Abstract
Mesenchymal/medicinal stem/signaling cells (MSCs), well known for regenerative potential, have been involved in hundreds of clinical trials. Even if equipped with reparative properties, aging significantly decreases their biological activity, representing a major challenge for MSC-based therapies. Age-related joint diseases, such as osteoarthritis, are associated with the accumulation of senescent cells, including synovial MSCs. An impaired ability of MSCs to self-renew and differentiate is one of the main contributors to the human aging process. Moreover, senescent MSCs (sMSCs) are characterized by the senescence-messaging secretome (SMS), which is typically manifested by the release of molecules with an adverse effect. Many factors, from genetic and metabolic pathways to environmental stressors, participate in the regulation of the senescent phenotype of MSCs. To better understand cellular senescence in MSCs, this review discusses the characteristics of sMSCs, their role in cartilage and synovial joint aging, and current rejuvenation approaches to delay/reverse age-related pathological changes, providing evidence from in vivo experiments as well.
Keywords: senescence, senescent mesenchymal/medicinal stem/signaling cells, senescence-messaging secretome, aging joint, rejuvenation
Introduction
Mesenchymal/medicinal stem/signaling cells (MSCs) are multipotent progenitor stem cells derived from adult tissues/fluids such as bone marrow 1 , adipose tissue 2 , skeletal muscle tissues 3 , synovial membrane 4 , and fluid 5 , urine 6 , and others. In fact, MSCs belong to highly heterogeneous cell populations. Therefore, their biological features can vary between individuals, tissues, or even within a single cell colony 7 . Introducing such ambiguously characterized cells into clinical trials can be complicated and may jeopardize the safety of MSC-based therapies. Consequently, a single set of rules allowing for the identification of human MSCs was created and approved in 2006 by the International Society for Cellular Therapy. The minimum criteria to define unique populations of human MSCs include the following: (1) plastic-adherence properties; (2) the expression of CD73, CD90, and CD105, and absence of CD14, CD19, CD34, CD45, and HLA-DR–specific surface markers; and (3) capability of multilineage differentiation in vitro 8 . Furthermore, MSCs are immune evasive due to the low expression of major histocompatibility complex (MHC) class I and the absence of MHC class II molecules (at the early passage) 9 . Their regenerative potential is principally based on the secretion of bioactive molecules. A cocktail of various growth factors, cytokines, chemokines, and enzymes has a reparative, anti-inflammatory, and immunomodulatory effect10,11. Noteworthily, the composition of secretome strictly depends on the MSCs’ origin and thus varies markedly between MSCs from different sources 12 .
The interest of scientists worldwide is enormous due to the remarkable MSCs’ therapeutic potential executed during the regeneration of various damaged tissues. Some of these defects are the result of injury, while others are associated with aging. Generally, aging is a continual and irreversible process, which affects all cells, including MSCs. Different internal13,14 and external factors 15 are responsible for the induction and/or acceleration of cell aging. Aging cells progressively lose their reparative and regenerative characteristics leading to increased tissue and cellular dysfunction. As an example, stress-induced accumulation of senescent cells, especially senescent chondrocytes and synovial MSCs (S-MSCs), can ultimately lead to the development of joint-related diseases such as osteoarthritis (OA). Recently, several studies showed that the removal of these cells from the joint enhanced the self-renewal and regeneration of cartilage 16 . Overall, the transplantation of young MSCs can be an innovative tool to prevent or slow the onset of age-related conditions. On the other hand, MSCs’ therapeutic efficacy may be skewed in an aging environment 17 . Therefore, optimizing strategies combating aging and cellular senescence is vitally important to minimize the pathological effects of age-related processes. In this review, we describe the characteristics of senescent MSCs (sMSCs), the factors causing senescence, and the role of senescent chondrocytes and sMSCs in cartilage and the synovial joint. We also provide a summary of current rejuvenation techniques.
Senescence at a Cellular and Molecular Level
MSCs, similarly to other cell types, go through age-related changes and become senescent over time. Indeed, both intrinsic [eg, reactive oxygen species (ROS)] and extrinsic stressors (eg, environmental toxins) are principal triggers of the aging process18,19. SMSCs are characterized by an irreversible cell growth arrest despite being metabolically active. Other identifiers of senescence are changes in cell shape, decreased proliferation, impaired differentiation ability, and reduction in capacity to form colonies14,20. In addition, sMSCs show elevated lysosomal β-galactosidase (SA-β-gal) levels compared with cells in early passages due to the increased lysosomal mass and altered pH21,22. SA-β-gal activity is commonly used as a senescence marker, but in some cases, it may produce false data. Of note, it cannot be detected in senescent cells with a defective lysosomal β-galactosidase 23 . Therefore, appropriate identification of senescent cells requires a combination of senescence markers. Alpha-fucosidase (SA-α-Fuc), so-called glycosidase, is another lysosomal enzyme observed in many senescent cells regardless of the senescence-inducing stimulus 14 . It is considered to be a more accurate senescence marker compared with SA-β-gal 24 . However, there are still doubts about its specificity when it comes to the identification of sMSCs.
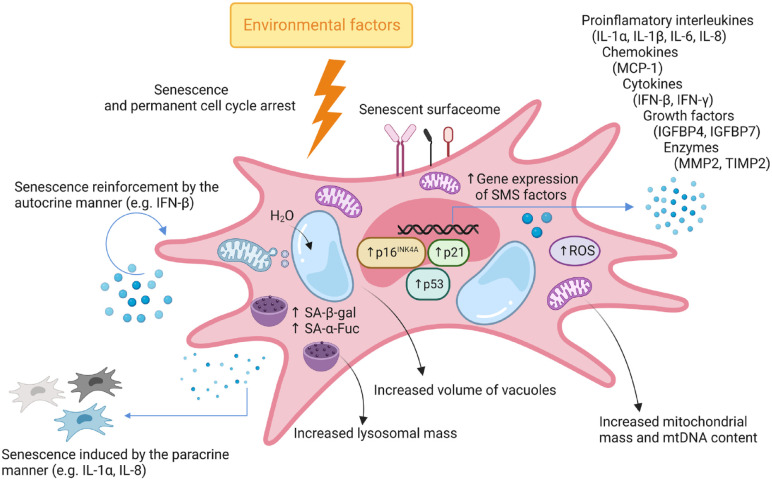
The expression of surface molecules undergoes dynamic changes during the aging of MSCs. Although these changes depend on the conditions under which MSC aging occurs. The reduced expression of MSCs’ markers, such as CD146 (known as melanoma cell adhesion molecule, MCAM) 25 , stromal cell surface marker-1 (STRO-1) 26 , or CD106 (known as VCAM-1) 27 , was detected during in vitro cultivation and in aged organisms. Moreover, the enhanced expression of CD264 was associated with sMSCs only during the long-term culture in vitro. Notably, CD264-positive MSCs also show increased SA-β-gal activity and reduced proliferation and differentiation ability. This suggests that CD264 may be considered another indicator of senescence 28 . SMSCs are also characterized by a senescence-messaging secretome (SMS), typically manifested by the release of molecules [interleukin (IL)-1α, monocyte chemoattractant protein (MCP)-1, insulin-like growth factor binding protein 4 (IGFBP4), etc] with an adverse effect on adjacent cells and their microenvironment 29 . Typical features of sMSCs are depicted in Fig. 1.
Figure 1.
Hallmarks of senescent mesenchymal stem cell (sMSC). Cell cycle arrest occurs due to the action of internal and environmental stressors that impair the proliferative capacity. Consequently, MSC acquires a senescent phenotype, which is manifested by an enlarged and flattened morphology, the presence of surface molecules associated with senescence, enlarged lysosomes with a raised level of enzymes (α-Fuc and β-gal), increased vacuolar volume, elevated content of mitochondrial DNA (mtDNA), and accumulation of DNA damage causing changes in gene expression. Subsequently, MSC secretory activity is shifted toward the senescence-messaging secretome (SMS). This is related to the increased release of factors (cytokines, growth factors, etc) and particles (eg, exosomes). The secreted molecules and particles can operate as paracrine factors triggering senescence in neighboring nonsenescent cells or autocrine factors augmenting the senescent phenotype within the same cell. Furthermore, the presence of dysfunctional mitochondria is linked to the massive production of ROS that further amplify the senescent status. IFN: interferon; IGFBP: insulin-like growth factor binding protein; IL: interleukin; MCP: monocyte chemoattractant protein; MMP: matrix metalloproteinase; mtDNA: mitochondrial DNA; ROS: reactive oxygen species; SA-α-Fuc: senescence-associated lysosomal α-l-fucosidase; SA-β-gal: senescence-associated beta-galactosidase; TIMP: tissue inhibitor of metalloproteinases.
To assess aging in MSCs, numerous assays evaluating cell morphology and density, proliferative potential, mitochondrial function, and secretome composition can be applied 30 . Experimentally, senescence is determined by measuring the activity of enzymes mentioned above 21 and length of telomeres 31 , monitoring the expression of selected markers (p16INK4a, p21Cdkn1a, and p53) 32 and alterations in gene methylation and epigenetic processes33,34, etc. In the following section, the cellular and molecular attributes of sMSCs will be discussed in more detail.
Morphology
SMSCs are characterized by a flattened, enlarged, and irregular shape. They also display granular cytoplasm with many cell inclusions, vacuoles, and lysosomes 14 . These exceptional morphological features are often used for sMSC recognition. Accordingly, it is possible to predict the biological behavior of MSCs under specific conditions. For example, Block et al. demonstrated that bone marrow–derived mesenchymal stem cells (BM-MSCs) isolated from elderly donors consisted of a small and a large cell subpopulation. Interestingly, the subpopulation of small-sized BM-MSCs possessed a similar level of ATP as BM-MSCs derived from young donors. The subpopulation of large BM-MSCs exhibited a decreased amount of ATP compared with BM-MSCs derived from young donors and a small subpopulation of BM-MSCs isolated from elderly people 35 . Besides altered cell size, senescence is accompanied by changes in the number, shape, and size of some organelles (eg, mitochondria, vacuoles, and nucleus). A typical characteristic of sMSCs is senescence-associated vacuole formation 21 . However, it still needs to be discovered what their exact cellular function is. The aging process is generally associated with modifications in cytoskeletal protein structure and/or expression. This causes disruption of the cytoskeleton architecture, which, in turn, leads to the morphological transformation of the cell membrane or organelles. Notably, the actin structure of senescent BM-MSCs was confirmed to be poorly organized, decreasing cell motility drastically. Behind the less dynamic cytoskeletal structure and slow actin turnover can, at least partially, stand the upregulated expression of actin-binding proteins, such as cortactin, α-actinin 2, and adducins, supporting the actin cytoskeleton stability and increasing cell rigidity. At the same time, a significant downregulation of proteins controlling microtubule dynamics [microtubule-associated proteins MAPRE1 (EB1), MAP1B, and MAP4] was detected in senescent BM-MSCs, indicating the extensive cell cytoskeleton rearrangement with senescence 36 . Moreover, the membranes of senescent cells exhibit altered expression of a wide range of proteins. For instance, caveolin-1 upregulation is linked to morphological alterations in senescent BM-MSCs as well37,38. Impaired mitochondrial dynamics (mtDNA damage, increased ROS production, reshaping, fusion, or fission) can also contribute to the altered mitochondrial morphology in sMSCs. Intriguingly, the process of mitochondrial fusion increases, whereas the fission decreases with senescence in BM-MSCs 39 . Conversely, Barilani et al. 40 detected a reduction in mitochondrial mass. The content of mtDNA, however, was elevated in aging BM-MSCs.
DNA Damage
DNA damage, if not removed by DNA reparative mechanisms, is one of the main factors that accelerate cell aging. DNA is frequently damaged as a consequence of increased oxidative stress. ROS are generated due to cellular metabolic pathways, and their low concentrations are necessary for the normal proliferation and differentiation of MSCs41,42. Although high ROS production is associated with sMSCs, confirmed in MSCs derived from bone marrow 43 , umbilical cord blood (UC-MSCs) 44 , and Wharton’s Jelly (WJ-MSCs) 45 . Incubation with N-acetylcysteine can prevent MSCs from entering the senescent stage as it serves as an oxygen scavenger and decreases the subsequent DNA damage 46 . Another option is the excessive stimulation of specific DNA repair pathways since their downregulation was identified in senescent human BM-MSCs 47 . Some studies have shown that spontaneous malignant transformations may occur during the long-term culture of MSCs 48 . Even though the study by Wang et al. 49 evidenced that long-term cultured human UC-MSCs developed genomic alterations, the malignant transformation was not confirmed.
Chromatin Reorganization and Epigenetic Modifications
DNA methylation and histone modifications (eg, acetylation) are the primary control mechanisms involved in chromatin remodeling. Epigenetic alterations are often found in aging MSCs and sMSCs. Changes in methylation were mainly observed in CpG sites in the promoter regions of homeobox genes and genes implicated in differentiation. The reduced expression of the homeobox gene distal-less homeobox 5, due to hypermethylation, supposedly led to impaired differentiation of BM-MSCs during long-term cultivation 33 . Furthermore, DNA methyltransferases (DNMTs) were shown to have an essential role in regulating senescence in UC-MSCs. These enzymes control not only the methylation status of DNA but also histone marks at genomic regions of polycomb group genes (PcGs)-targeting miRNAs and p16INK4a and p21CIP1/WAF1 promoter regions. DNMT inhibition triggered senescence via upregulation of p16INK4a and p21CIP1/WAF1 expression 50 . In addition, histone modifications are commonly present in sMSCs. As an example, histone deacetylase (HDAC) activity seems crucial for the self-renewal of adipose tissue–derived mesenchymal stem cells (AD-MSCs) and UC-MSCs. Properly functioning HDAC is presumably implicated in the balanced expression of PcGs and the jumonji domain-containing protein 3. Thus, senescence and loss of MSC self-renewal properties are related to the downregulation of HDACs, which ultimately leads to increased p16INK4a expression and elevated SA-β-gal activity 51 . Of important note, a different form of chromatin structure called senescence-associated heterochromatin foci was observed in sMSCs. The study by Gu et al. demonstrated that the nuclei of UC-MSCs in late passages (p11 and p17) displayed chromatin localized in small and condensed spots. These represent transcriptionally inactive chromatin, further underlining the senescence-associated attenuation of gene expression 52 .
Telomeres
Telomere shortening, another hallmark of sMSCs, occurs continuously from the first cell passage. Telomeres shorten with each round of cell division, eventually being too short, which induces cell cycle arrest. A recent study has found that the rejuvenation of sMSCs by overexpression of telomerase reverse transcriptase (TERT) results in telomere elongation in AD-MSCs and WJ-MSCs. TERT upregulation did not affect the karyotype 53 . Importantly, telomerase activity is related to sirtuins (SIRT), a group of deacetylases. Loss of SIRT1 in young MSCs stimulated cellular senescence and impaired cell proliferation, while its overexpression in aged MSCs reversed the senescent phenotype and activated cell proliferation. SIRT1-associated antiaging effects were mediated through the protection from age-related DNA damage, induction of TERT expression, and stimulation of telomerase activity. Moreover, SIRT1 upregulated the expression of tripeptidyl peptidase 1, which helps protect chromosome ends from DNA damage. Hence, the increased amount of SIRT1 can expand the cell lifespan and reverse the senescence in rat BM-MSCs 54 . Techniques detecting DNA ends in sMSCs could allow monitoring of their aging. On the other hand, it is crucial to take into account that telomere length can be variable and vary between individuals. Therefore, relying only on the measurement of this marker may not be sufficient.
Senescence-Messaging Secretome
From the initial signs of aging in cell culture to reaching complete senescence, it lasts approximately 10 days to 6 weeks. This process depends on the presence of inducers driving the cells to senescence as well as the type of cells. Almost 30%–70% of senescent cells are reported to develop a heterogeneous SMS, which is characterized by release of the immunomodulatory, pro-inflammatory, and pro-apoptotic cytokines [IL-1, IL-6, IL-8, IL-13, IL-15, interferon (IFN)-β, transforming growth factor β (TGF-β1), etc], chemokines (eotaxin 1-3, MCP-1/CCL2, MCP-2/CCL8, RANTES/CCL5, etc), tissue-destroying proteases such as matrix metalloproteinases (MMP1, MMP3, MMP9), ligands (eg, Fas ligand), growth factors [insulin-like growth factor 1 (IGF-1), IGFBP4, IGFBP7, hepatocyte growth factor (HGF), etc], and others55–58. Cells with SMS release even other factors involved in tissue necrosis, systemic inflammation, stem and progenitor cell dysfunction, fibrosis, and the spread of senescence to nonsenescent cells59,60. The composition of SMS can vary greatly and mainly depends on the cell type, inductors that trigger senescence (mechanical stress, chemotherapy, radiotherapy, etc), and the metabolism of the senescent cells. For example, senescence-induced mitochondrial dysfunction is characterized by SMS but without secretion of pro-inflammatory factors such as IL-161,62. In fact, SMS is involved in the propagation of senescence in a paracrine and autocrine fashion 63 . However, each type of senescence is stimulated by different SMS factors. TGF-β family members are mainly involved in the senescence of neighboring cells. Their downstream targets affect ROS production and the DNA damage response 64 . The findings of Acosta et al. 65 confirmed that some secreted factors, such as IL-1, can be involved in both autocrine and paracrine senescence. Accumulating only a small number of senescent cells can be harmful and may promote various age-related diseases66,67. Intraperitoneal transplantation of senescent AD-MSCs into young mice induced persistent physical dysfunction and spread of cellular senescence to host tissues 68 . Furthermore, the level of IGFBP4 has been shown to increase significantly in rat BM-MSCs with age. High IGFBP4 expression was associated with decreased osteogenic potential. This was also confirmed by IGFBP4 knockdown, which restored the osteogenic potency of aged BM-MSCs 69 . In addition, it has been reported that IGFBP4 and IGFBP7, harvested from the conditioned media of senescent BM-MSCs, are inevitable in the induction of senescence in young BM-MSCs 57 .
Aging Joint Environment and the Role of sMSCs
Stiffness, pain, and impaired joint mobility in older age are caused by a decrease in the amount of synovial fluid (SF), which ensures lubrication and smooth joint movement 70 . Due to its lack, mechanical friction in the end parts of the bones occurs, resulting in thinning of the cartilage surface layer. Moreover, the loss of chondrocytes and remodeling of extracellular matrix (ECM) proteins also accompany this process. On the other hand, water content increases with age-related diseases, especially in the early stages of OA71,72. A characteristic feature of aging cartilage is the development of excessive collagen cross-linking, leading to cartilage stiffness and impaired function 73 . Cartilage disruptors include enzymes that cause the loss of collagens (mainly type II) and aggrecans74–76. Degradation of collagens occurs due to increased levels of MMPs, particularly MMP13 77 . Eventually, all these factors, including metabolic and oxidative stress, disturb the cartilage environment. Thus, the aging of joints is reflected by the changes within SF composition. As an example, hyaluronic acid (HA) is the fundamental microenvironmental component of articular cartilage78,79. It has many physiological functions (lubrication, shock absorption, stabilization of joint structure, and regulation of water balance) and is involved in various cellular processes (eg, differentiation and proliferation)79,80. In the joint cavity, HA is synthesized by type B synoviocytes via the enzyme hyaluronan synthase (HAS), which exists in three isoforms. These produce HA polymers of various sizes. Therefore, healthy SF contains a mixture of different-sized HAs, low and high molecular weight (MW) ones 79 . With aging, the HA concentration and MW decrease. Reduction of high MW HA seems to be vitally important since these large molecules are primarily involved in boundary lubrication at articular cartilage–cartilage interfaces70,81. HA can interact with three main classes of cell surface receptors: (1) CD44 (a membrane glycoprotein), (2) receptor for hyaluronate-mediated motility, and (3) intercellular adhesion molecule 1. However, CD44 is the most widely distributed cell surface receptor recognized for HA binding. Some studies suggest that imbalanced HA polymers, with dominant low MW forms, may have a distinct biological impact on cellular and molecular pathways 82 . When high MW HA interacts with CD44, the pathway promoting the production of anti-inflammatory cytokines is activated. But when the low MW HA binds, the CD44-mediated signal has the opposite effect 83 . Also, Asari et al. 84 evidenced that low MW HA was involved in the induction and amplification of inflammation.
Undoubtedly, cartilage aging is closely related to structural changes in tissues and the presence of aging cells that influence homeostasis in joints. The SMS of senescent chondrocytes and resident MSCs contributes to the environmental imbalance by overproduction of chemokines, cytokines, and MMPs. These negatively affect cell susceptibility to growth factors and the ability to proliferate. As mentioned above, sMSCs express increased levels of the cell cycle inhibitor p16INK4a. Using the mouse collagenase-induced OA model, Malaise et al. 85 demonstrated that p16INK4a-positive sMSCs (ie, resident articular osteochondral progenitors) might damage cartilage and participate in the progression of OA. In OA synovial tissue, protein p16INK4a regulates the production of SMS-related factors that have a catabolic role86,87. Elevated levels of SMS factor IL-6 have been observed in SF of OA and rheumatoid arthritis (RA) patients88,89. Indeed, its long-term presence can prevent chondrogenic differentiation in SF-derived mesenchymal stem cells 90 . Another line of supporting evidence was provided by Wei et al. 91 Authors reported that IL-6 suppressed the differentiation of murine BM-MSCs into chondrocytes in a dose-dependent manner. Furthermore, human knee OA chondrocytes show impaired proteasomal function, which is probably caused by reduced autophagic function 92 . Autophagy is a necessary process for maintaining homeostasis in healthy cartilage. Its deregulation leads to the accumulation of damaged macromolecules that can promote degenerative changes. The recently published study demonstrated that enhanced autophagy in chondrocytes delayed the progression of OA 93 . The relationship between impaired autophagy and the increasing severity of OA has also been supported by other studies94,95.
Repair and restoration of articular cartilage mainly depend on the proper function of chondrocytes and resident MSCs. S-MSCs are characterized by higher chondrogenic potential compared with other MSCs 96 . Their cartilage rejuvenation properties rely on the production of hyaline cartilage matrices86,97. However, a large number of senescent S-MSCs (sS-MSCs) may have a negative impact on these processes. It should be noted that sS-MSCs have a longer survival time than healthy S-MSCs due to their resistance to apoptosis. Accumulation of sS-MSCs in joints, cartilage, synovial tissue, and SF has been observed in many pathological conditions. S-MSCs are easily activated and able to proliferate in an inflammatory environment. Although, persistent inflammation in the OA joint can lead to their depletion and induce replicative senescence in these otherwise rapidly proliferating cells. Cao et al. 98 showed that the synovium in the OA joint is the site with the high sS-MSC number. OA-related sS-MSCs displayed several characteristic features: cellular senescence, elevated pro-inflammatory molecule production, invasiveness, and reduced chondrogenic potential. However, the exact molecular mechanism by which resident sS-MSCs contribute to the disruption of homeostasis and loss of cartilage function is currently not well understood.
Of note, the number and size of extracellular vesicles (EVs) present in diseased/aged SF significantly differ from the young/healthy ones. Differences were also detected in the EV composition. Overall, the cargo of the young and old EVs primarily varies in the content of miRNAs, specifically the miR-183 cluster (miR-96/-182/-183) being strongly expressed in aged EVs. In the study conducted by Davis et al., mouse BM-MSCs were shown to easily engulf EVs isolated from the bone marrow interstitial fluid of old mice. These aged EVs suppressed the osteogenic differentiation in young BM-MSCs in vitro. Moreover, transfection of BM-MSCs with miR-183-5p decreased cell proliferation and osteogenic differentiation and favored senescence 99 . Indeed, EV production is several times higher in senescent cells100,101. Senescent EV-overloaded SFs also show an imbalance in levels of other miRNAs (eg, miR-199b, miR-185-5p, miR-661) 102 . Moreover, it was found that miR-34a in EVs derived from senescent chondrocytes can induce senescence in a paracrine way 103 . Elevated levels of miR-31 in plasma have also been reported in patients with osteoporosis 104 . Therefore, the in-depth analysis of EV cargo could be crucial for identifying senescent cells/environment and verifying diagnostic markers.
Rejuvenation
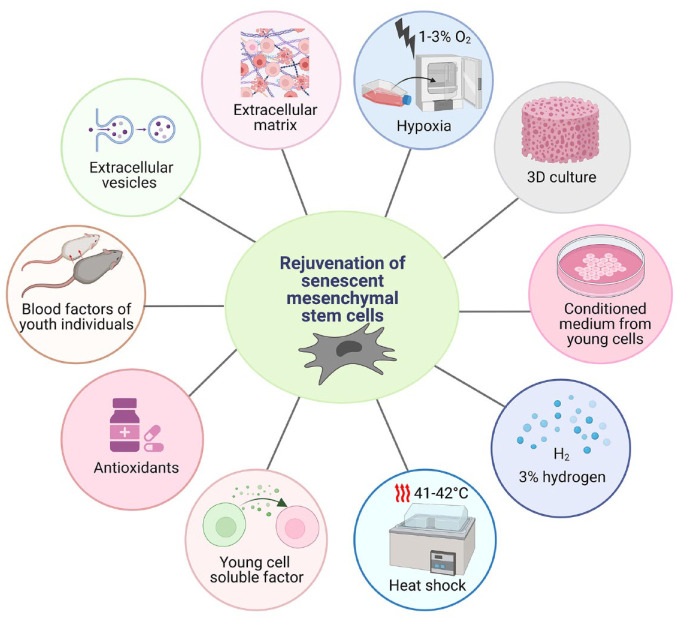
With increasing age, the extracellular environment is changing and affecting the fate of many cells. Pathological factors present in the microenvironment damage all cells, including resident MSCs. A better understanding of mechanisms involved in microenvironmental aging could contribute to its rejuvenation, maintaining the function of resident MSCs and preserving the healthy environment in many tissues at an older age. Several methods for sMSC rejuvenation are depicted in Fig. 2 and will be described in the following paragraphs.
Figure 2.
Techniques for the rejuvenation of senescent MSCs (sMSCs). This illustration shows various ways to maintain the stemness features in MSCs, possibly delaying/preventing the senescence-related adverse effects.
Rejuvenation of sMSCs by ECM, Antioxidants, Hypoxia, and Heat Shock
Generally, the niche is the local microenvironment in which MSCs naturally occur. It comprises ECM and bioactive molecules (growth factors, chemokines, cytokines, etc) 105 . The ECM fills the space between the cells and provides support. Also, it affects cells’ behavior, growth, differentiation, and metabolism 106 . Its exceptional composition allows MSCs to be kept in a quiescent state throughout life unless they are required to differentiate or repopulate damaged tissues. On the other hand, it can induce aging in MSCs, since the ECM composition changes with aging. Thus, through the modulation of ECM composition, the MSC biological activity may be retained until high cell passages. Lai et al. evidenced that the loss of BM-MSC characteristics was prevented when seeded on the human marrow stromal cell-derived ECM. This strongly enhanced cell proliferation and reduced the level of ROS. Furthermore, in vivo experiment confirmed that BM-MSCs grown on the ECM for multiple passages still retained the same ability for skeletogenesis. These findings suggest that large-scale BM-MSCs expanding on the marrow stromal cell-derived ECM is a promising technique for obtaining a high yield of highly functional BM-MSCs. Importantly, the establishment of a unique tissue-specific ECM can be utilized to control the MSC biological performance toward their desirable application 107 . Currently, much attention is paid to HA, as it is the most prevalent glycosaminoglycan in cartilage and its level reduces with age 108 . HA seems to have antiaging properties due to its ability to delay aging in murine AD-MSCs 109 . Moreover, Wong et al. demonstrated that placenta-derived MSCs (PD-MSCs) treated with HA could preserve the expression of stemness markers (CD105, CD90, and CD73) and osteogenic potential for 19 passages when compared with PD-MSCs cultured only on normal cell culture surface. The long-term HA treatment maintained the replicative capacity of PD-MSCs even after their transfer to the standard cell culture surface. Authors suggest that HA-related positive effects may be executed via the regulation of cytoskeleton protein distribution 110 . HA also protects MSCs from DNA damage caused by oxidative stress. And presumably, it is responsible for the decreased ROS formation in H2O2-treated PD-MSCs.
Further, in vitro experiments have shown that exposure of BM-MSCs to 3% hydrogen leads to an increased number of cell cycles without any loss in differentiation potential and paracrine activity. However, 3% hydrogen gas treatment did not reduce hydroxyl radical, protein carbonyl, and 8-hydroxydeoxyguanosine, indicating that scavenging hydroxyl radical might not be the primary mechanism behind the antiaging effect. In addition, hydrogen exposure is linked to the delay of senescence, thanks to decreased expression of SA-β-gal and p16INK4a, and altered paracrine activity in BM-MSCs. Even though hydrogen treatment did not reduce the paracrine activity of BM-MSCs, it changed the level of secreted components. For instance, increased secretion of basic FGF, HGF, and indoleamine 2,3-dioxygenase was detected 111 . Other antioxidants, such as ascorbic acid (AA) 112 , Cirsium setidens 113 , and lactoferrin 114 were confirmed to suppress ROS production and reduce the signs of aging in MSCs as well.
Notably, a hypoxic environment also shows a significant antisenescence effect on MSCs. Hypoxia-exposed MSCs can preserve stemness characteristics without increasing the risk of tumorigenicity 115 . So far, its beneficial effects have been demonstrated on MSCs derived from different tissues. For example, human amniotic fluid mesenchymal stem cells (AF-MSCs) cultured at low oxygen concentration (1% O2) preserved features of stemness, proliferation, and osteogenic potential. Hypoxic AF-MSCs underwent a metabolic shift and showed increased resistance to pro-apoptotic stimuli 116 . Under hypoxic conditions, AD-MSCs displayed more efficient cell proliferation with a faster population doubling rate and enhanced secretion of multiple angiogenic growth factors. And hypoxia-inducible factor (HIF) is considered the main executor of hypoxic response 117 . Similarly, a study by Sheng et al. proved the stimulatory effect of 2% hypoxia on proliferation, differentiation into endothelial cells, and vascular endothelial growth factor (VEGF) expression in BM-MSCs. The PI3K/Akt pathway was shown to have an essential role in this hypoxia-triggered boost 118 . Moreover, in mouse BM-MSCs, hypoxia (3% O2) contributes to the evaluated expression of chemokine receptors CXCR4 and CXCR7 implicated in cell migration and survival in vitro. Hypoxia preconditioning not only facilitated MSC chemotaxis and viability but also promoted the release of proangiogenic and mitogenic factors. The increased homing of hypoxia-exposed BM-MSCs was related to higher functional recovery, enhanced mitogenic response, and decreased apoptotic cell death in vivo 119 . In addition, exposure of olfactory mucosa-derived mesenchymal stem cells (OM-MSCs) to 3% hypoxia delayed senescence, enhanced survival after transplantation, and improved the neuroprotective effects in an intracerebral hemorrhage mouse model. Hypoxia ameliorated the function of OM-MSCs by upregulating the miR-326/PTBP1/PI3K-mediated autophagy 120 .
A prolonged exposure to heat shock (HS) can be harmful and trigger premature cellular senescence in human MSCs derived from menstrual blood (MB-hMSCs). HS-treated MB-hMSCs exhibited altered morphology (enlarged and flattened shape) as well as other senescence-related features, such as increased SA-β-gal activity and expression of cell cycle inhibitors 121 . However, other studies showed that HS can have also positive effect and improve the biological performance of aging MSCs. Exposure of AD-MSCs to HS (41°C for 60 min) once in a week led to increased expansion and differentiation potential, particularly at higher passages (eight passages cultured for approximately 7 weeks). Stressed AD-MSCs had elevated levels of SIRT-1, which might be related to the delayed senescence in these cells 122 . Similarly, in BM-MSCs, Wang et al. evidenced that HS pretreatment (42°C for 60 min) inhibited the apoptosis and enhanced the survival under cisplatin-induced chemotherapy environment. Most likely, this effect was associated with the increased expression of HSP70 and HSP90 123 . The antiapoptotic effect of HSPs may be executed via binding the caspase-recruitment domain of apoptotic protease-activating factor-1 and the caspase-independent death effector apoptosis-inducing factor leading to the suppression of the apoptotic pathway123,124. Interestingly, addition of exogenous HSP70 supported growth of aged but not young murine AD-MSCs. The stimulatory effect was observed also after application of a mild HS (42°C for 5 min). Importantly, aged murine AD-MSCs were significantly more responsive to higher heat stress compared with the young cells 125 .
Rejuvenation by Soluble Factors
Multicellular organisms do not have the ability and are not equipped with mechanisms to avoid the natural aging process. The best-known technique used to study vertebrate aging is heterochronic parabiosis, which represents the surgical connection of the circulatory system of old and young mice. This technique was discovered in 1864 by physiologist Paul Bert and later used to elucidate the improved stem cell aging in old mice after exposure to the young mouse circulatory system. It was found that factors in young blood contributed to the activation of biochemical pathways in aged stem cells and boosted their regenerative properties 126 . The ability of blood derivatives to affect the regeneration of stem cells and tissues is also consistent with the findings of several other studies127,128. As an example, platelet-rich fibrin and platelet-rich plasma include a wide range of mediators [eg, fibroblast growth factor 2 (FGF-2), IGF-1, platelet-derived growth factor (PDGF), and VEGF] that can promote MSC proliferation, differentiation, and maintenance of stemness characteristics129,130. The rejuvenation of long-term cultured senescent human bone–derived MSCs by human platelet lysate enhanced cell phenotype and proliferative activity 127 . Further research also confirmed the interaction of circulatory factors with signaling pathways involved in the activation or inhibition of tissue-specific MSCs. The reduced activity of MSCs in old mice is probably related to the dominance of inhibitory factors circulating in their blood. For instance, TGF-β is increased in the plasma of aged mice 131 . Gurung et al. demonstrated that TGF-β receptor (TGF-βR) inhibitor promoted endometrial mesenchymal stem cells (eMSCs) proliferation in the undifferentiation state during prolonged in vitro cultivation. Suppression of TGF-βR signaling further increased cell proliferation, and prevented apoptosis and senescence in eMSCs 132 . It is plausible that due to the high level of circulating TGF-β in the blood of old mice, aged subjects have a reduced ability to regenerate damaged tissue compared with young ones133,134.
Several lines of evidence indicate that all molecular and phenotypic features of aging are reversible and thus can be rescued by applying soluble factors and cells from a young circulating environment 135 . The overexpression or supplementation of systemic growth differentiation factor 11 (GDF-11), which normally decreases with age, improved homing of endothelial progenitor cells and angiogenesis in old ischemic hearts, enhanced muscle structural and functional features, and supported strength and endurance exercise capacity in aged mice131,136. Nevertheless, some studies have reported contradicting results regarding the positive effect of GDF-11, especially on skeletal muscle stem cells137,138. Delayed senescence of BM-MSCs was also achieved by stimulation with factors such as AA, epidermal growth factor (EGF), FGF-2, and PDGF. Although BM-MSCs exhibited an elevated in vitro expansion, their differentiation potential was reduced before reaching senescence. The expression of stem cell surface markers was not affected by the loss of differentiation capacity 139 . In general, growth factors, such as FGF-2, show pleiotropic effects, and their application may generate ambiguous data. Several studies have demonstrated a dual effect of FGF-2, both stimulatory and inhibitory, on the differentiation of MSCs. Hanada et al. 140 showed that FGF-2 markedly augmented cell growth and triggered osteoblastic differentiation in rat BM-MSCs. Moreover, its positive effects on chondrogenic differentiation in human BM-MSCs have also been reported 141 . On the other hand, the study by Baddoo et al. demonstrated an inhibitory effect of FGF-2 on multilineage differentiation of mouse BM-MSCs. Its removal from culture restored the differentiation potential of BM-MSCs. Therefore, FGF-2 may be used selectively when the expansion of undifferentiated BM-MSCs is required 142 . The study by Coutu et al. evidenced that murine BM-MSCs cultured without FGF-2 exhibited distinct features of cellular senescence at very early passages. Interestingly, the senescent phenotype of BM-MSCs expanded without FGF-2 cannot be reversed by the subsequent stimulation with FGF-2. To further clarify the effect of FGF-2, cells cultured in a medium supplemented with FGF-2 for three passages were subsequently grown in its absence. FGF-2 removal immediately triggered growth arrest in BM-MSCs 143 . Although numerous factors were shown to rejuvenate MSCs, their mechanism of action is still unknown. It is hypothesized that these youth-stimulating factors may operate nondirectly. Their activity possibly depends on epigenetic mechanisms or interaction with other components 144 .
Rejuvenation by the Secretome of the Young MSCs
MSCs secrete various types of EVs: exosomes (40–150 nm), microvesicles (150–1,000 nm), apoptotic bodies (50–2,000 nm), and others. EVs are frequently enriched with bioactive molecules (serum proteins, angiogenic and growth factors, hormones, cytokines, ECM, etc). The composition of EVs and secretome affects the condition of niche and resident cells. It also plays a significant role in protection against various diseases, especially if it comes from young and healthy cells 145 . Indeed, young secretome has a noticeable rejuvenating effect. On the other hand, aging cells typically release harmful cargos that can cause inflammation, epigenetic changes, dysfunction of cell organelles, and cellular senescence. Old MSCs cultured with ECM or a conditioned medium from young MSCs showed improved differentiation and replication ability 146 . The study performed by Liang et al. demonstrated the positive impact of human UC-MSC secretome on the aging rat BM-MSCs. Human UC-MSC secretome loaded into silk fibroin-based hydrogels was proved to recover MSC potential and attenuate the local bone loss in vivo 147 . In addition, a cocktail of molecules, including EGF, FGF, VEGF, and IGF from the secretome of UC-MSCs, may prevent the onset of osteoporosis, thanks to bone remodeling and regeneration148,149. Several studies have demonstrated a significant rejuvenating potential of molecules that are part of EV cargo derived from young cells on aging human MSCs150–152. More specifically, after EV-containing nicotinamide phosphoribosyltransferase (NAMPT) internalization into the cell, NAD+ biosynthesis was elevated. Furthermore, supplementing extracellular NAMPT through EVs obtained from young mice markedly augments the wheel-running activity and expands the lifespan of aged mice suggesting a potential antiaging treatment in humans 153 . The favorable effect of secretome is supported also by the study conducted by Arasu et al. Authors showed that BM-MSCs secreted HA-coated EVs that carried mRNAs for CD44 and all HAS isoforms. This indicates that HA-coated EVs may be used mainly when tissue regeneration requires HA supplementation, as in the case of aging articular cartilage 154 . Currently, considerable attention is paid to EVs derived from urine-isolated stem cells (USCs-EVs) since these cells are easily available and do not require invasive collection methods. USCs-EVs carry several components in abundance, including collagen triple-helix repeat containing 1 and osteoprotegerin. These mitigate bone loss by supporting osteoblast and suppressing osteoclast activity in osteoporotic mice. Importantly, USC-EV properties were not affected by age, gender, or health condition of the USC donor. Data clearly indicate the medicinal potential of autologous USCs-EVs as well as show a new promising strategy in osteoporosis treatment 155 . A remarkable rejuvenating potential of antler stem cells (ASCs)-derived exosomes (ASCs-EVs) on human sMSCs was recently demonstrated. Generally, ASCs show high proliferative and regenerative capacity since they annually initiate the formation of the entire organ. After ASCs-EVs treatment, human MSCs showed attenuated signs of senescence. Also, intra-articular injection of ASCs-EVs reduced cartilage damage in the OA mouse model. Taken together, ASCs could serve as an inexhaustible source of EVs to support cell-free therapy 156 . The effect of EVs harvested from hypoxic nonsenescent dental pulp stem cells (DPSCs) on normoxic prematurely senescent DPSCs was also investigated. Upon EV treatment, normoxic senescent DPSCs exhibited suppressed features of senescence, promoted expression of stemness markers, and metabolic switch toward glycolysis. EV components increased miR-302b and HIF-1α levels in acceptor cells. Notably, miR-302b was also encapsulated in EVs derived from hypoxic nonsenescent DPSCs. It was proposed that the exogenous and endogenous upregulation of miR-302b may induce HIF-1α. And this pathway is likely behind the delaying DPSC aging 152 . Indeed, the properties of MSC-isolated EVs may be modulated by environmental factors, such as low oxygen tension or hydrodynamic culture in bioreactors. 3D aggregation of BM-MSCs significantly increased the quantity (by twofold) and miR-21 and miR-22 expression, and changed the protein composition (eg, upregulation of cytokines and anti-inflammatory factors) of EVs compared with 2D culture. 3D BM-MSC-EVs rejuvenated sMSCs and displayed promoted immunomodulatory capacity 157 . Application of EVs represents a relatively safe cell-free approach to rejuvenate senescent cells since it does not induce tumor formation and shows low immunogenicity. Despite having many advantages, several drawbacks, such as short lifespan, degradation, and inefficient targeting, significantly reduce the effectiveness of EV-based techniques. The production of artificial EVs could, at least partially, eliminate these shortcomings.
Rejuvenating Approaches in Animal Models
Even if the rejuvenating techniques have not reached the clinical phase yet, their application in in vivo models is established. To show that these approaches do have a potential to enter clinical research we provide data from several preclinical studies.
Exosomes derived from young rat BM-MSCs (BM-MSCs-Exos) were locally administered and their impact on bone regeneration was investigated in older rats. Significant acceleration in healing process and better mechanical properties were demonstrated upon BM-MSCs-Exos treatment 158 . EVs secreted from hypoxia-pretreated BM-MSCs (5% O2 for 48 h) exerted a remarkable therapeutic outcome in the rat OA model. The chondroprotective effect of hypoxic EVs may be mediated by the miRNA-18-3P/JAK-STAT or miRNA-181c-5p/MAPK signaling pathway, which might enhance chondrocyte proliferation and migration and inhibit chondrocyte apoptosis 159 .
Moreover, an increased anti-inflammatory and regulatory potential of allogeneic BM-MSCs primed with tumor necrosis factor-α and IFN-γ was reported in an equine model of chemically induced OA 160 . Recently, an innovative approach has been brought by Pei et al. Cell-derived decellularized extracellular matrix (C-dECM) rejuvenation improved the infrapatellar fat pad–derived stem cell (IPFSC) cartilage engineering and functional regeneration in a rabbit model of osteochondral defect. The profound effect was observed particularly for the decellularized ECM deposited by urine-derived stem cells (UdSCs). Authors suggested that mesenchymal–epithelial transition and inflammation-mediated macrophage activation and polarization are presumably implicated in the C-dECM–related enhancement of IPFSCs’ chondrogenic potential 161 .
Chromobox protein homologue 4 (CBX4) is downregulated in aged human MSCs. The CBX4 deficiency causes destabilized nucleolar heterochromatin, increased ribosome biogenesis, elevated protein translation, and premature aging. CBX4-overexpressing lentiviral vectors delivered by intra-articular injection dampened cartilage erosion, improved bone density, triggered the expression of bone growth and differentiation genes, and downregulated the expression of aging and inflammation molecules in the OA model 162 . In addition, yes-associated protein (YAP) is a well-known effector of Hippo signaling, which represses the senescence of human MSCs via activation of forkhead box D1 (FOXD1) transcription. The intra-articular delivery of lentiviruses encoding YAP or FOXD1 decreased the number of senescent cells and suppressed articular inflammation and cartilage erosion. Thus, gene therapy via the introduction of geroprotective factors leading to rejuvenating sMSCs may represent a new tool in the OA therapy in the future 163 .
Conclusion
MSC senescence is integral to the human aging process and is one of the underlying causes of joint-related diseases such as OA. In vitro and in vivo MSC senescence is a major obstacle, especially the development of SMS, which not only changes the MSC biological activity but also affects other resident cells and their microenvironment. Mechanisms involved in regulating MSC senescent phenotype and the pathophysiological impact of sMSC-mediated autocrine and paracrine signaling are still not completely understood. On the other hand, parabiosis has suggested over decades that factors derived from young blood may support the regeneration of diseased or aged tissues. Currently, many rejuvenation strategies (from cell free to gene therapy) used for delaying and/or reversing cellular senescence are being scrutinized. These can be a very powerful tool for maintaining the proper function of resident MSCs and boosting the therapeutical potential of autologous MSCs isolated from older people before transplantation. MSCs represent an essential source for cellular and cell-free therapies in managing many disorders, including degenerative and other aging-related diseases. Therefore, a better understanding of senescence itself enables control of the course of MSC-based treatments to maximize clinical benefits.
Acknowledgments
Figures 1 and 2 were created with BioRender.com.
Footnotes
Author Contributions: TS conceived the work, wrote the first draft, and designed the figures. MC contributed to draft writing, editing, and critically reviewed the manuscript drafts for important intellectual content. LD performed the final manuscript editing. All authors revised the final manuscript version, and approved its submission.
Availability of Data and Material: Not applicable.
Ethical Approval: This review did not require institutional review board approval.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Operational Programme Integrated Infrastructure for the following project: Increasing the capacities and competences of the Comenius University in research, development, and innovation 313021BUZ3, cofinanced from the resources of the European Regional Development Fund. This research was funded also by the Comenius University in Bratislava (grant number: UK/251/2023) and it is the result of the project implementation: Center for Advanced Therapies of Chronic Inflammatory Diseases of the Musculoskeletal System (CPT-ZOPA), ITMS2014+: 313011W410 supported by the Operational Programme Integrated Infrastructure funded by the European Regional Development Fund.
ORCID iDs: Lubos Danisovic  https://orcid.org/0000-0002-5074-9621
https://orcid.org/0000-0002-5074-9621
Michaela Cehakova  https://orcid.org/0000-0001-9530-6499
https://orcid.org/0000-0001-9530-6499
References
- 1. Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9(1):168. doi: 10.1186/s13287-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3. Testa S, Riera CS, Fornetti E, Riccio F, Fuoco C, Bernardini S, Baldi J, Costantini M, Foddai ML, Cannata S, Gargioli C. Skeletal muscle-derived human mesenchymal stem cells: influence of different culture conditions on proliferative and myogenic capabilities. Front Physiol. 2020;11:553198. doi: 10.3389/fphys.2020.553198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–42. doi: [DOI] [PubMed] [Google Scholar]
- 5. Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford). 2008;47(8):1137–43. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- 6. Burdeyron P, Giraud S, Hauet T, Steichen C. Urine-derived stem/progenitor cells: a focus on their characterization and potential. World J Stem Cells. 2020;12(10):1080–96. doi: 10.4252/wjsc.V12.i10.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLeod CM, Mauck RL. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater. 2017;34:217–31. doi: 10.22203/eCM.v034a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm Lond Engl. 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445–53. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shin S, Lee J, Kwon Y, Park KS, Jeong JH, Choi SJ, Bang SI, Chang JW, Lee C. Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and Wharton’s jelly. Int J Mol Sci. 2021;22(2):845. doi: 10.3390/ijms22020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fathi E, Charoudeh HN, Sanaat Z, Farahzadi R. Telomere shortening as a hallmark of stem cell senescence. Stem Cell Investig. 2019;6:7. doi: 10.21037/sci.2019.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. Senescence of mesenchymal stem cells (Review). Int J Mol Med. 2017;39(4):775–82. doi: 10.3892/ijmm.2017.2912. [DOI] [PubMed] [Google Scholar]
- 15. Liu H, Xia X, Li B. Mesenchymal stem cell aging: mechanisms and influences on skeletal and non-skeletal tissues. Exp Biol Med (Maywood). 2015;240(8):1099–106. doi: 10.1177/1535370215591828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–81. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fraile M, Eiro N, Costa LA, Martín A, Vizoso FJ. Aging and mesenchymal stem cells: basic concepts, challenges and strategies. Biology. 2022;11(11):1678. doi: 10.3390/biology11111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bashiri Dezfouli A, Salar-Amoli J, Pourfathollah AA, Yazdi M, Nikougoftar-Zarif M, Khosravi M, Hassan J. Doxorubicin-induced senescence through NF-κB affected by the age of mouse mesenchymal stem cells. J Cell Physiol. 2020;235(3):2336–49. doi: 10.1002/jcp.29140. [DOI] [PubMed] [Google Scholar]
- 19. Al-Azab M, Safi M, Idiiatullina E, Al-Shaebi F, Zaky MY. Aging of mesenchymal stem cell: machinery, markers, and strategies of fighting. Cell Mol Biol Lett. 2022;27(1):69. doi: 10.1186/s11658-022-00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geissler S, Textor M, Kühnisch J, Könnig D, Klein O, Ode A, Pfitzner T, Adjaye J, Kasper G, Duda GN. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS ONE. 2012;7(12):e52700. doi: 10.1371/journal.pone.0052700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE. 2008;3(5):e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weng Z, Wang Y, Ouchi T, Liu H, Qiao X, Wu C, Zhao Z, Li L, Li B. Mesenchymal stem/stromal cell senescence: hallmarks, mechanisms, and combating strategies. Stem Cells Transl Med. 2022;11(4):356–71. doi: 10.1093/stcltm/szac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187–95. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 24. Hildebrand DG, Lehle S, Borst A, Haferkamp S, Essmann F, Schulze-Osthoff K. α-Fucosidase as a novel convenient biomarker for cellular senescence. Cell Cycle Georget Tex. 2013;12(12):1922–27. doi: 10.4161/cc.24944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gnani D, Crippa S, Della Volpe L, Rossella V, Conti A, Lettera E, Rivis S, Ometti M, Fraschini G, Bernardo ME, Di Micco R. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell. 2019;18(3):e12933. doi: 10.1111/acel.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang YHK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9(1):131. doi: 10.1186/s13287-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung EM, Kwon O, Kwon KS, Cho YS, Rhee SK, Min JK, Oh DB. Evidences for correlation between the reduced VCAM-1 expression and hyaluronan synthesis during cellular senescence of human mesenchymal stem cells. Biochem Biophys Res Commun. 2011;404(1):463–69. doi: 10.1016/j.bbrc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 28. Madsen SD, Jones SH, Tucker HA, Giler MK, Muller DC, Discher CT, Russell KC, Dobek GL, Sammarco MC, Bunnell BA, O’Connor KC. Survival of aging CD264+ and CD264- populations of human bone marrow mesenchymal stem cells is independent of colony-forming efficiency. Biotechnol Bioeng. 2020;117(1):223–37. doi: 10.1002/bit.27195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lunyak VV, Amaro-Ortiz A, Gaur M. Mesenchymal stem cells secretory responses: senescence messaging secretome and immunomodulation perspective. Front Genet. 2017;8:220. doi: 10.3389/fgene.2017.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oja S, Komulainen P, Penttilä A, Nystedt J, Korhonen M. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res Ther. 2018;9(1):6. doi: 10.1186/s13287-017-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serakinci N, Cagsin H, Mavis M. Use of U-STELA for accurate measurement of extremely short telomeres. Methods Mol Biol. 2019;2045:217–24. doi: 10.1007/7651_2018_120. [DOI] [PubMed] [Google Scholar]
- 32. Cheng H, Qiu L, Ma J, Zhang H, Cheng M, Li W, Zhao X, Liu K. Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts. Mol Biol Rep. 2011;38(8):5161–68. doi: 10.1007/s11033-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 33. Bork S, Pfister S, Witt H, Horn P, Korn B, Ho AD, Wagner W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9(1):54–63. doi: 10.1111/j.1474-9726.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koch CM, Joussen S, Schellenberg A, Lin Q, Zenke M, Wagner W. Monitoring of cellular senescence by DNA-methylation at specific CpG sites. Aging Cell. 2012;11(2):366–69. doi: 10.1111/j.1474-9726.2011.00784.x. [DOI] [PubMed] [Google Scholar]
- 35. Block TJ, Marinkovic M, Tran ON, Gonzalez AO, Marshall A, Dean DD, Chen XD. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res Ther. 2017;8(1):239. doi: 10.1186/s13287-017-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghosh D, Mejia Pena C, Quach N, Xuan B, Lee AH, Dawson MR. Senescent mesenchymal stem cells remodel extracellular matrix driving breast cancer cells to a more-invasive phenotype. J Cell Sci. 2020;133(2):jcs232470. doi: 10.1242/jcs.232470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Volonte D, Galbiati F. Caveolin-1, a master regulator of cellular senescence. Cancer Metastasis Rev. 2020;39(2):397–414. doi: 10.1007/s10555-020-09875-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park JS, Kim HY, Kim HW, Chae GN, Oh HT, Park JY, Shim H, Seo M, Shin EY, Kim EG, Park SC, et al. Increased caveolin-1, a cause for the declined adipogenic potential of senescent human mesenchymal stem cells. Mech Ageing Dev. 2005;126(5):551–59. doi: 10.1016/j.mad.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 39. Li X, Hong Y, He H, Jiang G, You W, Liang X, Fu Q, Han S, Lian Q, Zhang Y. FGF21 mediates mesenchymal stem cell senescence via regulation of mitochondrial dynamics. Oxid Med Cell Longev. 2019;2019:4915149. doi: 10.1155/2019/4915149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barilani M, Lovejoy C, Piras R, Abramov AY, Lazzari L, Angelova PR. Age-related changes in the energy of human mesenchymal stem cells. J Cell Physiol. 2022;237(3):1753–67. doi: 10.1002/jcp.30638. [DOI] [PubMed] [Google Scholar]
- 41. Hu C, Zhao L, Peng C, Li L. Regulation of the mitochondrial reactive oxygen species: strategies to control mesenchymal stem cell fates ex vivo and in vivo. J Cell Mol Med. 2018;22(11):5196–207. doi: 10.1111/jcmm.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nugud A, Sandeep D, El-Serafi AT. Two faces of the coin: minireview for dissecting the role of reactive oxygen species in stem cell potency and lineage commitment. J Adv Res. 2018;14:73–79. doi: 10.1016/j.jare.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jeong SG, Cho GW. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem Biophys Res Commun. 2015;460(4):971–76. doi: 10.1016/j.bbrc.2015.03.136. [DOI] [PubMed] [Google Scholar]
- 44. Ko E, Lee KY, Hwang DS. Human umbilical cord blood-derived mesenchymal stem cells undergo cellular senescence in response to oxidative stress. Stem Cells Dev. 2012;21(11):1877–86. doi: 10.1089/scd.2011.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choo KB, Tai L, Hymavathee KS, Wong CY, Nguyen PN, Huang CJ, Cheong SK, Kamarul T. Oxidative stress-induced premature senescence in Wharton’s jelly-derived mesenchymal stem cells. Int J Med Sci. 2014;11(11):1201–207. doi: 10.7150/ijms.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang DY, Pan Y, Zhang C, Yan BX, Yu SS, Wu DL, Shi MM, Shi K, Cai XX, Zhou SS, Wang JB, et al. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem. 2013;374(1–2):13–20. doi: 10.1007/s11010-012-1498-1. [DOI] [PubMed] [Google Scholar]
- 47. Yu J, Shi J, Zhang Y, Zhang Y, Huang Y, Chen Z, Yang J. The replicative senescent mesenchymal stem / stromal cells defect in DNA damage response and anti-oxidative capacity. Int J Med Sci. 2018;15(8):771–81. doi: 10.7150/ijms.24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69(13):5331–39. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Zhang Z, Chi Y, Zhang Q, Xu F, Yang Z, Meng L, Yang S, Yan S, Mao A, Zhang J, et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013;4:e950. doi: 10.1038/cddis.2013.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. So AY, Jung JW, Lee S, Kim HS, Kang KS. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS ONE. 2011;6(5):e19503. doi: 10.1371/journal.pone.0019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jung JW, Lee S, Seo MS, Park SB, Kurtz A, Kang SK, Kang KS. Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cell Mol Life Sci. 2010;67(7):1165–76. doi: 10.1007/s00018-009-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gu Y, Li T, Ding Y, Sun L, Tu T, Zhu W, Hu J, Sun X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol Med Rep. 2016;13(6):5207–15. doi: 10.3892/mmr.2016.5169. [DOI] [PubMed] [Google Scholar]
- 53. Trachana V, Petrakis S, Fotiadis Z, Siska EK, Balis V, Gonos ES, Kaloyianni M, Koliakos G. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress-induced genomic damage. Cytotherapy. 2017;19(7):808–20. doi: 10.1016/j.jcyt.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 54. Chen H, Liu X, Zhu W, Chen H, Hu X, Jiang Z, Xu Y, Wang L, Zhou Y, Chen P, Zhang N, et al. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front Aging Neurosci. 2014;6:103. doi: 10.3389/fnagi.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gaur M, Wang L, Amaro Ortiz A, Dobke M, Jordan IK, Lunyak VV. Acute genotoxic stress-induced senescence in human mesenchymal cells drives a unique composition of senescence messaging secretome (SMS). J Stem Cell Res Ther. 2017;7:8. doi: 10.4172/2157-7633.1000396. [DOI] [Google Scholar]
- 56. Lee HJ, Choi B, Kim Y, Lee SE, Jin HJ, Lee HS, Chang EJ, Kim SW. The upregulation of toll-like receptor 3 via autocrine IFN-β signaling drives the senescence of human umbilical cord blood-derived mesenchymal stem cells through JAK1. Front Immunol. 2019;10:1659. doi: 10.3389/fimmu.2019.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 2013;4(11):e911. doi: 10.1038/cddis.2013.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chou LY, Ho CT, Hung SC. Paracrine senescence of mesenchymal stromal cells involves inflammatory cytokines and the NF-κB pathway. Cells. 2022;11(20):3324. doi: 10.3390/cells11203324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Malaquin N, Martinez A, Rodier F. Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp Gerontol. 2016;82:39–49. doi: 10.1016/j.exger.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 60. Tchkonia T, Palmer AK, Kirkland JL. New horizons: novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J Clin Endocrinol Metab. 2021;106(3):e1481–87. doi: 10.1210/clinem/dgaa728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016;23(2):303–14. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Y, Liu Y, Chen E, Pan Z. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. 2020;382(3):457–62. doi: 10.1007/s00441-020-03272-z. [DOI] [PubMed] [Google Scholar]
- 63. Hoare M, Narita M. Transmitting senescence to the cell neighbourhood. Nat Cell Biol. 2013;15(8):887–89. doi: 10.1038/ncb2811. [DOI] [PubMed] [Google Scholar]
- 64. Hubackova S, Krejcikova K, Bartek J, Hodny Z. IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine “bystander senescence. Aging (Albany NY). 2012;4(12):932–51. doi: 10.18632/aging.100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–90. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen H, Liu O, Chen S, Zhou Y. Aging and mesenchymal stem cells: therapeutic opportunities and challenges in the older group. Gerontology. 2022;68(3):339–52. doi: 10.1159/000516668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–56. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu J, Wang C, Miao X, Wu Y, Yuan J, Ding M, Li J, Shi Z. Age-related insulin-like growth factor binding protein-4 overexpression inhibits osteogenic differentiation of rat mesenchymal stem cells. Cell Physiol Biochem. 2017;42(2):640–50. doi: 10.1159/000477873. [DOI] [PubMed] [Google Scholar]
- 70. Lin W, Liu Z, Kampf N, Klein J. The role of hyaluronic acid in cartilage boundary lubrication. Cells. 2020;9(7):E1606. doi: 10.3390/cells9071606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pearson B, Espino DM. Effect of hydration on the frequency-dependent viscoelastic properties of articular cartilage. Proc Inst Mech Eng H. 2013;227(11):1246–52. doi: 10.1177/0954411913501294. [DOI] [PubMed] [Google Scholar]
- 72. Hosseininia S, Lindberg LR, Dahlberg LE. Cartilage collagen damage in hip osteoarthritis similar to that seen in knee osteoarthritis; a case-control study of relationship between collagen, glycosaminoglycan and cartilage swelling. BMC Musculoskelet Disord. 2013;14:18. doi: 10.1186/1471-2474-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mirahmadi F, Koolstra JH, Lobbezoo F, van Lenthe GH, Ghazanfari S, Snabel J, Stoop R, Everts V. Mechanical stiffness of TMJ condylar cartilage increases after artificial aging by ribose. Arch Oral Biol. 2018;87:102–109. doi: 10.1016/j.archoralbio.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 74. Cooke ME, Lawless BM, Jones SW, Grover LM. Matrix degradation in osteoarthritis primes the superficial region of cartilage for mechanical damage. Acta Biomater. 2018;78:320–28. doi: 10.1016/j.actbio.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 75. Mort JS, Geng Y, Fisher WD, Roughley PJ. Aggrecan heterogeneity in articular cartilage from patients with osteoarthritis. BMC Musculoskelet Disord. 2016;17:89. doi: 10.1186/s12891-016-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bank RA, Soudry M, Maroudas A, Mizrahi J, TeKoppele JM. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 2000;43(10):2202–10. doi: [DOI] [PubMed] [Google Scholar]
- 77. Hui W, Young DA, Rowan AD, Xu X, Cawston TE, Proctor CJ. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann Rheum Dis. 2016;75(2):449–58. doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Abatangelo G, Vindigni V, Avruscio G, Pandis L, Brun P. Hyaluronic acid: redefining its role. Cells. 2020;9(7):1743. doi: 10.3390/cells9071743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gupta RC, Lall R, Srivastava A, Sinha A. Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci. 2019;6:192. doi: 10.3389/fvets.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhao N, Wang X, Qin L, Guo Z, Li D. Effect of molecular weight and concentration of hyaluronan on cell proliferation and osteogenic differentiation in vitro. Biochem Biophys Res Commun. 2015;465(3):569–74. doi: 10.1016/j.bbrc.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 81. Temple-Wong MM, Ren S, Quach P, Hansen BC, Chen AC, Hasegawa A, D’Lima DD, Koziol J, Masuda K, Lotz MK, Sah R. Hyaluronan concentration and size distribution in human knee synovial fluid: variations with age and cartilage degeneration. Arthritis Res Ther. 2016;18:18. doi: 10.1186/s13075-016-0922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang C, Cao M, Liu H, He Y, Xu J, Du Y, Liu Y, Wang W, Cui L, Hu J, Gao F. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem. 2012;287(51):43094–107. doi: 10.1074/jbc.M112.349209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hu L, Nomura S, Sato Y, Takagi K, Ishii T, Honma Y, Watanabe K, Mizukami Y, Muto J. Anti-inflammatory effects of differential molecular weight hyaluronic acids on UVB-induced calprotectin-mediated keratinocyte inflammation. J Dermatol Sci. 2022;107(1):24–31. doi: 10.1016/j.jdermsci.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 84. Asari A, Kanemitsu T, Kurihara H. Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via toll-like receptor 4 in the intestinal epithelium. J Biol Chem. 2010;285(32):24751–58. doi: 10.1074/jbc.M110.104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Malaise O, Tachikart Y, Constantinides M, Mumme M, Ferreira-Lopez R, Noack S, Krettek C, Noël D, Wang J, Jorgensen C, Brondello JM. Mesenchymal stem cell senescence alleviates their intrinsic and seno-suppressive paracrine properties contributing to osteoarthritis development. Aging. 2019;11(20):9128–46. doi: 10.18632/aging.102379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Miura Y, Endo K, Komori K, Sekiya I. Clearance of senescent cells with ABT-263 improves biological functions of synovial mesenchymal stem cells from osteoarthritis patients. Stem Cell Res Ther. 2022;13(1):222. doi: 10.1186/s13287-022-02901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Diekman BO, Sessions GA, Collins JA, Knecht AK, Strum SL, Mitin NK, Carlson CS, Loeser RF, Sharpless NE. Expression of p16INK4a is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell. 2018;17(4):e12771. doi: 10.1111/acel.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6(2):71–79. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 89. Santos Savio A, Machado Diaz AC, Chico Capote A, Miranda Navarro J, Rodríguez Alvarez Y, Bringas Pérez R, Estévez del Toro M, Guillen Nieto GE. Differential expression of pro-inflammatory cytokines IL-15Ralpha, IL-15, IL-6 and TNFalpha in synovial fluid from rheumatoid arthritis patients. BMC Musculoskelet Disord. 2015;16:51. doi: 10.1186/s12891-015-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liu W, Sun Y, He Y, Zhang H, Zheng Y, Yao Y, Zhang Z. IL-1β impedes the chondrogenic differentiation of synovial fluid mesenchymal stem cells in the human temporomandibular joint. Int J Mol Med. 2017;39(2):317–26. doi: 10.3892/ijmm.2016.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wei H, Shen G, Deng X, Lou D, Sun B, Wu H, Long L, Ding T, Zhao J. The role of IL-6 in bone marrow (BM)-derived mesenchymal stem cells (MSCs) proliferation and chondrogenesis. Cell Tissue Bank. 2013;14(4):699–706. doi: 10.1007/s10561-012-9354-9. [DOI] [PubMed] [Google Scholar]
- 92. Serrano RL, Chen LY, Lotz MK, Liu-Bryan R, Terkeltaub R. Impaired proteasomal function in human osteoarthritic chondrocytes can contribute to decreased levels of SOX9 and aggrecan. Arthritis Rheumatol. 2018;70(7):1030–41. doi: 10.1002/art.40456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Luo P, Gao F, Niu D, Sun X, Song Q, Guo C, Liang Y, Sun W. The role of autophagy in chondrocyte metabolism and osteoarthritis: a comprehensive research review. Biomed Res Int. 2019;2019:5171602. doi: 10.1155/2019/5171602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Goldring MB, Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol. 2015;22:51–63. doi: 10.1016/j.coph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fernandes TL, Kimura HA, Pinheiro CCG, Shimomura K, Nakamura N, Ferreira JR, Gomoll AH, Hernandez AJ, Bueno DF. Human synovial mesenchymal stem cells good manufacturing practices for articular cartilage regeneration. Tissue Eng Part C Methods. 2018;24(12):709–16. doi: 10.1089/ten.TEC.2018.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jeyaraman M, Muthu S, Jeyaraman N, Ranjan R, Jha SK, Mishra P. Synovium derived mesenchymal stromal cells (Sy-MSCs): a promising therapeutic paradigm in the management of knee osteoarthritis. Indian J Orthop. 2022;56(1):1–15. doi: 10.1007/s43465-021-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cao X, Wang X, Zhang W, Xia G, Zhang L, Wen Z, He J, Wang Z, Huang J, Wu S. WNT10A induces apoptosis of senescent synovial resident stem cells through Wnt/calcium pathway-mediated HDAC5 phosphorylation in OA joints. Bone. 2021;150:116006. doi: 10.1016/j.bone.2021.116006. [DOI] [PubMed] [Google Scholar]
- 99. Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, Hill WD, Liu Y, Shi X, Fulzele S, Hamrick MW. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A. 2017;23(21–22):1231–40. doi: 10.1089/ten.TEA.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jeon OH, Wilson DR, Clement CC, Rathod S, Cherry C, Powell B, Lee Z, Khalil AM, Green JJ, Campisi J, Santambrogio L, et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. 2019;4(7):e125019. doi: 10.1172/jci.insight.125019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Oh C, Koh D, Jeon HB, Kim KM. The role of extracellular vesicles in senescence. Mol Cells. 2022;45(9):603–609. doi: 10.14348/molcells.2022.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, Isales CM, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):2029. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang W, Hsu P, Zhong B, Guo S, Zhang C, Wang Y, Luo C, Zhan Y, Zhang C. MiR-34a enhances chondrocyte apoptosis, senescence and facilitates development of osteoarthritis by targeting DLL1 and regulating PI3K/AKT pathway. Cell Physiol Biochem. 2018;48(3):1304–16. doi: 10.1159/000492090. [DOI] [PubMed] [Google Scholar]
- 104. Weilner S, Schraml E, Wieser M, Messner P, Schneider K, Wassermann K, Micutkova L, Fortschegger K, Maier AB, Westendorp R, Resch H, et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell. 2016;15(4):744–54. doi: 10.1111/acel.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840(8):2506–19. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Novoseletskaya E, Grigorieva O, Nimiritsky P, Basalova N, Eremichev R, Milovskaya I, Kulebyakin K, Kulebyakina M, Rodionov S, Omelyanenko N, Efimenko A. Mesenchymal stromal cell-produced components of extracellular matrix potentiate multipotent stem cell response to differentiation stimuli. Front Cell Dev Biol. 2020;8:555378. doi: 10.3389/fcell.2020.555378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lai Y, Sun Y, Skinner CM, Son EL, Lu Z, Tuan RS, Jilka RL, Ling J, Chen XD. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 2010;19(7):1095–107. doi: 10.1089/scd.2009.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hemmati-Sadeghi S, Ringe J, Dehne T, Haag R, Sittinger M. Hyaluronic acid influence on normal and osteoarthritic tissue-engineered cartilage. Int J Mol Sci. 2018;19(5):1519. doi: 10.3390/ijms19051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen PY, Huang LLH, Hsieh HJ. Hyaluronan preserves the proliferation and differentiation potentials of long-term cultured murine adipose-derived stromal cells. Biochem Biophys Res Commun. 2007;360(1):1–6. doi: 10.1016/j.bbrc.2007.04.211. [DOI] [PubMed] [Google Scholar]
- 110. Wong TY, Chang CH, Yu CH, Huang LLH. Hyaluronan keeps mesenchymal stem cells quiescent and maintains the differentiation potential over time. Aging Cell. 2017;16(3):451–60. doi: 10.1111/acel.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kawasaki H, Guan J, Tamama K. Hydrogen gas treatment prolongs replicative lifespan of bone marrow multipotential stromal cells in vitro while preserving differentiation and paracrine potentials. Biochem Biophys Res Commun. 2010;397(3):608–13. doi: 10.1016/j.bbrc.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 112. Yang M, Teng S, Ma C, Yu Y, Wang P, Yi C. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology. 2018;70(5):1301–13. doi: 10.1007/s10616-018-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lee JH, Jung HK, Han YS, Yoon YM, Yun CW, Sun HY, Cho HW, Lee SH. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol Med Rep. 2016;14(4):3777–84. doi: 10.3892/mmr.2016.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Park SY, Jeong AJ, Kim GY, Jo A, Lee JE, Leem SH, Yoon JH, Ye SK, Chung JW. Lactoferrin protects human mesenchymal stem cells from oxidative stress-induced senescence and apoptosis. J Microbiol Biotechnol. 2017;27(10):1877–84. doi: 10.4014/jmb.1707.07040. [DOI] [PubMed] [Google Scholar]
- 115. Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117(2):459–69. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 116. Casciaro F, Borghesan M, Beretti F, Zavatti M, Bertucci E, Follo MY, Maraldi T, Demaria M. Prolonged hypoxia delays aging and preserves functionality of human amniotic fluid stem cells. Mech Ageing Dev. 2020;191:111328. doi: 10.1016/j.mad.2020.111328. [DOI] [PubMed] [Google Scholar]
- 117. Garcia JP, Avila FR, Torres RA, Maita KC, Eldaly AS, Rinker BD, Zubair AC, Forte AJ, Sarabia-Estrada R. Hypoxia-preconditioning of human adipose-derived stem cells enhances cellular proliferation and angiogenesis: a systematic review. J Clin Transl Res. 2022;8(1):61–70. [PMC free article] [PubMed] [Google Scholar]
- 118. Sheng L, Mao X, Yu Q, Yu D. Effect of the PI3K/AKT signaling pathway on hypoxia-induced proliferation and differentiation of bone marrow-derived mesenchymal stem cells. Exp Ther Med. 2017;13(1):55–62. doi: 10.3892/etm.2016.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS ONE. 2012;7(4):e34608. doi: 10.1371/journal.pone.0034608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu J, He J, Ge L, Xiao H, Huang Y, Zeng L, Jiang Z, Lu M, Hu Z. Hypoxic preconditioning rejuvenates mesenchymal stem cells and enhances neuroprotection following intracerebral hemorrhage via the miR-326-mediated autophagy. Stem Cell Res Ther. 2021;12(1):413. doi: 10.1186/s13287-021-02480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Alekseenko LL, Zemelko VI, Domnina AP, Lyublinskaya OG, Zenin VV, Pugovkina NA, Kozhukharova IV, Borodkina AV, Grinchuk TM, Fridlyanskaya II, Nikolsky NN. Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells. Cell Stress Chaperones. 2014;19(3):355–66. doi: 10.1007/s12192-013-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Choudhery MS, Badowski M, Muise A, Harris DT. Effect of mild heat stress on the proliferative and differentiative ability of human mesenchymal stromal cells. Cytotherapy. 2015;17(4):359–68. doi: 10.1016/j.jcyt.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 123. Wang Q, Li X, Wang Q, Xie J, Xie C, Fu X. Heat shock pretreatment improves mesenchymal stem cell viability by heat shock proteins and autophagy to prevent cisplatin-induced granulosa cell apoptosis. Stem Cell Res Ther. 2019;10(1):348. doi: 10.1186/s13287-019-1425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3(9):839–43. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 125. Andreeva NV, Zatsepina OG, Garbuz DG, Evgen’ev MB, Belyavsky AV. Recombinant HSP70 and mild heat shock stimulate growth of aged mesenchymal stem cells. Cell Stress Chaperones. 2016;21(4):727–33. doi: 10.1007/s12192-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Conboy IM, Rando TA. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle Georget Tex. 2012;11(12):2260–67. doi: 10.4161/cc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Griffiths S, Baraniak PR, Copland IB, Nerem RM, McDevitt TC. Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy. 2013;15(12):1469–83. doi: 10.1016/j.jcyt.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 128. Lohmann M, Walenda G, Hemeda H, Joussen S, Drescher W, Jockenhoevel S, Hutschenreuter G, Zenke M, Wagner W. Donor age of human platelet lysate affects proliferation and differentiation of mesenchymal stem cells. PLoS ONE. 2012;7(5):e37839. doi: 10.1371/journal.pone.0037839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Masoudi E, Ribas J, Kaushik G, Leijten J, Khademhosseini A. Platelet-rich blood derivatives for stem cell-based tissue engineering and regeneration. Curr Stem Cell Rep. 2016;2(1):33–42. doi: 10.1007/s40778-016-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PLoS ONE. 2014;9(8):e104662. doi: 10.1371/journal.pone.0104662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344(6184):649–52. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gurung S, Werkmeister JA, Gargett CE. Inhibition of Transforming Growth Factor-β Receptor signaling promotes culture expansion of undifferentiated human Endometrial Mesenchymal Stem/stromal Cells. Sci Rep. 2015;5:15042. doi: 10.1038/srep15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–64. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 134. Yousef H, Conboy MJ, Morgenthaler A, Schlesinger C, Bugaj L, Paliwal P, Greer C, Conboy IM, Schaffer D. Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget. 2015;6(14):11959–78. doi: 10.18632/oncotarget.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Conese M, Carbone A, Beccia E, Angiolillo A. The fountain of youth: a tale of parabiosis, stem cells, and rejuvenation. Open Med (Wars). 2017;12:376–83. doi: 10.1515/med-2017-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Du GQ, Shao ZB, Wu J, Yin WJ, Li SH, Wu J, Weisel RD, Tian JW, Li RK. Targeted myocardial delivery of GDF11 gene rejuvenates the aged mouse heart and enhances myocardial regeneration after ischemia-reperfusion injury. Basic Res Cardiol. 2017;112(1):7. doi: 10.1007/s00395-016-0593-y. [DOI] [PubMed] [Google Scholar]
- 137. Hinken AC, Powers JM, Luo G, Holt JA, Billin AN, Russell AJ. Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells. Aging Cell. 2016;15(3):582–84. doi: 10.1111/acel.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22(1):164–74. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012;1(11):771–82. doi: 10.5966/sctm.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res. 1997;12(10):1606–14. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- 141. Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 142. Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89(6):1235–49. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 143. Coutu DL, François M, Galipeau J. Inhibition of cellular senescence by developmentally regulated FGF receptors in mesenchymal stem cells. Blood. 2011;117(25):6801–12. doi: 10.1182/blood-2010-12-321539. [DOI] [PubMed] [Google Scholar]
- 144. Spehar K, Pan A, Beerman I. Restoring aged stem cell functionality: current progress and future directions. Stem Cells. 2020;38(9):1060–77. doi: 10.1002/stem.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Janockova J, Slovinska L, Harvanova D, Spakova T, Rosocha J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J Biomed Sci. 2021;28(1):39. doi: 10.1186/s12929-021-00736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sun Y, Li W, Lu Z, Chen R, Ling J, Ran Q, Jilka RL, Chen XD. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25(5):1474–85. doi: 10.1096/fj.10-161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Liang M, Liu W, Peng Z, Lv S, Guan Y, An G, Zhang Y, Huang T, Wang Y. The therapeutic effect of secretome from human umbilical cord-derived mesenchymal stem cells in age-related osteoporosis. Artif Cells Nanomed Biotechnol. 2019;47(1):1357–66. doi: 10.1080/21691401.2019.1596945. [DOI] [PubMed] [Google Scholar]
- 148. Bai L, Li D, Li J, Luo Z, Yu S, Cao S, Shen L, Zuo Z, Ma X. Bioactive molecules derived from umbilical cord mesenchymal stem cells. Acta Histochem. 2016;118(8):761–69. doi: 10.1016/j.acthis.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 149. Yun YR, Jang JH, Jeon E, Kang W, Lee S, Won JE, Kim HW, Wall I. Administration of growth factors for bone regeneration. Regen Med. 2012;7(3):369–85. doi: 10.2217/rme.12.1. [DOI] [PubMed] [Google Scholar]
- 150. Zhang N, Zhu J, Ma Q, Zhao Y, Wang Y, Hu X, Chen J, Zhu W, Han Z, Yu H. Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res Ther. 2020;11(1):273. doi: 10.1186/s13287-020-01782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhang Y, Xu J, Liu S, Lim M, Zhao S, Cui K, Zhang K, Wang L, Ji Q, Han Z, Kong D, et al. Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics. 2019;9(23):6976–90. doi: 10.7150/thno.35305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Gimeno-Mallench L, Inglés M, Viña J, Borrás C. Extracellular vesicles from healthy cells improves cell function and stemness in premature senescent stem cells by miR-302b and HIF-1α activation. Biomolecules. 2020;10(6):957. doi: 10.3390/biom10060957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, Wong M, Apte RS, Imai SI. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 2019;30(2):329–42.e5. doi: 10.1016/j.cmet.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Arasu UT, Kärnä R, Härkönen K, Oikari S, Koistinen A, Kröger H, Qu C, Lammi MJ, Rilla K. Human mesenchymal stem cells secrete hyaluronan-coated extracellular vesicles. Matrix Biol. 2017;64:54–68. doi: 10.1016/j.matbio.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 155. Chen CY, Rao SS, Tan YJ, Luo MJ, Hu XK, Yin H, Huang J, Hu Y, Luo ZW, Liu ZZ, Wang ZX, et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019;7:18. doi: 10.1038/s41413-019-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lei J, Jiang X, Li W, Ren J, Wang D, Ji Z, Wu Z, Cheng F, Cai Y, Yu ZR, Belmonte JCI, et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2022;13(3):220–26. doi: 10.1007/s13238-021-00860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yuan X, Sun L, Jeske R, Nkosi D, York SB, Liu Y, Grant SC, Meckes DG, Jr, Li Y. Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells. J Extracell Vesicles. 2022;11(6):e12235. doi: 10.1002/jev2.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Jia Y, Qiu S, Xu J, Kang Q, Chai Y. Exosomes secreted by young mesenchymal stem cells promote new bone formation during distraction osteogenesis in older rats. Calcif Tissue Int. 2020;106(5):509–17. doi: 10.1007/s00223-019-00656-4. [DOI] [PubMed] [Google Scholar]
- 159. Zhang B, Tian X, Qu Z, Hao J, Zhang W. Hypoxia-preconditioned extracellular vesicles from mesenchymal stem cells improve cartilage repair in osteoarthritis. Membranes. 2022;12(2):225. doi: 10.3390/membranes12020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Barrachina L, Remacha AR, Romero A, Vitoria A, Albareda J, Prades M, Roca M, Zaragoza P, Vázquez FJ, Rodellar C. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet Res. 2018;14(1):241. doi: 10.1186/s12917-018-1556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pei M, Pei YA, Zhou S, Mikaeiliagah E, Erickson C, Giertych B, Akhter H, Wang L, Stewart A, Parenti J, Wang B, et al. Matrix from urine stem cells boosts tissue-specific stem cell mediated functional cartilage reconstruction. Bioact Mater. 2023;23:353–67. doi: 10.1016/j.bioactmat.2022.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ren X, Hu B, Song M, Ding Z, Dang Y, Liu Z, Zhang W, Ji Q, Ren R, Ding J, Chan P, et al. Maintenance of nucleolar homeostasis by CBX4 alleviates senescence and osteoarthritis. Cell Rep. 2019;26(13):3643–56.e7. doi: 10.1016/j.celrep.2019.02.088. [DOI] [PubMed] [Google Scholar]
- 163. Fu L, Hu Y, Song M, Liu Z, Zhang W, Yu FX, Wu J, Wang S, Izpisua Belmonte JC, Chan P, Qu J, et al. Up-regulation of FOXD1 by YAP alleviates senescence and osteoarthritis. PLoS Biol. 2019;17(4):e3000201. doi: 10.1371/journal.pbio.3000201. [DOI] [PMC free article] [PubMed] [Google Scholar]