Abstract
Background
The increased growth rate of young animals can lead to higher lactation performance in adult goats; however, the effects of the ruminal microbiome on the growth of young goats, and the contribution of the early-life rumen microbiome to lifelong growth and lactation performance in goats has not yet been well defined. Hence, this study assessed the rumen microbiome in young goats with different average daily gains (ADG) and evaluated its contribution to growth and lactation performance during the first lactation period.
Results
Based on monitoring of a cohort of 99 goats from youth to first lactation, the 15 highest ADG (HADG) goats and 15 lowest ADG (LADG) goats were subjected to rumen fluid microbiome and metabolome profiling. The comparison of the rumen metagenome of HADG and LADG goats revealed that ruminal carbohydrate metabolism and amino acid metabolism function were enhanced in HADG goats, suggesting that the rumen fluid microbiome of HADG goats has higher feed fermentation ability. Co-occurrence network and correlation analysis revealed that Streptococcus, Candidatus Saccharimonans, and Succinivibrionaceae UCG-001 were significantly positively correlated with young goats’ growth rates and some HADG-enriched carbohydrate and protein metabolites, such as propionate, butyrate, maltoriose, and amino acids, while several genera and species of Prevotella and Methanogens exhibited a negative relationship with young goats’ growth rates and correlated with LADG-enriched metabolites, such as rumen acetate as well as methane. Additionally, some functional keystone bacterial taxa, such as Prevotella, in the rumen of young goats were significantly correlated with the same taxa in the rumen of adult lactation goats. Prevotella also enriched the rumen of LADG lactating goats and had a negative effect on rumen fermentation efficiency in lactating goats. Additional analysis using random forest machine learning showed that rumen fluid microbiota and their metabolites of young goats, such as Prevotellaceae UCG-003, acetate to propionate ratio could be potential microbial markers that can potentially classify high or low ADG goats with an accuracy of prediction of > 81.3%. Similarly, the abundance of Streptococcus in the rumen of young goats could be predictive of milk yield in adult goats with high accuracy (area under the curve 91.7%).
Conclusions
This study identified the keystone bacterial taxa that influence carbohydrate and amino acid metabolic functions and shape the rumen fluid microbiota in the rumen of adult animals. Keystone bacteria and their effects on rumen fluid microbiota and metabolome composition during early life can lead to higher lactation performance in adult ruminants. These findings suggest that the rumen microbiome together with their metabolites in young ruminants have long-term effect on feed efficiency and animal performance. The fundamental knowledge may allow us to develop advanced methods to manipulate the rumen microbiome and improve production efficiency of ruminants.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-023-01652-5.
Keywords: Rumen microbiome, Microbial interaction, Keystone bacteria, Rumen metabolome, Long-term effects and prediction, Goats
Background
There is an increasing demand to produce goat milk because it consists of more medium- and short-chain fatty acids, vitamins, β-casein, and trace minerals, and less α-casein and allergens than dairy cows’ milk and is more suitable for infant nutrition [1, 2]. Additionally, goat milk and its products provide important daily food sources of protein, phosphate, and calcium for people in the developing world [3]. Many factors can affect dairy goat milk production and quality, including genetics [4, 5], management [6], and feeding strategy [7]. The rumen serves as a bioreactor that enables animals to digest complex plant fibers and polysaccharides and produce volatile fatty acids (VFAs), microbial proteins, and vitamins [8]. Recent research has revealed that the rumen microbiome plays a vital role in affecting production traits of dairy cows, such as feed efficiency [9, 10], methane yield [11, 12], and milk production [13, 14]. However, the contribution of the rumen microbiome to lactation performance in goats is less understood.
Early-life rumen microbiome plays key roles in rumen development and microbial fermentation, which subsequently affects the growth and feed efficiency of young ruminants [15–17]. The VFAs produced by the rumen microbiome are vital to stimulate rumen tissue metabolism and epithelium development during early life [18]. It has been reported that rumen microbiota transplantation could alter the endogenous microbiota and lead to improved growth performance in calves and lambs [19–21]. Importantly, the preweaning and prepubertal ADG of heifers or goat kids have been reported to be highly correlated with adult milk production [22–24]. Similarly, a study revealed that the initial microbiota after birth has a long-lasting effect on the assembly process and adult composition of the ruminal microbiome based on the observations during 3 years of the cows’ life [25]. Early-life dietary interventions in dairy calves can also affect microbial colonization, with long-term consequences for the adult cow rumen microbiota and fermentation [26]. Although recent studies have remarkably expanded our understanding of the early-life rumen microbiome, there is a knowledge gap about whether and how highly efficient phenotypes (e.g., growth performance)-related ruminal microbiota in the early or adolescent life of goats could affect microbial community dynamic succession, and subsequently affect host lactation performance. In addition, a comprehensive understanding of the mechanisms of the rumen microbiome driving growth in dairy goats is still lacking.
The ruminal microbiota composition in young ruminants on subsequent ruminal microbiota succession in adult ruminants may serve as the potential mechanism to determine the link between the higher growth performance, such as ADG, in the youth period and the higher lactation performance in the adult period. In the present study, 6-month-old dairy goats with different ADG were used as a model to monitor their growth and performance until first lactation and to assess the rumen fluid microbiota, microbiome, and metabolome using metataxonomics, metagenomics, and metabolomics, respectively, aiming to (i) identify the contribution of the rumen fluid microbiome and their metabolome to the growth performance of young goats; (ii) identify whether the rumen fluid microbiota of young goats has a long-term effect on rumen microbiota colonization of adult goats and then contributes to regulating lactation performance; and (iii) explore rumen fluid microbes during the youth period that can be used as biological markers for predicting future milk production.
Results
Young dairy goats had differential growth rates and rumen fermentation
This study monitored the growth performance of 99 young goats, 84 of which successfully entered the lactation phase (Fig. 1). The growth performance parameters of 99 young dairy goats (187.9 ± 0.3 days of age [mean ± standard error (SE)], Table S1), including body weight (23.6 ± 0.3 kg, [coefficient of variation (CV)] = 12.01%), weight gain (20.6 ± 0.3 kg, [CV] = 13.00%), and ADG (109.47 ± 1.46 g/day, CV = 13.21%), were highly correlated (Spearman’s correlation coefficient > 0.97, p < 0.01).
Fig. 1.
The flowchart of this study. All animals were fed the same diet, kept under the same conditions, and housed together from birth (Table S9). During the preweaning phase, all kids were fed milk, alfalfa hay and concentrate mixture, and the weaned animals (3 months old to ~ 13 months old) were fed total mixed ration (TMR) with a forage to concentrate ratio of 60:40. After delivery, goats were fed TMR with forage:concentrate rations of 50:50. 1,2 From 99 young goats enrolled in this study, 15 goats with the highest average daily gain (ADG) were selected as the HADG group (ADG: 132.5 ± 1.5 g/day); 15 goats with the lowest ADG were selected as the LADG group (ADG: 88.2 ± 1.2 g/day). 3,4 After delivery of their newborn offspring kids, 84 of 99 healthy lactating goats remained, and 15 goats were excluded. In the HAL group, 13 lactating goats of 15 HADG goats remained and were renamed the HAL group; in the LAL group, 12 lactating goats of 15 LADG goats remained and were renamed the LAL group. 5,6 Among these 84 lactating goats, 15 lactating goats with the highest milk yield were selected as the HMP group (average daily milk yield: 2.82 ± 0.04 kg/day), and 15 lactating goats with the lowest milk yield were selected as the LMP group (average daily milk yield: 1.51 ± 0.03 kg/day), according to the milk yield recorded every 7 days over the first whole lactation period ADG: average daily gain, AA: amino acid, HADG: young goats with high average daily gain, LADG: young goats with low average daily gain, HAL: lactating goats of HADG, LAL: lactating goats of LADG, HMP: lactating goats with high milk yield, LMP: lactating goats with low-milk yield
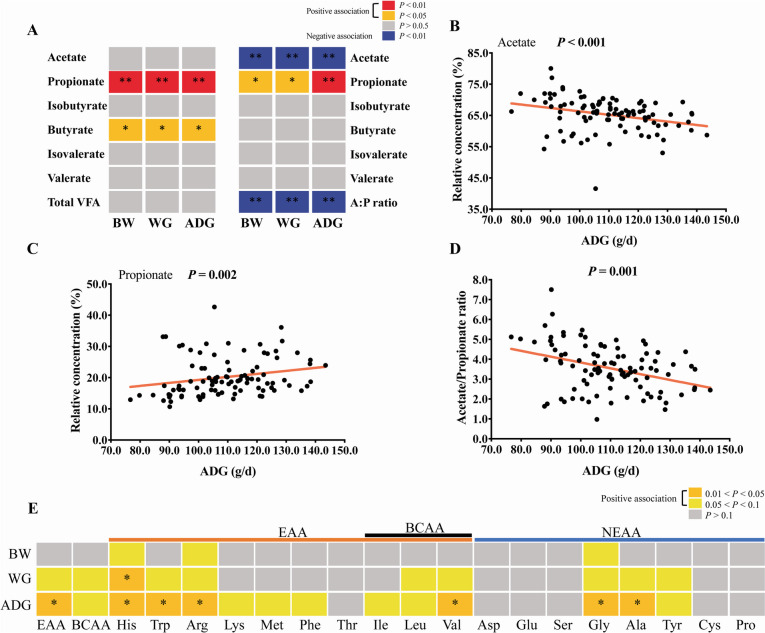
The analysis of the relationship between growth performance traits and rumen VFA profiles (n = 99) showed that several VFAs were significantly associated with these growth performance traits (Fig. 2a–d; Table S2). Specifically, rumen propionate and butyrate concentrations were positively correlated with body weight, weight gain, and ADG (r > 0.20, p < 0.05; Fig. 2a). The molar percentage of propionate was positively correlated with body weight, weight gain, and ADG (r > 0.25, p < 0.05; Fig. 2a, c), while the molar percentage of acetate, acetate to propionate ratio were negatively correlated with these traits (r < − 0.28, p < 0.01; Fig. 2a, b, d). Analysis of Spearman correlations between growth performance traits and rumen free amino acids measurements (n = 99) showed that ADG was significantly positively correlated with the rumen fluid concentrations of total essential amino acids, His, Trp, Arg, Val, Gly, and Ala (r > 0.2, p < 0.05; Fig. 2e). ADG had a weak positive correlation with the rumen fluid concentrations of total branched chain amino acids, Lys, Met, Phe, Ile, Leu, and Tyr (p < 0.1).
Fig. 2.
Rumen fermentation productions and their relationship with growth performance traits (n = 99). A Associations between rumen VFAs and growth performance traits. Left: absolute concentrations of VFAs, Right: relative abundance of VFAs. The molar percentage of acetate (B), propionate (C), and the acetate to propionate ratio (D) were significantly associated with ADG (Spearman’s correlation, p < 0.05). E Heatmap showing the association between rumen fluid free amino acids and growth performance traits (Spearman’s correlation, p < 0.05). *p < 0.05, **p < 0.01 BW: body weight, WG: weight gain, ADG: average daily gain, EAA: essential amino acid, BCAA: branched chain amino acid, NEAA: non-essential amino acid
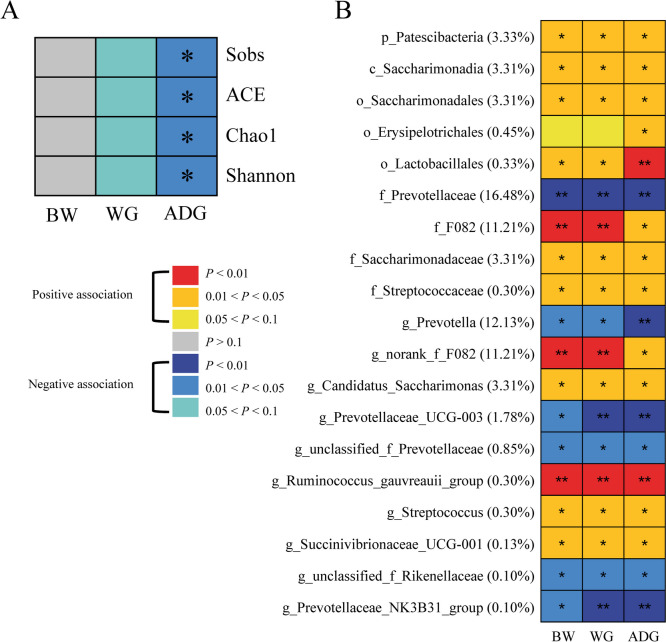
Varied ruminal microbiota diversity and key bacteria related to growth rates in young dairy goats
The metataxonomic analysis of rumen fluid samples collected from young dairy goats (n = 99) using 16S rRNA gene amplicon sequencing revealed a moderate negative association (r < − 0.20, p < 0.05) between ADG and Sobs, ACE, Chao1, and Shannon indices (Fig. 3a). The correlation between rumen fluid bacteria and growth performance traits (body weight, weight gain, and ADG) of young dairy goats (n = 99) was also identified (Fig. 3b; Table S3). At the family level (relative abundance ≥ 0.1%), the abundances of F082, Saccharimonadaceae, and Streptococcaceae were positively correlated with body weight, weight gain, and ADG, while Prevotellaceae were negatively correlated with these growth performance traits of young goats (r >|0.2|, p < 0.05). Among the bacterial genera (relative abundance ≥ 0.1%), the abundances of norank F082, Candidatus Saccharimonas, Ruminococcus gauvreauii group, Streptococcus, and Succinivibrionaceae UCG-001 showed significant positive associations with body weight, weight gain, and ADG, and the abundances of Prevotella, Prevotellaceae UCG-003, unclassified Prevotellaceae, unclassified Rikenellaceae, and Prevotellaceae NK3B31 group showed significant negative associations with these growth performance traits (r >|0.2|, p < 0.05).
Fig. 3.
The relationship between rumen fluid microbiota features and goat growth performance traits (n = 99). A Associations between alpha diversity of ruminal microbiota and growth performance traits. B Heatmap showing the association between bacterial taxa (average relative abundance ≥ 0.1%) and growth performance traits (Spearman’s correlation, p < 0.05, r >|0.2|), % the relative abundance of the taxa. *p < 0.05, **p < 0.01 BW: body weight, WG: weight gain, ADG: average daily gain
From this animal cohort (n = 99), two groups of goats with divergent ADG were identified: high ADG (HADG, n = 15) and low ADG (LADG, n = 15) (132.5 ± 1.5 g/day vs 88.2 ± 1.2 g/day, p < 0.001). When the rumen fluid microbiota was further compared between HADG and LADG goats, the rumen microbial community in HADG goats had lower alpha diversity indices (p < 0.05; Fig. S1a) and was clearly distinguished from that in LADG goats (ANOSIM R = 0.244, p = 0.001; Fig. S1b). In addition, compared to the LADG goats, the relative abundances of family F082, Saccharimonadaceae, Streptococcaceae, genus norank F082, Candidatus Saccharimonas, Ruminococcus gauvreauii group, Streptococcus, and Succinivibrionaceae UCG-001 were significantly higher in the rumen of HADG goats (abundance relative ≥ 0.1%; p < 0.05; Fig. S1c, d). In contrast, family Prevotellaceae, genus Prevotella, Prevotellaceae UCG-003, unclassified Prevotellaceae, and unclassified Rikenellaceae were significantly higher in LADG goats (abundance relative ≥ 0.1%; p < 0.05; Fig. S1c, d).
Microbial interactions differed between high and low performance goats
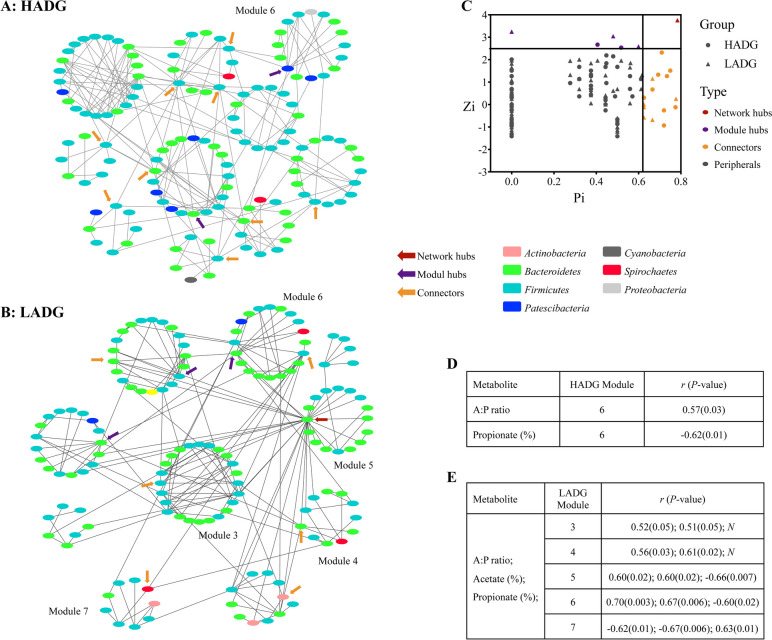
As bacteria-bacteria interactions are key modulators that shape the rumen microbiota, we further evaluated the potential microbial interactions (Fig. 4a, b) and identified the keystone bacterial taxa (Fig. 4c; Table S4) in the rumen fluid microbiota of HADG and LADG goats using random matrix theory (RMT)-based network analysis. A total of two nodes were highly connected species within their own module (a high Zi > 2.5 and a low Pi ≤ 0.62), which may act as module hubs in the HADG network (Fig. 4c; Table S4). ASV752 assigned to Candidatus Saccharimonas, and ASV4676 assigned to Oscillospiraceae NK4A214 group, may act as module hubs. Nine nodes had low Zi (≤ 0.25) and high Pi (> 0.62) values, linked several modules together and acted as connector species in the HADG network (Fig. 4c; Table S4). For example, ASV806, and ASV1105 were annotated to Oscillospiraceae NK4A214 group, and ASV4527 was annotated to Christensenellaceae R-7 group, which may act as connectors in the HADG network. In addition, three module hubs, two of which were annotated to Prevotellaceae UCG-003 and Christensenellaceae R-7 group, were identified in the LADG network. Notably, ASV2928 (annotated to Prevotella) had high Zi (> 2.5) and Pi (> 0.62) values, was highly connected species within its own module and linked several modules together, acting as a supergeneralist (network hub) that was detected in the LADG network (Fig. 4c; Table S4).
Fig. 4.
Co-occurrence network of ASVs in HADG and LADG goats, and network modules associated with rumen VFAs. Co-occurrence network of ASVs in HADG (A) and LADG goats (B). Nodes represent an ASV, and only significant (Pearson’s correlation, p < 0.05) relationships are shown in solid lines. C The scatter plot shows the distribution of ASVs based on their network roles. Network modules associated with rumen VFAs in HADG (D) and LADG goats (E). N: no significant difference HADG: young goats with high average daily gain, LADG: young goats with low average daily gain, Zi: within-module connectivity, Pi: among-module connectivity
Correlation analyses between module-based eigengenes and rumen VFAs were conducted to understand the relationship between individual modules and rumen fermentation (Fig. 4d, e). In the rumen microbial community of the LADG goats, at least five modules were significantly correlated with the VFAs or one of their two major components, acetate, and propionate. Module 3, 4, 5, and 6 were positively correlated with both the molar percentage of acetate, and the acetate to propionate ratio (r > 0.52, p < 0.05), while the latter two modules were negatively correlated with the molar percentage of propionate (r < -0.60, p < 0.05). Notably, the genus Prevotella was a predominant feature in module 3, including the network hub (ASV2928). In addition, 4 out of 11 or 4 out of 16 nodes in modules 4 and 5 were assigned to the family Prevotellaceae. In the HADG network, only module 6 was negatively associated with the molar percentage of propionate, and positively correlated with the acetate to propionate ratio (r >|0.57|, p < 0.05).
Functional variation in the rumen fluid microbiome related to the growth performance of young goats
We then compared the functional capacities of the rumen fluid microbiome between goats with high (n = 8) and low (n = 8) growth performance using metagenomic analysis. From a total of 1,200,051,228 metagenome reads (75,003,202 ± 965,517 per sample), 10,278,318 contigs were annotated (642,395 ± 26,180 per sample).
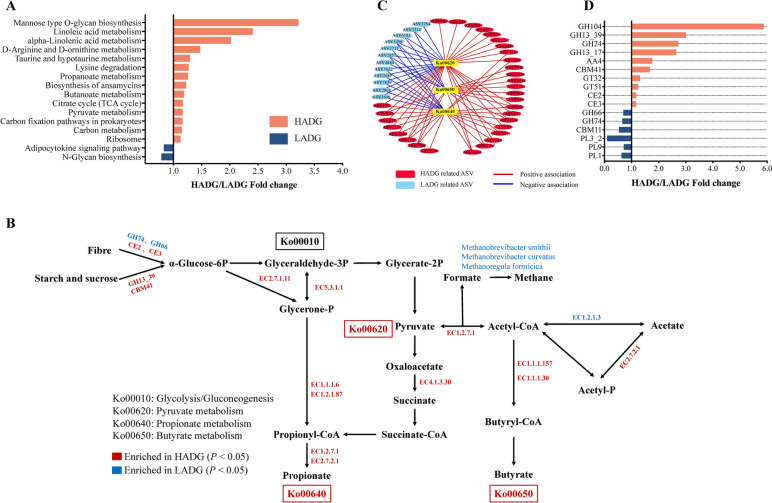
In total, 16 significantly different KEGG pathways were identified, 14 of which were enriched in the rumen microbiome of HADG goats (p < 0.05; Fig. 5a). Among them, 13 pathways were related to “metabolism” (first-level KEGG functions), including four “carbohydrate metabolism” pathways (TCA cycle, pyruvate metabolism, butanoate metabolism, and propionate metabolism), three “amino acid metabolism” pathways (taurine and hypotaurine metabolism, lysine degradation, and D-arginine and D-ornithine metabolism), two “lipid metabolism” pathways (linoleic acid metabolism, and alpha-linoleic acid metabolism), “energy metabolism” pathway (carbon fixation pathways in prokaryotes), “global and overview map” pathway (carbon metabolism), and “glycan biosynthesis and metabolism” pathway (mannose type O-glycan biosynthesis). In the rumen fluid microbiome of LADG goats, N-glycan biosynthesis and adipocytokine signaling pathways were enriched.
Fig. 5.
Difference in functional capacities of the rumen microbiome between HADG and LADG goats (n = 8 each group). A Differences in level 3 KEGG microbial pathways between HADG and LADG goats (Wilcoxon rank-sum test, p < 0.05). B Reconstruction of the metabolic pathway associated with VFA biosynthesis and methanogenesis. C Spearman’s rank correlations between the ADG-associated ASVs and the VFA-related KEGG pathways (p < 0.05). D Differential CAZyme functions between HADG and LADG goats based on family-level enzymes (Wilcoxon rank-sum test, p < 0.05) HADG: young goats with high average daily gain, LADG: young goats with low average daily gain
Because 4 out of 16 differentially abundant KEGG pathways were “carbohydrate metabolism,” which produce rumen VFAs, the main energy source, we then analyzed genes encoding enzymes involved in the metabolic pathways associated with VFA biosynthesis (glycolysis/gluconeogenesis, pyruvate metabolism, propionate metabolism, and butyrate metabolism). Of these identified enzymes that were significantly enriched in the rumen of HADG goats (p < 0.05; Fig. 5b; Table S5), 6-phosphofructokinase (EC2.7.1.11) and triose-phosphate isomerase (EC5.3.1.1) were involved in translating glucose-6P to pyruvate. Glycerol dehydrogenase (EC1.1.1.6) and propanal dehydrogenase (CoA-propanoylating, EC1.2.1.87) were involved in translating glycerone-P to propanoyl-CoA. Furthermore, methylisocitrate lyase (EC4.1.3.30), pyruvate synthase (EC1.2.7.1), and acetate kinase (EC2.7.2.1) were involved in translating pyruvate to propionate. Notably, methylisocitrate lyase (EC4.1.3.30), which converts methylisocitrate to succinate, was significantly higher in the HADG group. The results showed that 3-hydroxybutyryl-CoA dehydrogenase (EC1.1.1.157), and 3-hydroxybutyrate dehydrogenase (EC1.1.1.30), which can translate acetyl-CoA to butyrate, were also significantly higher in the HADG group. In contrast, aldehyde dehydrogenase (NAD +) (EC1.2.1.3), which is involved in translating acetyl-CoA to acetate, was enriched in the rumen microbiome of LADG goats (p = 0.031; Fig. 5b; Table S4). A Spearman’s rank correlation network further revealed varied relationships between ADG-associated ASVs and KEGG pathways (pyruvate metabolism, propionate metabolism, and butanoate metabolism) in the rumen fluid microbiome of HADG and LADG goats. A total of 43 ADG-related ASVs showed a significant correlation with these three pathways; 31 HADG-enriched ASVs, including some belonged to Candidatus Saccharimonas and Ruminococcus gauvreauii, had a positive correlation with these three pathways; and 12 LADG-enriched ASVs, such as ASV2717 belonged to Prevotellaceae UCG-003, had negative relationships with the propionate pathway and butyrate pathway (Fig. 5c; Table S6).
In addition, a total of 16 significantly differential CAZyme gene families were identified between the rumen fluid microbiome of HADG and LADG goats (p < 0.05; Fig. 5d). Among them, 10 CAZymes that were involved in the degradation of starch (such as GH13_39 and CBM41), xylan (CE2 and CE3), lignin (AA4), and peptidoglycan (GH24 and GH104) were enriched in the rumen fluid microbiome of HADG goats, while 6 CAZymes that were involved in the metabolism of cellulose (GH74 and GH66) and pectin (PL1, PL3_2 and PL9) were enriched in the rumen fluid microbiome of LADG goats.
Methanogenesis differed between high and low ADG goats
The metagenomics analysis revealed that several methane production-related archaeal species, including Methanobrevibacter smithii, Methanobrevibacter curvatus, and Methanoregula formicica, were significantly higher in the rumen of LADG goats (p < 0.05; Fig. 5b; Fig. S2). We further verified the effects of the rumen fluid microbiome on rumen fermentation and methane production using in vitro incubation analysis using rumen fluid from HADG and LADG young goats (n = 15 each group). The results showed that HADG goats produced significantly more propionate, less molar percentage of acetate, less acetate to propionate ratio, and less methane than LADG animals (p < 0.05; Fig. S3).
Rumen metabolome profiling differed between high and low performance goats
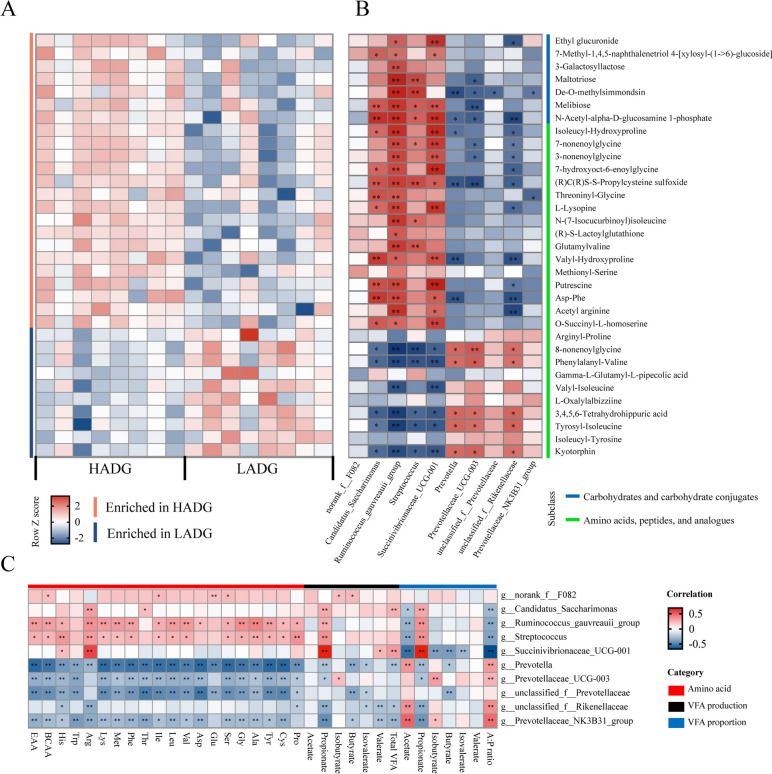
Further comparison of rumen metabolome profiles between HADG and LADG goats revealed 43 metabolites belonged to carbohydrates and carbohydrate conjugates, and amino acids, peptides, and analogs showed significant differences between HADG and LADG (p < 0.05; Table S7). Of these, 27 metabolites were significantly higher in the rumen of HADG goats, and the relative concentrations of 16 metabolites were significantly higher in the rumen of LADG goats (p < 0.05; Table S7). Then, correlation analysis between these different metabolome data and ADG was performed using Spearman’s rank correlation. The results showed that 33 metabolites were significantly associated with ADG (r >|0.5|, p < 0.05; Fig. 6a); 23 of them were significantly higher in HADG and positively associated with ADG, including L-lysopine, glutamylvaline, isoleucyl-hydroxyproline, threoninyl-glycine, valyl-hydroxyproline, methionyl-serine, and O-succinyl-l-homoserine, which belonged to amino acids, short peptides and analogs, and maltoriose, melibiose, and 3-galactosyllactose, which belonged to carbohydrates and carbohydrate conjugates (HADG-associated metabolites, p < 0.05). Meanwhile, 10 metabolites, including pheylalanyl-valine, valyl-isoleucine, and tyrosyl-isoleucine, were significantly higher in LADG and negatively associated with ADG (LADG-associated metabolites, p < 0.05).
Fig. 6.
The relationship between the ADG-associated microbiota and ruminal metabolites. A Ruminal metabolites that differed in normalized abundance between HADG and LADG goats (n = 8 each group) and were significantly associated with ADG (Spearman’s correlation, p < 0.05). B Correlations between the ADG-associated metabolites and the ADG-associated microbiota (n = 99, Spearman’s correlation). * p < 0.05, ** p < 0.01 HADG: young goats with high average daily gain, LADG: young goats with low average daily gain
The correlation analysis between ADG-related taxa and differential ruminal fermentation measures (Fig. 6c) showed that HADG-associated bacteria (Candidatus Saccharimonas, Ruminococcus gauvreauii group, Streptococcus, and Succinivibrionaceae UCG-001) were positively correlated with the concentration of propionate, and the molar percentage of propionate, and were negatively correlated with the molar percentage of acetate, and the acetate to propionate ratio (r >|0.2|, p < 0.05). Notably, the abundance of Ruminococcus gauvreauii group, Streptococcus also had a positive association with rumen amino acid concentration (r >|0.2|, p < 0.05). LADG-associated bacteria (Prevotella, Prevotellaceae UCG-003, unclassified Rikenellaceae, Prevotellaceae NK3B31 group) were positively correlated with the molar percentage of acetate, the acetate to propionate ratio, were negatively correlated with the concentration of propionate, and the molar percentage of propionate (r >|0.2|, p < 0.05); Prevotella, Prevotellaceae UCG-003, unclassified Prevotellaceae, and Prevotellaceae NK3B31 group were negatively correlated with the concentration of amino acids (r >|0.2|, p < 0.05).
Further analysis between ADG-associated microbiota and ADG-associated rumen metabolome (Fig. 6b) revealed that some HADG-enriched bacteria, such as Ruminococcus gauvreauii group, were positively correlated with nearly all HADG-associated metabolites; Candidatus Saccharimonas and Succinivibrionaceae UCG-001 were positively correlated with more than 50% HADG-associated metabolites, and Streptococcus was positively correlated with ~ 35% HADG-associated metabolites, and those bacteria were negatively correlated with ≥ 50% LADG-associated metabolites (p < 0.05). In contrast, some LADG-enriched bacteria were significantly negatively correlated with ADG-associated metabolites (p < 0.05). For example, Prevotella was negatively correlated with isoleucyl-hydroxyproline, and valyl-hydroxyproline; Prevotellaceae UCG-003 was negatively correlated with melibiose and maltotriose (p < 0.05).
Rumen fluid microbiota features and milk yield in adult goats with varied growth rates during early life
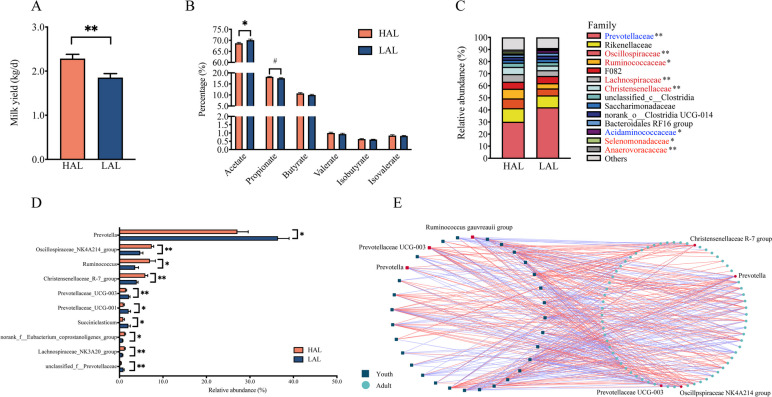
To determine whether the rumen fluid microbiota of young animals has long-term impacts on rumen microbiota features and host performance, we then measured the milk production, rumen fermentation, and microbiota of HADG lactating goats (HAL, n = 13) and LADG lactating goats (LAL, n = 12). The growth rate of these young goats (n = 25) had a significant positive correlation with their subsequent milk yield (r = 0.36, p = 0.048). Moreover, compared to the LAL group, the milk yield, milk fat, protein, and lactose yields were significantly higher in the HAL group (p < 0.05; Fig. 7a; Fig. S4a). Compared to the LAL goats, the HAL goats also had a lower molar percentage of acetate, and acetate to propionate ratio (p < 0.05; Fig. 7b; Fig. S4b).
Fig. 7.
The influence of the rumen fluid microbiome of young goats on the rumen fluid microbiota composition and milk performance of adult goats. A Comparison of milk yield between the HAL and LAL groups. B The difference in the percentage of ruminal VFAs between HAL and LAL goats. C Distribution of abundant bacterial families. D Top 10 different genera identified by the Wilcoxon rank-sum test between HAL and LAL goats. Bars represent the mean ± SE. E Correlation networks showed association in the microbiota between young and lactating goats. Edge color represents either positive or negative associations between bacteria (p < 0.05). The genera of young goats with more than 5 edges (links) were retained. A, B Student’s t test, data are presented as the mean ± SE. D Wilcoxon rank-sum test. E Spearman’s rank correlation. # 0.1 < p < 0.05 *p < 0.05, **p < 0.01 HAL: lactating goats of HADG, LAL: lactating goats of LADG
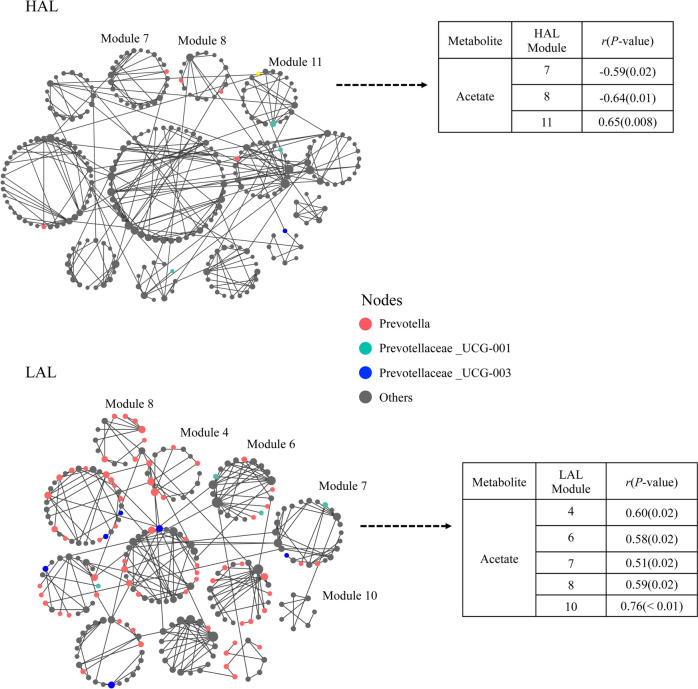
Although there was no significant difference in Chao1 and Shannon indices of rumen fluid microbiota between these two groups (Fig. S5a, b), the microbiota profiles of the HAL group clustered significantly different from that of the LAL group (ANOSIM R = 0.178, p = 0.002; Fig. S5c). Moreover, the family Prevotellaceae, genus Prevotella, and Prevotellaceae UCG-003 were significantly higher in the LAL group (p < 0.05; Fig. 7c, d). The abundance of Oscillpspiraceae NK4A214 group, Ruminococcus, and Christensenellaceae R-7 group were significantly higher in the HAL group (p < 0.05; Fig. 7c, d), with Prevotella, Prevotellaceae UCG-003, Oscillpspiraceae NK4A214 group, and Christensenellaceae R-7 group were the keystone bacteria in young goats (Table S4). Network module-trait relationship analysis revealed that in the LAL group, 5 modules (4, 6, 7, 8, and 10) were positively correlated with the molar percentage of acetate (r >|0.58|, p < 0.05; Fig. 8). The majority of nodes (ASVs) in two modules (4, and 8) belonged to the genus Prevotella (module 4: 6 out of 16, module 8: 7 out of 14). In contrast, in the HAL network, module 7 and module 8 were negatively correlated with the molar percentage of acetate (r < − 0.59, p < 0.02).
Fig. 8.
Co-occurrence network of rumen ASVs in HAL and LAL goats, and the network modules associated with the percent of acetate. Nodes represent an ASV, and only significant (Pearson’s correlation, p < 0.05) relationships are shown in solid lines HAL: lactating goats of HADG, LAL: lactating goats of LADG
The rumen keystone bacteria of young goats further affected host rumen microbiota features
Furthermore, the correlation between the bacteria in the rumen of young goats and adult goats was identified (genera with relative abundance ≥ 0.1%; Fig. 7e; Table S8). Notably, some ADG-related bacteria in the youth period were significantly correlated with bacteria in adult goats, such as Prevotella, LADG-enriched bacteria in the youth period, had a positive correlation with the abundance of Prevotella, had a negative correlation with Ruminococcus, and Christensenellaceae R-7 group (r >|0.4|, p < 0.05) in the lactation period. Prevotellaceae UCG-003, the LADG-enriched bacteria of young goats, was significantly correlated with more than 20 genera of adult goats, including Oscillpspiraceae NK4A214 group, Christensenellaceae R-7 group, and Lachnospiraceae NK3A20 group (r >|0.5|, p < 0.05). Ruminococcus gauvreauii group, the HADG-enriched bacteria in the youth period, had a positive correlation with Ruminococcus, Oscillpspiraceae NK4A214 group, Christensenellaceae R-7 group in the lactation period, had a negative correlation with Prevotella, and Prevotellaceae UCG-001 in the lactation period (r >|0.4|, p < 0.05).
Rumen microbiota and metabolites of young goats with high prediction accuracy for ADG and milk production traits
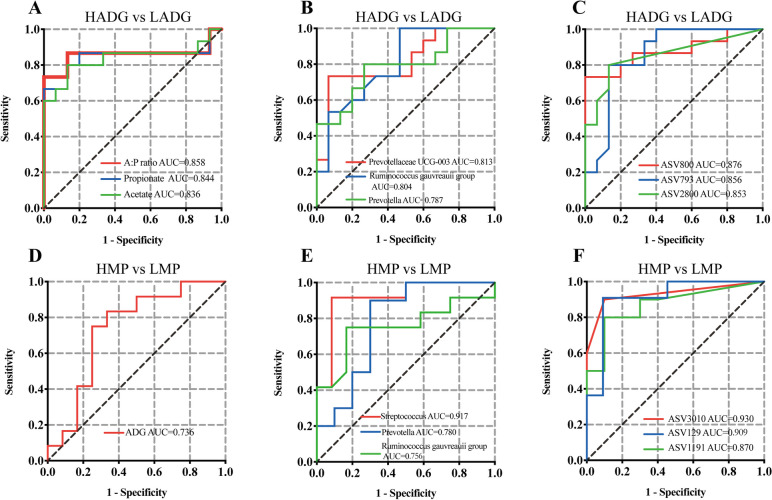
We then explored whether differential rumen fluid microbiota and metabolites associated with ADG in young goats can be used to predict ADG in youth, and/or milk production in adult animals using the random forest model. Rumen VFAs, such as the acetate to propionate ratio, the molar percentage of acetate, and propionate, classified the HADG and LADG goats with high accuracy (AUC > 0.830; Fig. 9a). A total of 30 differential metabolites could classify high and low ADG goats with AUC > 0.80 (Table S7). Of these, isoleucy-tyrosine, L-lysopine, and 3-nonenoylglycine were the top three features with AUC > 0.90 of rumen metabolome profiles (Fig. S6). Notably, we found that Prevotellaceae UCG-003, Ruminococcus gauvreauii group, and Prevotella had AUC values of 0.813, 0.804, and 0.787 in classified HADG and LADG goats (Fig. 9b), and the top 3 ASVs (ASV800: Candidatus Saccharimonas, ASV793: norank F082, ASV2800: Prevotellaceae UCG-003) with AUC values > 0.850 (Fig. 9c).
Fig. 9.
Prediction analyses based on the random forest model. Classification of host ADG using rumen metabolites (A) and microbiota (B, C) of young goats (n = 30), HADG vs LADG. Prediction of milk yield using ADG (D), and rumen microbiota (E, F) of young goats (n = 30), HMP vs LMP. ASV800: Candidatus Saccharimonas, ASV793: norank F082, ASV2800: Prevotellaceae UCG-003, ASV3010: unclassified Oscillospirales, ASV129: Streptococcus, ASV1191: Prevotella HADG: young goats with high average daily gain, LADG: young goats with low average daily gain, HMP: lactating goats with high milk yield, LMP: lactating goats with low-milk yield
Next, we explored whether ADG and rumen fluid microbiota of young goats could potentially serve as markers to predict adult milk yield. The results showed that the ADG of young goats had an AUC of 0.736 for classifying lactating goats with high- or low-milk yield (Fig. 9d). Rumen fluid bacteria, such as the top genus (Streptococcus) and ASV feature (ASV3010: unclassified Oscillospirales), had AUC values of 0.917 and 0.930, respectively, to predict milk yield. Moreover, the ADG-related genera, Ruminococcus gauvreauii group, and Prevotella, also showed AUC value > 0.750 (Fig. 9e, f) for milk yield prediction.
Discussion
In the present study, a large number of goats under the same dietary conditions and management were used to untangle the relationship between the rumen fluid microbiome and host growth rate, and revealed the microbiota and its metabolites of young ruminants affected their ADG and could have long-term implications and predict milk production during first lactation in dairy goats.
First, the current study identified varied complex interactions among rumen fluid microorganisms, and keystone taxa as well as their relationships with rumen fermentation and ADG in young goats. Compared with the studies conducted in dairy cows, very few studies have explored the rumen microbial composition and function in dairy goats. These few studies revealed that rumen microbial communities of goats play important roles in feed degradation and rumen fermentation [27] and undergo significant changes in response to shifts in age and diet [28, 29]. Many recent studies have investigated the potential impact of the rumen microbiome on cattle phenotypes (such as feed efficiency and milk production) [9, 13]. However, the microbial community structure, composition, and function differ between goats and cattle [27, 30]. Henderson et al. [30] reported that microbial communities of goats clustered separately from cattle in the rumen, with bacteria being the main drivers behind the observed differences. For example, unclassified Veillonellaceae were proportionally more abundant in the rumen of goats, while the abundance of Fibrobacter was enriched in the rumen of cattle. This suggests that the influence of the rumen microbiome on goat growth could differ from that reported for cattle. The current study is a large-scale systemic study to reveal the rumen microbiome and its relationship with goat growth, and milk production traits.
Most previous studies on the biodiversity of microbial communities have been focused on the number of species and the abundance of species, but not interactions among species. However, microbiota interactions could be more important to ecosystem functioning than bacterial richness and abundance in complex ecosystems [31, 32]. Co-occurrence network analysis of the rumen microbiome of dairy cows revealed differential microbial interaction patterns between animals with different feed efficiency [33]. Previous studies in the rumen microbiome showed that the keystone species play an exceptionally important role in determining the structure and function of ecosystems [12, 34]. In the present study, we found that more network modules in LADG and LAL goats than in HADG or HAL goats were positively correlated with the molar percentage of acetate. Based on network topology, we also found that HADG-enriched Candidatus Saccharimonas (module hub), LADG-enriched Prevotella (network hub) and Prevotellaceae UCG-003 (module hub) serve as keystone taxa of microbial networks in the rumen of HADG or LADG goats. These ADG-related keystone taxa together with non-ADG-related keystone taxa (Oscillospiraceae NK4A214 group, and Christensenellaceae R-7 group [module hubs and connectors]) may play important roles in microbiota structure and rumen function. From ecological perspectives, module hubs and connectors were close to generalists and network hubs were supergeneralists [35]. In this study, identifications of generalists and supergeneralists furthered the understanding of microbial community structure and differential microbial interactions, which play an empirical rather than theoretical role among microbial interactions [36]. Similar to our findings, a recent study found that Christensenellaceae R-7 group, and Prevotella were the keystone species in the network, and they may play important roles in the rumen of yaks at different growth stages [34]. The present study is among the first to document the importance of bacterial interactions and keystone species in shaping the rumen microbial communities and rumen functioning of goats.
In the lactating period, these keystone bacteria, including Prevotella, Christensenellaceae R-7 group, Oscillospiraceae NK4A214 group, and Prevotellaceae UCG-003, were also different between the HAL and LAL groups. Some specific bacteria, such as Prevotella, and Prevotellaceae UCG-003 of young goats, were significantly correlated with bacteria of adult animals, such as Prevotella, Oscillospiraceae NK4A214 group and Christensenellaceae R-7 group. Christensenellaceae, Oscillospiraceae, and Prevotellaceae and their genera were demonstrated to be keystone species for complex plant polysaccharides and were positively correlated with VFA concentrations [37–40]. These keystone species have similar reaction substrates and might regulate microbial interactions through nutritional supplementation or competition. Similar to our findings, Braga et al. [41] reported that microbes can interact with each other for the purpose of co-evolution leading to adaptation and specialization of certain microbial taxa, which promote future alterations in the microbial community. Recent studies found that increased growth and lactation performance could be achieved by increasing the abundance of rumen Oscillospiraceae NK4A214 group and Christensenellaceae R-7 group [42–44]. These genera have been reported to be associated with the degradation of a diverse array of structural carbohydrates as well as the production of propionate and butyrate [45, 46], which may provide more energy and a healthy rumen environment for host. Furthermore, Prevotella was the predominant, early colonizer and occupied various ecological niches within the rumen [47–49], and early arriving species exerted strong priority effects, having long-lasting impacts on the development of animal microbiomes [25].
In the present study, ADG-related genera played important roles in rumen fermentation (Fig. 6b, c) and had effects on goat growth. The abundance of Prevotellaceae family and its genera, which utilize various substrates (such as cellulose, starch, and protein) to mainly produce acetate and succinate [39, 40], have shown a negative relationship with goat growth rate. Moreover, the abundance of Prevotella was found to be negatively correlated with milk performance [8, 50], and feed efficiency [51] in ruminants. Indeed, our finding that the abundance of these Prevotella genera, and modules mainly composed of Prevotella have positive relationship with rumen acetate percentage provided further support for this notion (Fig. 4d, e; Fig. 6c; Fig. 8). However, in contrast to these findings, some studies also identified various Prevotella species associated with higher feed efficiency [52], and milk production [13, 53]. These conflicting results could be related to the large diversity of Prevotella with different functions, and the different metabolites shaped by microbe‒microbe interactions (the interaction between Prevotella with other genera). These findings suggested that strong microbial interactions of genus Prevotella with other others may increase rumen acetate production in LADG and LAL goats.
Candidatus saccharimonas, Ruminococcus gauvreauii group, Streptococcus, and Succinivibrionaceae UCG-001, HADG-related genera, also play important roles in feed digestion. Streptococcus and Candidatus Saccharimonas have been shown to be involved in amino acid biosynthesis and the metabolism of energy substrates [37, 54–56]. Furthermore, as a growth-related taxon in pigs [57], Streptococcus may also play an important role in promoting goat growth performance. Members of Succinivibrionaceae family compete with methanogens for hydrogen to produce succinate (a precursor of propionate) rather than methane [58], and were found to be associated with low methane yield [59], high ADG [60], and low abundance of methanogens [58] in ruminates. Meanwhile, our metagenome data revealed that fewer methanogens, such as Methanobrevibacter smithii, Methanobrevibacter curvatus, and Methanoregula formicica, were detected in HADG goats. Together, these bacteria are therefore candidates for further investigation to elucidate their association with growth, which might provide insight into whether these microbes could be manipulated to improve feed efficiency and the performance of goats.
Together, rumen keystone bacteria and early colonizers may exert priority effects, occupy ecological niches, and form complex microbial interactions with others, which will have long effects on microbiota succession, rumen fermentation, and host growth phenotypes. Feed intake is one of the key factors that affects animal growth performance, feed efficiency, and rumen microbiome. Long-term experiments should be conducted to further determine the relationship between feed intake, rumen microbiome, and feed efficiency of ruminants. Inoculating keystone species associated with feed efficiency or animal performance as precision probiotics in early life may modify microbiota colonization or composition, and subsequently improve animal performance. The present study investigated the potential role of rumen fluid microbiome in goats at early stage in regulating rumen fermentation and production performance in adult dairy goats. However, in addition to the liquid-associated microbiome, rumen particle- and epithelium-associated microbiome also play a key role in regulating rumen function [61, 62]. Future research activities will be carried out to comprehensively understand the role of different kinds of microbiome in the rumen, and how different members of the rumen microbiota may influence each other, and effective microbial manipulation tools and techniques.
There are considerable benefits associated with understanding rumen function, as rumen dynamics are almost solely responsible for providing nutrients to the host animal [63]. The genes encoding CAZymes involved in deconstructing carbohydrates, such as starch (GH13_39 and CBM41) and xylan (CE2 and CE3), and KEGG functions in carbohydrate metabolism were enriched in the rumen of HADG goats, including “TCA cycle, pyruvate metabolism, butanoate metabolism, and propionate metabolism.” The genes encoding relevant enzymes, such as EC2.7.1.11, which were involved in the glycolysis pathway, EC4.1.3.30, EC1.1.157, and EC1.2.7.1, which were involved in the pyruvate, propionate, and butyrate metabolism pathways, were enriched in HADG goats. EC1.2.13, which was involved in translating acetyl-Coa to acetate, was less abundant in HADG goats. These results indicated that the microbiome of HADG goats has a stronger ability to degrade carbohydrates to produce pyruvate, and subsequently used for more propionate and butyrate production, rather than methane and acetate biosynthesis. Together, these findings clearly indicated that the specific microbiome functional potential related to carbohydrate degradation and VFA biosynthesis significantly changes in goats with different growth rates, which advances the understanding of the functional roles of the rumen microbiome in contributing to rumen fermentation and goat growth.
The present study was one of the first to explore the relationship between rumen metabolites, microbiota taxa, and growth performance of dairy goats. These findings of rumen metabolite data are in good agreement with the rumen metagenome that more disaccharides, such as maltoriose, melibiose, and 3-galactosyllactose, which are intermediates in carbohydrate metabolism, and propionate and butyrate production were enriched in HADG goats, methane yield and acetate biosynthesis were enriched in LADG goats (Fig. 2 and Fig. 6). These disaccharides can be hydrolyzed to produce glucose for growth and development of the body [64]. Furthermore, in rumen VFA fermentation, propionate fermentation is the most energy efficient, due to assimilating energy from hydrogen and being the main precursor of gluconeogenesis in animals, whereas acetate production is accompanied by hydrogen production [65, 66]. For host animals, the energy recovery efficiency is increased by 9% when the substrate (glucose) is fermented into propionate but reduced by 22% and 38% when fermented into butyrate and acetate, respectively [67]. Indeed, studies have revealed that efficient animals tend to have higher ruminal propionate and butyrate concentrations [9, 68]. Together, our data suggest that the microbiome of HADG goats has a higher feed efficiency with a higher ability and efficiency to degrade carbohydrates to produce more propionate and butyrate, less acetate and methane, to support the host’s energy requirements. Furthermore, amino acids and peptides are extremely important factors affecting rumen microbial growth and microbial protein synthesis [69], in which microbial protein contributes 60–90% of the protein absorbed at the duodenum [8, 70]. Compared to the LADG goats, 3 amino acid metabolism pathways, taurine and hypotaurine metabolism, lysine degradation, and D-arginine and D-ornithine metabolism, were enriched in the HADG goat microbiome. We also found that some amino acids and peptides were enriched in HADG goats, including BCAAs and some EAAs, which (such as His, Trp, and Arg) are the limiting AAs for growing goats and cows [71, 72]. Moreover, we identified the associations between rumen microbiota and rumen microbial metabolomes and found that ADG-associated microbial taxa were significantly correlated with carbohydrate and protein metabolism. Overall, our data revealed that some specific rumen taxa, such as Prevotella, and Streptococcus, may play important roles in producing small molecule metabolites and contributing to goat growth, which has rarely been reported in dairy goats previously.
Furthermore, the development of sequencing techniques and machine learning methods facilitates the application of the microbiota and microbial metabolites to predict host phenotypes, including for the prediction of disease risk [73, 74] and performance [33]. In the present study, we found that rumen metabolites and microbes could predict host growth performance with high accuracy (Fig. 9a–c). Moreover, the rumen microbiota of young goats can also predict further milk yield with high accuracy (Fig. 9d,e). Notably, the random forest machine learning algorithm revealed that Streptococcus in young goats can be a key microbial marker that can differentiate high- and low-milk yield goats, with an accuracy of 91.7%. To our knowledge, it is still challenging to predict ruminant performance using only early-life microbial markers in practice. Further studies are required to integrate various key data, such as host genetic data, microbiome features, and metabolites, and find the best combined features to improve the accuracy and robustness of prediction, which could help us apply our findings in practice in the future.
The rumen microbial community is influenced by multiple factors, including diet, environment, and host genotype. In our study, all the goats were fed the same diet and raised under the same feeding and management regimes, the interanimal variations in the rumen microbiome may be directly or indirectly affected by animal genetics. Several studies have revealed that some rumen microbiota are heritable and associated with host phenotypes [12, 75, 76]. For example, a recent study found that Prevotella was related to several loci on cattle chromosomes 2, 6, 9, 19, 23, and 27 [76]. Future studies focus on revealing the relationship between rumen microbiota and genetic markers, which will help us use marker-assisted selection and management to improve feed efficiency, and animal performance.
Conclusion
In summary, ADG-related microbiota and microbial interactions affect rumen fermentation and feed efficiency, and subsequently affect the energy and nutrients that are supplied to the host. Our study strengthens the notion that rumen microbial variations can lead to variations in feed efficiency, which will affect host performance. Overall, rumen microbiome features (such as diversity, structure, composition, and function) were different among young goats with different growth rates. Some bacteria and archaeal species, such as members of Prevotellaceae family, Streptococcus, and Candidatus Saccharimonans, were identified for their significant associations with animal growth. These rumen microbial variations contribute to carbohydrate and protein metabolism functions, and ruminal propionate, butyrate, and ruminal amino acid production in high ADG goats, which could provide more energy and nutrients for goats. Additionally, high ADG goats have more milk yield in the lactation stage. Some keystone bacteria may have long effects on microbiota succession, feed fermentation, and host phenotypes. The rumen microbiota of young goats identified by random forest analysis could be used as effective biomarkers for predicting animal performance (growth rate and milk yield). Thus, our results provide a deeper understanding of the potential influence of the rumen microbiome on feed efficiency and animal performance, highlighting the long-term role of keystone bacteria in microbe‒microbe interactions and in shaping microbiota composition and rumen fermentation patterns, and may aid in developing strategies to improve feed efficiency and animal performance.
Methods
Animals, grouping, and sampling
All the goats, which were fed the same diet, kept under the same conditions, and housed together during the whole experimental period, were raised in a Saanen goat farm in Baoji, Shaanxi (34° 41′ N, 109° 09′ E). The detailed feeding programs are shown and described in Supplementary Table S8. Animals that had been administered antimicrobial agents (antibiotics, antifungals or antivirals) within 3 months before sampling or had a history of an infectious disease or other force majeure were excluded from the experiment.
A total of 99 healthy female Guanzhong goats (born in late January 2020) were used for the experiment (Fig. 1). The birth weight of all goat kids was recorded immediately after birth. Herein, at the age of 6 months old (187.9 ± 0.3 [mean ± SE] days of age), the body weight of all selected goats was weighed at least two times, the mean body weight was calculated for each goat, and rumen fluid and blood samples were collected before the morning feeding. Individual weight gain was calculated as the difference between 6-month body weight and birth weight, and individual ADG was calculated as weight gain divided by the number of days.
The feed intake of HADG and LADG goats (n = 15 each group) was measured 2 weeks before weighing (174.2 ± 0.4 days). In brief, feed offered to and refused by each goat was recorded continuously for 7 days. The feed samples were dried at 65°C for 48 h to obtain the dry matter content of the ration. Daily dry matter intake (DMI) per goat was calculated by multiplying daily as-fed intake by the dry matter content of the ration. There was no significant difference in DMI between the HADG and LADG (1.03 ± 0.03 vs 1.01 ± 0.02, P = 0.735, t test).
Then, these dairy goats were naturally oestrus and mated in late September (8 months old). A total of 15 goats, who were not pregnant, or had been administered antimicrobial agents within 3 months before sampling, or had a history of infectious disease, or some other force majeure were excluded from the sampling. Then, since kidding in late February 2021, 84 remaining dairy goats entered the lactation period. The lactation performance of all dairy goats was recorded. Briefly, all 84 goats were milked twice daily at 0630 and 1600 h. The milk yield was recorded every 7 days over the first whole lactation period, the milk sample (2/3 from the morning and 1/3 from the evening milking were pooled as a daily sample) was collected for the milk composition analysis, and the average milk yield, milk composition of each goat was used for the following analysis. Meanwhile, the rumen fluid sample was also collected at 2 to 3 h after the morning feeding of the 23rd week of lactation (19 months old).
Briefly, rumen fluid samples were collected via esophageal tubing. The first 50 mL of rumen fluid was discarded to avoid saliva contamination, and the next 50 mL rumen fluid was strained through four layers of sterile cheesecloth under a constant flux of CO2.
According to the calculated ADG from birth to 6 months old, the 15 highest ADG goats of these 99 goats were selected as the HADG group (ADG: 132.5 ± 1.5 g/day), and the 15 lowest ADG goats were selected as the LADG group (ADG: 88.2 ± 1.2 g/day). After delivery of their newborn offspring kids, 84 of 99 lactating goats remained. Among these 84 lactating goats, 13 lactating goats of 15 HADG goats remained as the HAL group; 12 lactating goats of 15 LADG goats remained as the LAL group. Furthermore, in order to explore whether the rumen fluid bacteria, VFAs and ADG of young goats could serve as useful biomarkers to predict the milk yield in adult animals, the 15 lactating goats of these 84 lactating goats with the highest milk yield were selected and named the HMP group (average daily milk yield: 2.82 ± 0.04 kg/day), and 15 lactating goats with the lowest milk yield were selected and named the LMP group (average daily milk yield: 1.51 ± 0.03 kg/day), according to the milk yield recorded every 7 days over the first whole lactation period.
Estimation of methane production using an in vitro experiment using rumen fluid of young goats
To estimate the ruminal methane production of young goats (6 months old) with different growth rates, rumen fluid samples of the HADG and LADG groups (n = 15 each group) were collected on the same day and used for the in vitro rumen fermentation study immediately.
The in vitro experiment was conducted following the procedure of Mauricio et al. [77], and artificial saliva (buffer) was prepared according to the formula provided by Menke et al. [78]. Briefly, under anaerobic condition, approximately 1.0 g total mixed ration (TMR) of young goats (DM basis, shown in Table S9) was weighed into individual 135 mL fermentation bottles, and then 20 mL collected rumen fluid from each goat, and 40 mL buffer was added into the fermentation bottle. Fermentation bottles, which contained the differential rumen fluid from different young goats of the HADG and LADG groups (n = 15 each group), were sealed with butyl rubber stoppers and aluminum crimp caps and placed in an incubator at 39°C for 24 h in a simulative incubator under anaerobic condition (ANKOM DAISY II, Ankom Technology Macedon, New York, USA).
At 4, 8, 12, 18, and 24 h of incubation, the total gas of each bottle was separately collected. The total gas of the same incubation tubes collected at different times was mixed. The volume of the total gas produced was determined using a glass syringe. Then, approximately 15 mL of gas sample was taken from the collected total gas and injected into gas chromatography to determine the CH4 concentration [79, 80], and the total CH4 production was calculated as CH4 concentration × total gas production. Furthermore, after 24 h of incubation, fermentation was terminated by placing bottles on ice. After opening the bottles, 5 mL fermentation broths were collected and stored at − 80°C until for VFA analysis.
VFAs, amino acids, milk composition measurement
The concentrations of VFAs (acetate, propionate, butyrate, valerate, isobutyrate, and isovalerate) were determined using gas chromatography (Agilent 7820A, Santa Clara, CA, USA) with a capillary column (AE-FFAP of 30 m × 0.25 mm × 0.33 μm; ATECH Technologies Co., Lanzhou, China) according to the method described by Li et al. [81]. In brief, the thawed rumen fluid samples were centrifuged for 10 min at 16,000 × g at 4°C. Two milliliters of the supernatant were mixed with 25% metaphosphoric acid (400 μL). After standing for 4 h at 4°C, the mixture was centrifuged for 10 min at 16,000 × g at 4°C. Two hundred microliters of crotonic acid (10 g/L) were added to an aliquot (200 μL) of the supernatants and then filtered through a 0.45-μm filter. The injector and detector temperatures were set at 200°C and 250°C, respectively. The column temperature was increased from 45 to 150°C at 20°C/min and held for 5 min.
Rumen free amino acid abundance analysis was performed through separation and quantification by liquid chromatography‒mass spectrometry (LC‒MS) (Exion LC AC, QTRAP 5500, AB SCIEX, Framingham, MA, USA) with a phase column (4.6 mm × 100 mm × 2.7 μm Infinity Lab Poroshell 120 EC-C18, Agilent, Santa Clara, CA, USA) [82]. The MS is equipped with electrospray (ESI) as an ion source and is an ion mode of ESI + . The main parameters were as follows: curtain gas, 40 psi; collision gas, medium; ion spray voltage, 5500 V; temperature, 650°C; ion source gas 1, 60 psi; ion source gas 2, 60 psi. The LC conditions were as follows: column temperature, 30°C; flow rate, 0.8 mL/min; automatic sampler temperature, 15°C; injection volume, 5 μL; mobile phase A, 0.1% acetic acid + water; mobile phase B, 0.1% acetic acid + acetonitrile.
Milk samples were analyzed for fat, protein, and lactose using a milk composition analyzer (MilkoScan FT1, FOSS, Denmark).
DNA extraction and 16S rRNA gene sequencing
Total DNA was extracted from rumen fluid samples of 99 young goats and 25 lactating goats (HAL: n = 13, LAL: n = 12; Fig. 1) using the QIAamp DNA Stool Mini kit (QIAGEN, Germany) according to the manufacturer’s protocol. The DNA concentration was measured with a Nanodrop-2000 (Thermo Fisher Scientific, USA), and the quality was assessed by 1% agarose gel electrophoresis. Bacterial 16S rRNA gene fragments (V3-V4) were amplified from the extracted DNA using the forward primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and the reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). All amplicons were sequenced using the paired-end (2 × 300 bp) method on a MiSeq platform (Illumina, USA) following standard protocols [83].
The raw sequences were merged with FLASH (v1.2.11) [84] and quality filtered with fastp (0.19.6) [85]. Sequences were imported into QIIME2 v2021.8 for demultiplexing and the construction of an amplicon sequence variant (ASV) table using DADA2 [86]. Bacterial 16S ASVs were assigned a taxonomy using the SILVA database (version 138) as the reference. The relative abundance of a taxon in the sample was the fraction of the taxon observed in the ASV table relative to the sum of all observed taxa corresponding to the sample in the ASV table. Alpha diversity indices, including the Sobs, ACE, Chao1 richness estimate, and Shannon diversity index, were calculated using QIIME2, and analyzed at the ASV level. Principal coordinate analysis (PCoA) was performed based on Bray‒Curtis distance, and statistical significance was determined using analysis of similarities (ANOSIM) with 999 permutations at the ASV level. Taxa with relative abundance ≥ 0.1% for downstream analysis of correlation and comparison.
Metagenome sequencing
DNA extract of HADG and LADG (n = 8 each group) was fragmented to an average size of approximately 400 bp using Covaris M220 (Gene Company Limited, China) for paired-end library construction. Individual sequencing libraries were prepared using the TruSeqTM DNA Sample Prep Kit (Illumina, San Diego, CA, USA). Metagenome library sequencing was performed on an Illumina HiSeq4000 platform (150 bp paired-end sequencing; Illumina Inc., San Diego, CA, USA).
Adapter sequences were trimmed off from the paired-end reads using SeqPre (v1.1). Low-quality reads (quality scores < 20 or length < 50 or having N bases) were removed using Sickle (v1.33). Host reads were filtered by aligning reads against the Capra hircus genome with BWA (v0.7.9a) and removing reads with high-scoring alignments host. These high-quality reads were then assembled into contigs using Megahit (v1.1.2). The assembled contigs were subjected to prediction of open reading frames (ORFs) using MetaGeneMark (v2.10) [87]. Non-redundant contigs were identified using CD-HIT (v4.6.1) at 95% sequence identity and 90% coverage [88]. The quality-filtered sequence reads were mapped to the representative sequences with 95% identity using SOAPaligner (v2.2.1) [89], and the gene abundance in each sample was calculated as reads per kilobase per million mapped reads (RPKM).
Representative sequences of non-redundant gene catalog were aligned to the NCBI NR database using Diamond (v0.8.35) for taxonomic annotations using the Best-hit method [90]. KEGG annotation was conducted using Diamond against the Kyoto Encyclopedia of Genes and Genomes database (v94.2). Carbohydrate-active enzyme annotation was conducted using hmmscan against the CAZy database [91]. All these databases had an E-value cut-off of 1e−5 while annotating ORFs.
Analysis of rumen metabolome
The rumen fluid samples of young goats were thawed at 4°C and extracted using methanol/acetonitrile (1:1, v/v) buffer at 1:2 of sample: buffer. The mixtures were vortexed for 30 s, and then extracted in an ultrasonic bath (Kunshan Ultrasonic Instrument Co. Ltd., China) at 5°C with an ultrasonic frequency of 40 kHz for 30 min and incubated at − 20°C for 30 min. After centrifugation at 13,000 × g for 15 min at 4°C to precipitate the protein, the supernatants were transferred and dried using a vacuum evaporator. The concentrated product was resuspended in 100 μL of water/acetonitrile (1:1, v/v), and then subjected to LC‒MS analysis using an UHPLC system (Q-Exactive, Thermo Fisher Scientific, USA). Quality control was performed via a pooled QC sample by mixing equal volumes (20 μL) of each sample. Chromatographic separations were performed on an ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm) (Waters Co., USA). The column temperature was maintained at 40°C, and the injection volume was 2.0 μL. Eluent A was prepared by mixing water/acetonitrile (5:95, v/v), and eluent B was prepared by mixing acetonitrile/2-propanol/water (47.5:47.5:5, v/v/v). The mass spectrometric data in both positive and negative modes were collected using an electrospray ionization source.
The LC‒MS data were processed using Progenesis QI software to extract raw peaks, filter and calibrate baseline, align peaks, deconvolute, identify peaks, and integrate peak areas. Rumen metabolite that was present in < 50% of samples or with a relative standard deviation > 30% were removed [92]. The metabolites were annotated into “carbohydrates and carbohydrate conjugates,” and “amino acids, peptides, and analogs” (subclass) using the human metabolome databases [93] for downstream analysis.
Construction of microbial co-occurrence networks based on random matrix theory
Microbial co-occurrence networks were constructed using a random matrix theory (RMT)-based pipeline with default parameters as described by Deng et al. [94] to identify microbial interactions. Briefly, the data matrix of standardized relative abundance, multiplied by 106 to satisfy the requirements of the pipeline, was uploaded to construct the network with default settings, including (1) the ASVs detected in ≤ 50% of all samples were excluded due to a drastic effect of ASV sparsity on the precision and sensitivity of network inference [95]; (2) only filling with 0.01 in blanks with paired valid values. The fast-greedy modularity optimization procedure was used for module separation. After modules were determined, eigengene analysis was used to reveal higher-order organizations in the network structure [94, 96]. In eigengene analysis, each module is represented by its singular value decomposition of the abundance profile called the module eigengene [96]. The within-module degree (Zi) and among-module connectivity (Pi) were calculated and plotted to generate a scatter plot for each network. The relationship between module-based eigengenes and environmental traits was analyzed using Pearson correlation coefficients. The visualization of the network structure was performed using Cytoscape v3.8.0. In this study, we used the simplified classification as follows: (i) peripheral nodes (Zi ≤ 2.5, Pi ≤ 0.62), which had only a few links and almost always to the nodes within their modules, (ii) connectors (Zi ≤ 2.5, Pi > 0.62), and (iii) module hubs (Zi > 2.5, Pi ≤ 0.62). Species with either a high value of Zi or Pi were generalists. These included module hubs, i.e., highly connected species linked to many species within their own module, and connectors highly linked to several modules. (iv) Network hubs or supergeneralists (Zi > 2.5, Pi > 0.62), acted as both module hubs and connectors [35]. These connectors, module hubs, and network hubs of the microbial network may play keystone roles in the microbial communities, which were called keystone species in this study.
Construction of random forest classifier
The random forest package in R was used for the random forest analysis [97], with the rumen fluid bacteria, rumen metabolites, and ADG of young goats (HADG and LADG, HMP and LMP) being used as the inputs of the random forest model to classify high or low ADG goats, and/or predict milk yield in adult goats. Each genus, ASV from 16S rDNA sequence data (relative abundance ≥ 0.1%), metabolite from metabolome data, and each VFA were considered as a feature. The machine learning design was performed according to measure the description by Verhaar et al. [98]. To present overfitting, we used a nested cross-validation design in performing these models. In each of the 30 iterations, the dataset was randomly split into a test set containing 30% of the subjects and a training set with the remaining 70%. Within the training set, fivefold cross-validation was performed to optimize the model hyperparameters. The resulting model was evaluated on the test set, which yielded an area under the receiver-operator curve (AUC). These were recorded for each iteration and were averaged across 30 iterations. The random forest analysis was implemented in R using the randomForest package with default parameters. ROC curve results were plotted manually by the true positive rate against the false positive rate. ROC curves were constructed, and the AUC was used to designate the ROC effect.
Statistical analysis
The 16S rDNA sequence data (rumen microbial alpha diversity, phyla, families, and genera) and metabolome data (KEGG pathways, KEGG enzymes, CAZymes, and rumen archaea) were compared between the two compare groups (HADG vs LADG, HAL vs LAL) which were compared using the Wilcoxon rank-sum test with the FDR adjusted p value < 0.05 considered significantly different. Phenotypic data (milking traits, and rumen VFAs) were compared between the two groups using t test, and p value < 0.05 was considered significant. Each metabolite from rumen metabolome data was compared using the Wilcoxon rank-sum test between two groups, with the FDR adjusted p value < 0.05, and the variable importance in projection (VIP) from orthogonal partial least squares discriminant analysis > 1 being considered significantly different metabolites.
Correlation analysis between rumen bacterial taxa, alpha diversity indices, rumen VFAs, AAs, and growth performance traits (n = 99) was performed using Spearman’s rank correlation, with coefficient >|0.2|, p value < 0.05 considered significant. Correlation analysis between ADG-related bacteria and metabolome profiles, ADG-related ASV and KEGG pathways (n = 16) was performed using Spearman’s rank correlation, with coefficient >|0.4|, as well as p value < 0.05 that was considered significant. Pairwise correlations (Spearman’s correlation, p < 0.05) were used to generate genus-level co-occurrence networks.
Supplementary Information
Acknowledgements
The authors acknowledge the members of the Innovative Research Team of Animal Nutrition & Healthy Feeding of Northwest A&F University for their assistance in the field sampling.
Abbreviations
- ADG
Average daily gain
- ANOSIM
Analysis of similarities
- ASV
Amplicon sequence variant
- AUC
Area under the receiver operating characteristic curve
- BW
Body weight
- CAZyme
Carbohydrate-active enzymes
- CV
Coefficient of variation
- DMI
Daily dry matter intake
- FDR
False discovery rate
- GH
Glycoside hydrolases
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- PCoA
Principal coordinate analysis
- PERMANOVA
Permutational multivariate analysis of variance
- SE
Standard error
- VFAs
Volatile fatty acids
- WG
Weight gain
Author’s contributions
Dangdang Wang, Junhu Yao, Le Luo Guan, and Shengru Wu conceived and designed the research. Dangdang Wang, Luyu Chen, Guangfu Tang, JunjianYu, and Jie Chen collected the rumen and serum samples and performed the experiment. Shengru Wu, Zongjun Li, Yangchun Cao, and Xinjian Lei discussed the data and gave advice during the experiments. Dangdang Wang and Luyu Chen analyzed the data. Shengru Wu, Le Luo Guan, and Lu Deng helped with the bioinformatics and statistical analysis. Dangdang Wang wrote the manuscript, and all other authors revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (award number: 32272829) and Chinese Universities Scientific Fund (award number: 2452022252).
Availability of data and materials
Raw sequencing data of all 16S rRNA sequences and metagenomes have been deposited into the NCBI Sequence Read Archive (SRA) under accession numbers PRJNA836638 and PRJNA836913, respectively.
Declarations
Ethics approval and consent to participate
Animal care and experimental procedures were approved by the Animal Care Committee of Northwest A&F University (Yangling, China) and followed the university’s guidelines for animal research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shengru Wu, Email: yaojunhu2004@sohu.com.
Le Luo Guan, Email: leluo.guan@ualberta.ca.
Junhu Yao, Email: wushengru2013@163.com.
References
- 1.Verruck S, Dantas A, Prudencio ES. Functionality of the components from goat’s milk, recent advances for functional dairy products development and its implications on human health. J Funct Foods. 2019;52:243–257. doi: 10.1016/j.jff.2018.11.017. [DOI] [Google Scholar]
- 2.Park YW. Goat milk–chemistry and nutrition. In: Park YW, Haenlein GFW, editors. Handb Milk Non-Bovine Mamm. Oxford: Blackwell Publishing; 2017. pp. 42–83. [Google Scholar]
- 3.Haenlein GFW. Goat milk in human nutrition. Small Ruminant Res. 2004;51:155–163. doi: 10.1016/j.smallrumres.2003.08.010. [DOI] [Google Scholar]
- 4.Mucha S, Mrode R, Coffey M, Kizilaslan M, Desire S, Conington J. Genome-wide association study of conformation and milk yield in mixed-breed dairy goats. J Dairy Sci. 2018;101:2213–2225. doi: 10.3168/jds.2017-12919. [DOI] [PubMed] [Google Scholar]
- 5.Vacca GM, Stocco G, Dettori ML, Pira E, Bittante G, Pazzola M. Milk yield, quality, and coagulation properties of 6 breeds of goats: environmental and individual variability. J Dairy Sci. 2018;101:7236–7247. doi: 10.3168/jds.2017-14111. [DOI] [PubMed] [Google Scholar]
- 6.Marnet PG, Komara M. Management systems with extended milking intervals in ruminants: regulation of production and quality of milk. J Anim Sci. 2008;86:47–56. doi: 10.2527/jas.2007-0285. [DOI] [PubMed] [Google Scholar]
- 7.Argov-Argaman N, Glasser T, Glasser T, Muklada H, Hadaya O, Mesilati-Stahy R, Raz C, et al. Lipidome changes, with a focus on phospholipids, due to feeding systems and processing in goat milk. Food Chem. 2021;340:127938. doi: 10.1016/j.foodchem.2020.127938. [DOI] [PubMed] [Google Scholar]
- 8.Russell JB, Rychlik JL. Factors that alter rumen microbial ecology. Science. 2001;292:1119–1122. doi: 10.1126/science.1058830. [DOI] [PubMed] [Google Scholar]
- 9.Shabat SKB, Sasson G, Doron-Faigenboim A, Durman T, Yaacoby S, Berg Miller ME, et al. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016;10:2958–2972. doi: 10.1038/ismej.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Hitch TC, Chen Y, Creevey CJ, Guan LL. Comparative metagenomic and metatranscriptomic analyses reveal the breed effect on the rumen microbiome and its associations with feed efficiency in beef cattle. Microbiome. 2019;7:6. doi: 10.1186/s40168-019-0618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamke J, Kittelmann S, Soni P, Li Y, Tavendale M, Ganesh S, et al. Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome. 2016;4:56. doi: 10.1186/s40168-016-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace RJ, Sasson G, Garnsworthy PC, Tapio I, Gregson E, Bani P, et al. A heritable subset of the core rumen microbiome dictates dairy cow productivity and emissions. Sci Adv. 2019;5:eaav8391. doi: 10.1126/sciadv.aav8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue MY, Sun HZ, Wu XH, Liu JX, Guan LL. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome. 2020;8:64. doi: 10.1186/s40168-020-00819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue MY, Sun HZ, Wu XH, Guan LL, Liu JX. Assessment of rumen bacteria in dairy cows with varied milk protein yield. J Dairy Sci. 2019;102:5031–5041. doi: 10.3168/jds.2018-15974. [DOI] [PubMed] [Google Scholar]
- 15.Yáñez-Ruiz DR, Abecia L, Newbold CJ. Manipulating rumen microbiome and fermentation through interventions during early life: a review. Front Microbiol. 2015;6:1133. doi: 10.3389/fmicb.2015.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arshad MA, Hassan FU, Rehman MS, Huws SA, Cheng Y, Din AU. Gut microbiome colonization and development in neonatal ruminants: strategies, prospects, and opportunities. Anim Nutr. 2021;7:883–895. doi: 10.1016/j.aninu.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muscato T, Tedeschi L, Russell J. The effect of ruminal fluid preparations on the growth and health of newborn, milk-fed dairy calves. J Dairy Sci. 2002;85:648–656. doi: 10.3168/jds.S0022-0302(02)74119-2. [DOI] [PubMed] [Google Scholar]
- 18.Malmuthuge N, Liang G, Guan LL. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019;20:172. doi: 10.1186/s13059-019-1786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong RZ, Sun HX, Li GD, Liu HW, Zhou DW. Effects of inoculation with rumen fluid on nutrient digestibility, growth performance and rumen fermentation of early weaned lambs. Livest Sci. 2014;162:154–158. doi: 10.1016/j.livsci.2013.12.021. [DOI] [Google Scholar]
- 20.Yu S, Zhang G, Liu Z, Wu P, Yu Z, Wang J. Repeated inoculation with fresh rumen fluid before or during weaning modulates the microbiota composition and co-occurrence of the rumen and colon of lambs. BMC Microbiol. 2020;20:29. doi: 10.1186/s12866-020-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belanche A, Palma-Hidalgo JM, Nejjam I, Jiménez E, Martín-García AI, Yáñez-Ruiz DR. Inoculation with rumen fluid in early life as a strategy to optimize the weaning process in intensive dairy goat systems. J Dairy Sci. 2020;103:5047–5060. doi: 10.3168/jds.2019-18002. [DOI] [PubMed] [Google Scholar]
- 22.Soberon F, Raffrenato E, Everett RW, Van Amburgh ME. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J Dairy Sci. 2012;95:783–793. doi: 10.3168/jds.2011-4391. [DOI] [PubMed] [Google Scholar]
- 23.Gelsinger SL, Heinrichs AJ, Jones CM. A meta-analysis of the effects of preweaned calf nutrition and growth on first-lactation performance. J Dairy Sci. 2016;99:6206–6214. doi: 10.3168/jds.2015-10744. [DOI] [PubMed] [Google Scholar]
- 24.Zanton GI, Heinrichs AJ. Meta-analysis to assess effect of prepubertal average daily gain of Holstein heifers on first-lactation production. J Dairy Sci. 2005;88:3860–3867. doi: 10.3168/jds.S0022-0302(05)73071-X. [DOI] [PubMed] [Google Scholar]
- 25.Furman O, Shenhav L, Sasson G, Kokou F, Honig H, Jacoby S, et al. Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat Commun. 2020;11:1904. doi: 10.1038/s41467-020-15652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dill-McFarland KA, Weimer PJ, Breaker JD, Suen G. Diet influences early microbiota development in dairy calves without long-term impacts on milk production. Appl Environ Microbiol. 2019;85:e02141–e2218. doi: 10.1128/AEM.02141-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giger-Reverdin S, Domange C, Broudiscou LP, Sauvant D, Berthelot V. Rumen function in goats, an example of adaptive capacity. J Dairy Res. 2020;87:45–51. doi: 10.1017/S0022029920000060. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Xu Q, Kong F, Yang Y, Wu D, Mishra S, et al. Exploring the goat rumen microbiome from seven days to two years. PLoS One. 2016;11:e0154354. doi: 10.1371/journal.pone.0154354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen J, Zheng L, Chen X, Han X, Cao Y, Yao J. Metagenomic analyses of microbial and carbohydrate-active enzymes in the rumen of dairy goats fed different rumen degradable starch. Front Microbiol. 2020;11:1003. doi: 10.3389/fmicb.2020.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson G, Cox F, Ganesh S, Jonker A, Young W, Colloaborators GRC, et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015;5(1):14567. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon R, Wein T, Levy B, Eshed S, Dror R, Reiss V, et al. Protozoa populations are ecosystem engineers that shape prokaryotic community structure and function of the rumen microbial ecosystem. ISME J. 2022;16(4):1187–1197. doi: 10.1038/s41396-021-01170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukami T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Syst. 2015;46:1–23. doi: 10.1146/annurev-ecolsys-110411-160340. [DOI] [Google Scholar]
- 33.Xue MY, Xie YY, Zhong Y, Ma XJ, Sun HZ, Liu JX. Integrated meta-omics reveals new ruminal microbial features associated with feed efficiency in dairy cattle. Microbiome. 2022;10:32. doi: 10.1186/s40168-022-01228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo W, Zhou M, Ma T, Bi S, Wang W, Zhang Y, et al. Survey of rumen microbiota of domestic grazing yak during different growth stages revealed novel maturation patterns of four key microbial groups and their dynamic interactions. Anim Microbiome. 2020;2:23. doi: 10.1186/s42523-020-00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olesen JM, Bascompte J, Dupont YL, Jordano P. The modularity of pollination networks. Proc Natl Acad Sci. 2007;104:19891–19896. doi: 10.1073/pnas.0706375104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan Z, Chen Y, Zhou M, McAllister TA, Guan LL. Microbial interaction-driven community differences as revealed by network analysis. Comput Struct Biotechnol J. 2021;19:6000–8. doi: 10.1016/j.csbj.2021.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogunade I, Schweickart H, McCoun M, Cannon K, McManus C. Integrating 16S rRNA sequencing and LC–MS-based metabolomics to evaluate the effects of live yeast on rumen function in beef cattle. Animals. 2019;9:28. doi: 10.3390/ani9010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascual J, Hahnke S, Abendroth C, Langer T, Ramm P, Klocke M, et al. Draft genome sequence of a new Oscillospiraceae bacterium isolated from anaerobic digestion of biomass. Microbiol Resour Announc. 2020;9:e00507–e520. doi: 10.1128/MRA.00507-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah HN, Chattaway MA, Rajakurana L, Gharbia SE. Prevotella. In: Whitman WB, editor. Bergey's Man Syst Archaea Bact. New York: John Wiley & Sons; 2015. pp. 1–25. [Google Scholar]
- 40.Qi K, Men X, Wu J, Deng B, Xu Z. Effects of growth stage and rearing pattern on pig gut microbiota. Curr Microbiol. 2022;79:136. doi: 10.1007/s00284-022-02828-2. [DOI] [PubMed] [Google Scholar]
- 41.Braga RM, Dourado MN, Araújo WL. Microbial interactions: ecology in a molecular perspective. Braz J Microbiol. 2016;47:86–98. doi: 10.1016/j.bjm.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong Y, Guo C, Wang L, Chen F, Dong X, Li X, et al. Effects of paper mulberry silage on the growth performance, rumen microbiota and muscle fatty acid composition in hu lambs. Fermentation. 2021;7(4):286. doi: 10.3390/fermentation7040286. [DOI] [Google Scholar]
- 43.Huang C, Ge F, Yao X, Guo X, Bao P, Ma X, et al. Microbiome and metabolomics reveal the effects of different feeding systems on the growth and ruminal development of yaks. Front Microbiol. 2021;12:682989. doi: 10.3389/fmicb.2021.682989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tong J, Zhang H, Yang D, Zhang Y, Xiong B, Jiang L. Illumina sequencing analysis of the ruminal microbiota in high-yield and low-yield lactating dairy cows. Plos One. 2018;13(11):e0198225. doi: 10.1371/journal.pone.0198225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morotomi M, Nagai F, Watanabe Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int J Syst Evol Microbiol. 2012;62(1):144–149. doi: 10.1099/ijs.0.026989-0. [DOI] [PubMed] [Google Scholar]
- 46.Weinert-Nelson JR, Biddle AS, Williams CA. Fecal microbiome of horses transitioning between warm-season and cool-season grass pasture within integrated rotational grazing systems. Anim Microbiome. 2022;4(1):41. doi: 10.1186/s42523-022-00192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue MY, Sun HZ, Wu XH, Guan LL, Liu JX. Assessment of rumen microbiota from a large dairy cattle cohort reveals the pan and core bacteriomes contributing to varied phenotypes. Appl Environ Microbiol. 2018;84:e00970–e1018. doi: 10.1128/AEM.00970-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Zhang K, Zhang C, Feng Y, Zhang X, Wang X, et al. Dynamics and stabilization of the rumen microbiome in yearling Tibetan sheep. Sci Rep. 2019;9:19620. doi: 10.1038/s41598-019-56206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moraïs S, Mizrahi I. Islands in the stream: from individual to communal fiber degradation in the rumen ecosystem. FEMS Microbiol Rev. 2019;43:362–379. doi: 10.1093/femsre/fuz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jami E, White BA, Mizrahi I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. Plos One. 2014;9:e85423. doi: 10.1371/journal.pone.0085423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carberry CA, Kenny DA, Han S, McCabe MS, Waters SM. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl Environ Microbiol. 2012;78:4949–4958. doi: 10.1128/AEM.07759-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jewell KA, McCormick CA, Odt CL, Weimer PJ, Suen G. Ruminal bacterial community composition in dairy cows is dynamic over the course of two lactations and correlates with feed efficiency. Appl Environ Microbiol. 2015;81:4697–4710. doi: 10.1128/AEM.00720-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Indugu N, Vecchiarelli B, Baker LD, Ferguson JD, Vanamala JK, Pitta DW. Comparison of rumen bacterial communities in dairy herds of different production. BMC Microbiol. 2017;17:190. doi: 10.1186/s12866-017-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong J, Zhang H, Yang D, Zhang Y, Xiong B, Jiang L. Illumina sequencing analysis of the ruminal microbiota in high-yield and low-yield lactating dairy cows. Plos One. 2018;13:e0198225. doi: 10.1371/journal.pone.0198225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin D, Zhao S, Wang P, Zheng N, Bu D, Beckers Y, et al. Insights into abundant rumen ureolytic bacterial community using rumen simulation system. Front Microbiol. 2016;7:1006. doi: 10.3389/fmicb.2016.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kakimoto S, Okazaki K, Sakane T, Imai K, Sumino Y, Akiyama S, et al. Isolation and taxonomie characterization of acid urease-producing bacteria. Agric Biol Chem. 1989;53:1111–1117. doi: 10.1080/00021369.1989.10869439. [DOI] [Google Scholar]
- 57.Wang X, Tsai T, Deng F, Wei X, Chai J, Knapp J, et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome. 2019;7:109. doi: 10.1186/s40168-019-0721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCabe MS, Cormican P, Keogh K, O’Connor A, O’Hara E, Palladino RA, et al. MiSeq phylogenetic amplicon sequencing shows a large reduction of an uncharacterised Succinivibrionaceae and an increase of the Methanobrevibacter gottschalkii clade in feed restricted cattle. Plos One. 2015;10:e0133234. doi: 10.1371/journal.pone.0133234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pope PB, Smith W, Denman SE, Tringe SG, Barry K, Hugenholtz P, et al. Isolation of Succinivibrionaceae implicated in low methane emissions from Tammar wallabies. Science. 2011;333:646–648. doi: 10.1126/science.1205760. [DOI] [PubMed] [Google Scholar]
- 60.Daghio M, Ciucci F, Buccioni A, Cappucci A, Casarosa L, Serra A, et al. Correlation of breed, growth performance, and rumen microbiota in two rustic cattle breeds reared under different conditions. Front Microbiol. 2021;12:652031. doi: 10.3389/fmicb.2021.652031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strachan CR, Yu XA, Neubauer V, Mueller AJ, Wagner M, Zebeli Q, et al. Differential carbon utilization enables co-existence of recently speciated Campylobacteraceae in the cow rumen epithelial microbiome. Nat Microbiol. 2023;8(2):309–320. doi: 10.1038/s41564-022-01300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Mulder T, Goossens K, Peiren N, Vandaele L, Haegeman A, De Tender C, et al. Exploring the methanogen and bacterial communities of rumen environments: solid adherent, fluid and epimural. FEMS Microbiol Ecol. 2017;93(3):fiw251. doi: 10.1093/femsec/fiw251. [DOI] [PubMed] [Google Scholar]
- 63.Krehbiel CR. Invited review: applied nutrition of ruminants: fermentation and digestive physiology. Prof Anim Sci. 2014;30:129–39. doi: 10.15232/S1080-7446(15)30100-5. [DOI] [Google Scholar]
- 64.Navarro DMDL, Abelilla JJ, Stein HH. Structures and characteristics of carbohydrates in diets fed to pigs: a review. J Anim Sci Biotechnol. 2019;10:39. doi: 10.1186/s40104-019-0345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goopy JP, Woodgate R, Donaldson A, Robinson DL, Hegarty RS. Validation of a short-term methane measurement using portable static chambers to estimate daily methane production in sheep. Anim Feed Sci Technol. 2011;166:219–226. doi: 10.1016/j.anifeedsci.2011.04.012. [DOI] [Google Scholar]
- 66.Janssen PH. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim Feed Sci Technol. 2010;160:1–22. doi: 10.1016/j.anifeedsci.2010.07.002. [DOI] [Google Scholar]
- 67.Owens FN, Basalan M. Ruminal Fermentation. In: Millen DD, Arrigoni MDB, Pacheco RDL, editors. Rumenology. Switzerland: Springer International Publishing; 2016. pp. 63–103. [Google Scholar]
- 68.Guan LL, Nkrumah JD, Basarab JA, Moore SS. Linkage of microbial ecology to phenotype: correlation of rumen microbial ecology to cattle's feed efficiency. FEMS Microbiol Lett. 2008;288:85–91. doi: 10.1111/j.1574-6968.2008.01343.x. [DOI] [PubMed] [Google Scholar]
- 69.Argyle JL, Baldwin RL. Effects of amino acids and peptides on rumen microbial growth yields. J Dairy Sci. 1989;72:2017–2027. doi: 10.3168/jds.S0022-0302(89)79325-5. [DOI] [PubMed] [Google Scholar]
- 70.Wallace RJ, Onodera R, Cotta MA. Metabolism of nitrogen-containing compounds. In: Hobson PN, Stewart CS, editors. Rumen Microb Ecosyst. London: Chapman & Hall; 1997. pp. 283–328. [Google Scholar]
- 71.Shan JG, Tan ZL, Sun ZH, Hu JP, Tang SX, Jiang HL, et al. Limiting amino acids for growing goats fed a corn grain, soybean meal and maize stover based diet. Anim Feed Sci Technol. 2007;139:159–169. doi: 10.1016/j.anifeedsci.2007.01.019. [DOI] [Google Scholar]
- 72.Abe M, Iriki T, Funaba M, Onda S. Limiting amino acids for a corn and soybean meal diet in weaned calves less than three months of age. J Anim Sci. 1998;76:628–636. doi: 10.2527/1998.762628x. [DOI] [PubMed] [Google Scholar]
- 73.Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25:968–976. doi: 10.1038/s41591-019-0458-7. [DOI] [PubMed] [Google Scholar]
- 74.Ma T, Villot C, Renaud D, Skidmore A, Chevaux E, Steele M, et al. Linking perturbations to temporal changes in diversity, stability, and compositions of neonatal calf gut microbiota: prediction of diarrhea. ISME J. 2020;14:2223–2235. doi: 10.1038/s41396-020-0678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sasson G, Kruger Ben-Shabat S, Seroussi E, Doron-Faigenboim A, Shterzer N, Yaacoby S. Heritable bovine rumen bacteria are phylogenetically related and correlated with the cow’s capacity to harvest energy from its feed. mBio. 2017;8:e00703–17. doi: 10.1128/mBio.00703-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbas W, Howard JT, Paz HA, Hales KE, Wells JE, Kuehn LA, et al. Influence of host genetics in shaping the rumen bacterial community in beef cattle. Sci Rep. 2020;10:15101. doi: 10.1038/s41598-020-72011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mauricio RM, Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim Feed Sci Technol. 1999;79:321–330. doi: 10.1016/S0377-8401(99)00033-4. [DOI] [Google Scholar]
- 78.Menke KH, Raab L, Salewski A, Steingass H, Fritz D, Schneider W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J Agric Sci. 1979;93:217–222. doi: 10.1017/S0021859600086305. [DOI] [Google Scholar]
- 79.Navarro-Villa A, O’brien M, López S, Boland TM, O’kiely P. Modifications of a gas production technique for assessing in vitro rumen methane production from feedstuffs. Anim Feed Sci Technol. 2011;166:163–74. doi: 10.1016/j.anifeedsci.2011.04.064. [DOI] [Google Scholar]
- 80.Friedman N, Shriker E, Gold B, Durman T, Zarecki R, Ruppin E, et al. Diet-induced changes of redox potential underlie compositional shifts in the rumen archaeal community. Environ Microbiol. 2017;19:174–184. doi: 10.1111/1462-2920.13551. [DOI] [PubMed] [Google Scholar]
- 81.Li F, Yang X, Cao Y, Li S, Yao J, Li Z, et al. Effects of dietary effective fiber to rumen degradable starch ratios on the risk of sub-acute ruminal acidosis and rumen content fatty acids composition in dairy goat. Anim Feed Sci Technol. 2014;189:54–62. doi: 10.1016/j.anifeedsci.2013.12.011. [DOI] [Google Scholar]
- 82.Chen J, Lei XJ, Wang L, Zhang YL, Wang DD, Zhao LC, et al. Effects of rumen-protected leucine on production performance and starch digestion in the small intestine of lactating goats. Anim Feed Sci Technol. 2022;287:115270. doi: 10.1016/j.anifeedsci.2022.115270. [DOI] [Google Scholar]
- 83.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu W, Lomsadze A, Borodovsky M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010;38:e132. doi: 10.1093/nar/gkq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24:713–714. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- 90.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 91.Yin Y, Mao X, Yang J, Chen X, Mao F, Xu Y. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40(W1):W445–W451. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011;6:1060–1083. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- 93.Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46:D608-D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deng Y, Jiang YH, Yang Y, He Z, Luo F, Zhou J. Molecular ecological network analyses. BMC Bioinformatics. 2012;13:113. doi: 10.1186/1471-2105-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weiss Y, Class C, Goldstein SL, Hanyu T. Key new pieces of the HIMU puzzle from olivines and diamond inclusions. Nature. 2016;537:666–670. doi: 10.1038/nature19113. [DOI] [PubMed] [Google Scholar]
- 96.Langfelder P, Horvath S. Eigengene networks for studying the relationships between co-expression modules. BMC Syst Biol. 2007;1:1–17. doi: 10.1186/1752-0509-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Breiman L. Random forests. Mach Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 98.Verhaar BJ, Hendriksen HM, de Leeuw FA, Doorduijn AS, van Leeuwenstijn M, Teunissen CE, et al. Gut microbiota composition is related to AD pathology. Immunol. 2022;12:794519. doi: 10.3389/fimmu.2021.794519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data of all 16S rRNA sequences and metagenomes have been deposited into the NCBI Sequence Read Archive (SRA) under accession numbers PRJNA836638 and PRJNA836913, respectively.