Abstract
Background
Complex surgical resection and reconstruction for rare thoracic cancers (RTCs) represent a major challenge, given their very low frequency, extreme variability of presentation, multi-modality treatment options and inadequate outcome prediction. We analysed the experience of a tertiary referral centre on a consecutive series of patients with thoracic germ cell tumours, thymomas and sarcomas, with the aim of reporting the long-term outcome by cancer type and complexity of surgical procedures.
Methods
From Jan 2003 to Dec 2018, 768 surgical procedures were performed with curative intent on 644 RTC patients. Study endpoints were: post-operative hospital stay (Pod), 30-day and 90-day mortality, 5-year and 10-year overall survival (OS). Median follow-up of alive patients was 7.2 years.
Results
Median Pod was 7 days, with a 1.2% 30-day and 2.9% 90-day mortality. OS was 90.8% at one year, 74.2% at five years and 62.8% at 10 years. Ten-year OS was 73.0% in low, 65.3% in intermediate, and 55.6% in high complexity score (Log-rank tests p<0.0001); 66.6% in patients with one or two reconstructions and 46.4% in patients with three or more reconstructions (p<0.0001); 46.0% with vascular and 50.0% with chest wall reconstruction; 71.8% in germ cell tumours, 64.6% in thymoma and 51.3% in sarcoma (p<0.0001).
Conclusion
Complex surgical resection and reconstruction was associated with acceptable 90-day mortality and good 10-year survival in all RTC types. A predictive score based on surgical complexity and cancer type can help the clinical decision making.
Keywords: Rare thoracic cancer, complex surgical resection, post-operative mortality, 10-years survival
Introduction
Rare cancers encompass a very heterogeneous group of diseases with an incidence of fewer than six cases per 100,000 individuals per year 1 and accounting about 25% of all malignancies worldwide. 2 Specifically, rare thoracic cancers (RTCs) represent 8% of all tumours of the chest cavity 3 with a crude incidence in Europe and in Italy of 6.8 and 5.4 per 100 000 people per year, respectively.2,3
According to the results of RARECAREnet and SEER databases, in the European Union (EU) and USA the five-year survival of all rare cancers is lower (48.5 and 55%, respectively) compared with all common cancers (63.4 and 74%, respectively).2,3 The reasons for this worse survival may be related to the lack of effective and standardised treatments as well as delays in diagnosis. 4 On the other hand, RTCs represent an exception in terms of survival, confirming a better outcome in EU and Italian population (13.4% and 17%, respectively) compared with their more common counterparts with a five-year survival of 10.1%.2,3
In the setting of RTCs, the SEER database (from 2009 to 2013) reports an incidence of 0.22-0.25 cases per 100,000 per year for thymomas, 0.04-0.09 for mediastinal germ-cell tumours, and 0.03-0.80 for thoracic sarcomas. 1 Such a low incidence results in a limited source of data from the literature, with small case series, no prospective studies5-7 and very rare registry studies reported.8-11 Given their very low frequency, extreme variability of clinical presentation, different treatment options and limited individual expertise, RTCs represent a challenge for most surgeons who could face troubles in surgical decision making and choice of multimodality strategy. The available literature indicates the need to centralise diagnostic work-up and treatment in reference centres with high volume, specific expertise, and multidisciplinary approach.
In this scenario, we reported our experience of a tertiary referral centre on an unselected series of RTC patients to provide more solid evidence on surgical management, perioperative and long-term outcome with a new predictive model, based on the complexity of surgical procedures.
Methods
Study design and participants
From January 2003 to December 2018, 1265 consecutive patients admitted to the IRCCS Istituto Nazionale dei Tumori of Milan with a diagnosis of RTC were retrospectively evaluated, and 644 undergoing a curative resection for germ-cell tumour, thymoma or sarcoma were included in this analysis, 487 for primary tumour and 157 for metastatic disease. Among metastatic patients, only thymomas or germ-cell tumours with pulmonary, pleural or mediastinal metastases were included. Lung metastasectomies for sarcomas were not included. All available information on tumour type, description of surgery (date, type, complexity, number and type of reconstructions), post-operative stay and follow-up were retrieved.
Complexity of surgical procedure was classified in Low, Intermediate and High, (as reported in the online Supplementary Material) according to the extension of surgical resection and kind of associated reconstruction. Patients with unresectable tumour, macroscopic (R2) residual resection or undergoing biopsy alone were excluded from the analysis. Demographic information was collected at the hospitalisation and the vital status was obtained through the Istituto Nazionale di Statistica (ISTAT, SIATEL 2.0 platform), which provides the exact date of death within three months of occurrence. The follow-up was closed at June 2020, with a median observation time of 7.2 years for alive patients.
The study was approved by the appropriate institutional review committee of the Fondazione IRCCS Istituto Nazionale dei Tumori of Milan (INTM) and meets the guidelines of their responsible governmental agency.
Statistical analysis
The study endpoints were: a) post-operative hospital stay; b) post-operative 30-day and 90-day mortality; c) post-operative one-year, five-year and 10-year survival (OS). Follow-up time was calculated from the date of surgery to the date of death from any cause or last follow-up. Proportions were compared by Chi-square test or Fisher’s exact test, as appropriate. Survival functions were estimated using Kaplan–Meier Curves and comparisons were tested by Log-rank test. Multivariate Cox regression models were performed to estimate hazard ratios (HRs) and 95% Confidence Interval (CI) of complexity score and number of reconstructions, adjusted for age, sex and type of tumour. Long-term survival curves were truncated at 10 years. All analyses were performed using the Statistical Analysis System Software (Release SAS:9.04; SAS Institute, Cary, North Carolina, USA).
Results
Patient characteristics and surgical information
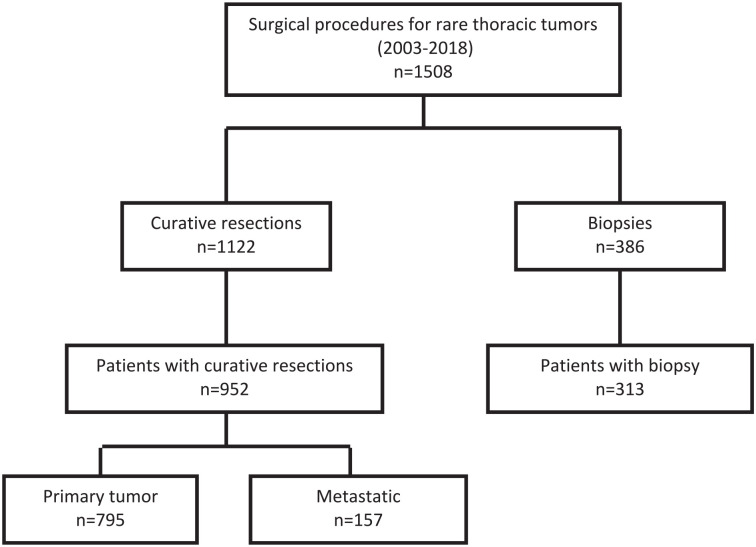
A total of 768 surgical procedures performed from January 2003 to December 2018 for germ-cell tumour, thymoma or sarcoma were included in this analysis: 539 (70.1%) resections for primary tumour and 229 (29.8%) for metastatic disease. (Figure 1). A total of 676 procedures for mesothelioma, esophageal cancer and other rare tumours were excluded.
Figure 1.
Consort diagram.
Patient’s characteristics are summarised in Table 1: median age was 47 years (IQR 33-61) and 33.2% were female. Patients with germ cell tumour were younger than other types, with median age of 32 (IQR 26-39) and most of them were males (195/205, 95%). Conversely, most of the older patients (⩾ 65 years) had thymoma (51.5%) or sarcoma (47.7%). Females were mostly affected by thymoma (47.2%) or sarcoma (48.1%), and 61.2% had a high complexity score. Complexity score and number of reconstructions were higher in older patients. Details on the numbers of reconstructions made according to tumour type were reported in online Supplemental Table S1. The median age of patients who underwent vascular reconstructions was 48, and of chest wall reconstruction 54.
Table 1.
Patients’ characteristics according to tumour and surgical information.
| Age | Sex | ||||
|---|---|---|---|---|---|
| Total | < 40 | ⩾ 65 | Median (Qrange) | Female | |
| 644 | 232 (36.0%) | 132 (20.5%) | 47 (33-61.5) | 214 (33.2%) | |
| Type | |||||
| Sarcoma | 227 | 53 (22.8%) | 63 (47.7%) | 53 (40-66) | 103 (48.1%) |
| Thymoma | 212 | 22 (9.5%) | 68 (51.5%) | 58.5 (49-66) | 101 (47.2%) |
| Germ Cell | 205 | 157 (67.7%) | 1 (0.8%) | 32 (26-39) | 10 (4.7%) |
| Primary tumour | 487 | 127 (54.7%) | 128 (97.0%) | 53 (39-65) | 203 (94.9%) |
| Metastatic disease | 157 | 105 (45.3%) | 4 (3.0%) | 35 (28-43) | 11 (5.1%) |
| Complexity score | |||||
| L | 220 | 117 (50.4%) | 22 (16.7%) | 38 (29-53) | 42 (19.6%) |
| I | 86 | 16 (6.9%) | 19 (14.4%) | 55 (43-64) | 41 (19.2%) |
| H | 338 | 99 (42.7%) | 91 (68.9%) | 52 (37-65) | 131 (61.2%) |
| Reconstructions | |||||
| None | 297 | 136 (58.6%) | 39 (29.6%) | 42 (30-56) | 76 (35.5%) |
| 1 or 2 | 185 | 53 (22.8%) | 46 (34.9%) | 52 (37-64) | 77 (36.0%) |
| ⩾ 3 | 162 | 43 (18.5%) | 47 (35.6%) | 52.5 (38-66) | 61 (28.5%) |
| Vascular reconstructions | 71 | 21 (9.1%) | 15 (11.4%) | 48 (38-62) | 20 (9.3%) |
| Chest Wall reconstructions | 143 | 35 (15.15) | 43 (32.6%) | 54 (40-66) | 62 (29.0%) |
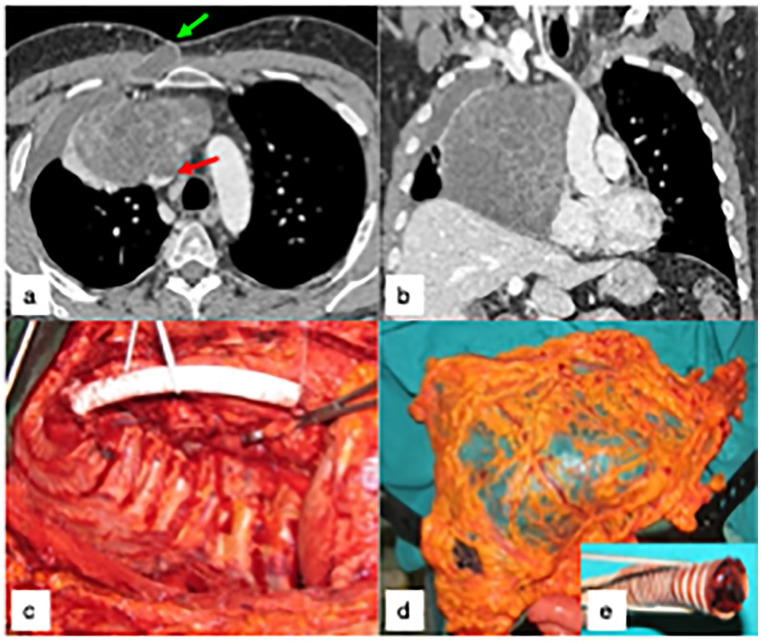
Figure 2 illustrates a case of primary mediastinal germ cell tumour, treated by extended surgical resection and reconstruction, followed by salvage surgery for severe postoperative complications, and excellent long-term outcome.
Figure 2.
(a-b) shows the preoperative CT of a 35 years-old male with primary mediastinal germ cell neoplasm invading the superior vena cava (SVC, red arrow), previously treated in another hospital by two cycles of PEB chemotherapy and 36Gy of external RT, with normalization of blood markers and apparent tumor size increase, presenting with a severe septic clinical picture, pleural empyema and cutaneous fistula (green arrow). The patient was treated by right extra-pleural pneumonectomy and SVC replacement with a PTFE prosthesis, through a thoraco-sternotomy approach (right hemi-clamshell, Figure 2c). Six months later, he was re-admitted for recurrent right pleural empyema with broncho-pleural fistula, and treated by a pedicled omental flap (Figure 2d) after removal of the infected PFE prosthesis (Figure 2e). The patient is alive and well, seventeen years later.
Post-operative mortality
In all 768 resections, median post-operative hospital stay (Pod) was seven days, with a 1.2% 30-day mortality and 2.9% 90-day mortality (Table 2). Length of hospital stay slightly increased with the increase of complexity and of the number of reconstructions, not reaching statistical significance.
Table 2.
Postoperative hospital stay and mortality in 768 resections (in 644 patients).
| N resections | Pod (median) |
30d MO | P-value | 90d MO | P-value | |
|---|---|---|---|---|---|---|
| Total | 768 | 7 | 1.2% | 2.9% | ||
| Type | ||||||
| Sarcoma | 259 | 8 | 1.5% | 0.2544 a | 2.7% | 0.9817 b |
| Thymoma | 237 | 6 | 1.7% | 3.0% | ||
| Germ Cell | 272 | 6 | 0.4% | 2.9% | ||
| Primary tumour | 539 | 8 | 1.7% | 0.0643 a | 3.7% | 0.0324 a |
| Metastatic disease | 229 | 5 | 0% | 0.9% | ||
| Complexity score | ||||||
| L | 308 | 5 | 0.7% | 0.4558 a | 1.9% | 0.3601 a |
| I | 95 | 6 | 1.1% | 2.1% | ||
| H | 365 | 9 | 1.6% | 3.8% | ||
| Reconstructions | ||||||
| None | 396 | 5 | 0.8% | 0.2939 a | 2.0% | 0.1302 b |
| 1 OR 2 | 193 | 7 | 1.0% | 2.6% | ||
| ⩾ 3 | 179 | 11 | 2.2% | 5.0% | ||
| Vascular reconstructions | 76 | 12 | 5.3% | 7.9% | ||
| Chest Wall reconstructions | 151 | 9 | 1.3% | 3.3% | ||
Pod, post-operative day; MO, mortality.
Fisher exact test.
Chi-Squared test.
No statistically significant difference in 30-day mortality was found according to tumour types (p=0.2544): 0.4% in germ cell tumour, 1.5% in sarcoma, and 1.7% in thymoma. The same applied to 90-days mortality (p=0.9817): 2.7% in sarcoma, 2.9% in germ cell tumour, and 3.0% in thymoma. Both 30- and 90-days mortalities were not statistically different in strata of complexity score (p=0.4458 and p=0.3601 respectively): resections with a high complexity score had a higher 30-days mortality of 1.6% and 90-days mortality of 3.8%. Similarly, no statistically significant differences were found stratifying by the number of reconstructions (30-days p=0.2339, 90-days p=0.1302): patients with ⩾3 reconstructions had a 2.2% 30-days and 5.0% 90-days.
The 90-day post-operative mortality was significantly lower in metastatic disease than in primary tumours (0.9% vs. 3.7%, p=0.0324, Table 2).
The results of multivariate Cox models (online Supplementary Table S2) did not show a significant impact of complexity score and number of reconstructions on 30-day and 90-day post-operative mortality after the adjustment for age and sex (Model A) or age, sex and tumour type (Model B).
Long-term survival
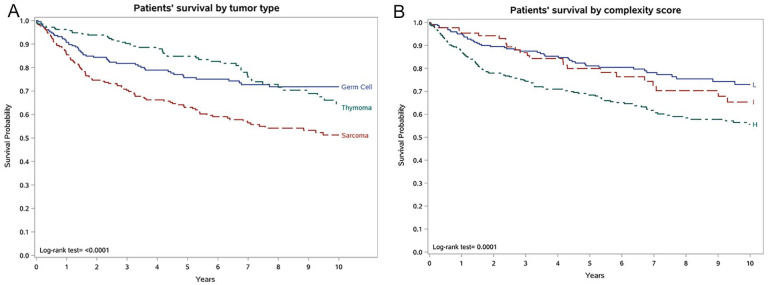
Overall survival (OS) was 90.8% at one year, 74.2% at five years and 62.8% at 10 year (Table 3). OS was significantly different among tumour types, with Log-rank test p<0.001 at one, five and 10 years (Table 3). Five-year OS were better in thymoma (84.8%) and in germ cell tumour (75.7%), and 10-year OS was better in germ cell tumours (71.8%), followed by thymomas (64.6%), sarcomas (51.3) (Table 3 and Figure 3 panel A). More complex surgery was associated with a worst OS, at one, five and 10 years. 10-year OS was statistically different in levels of complexity score (Log-rank tests p<0.0001): 73.0% in low, 65.3% in intermediate, and 55.6% in high complexity score (Table 3 and Figure 3 panel B). Similarly, OS was worst in patients undergoing the highest number of reconstructions, at one, five and 10-years (Log-rank tests p<0.0026). 10-year OS was 46.4% in patients with three or more reconstructions, 66.6% in patients with one or two reconstructions and 69.8% in patients with no reconstructions (Table 3 and online Supplemental Figure S1). 10-year OS was 46% in patients undergoing vascular reconstructions and 50% in chest wall reconstructions (Table 3).
Table 3.
Cumulative survival at one year, five years and 10 years of 952 patients.
| N patients |
1-year cumulative survival | Log-Rank P-value |
5-year Cumulative survival |
Log-Rank P-value |
10-year cumulative survival | Log-Rank P-value |
|
|---|---|---|---|---|---|---|---|
| Total | 644 | 90.8% | 74.2% | 62.8% | |||
| Type | |||||||
| Sarcoma | 227 | 85.8% | 0.0010 | 63.1% | <0.0001 | 51.3% | <0.0001 |
| Thymoma | 212 | 96.2% | 84.8% | 64.6% | |||
| Germ Cell | 205 | 90.7% | 75.7% | 71.8% | |||
| Primary tumour | 487 | 89.7% | 0.0848 | 74.0% | 0.5563 | 60.9% | 0.2056 |
| Metastatic disease | 157 | 94.3% | 74.9% | 69.3% | |||
| Complexity score | |||||||
| L | 220 | 95.0% | 0.0017 | 81.1% | 0.0006 | 73.0% | 0.0001 |
| I | 86 | 95.3% | 79.9% | 65.3% | |||
| H | 338 | 86.9% | 68.4% | 55.6% | |||
| Reconstructions | |||||||
| None | 297 | 94.6% | 0.0026 | 78.6% | <0.0001 | 69.8% | <0.0001 |
| 1 or 2 | 185 | 89.7% | 78.6% | 66.6% | |||
| ⩾ 3 | 162 | 85.1% | 61.5% | 46.4% | |||
| Vascular reconstructions | 71 | 83.1% | 67.4% | 46.0% | |||
| Chest Wall reconstructions | 143 | 85.3% | 59.2% | 50.0% | |||
Figure 3.
Patients’ survival by A) tumour type and B) complexity score.
Kaplan-Meier curves restricted to patients with three or more reconstructions showed 10-year OS in tumour types similar to that described above (Figure 4). 10-year survival curves of patients with any reconstructions stratified by tumour type are reported in online Supplementary Figure S2.
Figure 4.
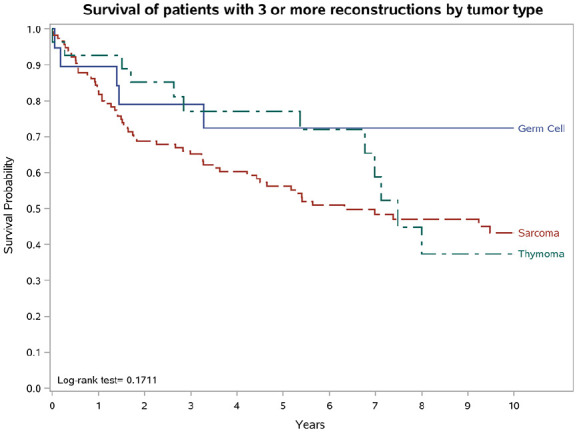
Survival of patients with three or more reconstructions by tumour type.
For germ cell tumours and for thymomas, online Supplemental Table S3 shows the one-year, five-year and 10-year survival, overall and stratified by tumour site, complexity score and reconstruction. The five-year and 10-year survival was similar in metastatic disease than in primary tumours (0.9% vs. 3.7%, p=0.0324, Table 2). In particular, for germ cell tumour five- and 10- year OS were respectively 75% and 73% in primary, 76% and 71% in metastatic disease (online Supplemental Figure S3A); for thymoma 87% and 65% respectively in primary, 63% and 52% in metastatic disease (online Supplemental Figure S3B).
The results of age and sex adjusted multivariate Cox models (online Supplemental Table S4) showed a nonsignificant impact of complexity score and number of reconstructions on five-year survival (Model A). However, after adjustment for age, sex and tumour type, the high complexity score showed a significantly poorer five-year survival compared to low score (HR=2.08, p=0.0064, online Supplemental Table S4, Model B). A similar risk profile was observed for 10-year survival (high complexity score HR=1.72, p=0.0225, online Supplemental Table S4, Model B).
Discussion
We analysed a large monocentric database of unselected RTC patients and, to our knowledge, reported for the first time a predictive analysis that estimated the surgical outcome in terms of postoperative mortality and overall survival at 10 years. In the literature, prognostic models are rarely reported in this setting and, specifically, only for single rare diseases such as for primary mediastinal germ-cell tumours, 11 thymoma, 10 while no predictive models (except for their retroperitoneal counterparts) 12 have been proposed for thoracic sarcomas. In addition, these scores mainly consider clinical or pathological features, leaving out the impact of surgical complexity on the life expectancy of these patients. To overcome the high heterogeneity of RTC diseases, we grouped patients according to the extension (and complexity) of surgical procedures. As shown in Figure 2 panel B, patients undergoing single resections (i.e., lobectomy, pleurectomy, L) had the best 10-year survival (64.8%), but even patients with extensive resections (H) showed a favourable outcome (42.4%).
Unexpectedly, the extension and complexity of surgical procedures had no significant impact on post-operative mortality, with multivariate Cox models even after adjustment for age, sex and tumour type (Table S2). On the contrary, age, sex and tumour type adjusted Cox models revealed that a high complexity score was associated with significantly poorer survival compared to low complexity score at 5 years (HR=2.08, p=0.0064, online Supplemental Table S4, Model B) as well as at 10 years (HR=1.72, p=0.0225).
In the literature, the prognostic impact of surgical extension rarely has been addressed, and only in terms of early outcome. 13 In the context of rare cancers, where specific staging systems are not comparable, this surgical complexity score may represent a simpler tool to categorise patients by anatomical extension, analogously to TNM system. This evidence is further supported by survival analyses according to the extent of reconstructions (Figure 4), where the degree of surgical invasiveness predicts the outcome of RTCs. In clinical practice, these results provide a valuable support to multidisciplinary preoperative evaluation (radiological and functional) of these rare patients, and guidance in the decision-making process. On the other hand, they confirm the need of RTC referral to highly qualified centres, to select who may benefit from extensive surgery, predict the outcome (by using the complexity score) and apply the appropriate intraoperative and postoperative management. As a matter of fact, when performed in high-volume centres, complex operations can be carried out with satisfactory outcomes. An example of long-term survival after extreme surgical resection are the four cases of total chest-wall resection of the whole emi-thorax and diaphragm en bloc with pneumonectomy (toraco-pleuro-pneumonectomy) for recurrent sarcomas, that are included in the present series.14,15
The role of vascular invasiveness and related surgery (resection and vascular reconstruction) in RTCs has also been a matter for debate over the past years. Especially for advanced thymic and germ-cell neoplasms, infiltration of mediastinal great vessels (innominate veins and superior vena cava, SVC) is common. In this context, vascular infiltration increases the technical complexity to achieve complete resection at the vascular site and requires an effective reconstructive strategy. In primary lung cancer (NSCLC), many surgeons mention bad experiences when facing the involvement of innominate veins or SVC, and poor survival even in the case of successful vascular reconstruction, with a five-year OS ranging from 15 to 40%.16-18 On the contrary, few authors have reported interesting outcomes in vascular resections for thymic neoplasms. Comacchio et al. 19 reported a favourable outcome in 144 vascular resections for thymomas (five- and 10-years OS rates of 75% and 56%, respectively), with a worse DFS in case of pathological vascular infiltration (49% and 41%, respectively). Similarly, a retrospective analysis performed by Yu et al. 20 reported a five- and 10-year OS of 93.94%, and 60.81% in a series of 45 patients undergoing vascular resection/reconstruction. Compared to NSCLC patients, RTCs outcome reflects the specific tumour biology as well as global patients’ features: younger age, less risk factors (smoking) and/or comorbidities (emphysema, coronary disease). Noteworthy, given the small numbers, it is hard to find a comparison of outcome in different RTCs undergoing vascular reconstruction.
Our results confirmed this favourable long-term survival (five- and 10-years OS rates of 74.2% and 62.8%, respectively), and gave us the opportunity to evaluate the role of extensive surgery in different RTC types, with or without vascular invasion. Of interest, the five- and 10-year survival were similar in metastatic disease and primary tumours, justifying the use of complex salvage surgery for advanced or recurrent disease. We are aware that further prospective investigations are needed to better understand this issue.
The results of our study also confirmed those reported by RARECAREnet and AIRTUM database for RTCs,2,3 showing a very good prognosis for resected thymomas, primary mediastinal germ-cell tumours and thoracic sarcoma, with a 5-yr survival of 84.8%, 75.7% and 63.1%, respectively.
Furthermore, our study underlines the role of surgery as a part of multimodality therapy in germ-cell tumours, where the cure rate strongly depends on setting and first-line chemotherapy (cisplatin-based combination chemotherapy) as well as on complete resection of residual mass, 21 due to the risk of persistent viable cells, teratoma and/or teratoma with malignant transformation.7,22 In mediastinal germ-cell tumours, surgery has proven to improve cure rates8,23 and predict survival by post-chemotherapy pathologic assessment. 24 Our results demonstrate that surgery for primary mediastinal germ-cell tumours is associated with a very good outcome even in patients with high complexity score (10-yr OS of 72%, online Supplemental Table S3).
In conclusion, the long-term survival observed in our unselected RTCs series, demonstrates that a careful preoperative evaluation combined with specific expertise in complex surgical resections and a multidisciplinary management provide the best chance of cure, especially for primary mediastinal germ-cell tumours, thymomas and sarcomas. We hope that in the future a multicentric pooling of surgical data can refine the long-term outcome prediction and improve selection of “tailored” multi-modality treatments for RTCs. A predictive score based on surgical complexity and cancer type can help the clinical decision making.
Supplemental Material
Supplemental material, sj-pdf-1-tmj-10.1177_03008916231154763 for Long term outcome of complex surgical resection and reconstruction for rare thoracic cancers by Ugo Pastorino, Giovanni Leuzzi, Federica Sabia, Paolo Girotti, Leonardo Duranti, Stefano Radaelli, Marco Fiore, Silvia Stacchiotti, Giannatempo Patrizia, Roberto Salvioni and Alessandro Gronchi in Tumori Journal
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Ugo Pastorino  https://orcid.org/0000-0001-9974-7902
https://orcid.org/0000-0001-9974-7902
Alessandro Gronchi  https://orcid.org/0000-0002-4703-3534
https://orcid.org/0000-0002-4703-3534
Supplemental material: Supplemental material for this article is available online.
References
- 1. DeSantis CE, Kramer JL, Jemal A. The burden of rare cancers in the United States. CA Cancer J Clin 2017; 67: 261-272 [DOI] [PubMed] [Google Scholar]
- 2. Gatta G, Capocaccia R, Botta L, et al. RARECAREnet working group. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol 2017; 18: 1022-1039. [DOI] [PubMed] [Google Scholar]
- 3. AIRTUM Working Group, Busco S, Buzzoni C, et al. Italian cancer figures–Report 2015: The burden of rare cancers in Italy. Epidemiol Prev 2016; 40: 1-120. [DOI] [PubMed] [Google Scholar]
- 4. Komatsubara KM, Carvajal RD. The promise and challenges of rare cancer research. Lancet Oncol 2016; 17: 136-138. [DOI] [PubMed] [Google Scholar]
- 5. Feldman DR, Voss MH, Jacobsen EP, et al. Clinical features, presentation, and tolerance of platinum-based chemotherapy in germ cell tumour patients 50 years of age and older. Cancer 2013; 119: 2574–2581. [DOI] [PubMed] [Google Scholar]
- 6. Kesler KA, Einhorn LH. Multimodality treatment of germ cell tumours of the mediastinum. Thorac Surg Clin 2009; 19: 63–69. [DOI] [PubMed] [Google Scholar]
- 7. Rosti G, Secondino S, Necchi A, et al. Primary mediastinal germ cell tumours. Semin Oncol 2019; 46: 107-111. [DOI] [PubMed] [Google Scholar]
- 8. Yang X, Zhao K, Mei J, et al. Primary mediastinal nonseminomas: a population-based surveillance, epidemiology, and end results analysis. J Surg Res 2021; 267: 25-36. [DOI] [PubMed] [Google Scholar]
- 9. Gao H, Zhou Y, Wang Z, et al. Clinical features and prognostic analysis of patients with chest wall chondrosarcoma. Medicine (Baltimore) 2019; 98: e17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao M, Yin J, Yang X, et al. Nomogram to predict thymoma prognosis: A population-based study of 1312 cases. Thorac Cancer 2019; 10: 1167-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Necchi A, Giannatempo P, Lo Vullo S, et al. A prognostic model including pre- and postsurgical variables to enhance risk stratification of primary mediastinal nonseminomatous germ cell tumours: the 27-year experience of a referral center. Clin Genitourin Cancer 2015; 13: 87-93.e1. [DOI] [PubMed] [Google Scholar]
- 12. Callegaro D, Barretta F, Swallow CJ, et al. Longitudinal prognostication in retroperitoneal sarcoma survivors: development and external validation of two dynamic nomograms. Eur J Cancer 2021; 157: 291-300. [DOI] [PubMed] [Google Scholar]
- 13. Woo J, Lee JH, Shim KN, et al. Does the difference of invasiveness between totally laparoscopic distal gastrectomy and laparoscopy-assisted distal gastrectomy lead to a difference in early surgical outcomes? A prospective randomized trial. Ann Surg Oncol 2015; 22: 1836-1843. [DOI] [PubMed] [Google Scholar]
- 14. Pastorino U, Duranti L, Scanagatta P, et al. Thoracopleuropneumonectomy with riblike reconstruction for recurrent thoracic sarcomas. Ann Surg Oncol 2014; 21: 1610-1615. [DOI] [PubMed] [Google Scholar]
- 15. Pastorino U, Scanagatta P, Girotti P, et al. Long-term results of thoraco-pleuro-pneumonectomy (TPP) for recurrent thoracic sarcomas. Ann Surg Oncol 2017; 24: S551–S554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chenesseau J, Mitilian D, Sharma G, et al. Superior vena cava prosthetic replacement for non-small cell lung cancer: is it worthwhile? Eur J Cardiothorac Surg 2021; 1195–1200. 10.1093/ejcts/ezab248 [DOI] [PubMed]
- 17. Spaggiari L, Thomas P, Magdeleinat P, et al. Superior vena cava resection with prosthetic replacement for non-small cell lung cancer: long-term results of a multicentric study. Eur J Cardiothorac Surg 2002; 21: 1080-1086 [DOI] [PubMed] [Google Scholar]
- 18. Spaggiari L, Magdeleinat P, Kondo H, et al. Results of superior vena cava resection for lung cancer. Analysis of prognostic factors. Lung Cancer 2004; 44: 339-346 [DOI] [PubMed] [Google Scholar]
- 19. Comacchio GM, Dell’Amore A, Marino MC, et al. Vascular involvement in thymic epithelial tumours: surgical and oncological outcomes. Cancers (Basel) 2021; 13: 3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu Z, Yu L, Yu T, et al. Surgical feasibility and long-term outcome of superior vena cava replacement for advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy. Thorac Cancer 2021; 12: 1074-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walsh GL, Taylor GD, Nesbitt JC, et al. Intensive chemotherapy and radical resections for primary mediastinal nonseminomatous germ cell tumours. Ann Thor Surg 2000; 69: 337–343 . [DOI] [PubMed] [Google Scholar]
- 22. Giannatempo P, Pond GR, Sonpavde G, et al. Treatment and clinical outcomes of patients with teratoma with somatic-type malignant transformation: an international collaboration. J Urol 2016; 196: 95-100. [DOI] [PubMed] [Google Scholar]
- 23. Bokemeyer C, Nichols CR, Broz J-P, et al. Extragonadal germ cell tumours of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002; 20: 1864–1873. [DOI] [PubMed] [Google Scholar]
- 24. Kesler KA, Stram AR, Timsina LR, et al. Outcomes following surgery for primary mediastinal nonseminomatous germ cell tumours in the cisplatin era. J Thorac Cardiovasc Surg 2021; 161: 1947-1959.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tmj-10.1177_03008916231154763 for Long term outcome of complex surgical resection and reconstruction for rare thoracic cancers by Ugo Pastorino, Giovanni Leuzzi, Federica Sabia, Paolo Girotti, Leonardo Duranti, Stefano Radaelli, Marco Fiore, Silvia Stacchiotti, Giannatempo Patrizia, Roberto Salvioni and Alessandro Gronchi in Tumori Journal