Abstract
Background:
Endoscopic approaches in the treatment of transmural esophageal defects, either after esophageal resection or due to perforation, have demonstrated convincing feasibility. Surgical options are limited and associated with high morbidity and mortality rates. Currently, internal endoscopic drainage with pigtail stents, self-expanding metal stent (SEMS), or endoscopic vacuum therapy (EVT) are options for first-line treatment. Here, we report the outcome of the recently developed combination of SEMS and EVT using the endoscopic Microtech®-VAC-Stent (EVS).
Methods:
Between June and July 2022, three consecutive patients (one female and two males) with esophageal transmural defects were treated with the Microtech®-VAC-Stent. Two patients suffered from an anastomotic leak after oncologic gastroesophageal surgery, and one patient presented with esophageal perforation due to Boerhaave syndrome.
Results:
Three consecutive patients were successfully treated with EVS. In one patient, one EVS treatment was sufficient, whereas the other two patients needed two and six EVS exchanges. Exchanges were scheduled every 7 days and no procedural adverse events were observed.
Conclusion:
In line with the former case series, EVS therapy is a promising new approach for the treatment of esophageal leaks. Exchange of the EVS seems feasible every 7 days reducing interventions for the individual patient. Prospective studies comparing EVS with other endoscopic therapies are needed to define the best therapeutic approach.
Keywords: endoscopic treatment, esophageal leakage, vacuum stent
Background
Esophageal leakage due to anastomotic failure is a major complication that occurs in over 20% of patients after esophageal resection. 1 Other causes are iatrogenic as well as spontaneous esophageal perforation (Boerhaave syndrome). Esophageal leakage is associated with high morbidity and mortality 1 and can cause life-threatening complications, including mediastinitis, pleural empyema, sepsis, or bronchial erosion. 2 Treatment options are limited and include re-operation with resection of the anastomosis, suture of the esophageal tear, or formation of an esophageal stoma. Endoscopic approaches are the insertion of double-pigtail stents, endoscopic vacuum therapy (EVT), or self-expanding metal stents (SEMS).2–5
In general, endoscopic treatment should be preferred over open approaches given its lower invasiveness, but the techniques used differ considerably. In a recent study, pigtail drainage was successful in all patients after a median treatment duration of 42 days. 3 By contrast, EVT was more rapid with 17-day treatment but achieved leak closure in 85% of patients despite more frequent interventions. 3 Apparently, EVT seemed not suitable for small lesions, but newer devices offer further options.6,7 However, large datasets evaluating the capability of such approaches are still lacking. Otherwise, SEMS is associated with increased complication rates, including perforation or stent migration. 8 The endoscopic vacuum stent (EVS) is a new technique combining both established principles of EVT and SEMS. It provides a vacuum sponge with negative pressure and continuous suction and sealing of the esophageal lumen with a cover. Published experience on patients’ outcomes after EVS therapy is still scarce, with promising reports having been published.9–12
In this case series, we present three consecutive patients treated with Vacuum (VAC)-Stent therapy with respect to technical success, peri-interventional complications, and leakage healing.
Methods
Data source and cohort definition
Data from all patients who received EVS for esophageal leakage at the Department of Internal Medicine I at University Hospital Halle were retrospectively collected from the medical charts. Baseline data included age, gender, American Society of Anesthesiologists (ASA) score, initial diagnosis, surgical treatment, and previous endoscopic treatment (Table 1). Informed consent was obtained from all patients presented in this case series.
Table 1.
Patient characteristics.
| Variable | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age (years) | 71 | 59 | 37 |
| Gender | Male | Female | Male |
| Diagnosis | Adenocarcinoma of the esophagus | Squamous cell carcinoma of the esophagus | Boerhaave Syndrome |
| Initial therapy | Ivor-Leweis resection | Ivor-Leweis resection | Oversuture and drain placement |
| ASA score | III | III | I |
| Complications | Anastomotic leakage | Anastomotic leakage | Suture insufficiency |
| Time from surgery to endoscopic treatment (days) | 21 | 5 | 5 |
| VAC-Stent therapies | 2 | 6 | 1 |
| Previous sponge (EVT) | Yes | No | No |
| Previous stent (SEMS) | Yes | No | No |
| Total duration of VAC-Stent therapy (days) | 5 | 32 | 5 |
| Hospitalization duration (total, days) | 92 | 53 | 21 |
| ICU treatment (days) | 67 | 53 | 14 |
| Hospitalization duration after end of VAC-Stent therapy (days) | 24 | 5 | 9 |
ASA, American Society of Anesthesiologists; EVT, endoscopic vacuum therapy; SEMS, self-expanding metal stent; VAC, Vacuum.
Endoscopic technique and definitions
Diagnosis of esophageal leakage was performed by endoscopy or CT scan. Anastomotic leak after esophagectomy was defined according to the Esophagectomy Complications Consensus Group. 13
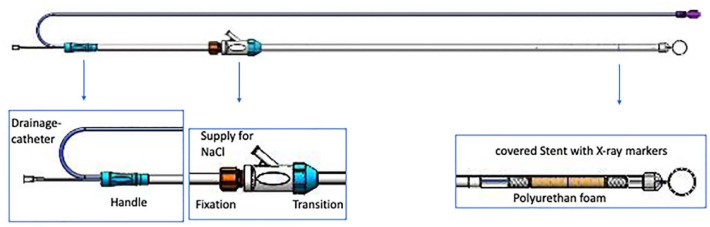
All endoscopies were performed by experienced interventional endoscopists. For EVS, the VAC Stent GI (Ref 00003820; MICRO TECH Europe GmbH, Düsseldorf, Germany) was used (Figure 1). First, the stent system was rinsed with 100 ml of sodium chloride solution. Hereafter, during gastroscopy, a guidewire was positioned and the leakage site was identified and marked using an X-ray with a metal pin attached to the patient’s thorax. The fully prepared VAC-Stent was deployed under radiographic control in the correct position and again rinsed to eliminate air in the system. After the final release of the stent in the correct position, the sponge was rinsed through the aspiration catheter to expand it. The final position of the VAC-Stent was checked endoscopically. Finally, the aspiration catheter was switched to transnasal and the vacuum was adjusted to a continuous negative pressure of 60–80 mmHg. The VAC-Stent was changed or removed after 6–8 days.
Figure 1.
Schematic view of the Microtech®-VAC-Stent in full length and special parts depicted in more detail. With permission of MICRO-TECH Europe GmbH.
Results
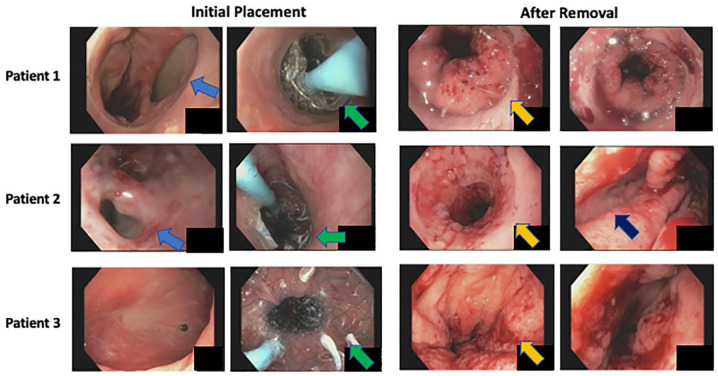
The first patient in this series was treated for an adenocarcinoma of the esophageal-gastric junction by an abdominal-thoracic resection (Ivor-Lewis resection). A total of 5 days after surgery anastomotic insufficiency was endoscopically confirmed. A small dehiscence was observed (Figure 2), whereas a CT scan showed no clear signs of an anastomotic insufficiency at that time point. Conservative treatment including positioning of a gastric tube, i.v. antibiotics, and pleural drainage was performed. Here, another option would have been endoluminal negative pressure therapy. 14 Two weeks later, laboratory inflammatory parameters were elevated and the size of the leak had increased. A therapeutic approach with EVT followed with in total of four interventions. EVT was performed with a polyurethane sponge (Eso-SPONGE® B. Braun, Melsungen, Germany) positioned in the cavity. Inflammation parameters were normalized and the patient presented in good clinical condition. The endoscopic leak diameter was too small to allow further intracavitary EVT with a not-modified system. In such cases, further options could be intraluminal EVT or a double-lumen open-pore film drain. 15 We implanted a SEMS without stent fixation that had to be repositioned twice. Due to the persistent drainage of pus over the pleural drainage still in place and rising inflammatory parameters, the SEMS was removed. Finally, EVS therapy was performed and after removing the stent after 7 days, the apparent dehiscence had nearly completely healed (Figure 2). The patient was in good clinical condition regarding the esophageal situation, the pleural effusion resolved, and he was discharged from the hospital 24 days after termination of EVS therapy. Limitations in the clinical situation were due to postoperative hyperactive delirium and a fracture of the femoral neck after falling out of bed during a hyperactive phase, which complicated and prolonged the hospital stay. The patient was able to eat and drink small portions of soft food but needed additional parenteral nutrition.
Figure 2.
Representative images of the endoscopic picture immediately before initial placement of the Microtech®-VAC-Stent (left) and after removal and termination of stent therapy (right) in all three patients (patient 1 on the top, patient 2 in the middle, and patient 3 at the bottom). Blue arrows show the insufficiency, green arrows mark the Microtech®-VAC-Stent, orange arrows mark the anastomosis region, and dark blue arrows show a residual cavity in patient 2.
The second patient was a 59-year-old woman with an anastomotic insufficiency following Ivor-Lewis resection of a squamous cell carcinoma of the esophagus. On the third postoperative day, she developed respiratory distress and the CT scan demonstrated an anastomotic leak as well as pneumonia. Two days later, she was reoperated due to respiratory failure requiring intubation. Intraoperatively, an intrathoracic abscess was removed, lavaged, and an intrathoracic drain was inserted. The anastomosis and the gastric tube had no obvious insufficiency. For additional information, an intraoperative endoscopy was performed showing a small dehiscence (Figure 2). After interdisciplinary discussion and with respect to the clinical, radiologic, intraoperative, and endoscopic findings, the first EVS was administered directly after surgery. This decision was made although alternative options like intraluminal pressure therapy are available as the capacity of the EVS seemed at least comparable to these and needed fewer exchanges following our standard operation procedures. 16
A total of 6 days later, the EVS was exchanged since the CT scan showed less, but still residual fluids. Another 6 days later, EVS was extracted. A CT scan and gastroscopy 4 days later depicted a relevant leak with the need for further EVS. After four EVS treatments, with changes every 6–7 days, no insufficiency was detectable in gastroscopy (Figure 2) and this was confirmed by a CT scan. The patient was discharged in good clinical condition 5 days after the EVS was removed (Figure 2). The patient was able to eat and drink small amounts of food which was added by parenteral feeding when the patient was transferred to a neurologic rehabilitation clinic.
The third patient was a 37-year-old man admitted with Boerhaave syndrome with mediastinal emphysema, seropneumothorax, and in septic condition. An emergency operation was performed, and the perforation was overseen. Five days later, reoperation due to a relapse of the leak with seropneumothorax and paraesophageal fluid collections had to be performed. After surgical oversewing of the anastomosis, an intraoperative endoscopy was performed showing no obvious defect, but in an interdisciplinary approach, it was decided for an EVS, which was administered to decrease the risk of further insufficiency (Figure 1). A possible alternative in this case would have been an intraluminal polyurethane foam for complete gastric secretion drainage. 17 As the EVS offers the advantage of circularly covering the vulnerable region and enables the patient to drink liquids, we decided to use the EVS in this case. After 8 days, the EVS was removed and endoscopy did not identify aspects of insufficiency. The patient was discharged 9 days after EVS removal (Figure 2). He had completed oral intake.
All patient characteristics are summarized in Table 1. Removal of the EVS was performed according to the manufacturer’s recommendations. Suction was stopped 120 min before removal. As expected in all cases, small (2–5 mm) erosions of the mucosa were seen. No sponge rupture was observed.
Discussion
Transmural esophageal defects are associated with high mortality and morbidity. 18 Whenever possible, endoscopic treatment should be considered as a first approach and novel technologies like EVS might improve outcomes.9,18,19 Until now, no randomized clinical trial investigated the superiority of distinct endoscopic techniques, and therefore the evidence of recommendations for the treatment is low. 20 A recent meta-analysis indicated EVT to be superior to SEMS for successful leak closure, mortality, adverse events, and the duration of treatment. 8 Nevertheless, EVT has limitations if the sponge shall be placed transmurally as this requires a certain diameter for its placement. Here, recent developments like the intraluminal open-pore film drainage are reasonable therapeutic options. 15
The combination of EVT and SEMS may combine the advantages of both systems with the potential for improved wound healing and the option of enteral nutrition. Furthermore, it may minimize complications like stent migration that cannot always be prevented by SEMS fixation. Data on EVS are still scarce with two case series reporting in total of 13 patients and a prospective, investigator-initiated single-center study with 20 patients thus far.9–12 The results of the presented three consecutive patients are in line with the previous case series. As presented here, the formerly reported technical success rate was 100% in all studies, whereas successful treatment was achieved in 70% (7/10) and 100% (3/3) in the case series and 60% (12/20) in the prospective study. The differences in success rates might be due to several reasons. First, the etiology of the leak, its diameter, and (potentially) corresponding cavity size were different in patients, which might influence outcomes. Furthermore, pretreatment of patients was distinct with EVS used as the primary strategy in 24/33 (72%) patients, which implies that several confounders might be relevant. In addition, combinatory treatment approaches have been reported that further highlight the difficulties in assessing the causality of treatment success in these patients. Compared to EVT (67–100%) and SEMS (70–81%) treatment, the overall clinical success rate of EVS seems comparable (25/36, 69%).9–12,20 However, the real efficacy of EVS remains speculative and prospective, and randomized trials are needed to identify the best treatment strategy.
Another possible advantage of EVS is to enable oral feeding of patients without the placement of a gastric tube. 12 This was challenged by a recent prospective study in that supplementation with high-energy drinks impacted vacuum therapy of EVS as the suction tube was obstructed. 10 The authors discussed too low suction power (−65 mmHg) and too brief exchange interval (3–5 days) as possible reasons. The here presented data showed that a higher negative pressure and a longer exchange interval were feasible and resulted in clinical success. Again, this observation warrants further investigation as, for instance, the viscosity of high-energy drinks might be too high and needs to be adapted to avoid such side effects. Still, it remains unclear whether a sufficient oral caloric intake by patients undergoing EVS can be achieved or if further enteral or parenteral nutrition is required. Another therapeutic option in such cases is therapy with intraluminal open-pore film drainage with simultaneous enteral feeding. 15
Performing EVS was straightforward, and deployment of the system was fast and did not require a huge number of resources but should most likely be performed under X-ray control. This is mirrored by the technical success rate of 100% in all reported patients. The best interval to exchange EVS is not well defined and was 3–7 days in the previously reported patients.10–12 In our case series, we exchanged EVS after 6–8 days and observed no complications or procedural difficulties. Cost calculations cannot be performed with the available data, but with EVS currently being a rather expensive procedure reduction in interventions would be of financial interest. Lastly, no sufficient data on long-term outcomes like esophageal strictures have been reported so far. Also, in the mid-term analysis of Chon et al., 10 no stenosis at the site of the leakage was detected in a follow-up time of 109.2 ± 93.13 days, and further data are needed in this regard. 10
Conclusion
In summary, our case series of three consecutively treated patients with EVS demonstrated comparable feasibility in line with former studies, but higher treatment success. Results, however, must be interpreted with caution as patient characteristics and treatment indications differed substantially. To clarify the role of EVS in esophageal leak treatment a randomized, prospective trial comparing all the above-mentioned methodologies is warranted.
Acknowledgments
The authors thank the patients for consenting to publish the data.
Footnotes
ORCID iDs: Michelle A. Klose  https://orcid.org/0000-0001-9871-6207
https://orcid.org/0000-0001-9871-6207
Ulrich Ronellenfitsch  https://orcid.org/0000-0003-1107-813X
https://orcid.org/0000-0003-1107-813X
Jonas Rosendahl  https://orcid.org/0000-0003-4513-0506
https://orcid.org/0000-0003-4513-0506
Contributor Information
Michelle A. Klose, Department of Internal Medicine I, Martin-Luther-University Halle-Wittenberg, Saale, Germany
Jens Walldorf, Department of Internal Medicine I, Martin-Luther-University Halle-Wittenberg, Saale, Germany.
Marko Damm, Department of Internal Medicine I, Martin-Luther-University Halle-Wittenberg, Saale, Germany.
Sebastian Krug, Department of Internal Medicine I, Martin-Luther-University Halle-Wittenberg, Saale, Germany.
Johannes Klose, Department of General, Visceral, Vascular and Endocrine Surgery, Martin-Luther-University Halle-Wittenberg, Saale, Germany.
Ulrich Ronellenfitsch, Department of General, Visceral, Vascular and Endocrine Surgery, Martin-Luther-University Halle-Wittenberg, Saale, Germany.
Joerg Kleeff, Department of General, Visceral, Vascular and Endocrine Surgery, Martin-Luther-University Halle-Wittenberg, Saale, Germany.
Patrick Michl, Department of Internal Medicine I, Martin-Luther-University Halle-Wittenberg, Saale, Germany.
Jonas Rosendahl, Department of Internal Medicine I, Martin-Luther-University Halle-Wittenberg, Ernst-Grube-Strasse 40, D-06120 Halle, Saale, Germany.
Declarations
Ethics approval and consent to participate: The local ethics committee approved this study.
Consent for publication: All patients provided written consent before data acquisition and publication.
Author contributions: Michelle A. Klose: Conceptualization; Data curation; Formal analysis; Writing – original draft.
Jens Walldorf: Writing – review & editing.
Marko Damm: Writing – review & editing.
Sebastian Krug: Investigation; Writing – review & editing.
Johannes Klose: Conceptualization; Formal analysis; Writing – review & editing.
Ulrich Ronellenfitsch: Writing – review & editing.
Joerg Kleeff: Data curation; Investigation, Writing – review & editing.
Patrick Michl: Supervision.
Jonas Rosendahl: Project administration; Supervision; Writing – original draft.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Data and materials can be obtained from the corresponding author upon request.
References
- 1. Fabbi M, Hagens ERC, van Berge Henegouwen MI, et al. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus 2021; 34: doaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hagens ERC, Reijntjes MA, Anderegg MCJ, et al. Risk factors and consequences of anastomotic leakage after esophagectomy for cancer. Ann Thorac Surg 2021; 112: 255–263. [DOI] [PubMed] [Google Scholar]
- 3. Jung CFM, Hallit R, Müller-Dornieden A, et al. Endoscopic internal drainage and low negative-pressure endoscopic vacuum therapy for anastomotic leaks after oncologic upper gastrointestinal surgery. Endoscopy 2022; 54: 71–74. [DOI] [PubMed] [Google Scholar]
- 4. Pross M, Manger T, Reinheckel T, et al. Endoscopic treatment of clinically symptomatic leaks of thoracic esophageal anastomoses. Gastrointest Endoscopy 2000; 51: 73–76. [DOI] [PubMed] [Google Scholar]
- 5. Raju GS. Endoscopic management of gastrointestinal leaks. Gastrointest Endoscopy Clin North Am 2007; 17: 487–503. [DOI] [PubMed] [Google Scholar]
- 6. Loske G, Schorsch T, Müller C. Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy 2011; 43: 540–544. [DOI] [PubMed] [Google Scholar]
- 7. Loske G, Schorsch T, Rucktaeschel F, et al. Open-pore film drainage (OFD): a new multipurpose tool for endoscopic negative pressure therapy (ENPT). Endoscopy Int Open 2018; 6: E865–E871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. do Monte Junior ES, de Moura DTH, Ribeiro IB, et al. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: systematic review and meta-analysis. Dig Endoscopy 2021; 33: 892–902. [DOI] [PubMed] [Google Scholar]
- 9. Chon SH, Bartella I, Bürger M, et al. VACStent: a new option for endoscopic vacuum therapy in patients with esophageal anastomotic leaks after upper gastrointestinal surgery. Endoscopy 2020; 52: E166–E167. [DOI] [PubMed] [Google Scholar]
- 10. Chon SH, Scherdel J, Rieck I, et al. A new hybrid stent using endoscopic vacuum therapy in treating esophageal leaks: a prospective single-center experience of its safety and feasibility with mid-term follow-up. Dis Esophagus 2022; 35: doab067. [DOI] [PubMed] [Google Scholar]
- 11. Chon SH, Töx U, Lorenz F, et al. A novel hybrid stent with endoscopic vacuum therapy for treating leaks of the upper gastrointestinal tract. Visc Med 2021; 37: 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lange J, Dormann A, Bulian DR, et al. VACStent: combining the benefits of endoscopic vacuum therapy and covered stents for upper gastrointestinal tract leakage. Endoscopy Int Open 2021; 9: E971–E976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg 2015; 262: 286–294. [DOI] [PubMed] [Google Scholar]
- 14. Neumann PA, Mennigen R, Palmes D, et al. Pre-emptive endoscopic vacuum therapy for treatment of anastomotic ischemia after esophageal resections. Endoscopy 2017; 49: 498–503. [DOI] [PubMed] [Google Scholar]
- 15. Loske G, Müller J, Schulze W, et al. Pre-emptive active drainage of reflux (PARD) in Ivor-Lewis oesophagectomy with negative pressure and simultaneous enteral nutrition using a double-lumen open-pore film drain (dOFD). Surg Endoscopy 2022; 36: 2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuehn F, Schiffmann L, Janisch F, et al. Surgical endoscopic vacuum therapy for defects of the upper gastrointestinal tract. J Gastrointest Surg 2016; 20: 237–243. [DOI] [PubMed] [Google Scholar]
- 17. Loske G, Albers K, Mueller CT. Endoscopic negative pressure therapy (ENPT) of a spontaneous oesophageal rupture (Boerhaave’s syndrome) with peritonitis – a new treatment option. Innov Surg Sci 2021; 6: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paspatis GA, Dumonceau JM, Barthet M, et al. Diagnosis and management of iatrogenic endoscopic perforations: European society of gastrointestinal endoscopy (ESGE) position statement. Endoscopy 2014; 46: 693–711. [DOI] [PubMed] [Google Scholar]
- 19. Kähler G. Anastomotic leakage after upper gastrointestinal surgery: endoscopic treatment. Visc Med 2017; 33: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tachezy M, Chon SH, Rieck I, et al. Endoscopic vacuum therapy versus stent treatment of esophageal anastomotic leaks (ESOLEAK): study protocol for a prospective randomized phase 2 trial. Trials 2021; 22: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]