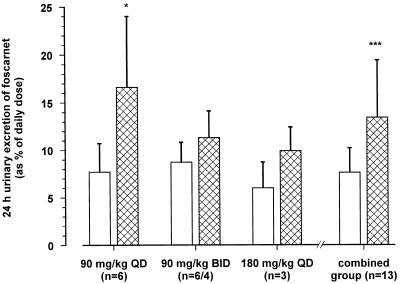
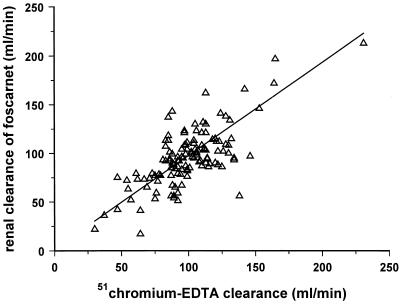
The mean 24-h urinary excretions of foscarnet on the 1st and 8th days of oral dosing are presented in Fig. 5. Drug excretion rose from 7.7% ± 3.0% (90 mg/kg QD), 8.7% ± 2.1% (90 mg/kg BID), and 6.0% ± 2.7% (180 mg/kg QD) on the day 1 of dosing to 16.6% ± 7.4%, 11.3% ± 2.8%, and 9.9% ± 2.5%, respectively, on day 8 of dosing. The overall mean urinary excretion for the entire study group, expressed as a percentage of the administered dose, was 7.8% ± 2.6% on day 1 and 13.4% ± 6.0% on day 8 (Fig. 5; P < 0.001). The total amount of drug excreted in the urine over the 24-h period following the administration of the intravenous dose of foscarnet (90 mg/kg; n = 13) was 95.0% ± 4.9%. The bioavailability of oral foscarnet estimated from urinary excretion data was lower, but it was not significantly different from the value calculated by using plasma AUC data (8.0% ± 2.8% versus 8.9% ± 2.4%; n = 13; P was not significant). The CLR of foscarnet was found to be the similar to GFR, determined by using 51Cr-EDTA clearance (98 ± 39 versus 100 ± 27 ml/min; P was not significant) for a total of 125 timed urine fractions collected at each visit from each subject. There was a linear correlation between the CLR of foscarnet and GFR (Fig. 6), with the slope of the regression line being not significantly different from unity (0.959; 95% confidence interval, 0.764 to 1.154). There were no changes in either GFR (51Cr-EDTA clearance or creatinine clearance) throughout the study following oral or intravenous dosing with foscarnet. Simultaneous determinations of creatinine clearance and CLR of foscarnet were undertaken for a total of 209 separate timed urine samples collected over the entire 24-hour period after drug infusion, and the overall CLR of foscarnet was found to be significantly lower than creatinine clearance (96 ± 35 versus 119 ± 29 ml/min; P < 0.001). A linear relationship was also observed between the CLR of foscarnet and creatinine clearance, but the slope was significantly less than unity (0.729; 95% confidence interval, 0.578 to 0.860; P < 0.001).
FIG. 5.
Mean ± SD urinary excretion of drug over 24 h on day 1 (open columns) and on day 8 (hatched columns) of oral dosing with foscarnet. Patients received the drug at dosages of 90 mg/kg QD (n = 6), 90 mg/kg BID (n = 6), and 180 mg/kg QD (n = 3). Two patients, both of whom were in the 90-mg/kg BID group, withdrew from the study, and data for day 8 were not available for these patients. Urinary excretion of the drug was higher on day 8 than on day 1 ∗, P < 0.05; ∗∗∗, P < 0.001 (day 8 versus day 1).
FIG. 6.
Linear relationship between CLR of foscarnet and GFR, as measured by using 51Cr-EDTA clearance (r = 0.660; n = 125; P < 0.001). Urine samples were collected over an 8-h period postdosing on days 1 and 8 for those on the oral dosing schedule and on the intravenous dosing day. The slope of the regression line (0.960; 95% confidence interval, 0.765 to 1.155) was not different from unity.
Tolerability of foscarnet.
The most common adverse effect was disturbance of the GI tract function (including diarrhea, bloating, and abdominal discomfort) seen in all subjects after oral foscarnet administration. This ranged from mild (soft stools with less than three bowel movements/day) at the lower dosages (90 mg/kg QD) to severe (watery, profuse diarrhea with more than five bowel movements/day) at the higher dosages of 180 mg/kg daily when given singly or in divided doses. In some subjects, GI disturbances appeared soon after ingestion of the first oral dose of foscarnet, while in other subjects these disturbances became apparent only after 2 to 3 days. In many patients symptoms were severe enough to restrict normal lifestyle because the induced diarrhea was not always adequately controlled by conventional antidiarrheal agents such as loperamide alone (6 of 15 subjects; 2 to 4 mg/day) or when combined with codeine phosphate (5 of 15 subjects; 60 mg/day). Two of the patients (90-mg/kg BID group) withdrew from the study (on days 3 and 5, respectively) because of the GI disturbances. Nausea (5 of 15 subjects), vomiting (5 of 15), headaches (4 of 15), and pharyngitis (2 of 15) were also reported during the course of the investigation, although these adverse effects were less consistent. No other clinical or biochemical abnormalities were observed. Intravenously administered foscarnet, given to each subject on only one occasion, was not associated with any adverse effects.
DISCUSSION
The Cmaxs of foscarnet occurred between 90 and 120 min postdosing and were similar (32 to 139 μM), irrespective of the dose; the concentrations then declined to reach trough levels of between 1 and 9 μM. Effective suppression of CMV replication requires circulating plasma foscarnet concentrations of between 100 and 500 μM (6, 16), and so it is clear that the maximum concentrations achieved in plasma in the present study, despite some 1.9-fold accumulation after 8 days of repeated dosing, were inadequate for effective disease suppression. By contrast, with ganciclovir, whose bioavailability is also very low (3 to 7%) (1, 11, 23), the concentrations that are achieved in plasma after oral dosing are sufficiently high to suppress virus replication.
Only about 10% of the orally administered foscarnet was absorbed, even though most of the patients had normal or nearly normal GI function. The extent of foscarnet absorption paralleled the absorption of d-xylose, a carbohydrate probe used to assess upper gut absorptive function (10). This suggests that the absorption of foscarnet was largely occurring in the upper GI tract. The bioavailability of the drug observed in the present study was considerably lower than the value of 17% (range, 12 to 22%) reported by Sjovall et al. (22) following oral ingestion of foscarnet solution. Those investigators calculated bioavailability using the cumulative urinary recovery after continuous infusion of foscarnet over a 72-h period (22). Such a regimen would result in a degree of drug accumulation and therefore would be likely to overestimate the true bioavailability. In the present study there was an increase in the urinary excretion of foscarnet from 7.8% (range, 3 to 11%) on the first day of dosing to 13.4% (range, 4 to 26%) on the last day of dosing. This degree of accumulation of foscarnet following 8 days of oral dosing was greater that that expected from the t1/2z reported. In order to achieve an accumulation ratio of 2, the drug must be administered at times equivalent to every half-life, provided that all other disposition characteristics remain unaltered. In our case foscarnet was administered after four half-lives for subjects receiving QD dosing and approximately every other half-life for subjects receiving BID dosing. The accumulation ratio of approximately 2 in both of these cases implies that a slowly equilibrating compartment, such as the bone (22, 23), may have contributed to the observed accumulation of foscarnet. An alternative or additional mechanism may be that the absorption characteristics of foscarnet may have been altered following repeated dosing. The relatively poor bioavailability of oral foscarnet means that any improvement in the absorption characteristics of subsequent doses of foscarnet may have a disproportionate effect on the determination of the accumulation ratio, especially because neither the CLR of foscarnet nor the GFR changed over this period.
With respect to the CLR of foscarnet, some earlier studies have shown that CLR is greater than creatinine clearance, suggesting the occurrence of significant tubular secretion of the drug (21, 22). If this were the case, then an agent such as probenecid, which inhibits the weak organic acid pathway in the proximal renal tubule, would be expected to interfere with the secretion of foscarnet. We have previously shown that there is no pharmacokinetic interaction between foscarnet and probenecid (14), implying a lack of tubular drug secretion. This is further confirmed in the present study, since the CLR of foscarnet, which averaged 98 ml/min, was not significantly different from the mean GFR of 100 ml/min.
The particular oral formulation of foscarnet used in the present study was chosen with a view to enhancing drug bioavailability. Unfortunately, the maximum bioavailability achieved was less than 10% and is likely to have contributed to the high incidence of dose-related GI disturbances. The failure to reach therapeutic concentrations in the plasma necessary for the effective suppression of CMV plus the high incidence of GI adverse effects are major drawbacks that clearly limit the therapeutic potential of this otherwise chemically unique antiviral agent.
ACKNOWLEDGMENTS
This project was funded, in part, by Astra USA Inc., Westborough, Mass., and by The Lant Trust for Medical Research, London, United Kingdom.
REFERENCES
- 1.Anderson R D, Griffy K G, Jung D, Dorr A, Hulse J D, Smith R B. Ganciclovir absolute bioavailability and steady-state pharmacokinetics after oral administration of two 3000 mg/d dosing regimens in human immunodeficiency virus-seropositive patients. Clin Ther. 1995;17:425–432. doi: 10.1016/0149-2918(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 2.Aweeka F, Gambertoglio J, Mills J, Jacobson M A. Pharmacokinetics of intermittently administered intravenous foscarnet in the treatment of acquired immunodeficiency syndrome patients with serious cytomegalovirus retinitis. Antimicrob Agents Chemother. 1989;33:742–745. doi: 10.1128/aac.33.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barditch-Crovo P, Petty B G, Gambertoglio J, Nerhood L J, Kuwahara S, Hafner R, Lietman P S, Kornhauser D M. Abstracts of the 7th International Conference on AIDS. 1991. The effect of increasing gastric pH upon bioavailability of orally administered phosphonoformic acid (foscarnet), abstr. WB 2115. [Google Scholar]
- 4.Bonsnes R W, Taussky H H. The colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem. 1945;158:581–591. [Google Scholar]
- 5.Brøchner-Mortensen J. Current status on assessment and measurement of glomerular filtration rate. Clin Physiol. 1985;5:1–17. doi: 10.1111/j.1475-097x.1985.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 6.Chrisp P, Clissold S P. Foscarnet. Drugs. 1991;41:104–129. doi: 10.2165/00003495-199141010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Cundy K C, Petty B G, Flaherty J, Fisher P E, Polis M A, Wachsman M, Lietman P S, Lalezari J P, Hitchcock M J M, Jaffe H S. Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1995;39:1247–1252. doi: 10.1128/aac.39.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixey J J, Noormohamed F H, Pawa J S, Lant A F, Brewerton D A. The influence of nonsteroidal anti-inflammatory drugs and probenecid on the renal response to and kinetics of piretanide in man. Clin Pharmacol Ther. 1988;44:531–539. doi: 10.1038/clpt.1988.190. [DOI] [PubMed] [Google Scholar]
- 9.Favre H R, Wing A J. Simultaneous 51Cr EDTA acid, inulin and endogenous creatinine clearances in 20 patients with renal disease. Br Med J. 1968;1:84–86. doi: 10.1136/bmj.1.5584.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haeney M R, Culank L S, Montgomery R D, Sammons H G. Evaluation of xylose absorption as measured in blood and urine: a one-hour blood xylose screening test in malabsorption. Gastroenterology. 1978;75:393–400. [PubMed] [Google Scholar]
- 11.Jacobson M A, de-Miranda P, Cederberg D M, Burnette T, Cobb E, Brodie H R, Mills J. Human pharmacokinetics of and tolerance of oral ganciclovir. Antimicrob Agents Chemother. 1987;31:1251–1254. doi: 10.1128/aac.31.8.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katlama, C., E., Dohin, E. Caumes, I. Cochereau-Massin, C. Brancon, M. Robinet, O. Rogeaux, R. Dahan, and M. Gentilini. 1992. Foscarnet induction therapy for cytomegalovirus retinitis in AIDS: comparison of twice-daily and three-times-daily regimes. J. Acquired Immune Defic. Syndr. 5(Suppl. 1):S18–S24. [PubMed]
- 13.Midha K K, Ormsby E D, Hubbard J W, McKay G, Hawes E M, Gavalas I, McGilveray I J. Logarithmic transformation in bioequivalence: application with two formulations of perphenazine. J Pharm Sci. 1993;82:138–144. doi: 10.1002/jps.2600820205. [DOI] [PubMed] [Google Scholar]
- 14.Noormohamed F H, Youle M S, Higgs C J, Gazzard B G, Lant A F. Renal excretion and pharmacokinetics of foscarnet in HIV sero-positive patients: effect of probenecid pretreatment. Br J Clin Pharmacol. 1997;43:112–115. doi: 10.1111/j.1365-2125.1997.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 14a.Noormohamed F H, Youle M S, Higgs C J, Martin-Munley S, Gazzard B G, Lant A F. Pharmacokinetics and bioavailability of oral foscarnet in HIV seropositive patients. J Clin Pharmacol. 1994;34:1013. doi: 10.1128/aac.42.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noormohamed F H, Youle M S, Tang B, Martin-Munley S, Gazzard B G, Lant A F. Foscarnet-induced changes in plasma concentrations of total and ionised calcium and magnesium in HIV-positive patients. Antivir Ther. 1996;1:172–179. [PubMed] [Google Scholar]
- 16.Oberg B. Antiviral effects of phosphonoformate (PFA, foscarnet sodium) Pharmacol Ther. 1983;19:387–415. doi: 10.1016/0163-7258(82)90074-2. [DOI] [PubMed] [Google Scholar]
- 17.Palestine A G, Polis M A, De Smet M D, Baird B F, Falloon J, Kovacs J A, Davey R T, Zurlo J J, Zunich K M, Davis M, Hubbard L, Brothers R, Ferris F L, Chew E, Davis J L, Rubin B I, Mellow S D, Metcalf J A, Manischewitz J, Minor J R, Nussenblatt R B, Masur H, Lane H C. A randomized, controlled trial of foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. J Intern Med. 1991;115:665–673. doi: 10.7326/0003-4819-115-9-665. [DOI] [PubMed] [Google Scholar]
- 18.Petterson K J, Nordgren T, Westerlund D. Determination of phosphonoformate (foscarnet) in biological fluids by ion-paired, reversed-phase liquid chromatography. J Chromatogr. 1989;488:447–455. doi: 10.1016/s0378-4347(00)82968-0. [DOI] [PubMed] [Google Scholar]
- 19.Polis M A, De Smet M D, Baird B F, Mellow S D, Falloon J, Davey R T, Kovacs J A, Palestine A G, Nussenblatt R B, Masur H, Lane H C. Increased survival of a cohort of patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis who received sodium phosphonoformate (foscarnet) Am J Med. 1993;94:175–180. doi: 10.1016/0002-9343(93)90180-w. [DOI] [PubMed] [Google Scholar]
- 20.Polis M A, Spooner K M, Baird B F, Manischewitz J F, Jaffe H S, Fisher P E, Falloon J, Davey R T, Jr, Kovacs J A, Walker R E, Whitcup S M, Nussenblatt R B, Lane H C, Masur H. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother. 1996;39:882–886. doi: 10.1128/aac.39.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjovall J, Bergdahl S, Movin G, Ogenstad S, Saarimaki M. Pharmacokinetics of foscarnet and distribution to cerebrospinal fluid after intravenous infusion in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1989;33:1023–1031. doi: 10.1128/aac.33.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjovall J, Karlson A, Ogenstad S, Sandstrom E, Saarimaki M. Pharmacokinetics and absorption of foscarnet after intravenous and oral administration to patients with human immunodeficiency virus. Clin Pharmacol Ther. 1988;44:65–73. doi: 10.1038/clpt.1988.114. [DOI] [PubMed] [Google Scholar]
- 23.Spector S A, Busch B F, Follansbee S, Squires K, Lalezari J P, Jacobson M A, Connor J D, Jung D, Shadman A, Mastre B, Buhles W, Drew W L AIDS Clinical Trial Group; Cytomegalovirus Cooperative Study Group. Pharmacokinetic, safety, and antiviral profiles of oral ganciclovir in persons infected with human immunodeficiency virus: a phase I/II study. J Infect Dis. 1995;171:1431–1437. doi: 10.1093/infdis/171.6.1431. [DOI] [PubMed] [Google Scholar]
- 24.Spector S A, McKinley G F, Lalezari J P, Samo T, Andruczk R, Follansbee S, Sparti P D, Havlir D V, Simpson G, Buhles W, Wong R, Stempien M J for the Roche Cooperative Oral Ganciclovir Study Group. Oral ganciclovir for the prevention of cytomegalovirus disease in persons AIDS. N Engl J Med. 1996;334:1491–1497. doi: 10.1056/NEJM199606063342302. [DOI] [PubMed] [Google Scholar]
- 25.Taburet A-M, Katlama C, Blanshard C, Gorza G, Gazzard D, Dohin E, Gazzard B G, Frostegard C, Singlas E. Pharmacokinetics of foscarnet and distribution to cerebrospinal fluid after intravenous infusion in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1992;36:1821–1824. doi: 10.1128/aac.36.9.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]