ABSTRACT
Background
There is epidemiological evidence which suggests an association between 25-hydroxyvitamin D [25(OH)D] levels and bone and muscle function; however, it is unclear whether vitamin D supplementation has an added benefit beyond bone health. Here, we investigated the effects of vitamin D3 supplementation (1 month) on physical performance in Chinese university students in winter.
Methods
One hundred and seventeen eligible subjects with 25(OH)D (19.2 ± 7.8 ng/mL) were randomly assigned to either vitamin D3 supplement (N = 56; 1000 IU/day) or the control (N = 61) group for 1 month. Pre- and post-measurements included: 1) serum levels of 25(OH)D; 2) musculoskeletal and pulmonary function [vertical jump height (VJH) and right handgrip strength (RHS), forced vital capacity (FVC), and forced expiratory volume at 1s (FEV1)]; 3) bone turnover markers [parathyroid hormone (PTH), n-terminal osteocalcin (N-MID), and calcium]; 4) hemoglobin-related parameters [hemoglobin (Hb), hematocrit (HCT), red blood cells (RBC), and red cell distribution width (RDW)]; 5) lipid parameters [total triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)]; 6) Fatigue-related indicators [serum creatine kinase (CK), lactate dehydrogenase (LDH), and total testosterone (T)]. In addition, aerobic capacity was assessed by measuring maximal oxygen uptake (VO2max) at baseline.
Results
During wintertime, supplementation with 1000 IU/d of vitamin D3 significantly increased serum 25(OH)D levels (from 18.85 ± 7.04 to 26.98 ± 5.88 ng/mL, p < 0.05), accompanied by a decrease of PTH (p < 0.05). However, vitamin D3 supplementation did not significantly impact the physical performance, serum lipid parameters, and bone turnover markers of students. Furthermore, 25(OH)D was found to be positively correlated with VJH and negatively correlated with PTH and TC at the beginning and end of the study (p < 0.05). In addition, the multiple linear regression analysis showed that 25(OH)D combined with athletic, gender, height, weight, Hb, and FVC could account for 84.0% of the VO2max value.
Conclusions
The study demonstrated that one-month of 1000 IU/d of vitamin D3 supplementation during the winter had beneficial effects on 25(OH)D status and PTH. However, vitamin D3 intervention was not sufficient to improve physical performance. Furthermore, 25(OH)D levels combined with athletic, Hb and FVC could be a predictor of VO2max.
KEYWORDS: Vitamin D, physical performance, maximal oxygen uptake, university students, winter supplementary
1. Introduction
Vitamin D3 is a prohormone that humans obtain primarily by exposure to sunlight and secondarily from diet or dietary supplements [1]. Recent research revealed that low vitamin D levels may lead to a wide range of extra-skeletal health outcomes, including impaired muscular strength [1–3], poor pulmonary function [4], poor cardiorespiratory fitness [5,6], and cardiovacular diseases [7]. Vitamin D deficiency, insufficiency, and sufficiency were defined as a serum 25(OH)D value of <20 ng/mL (50 nmol/L), 21–29 ng/mL (50–75 nmol/L) and >30 ng/mL (75 nmol/L), respectively [8]. The worldwide prevalence of vitamin D deficiency and insufficiency ranges from −5% to 18% and 24% to 49%, respectively [9]. For Chinese university students, low vitamin D status is prevalent as well [10–12] during the winter months, owing to the lower consumption frequency of vitamin D-rich foods and lower UV exposure [11].
Given that low vitamin D status has become a worldwide problem, a series of vitamin D supplementation studies have been conducted to explore the skeletal and extra-skeletal benefits of vitamin D interventions. Some studies suggest that vitamin D supplementation is beneficial to physical performance and cardiovascular health, partly by atherogenic blood lipids [10,13–16]. For instance, there is growing evidence that vitamin D status may modulate musculoskeletal function [2,17], since 1,25-hydroxyvitamin D3 (1,25(OH)2D3), the active form of vitamin D, participates in cell proliferation and differentiation [18,19], regulation of protein synthesis [20] and mitochondrial function [15,21] through activation of various cellular signaling cascades [18,20,22]. In previous studies, it has been demonstrated that vitamin D supplementation appears to be a reasonable strategy for enhancing the vertical jump height among young ballet dancers [23,24]. Furthermore, some studies have reported positive associations between 25(OH)D concentrations and maximal oxygen uptake (VO2max), which is an accurate measure of aerobic capacity and cardiopulmonary health [5,25–27]. And a recent study found that 6,000 IU of vitamin D3 per day increased VO2max in rowers[28]. In addition, vitamin D intervention also showed various benefits for blood lipids for adolescents [29,30]. In contrast, some studies investigated the association between vitamin D supplementation and possible improvements in physical performance in different populations, with conflicting results [31–33]. For instance, vitamin D administration for 12 weeks did not have any effect on changes in lipid parameters in healthy adults [34]. Therefore, the effect of vitamin D intervention on physical performance remains ambiguous due to numerous factors could impact physical performance improvements, including fitness level, physical activity level, type of practiced sport, ethnicity, vitamin D status, and intervention dosages [26]. Nevertheless, there are currently limited data on the effects of vitamin D intake in young healthy adults.
The objectives of this study were to examine the benefits of winter vitamin D3 supplementation on the serum 25(OH)D levels, physical performance, bone turnover, and serum lipids in university students.
2. Materials and methods
2.1. Participants
Participants were recruited from the campus of the Soochow University. The exclusion criteria included the presence of serious diseases (cardiac diseases, type 1 or 2 diabetes, hepatic disease, chronic renal failure, sickle cell anemia, megaloblastic anemia, and inflammatory diseases), medicines affecting vitamin D metabolism, ongoing treatment with vitamin D, and inability to perform the physical fitness test. The purpose, procedures, and risks of the study were explained to each participant before inclusion, and all of the participants were enrolled with written informed consent. The study was approved by the Ethics Committee of Soochow University. (Reg. No. SUDA20211117A01)
2.2. Study design
We conducted a 1-month, randomized parallel-arm trial to investigate the effect of 1000 IU/d of oral vitamin D3 versus controls on the physical performance of Chinese undergraduates between November of 2021 and January of 2022.
Eligible participants were randomized by a stratified random sample (based on major specificity) into two groups: 1000 IU/d of oral vitamin D3 (intervention group) and blank control group. All participants and researchers were blinded to the allocations until completion of the study and subsequent data analysis. Recruitment, the randomization scheme, and final sample distributions group are presented in the consolidated standards of reporting trials (CONSORT) diagram (Figure 1).
Figure 1.
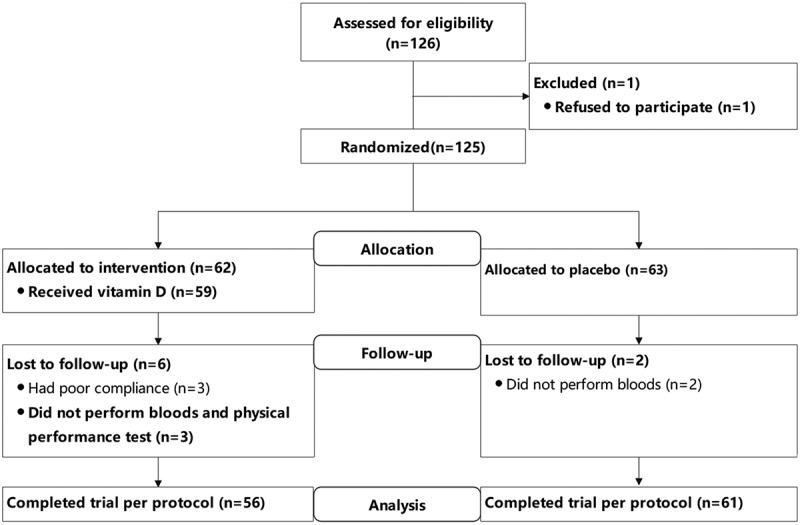
Flow diagram of the study.
Among studied participants, 117 (93.6%) completed blood, vertical jump, hand grip, FVC, and FEV1 analysis (vitamin D group n = 56 and control n = 61). At baseline, 104 participants completed VO2max test.
2.3. For primary outcomes
The serum concentrations of 25(OH)D, including 25(OH)2 and 25(OH)D3, were examined with chemiluminescent immunoassay.
Vertical jump height (VJH) (cm) was measured using a calibrated electronic jump mat (Beijing Xindong Huateng Sports Equipment Co. Ltd, Beijing, China). Participants performed three adversarial movement jumps and the best recorded jump height was taken for analysis. Handgrip strength (RHS) (kg) was measured using a hand-held dynamometer (Jamar Plus+, Patterson Medical, Warrenville, IL, China). Participants held the device next to their body and grasped it maximally for a total of three times. The maximum handgrip strength was calculated for the analysis. Lung function was measured using a calibrated Micro Lab portable pulmonary function equipment (Cosmed, Panova di Albano, Rome, Italy) [35]. Forced vital capacity (FVC) and forced expiratory volume at 1s (FEV1) were quantified by exhaling maximally into a one-way disposable mouthpiece, and a minimum of three repeats was performed in order to derive the best value.
VO2max testing was performed using a treadmill (MERC-C, WOODWAY GmbH, Germany) [33] and a metabolic cart (Metallizer 3B, CORTEX Biophysik GmbH, Germany) [35]. Participants walked on the treadmill until they reached physical exhaustion. The Bruce protocol treadmill test was used. The test was valid when any three of the four following criteria were reached: a respiratory exchange ratio (RER) >1.15; oxygen consumption remains at a steady state despite a further increase in workload [33]; volitional fatigue, as indicated by the inability to maintain a set rate despite verbal encouragement; and a posttest blood lactate concentration >9 mmol/L in males or >7 mmol/L in females. The posttest blood lactate concentrations were measured using a lactate pro device as per the manufacturers’ recommendations (Arkray Inc, Kyoto, Japan). At 1 min, following cessation of the VO2max test, a capillary blood sample was obtained for blood lactate analysis.
2.4. For secondary outcomes
The blood samples were analyzed using an automatic biochemical analyzer, and these biochemical indicators including: parathyroid hormone (PTH), n-terminal osteocalcin (N-MID), calcium, the level of hemoglobin (Hb), hematocrit (HCT), red blood cells (RBC), red cell distribution width (RDW), glycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), free fatty acids (FFA), creatine kinase (CK), lactate dehydrogenase (LDH), total testosterone (T), total bilirubin (TBIL), serum creatinine (CREA), uric acid (UA), urea (UREA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT). The intra-assay coefficient of variation (CV) was under 10% for all tests.
Height (cm) was measured by a portable altimeter (seca213, seca Zhong guo, Hangzhou, China), while weight (kg) and body mass index (BMI) were assessed by a performed by electrical impedance analysis (MF-BIA) using the In Body 770 (Bio-space Co., Ltd., Seoul, Korea).
Physical activity was measured using the International Physical Activity Questionnaire (IPAQ) and was completed before intervention [36]. Throughout the study, participants were instructed to maintain their daily physical activity and not to modify their lifestyle.
2.5. Statistical analysis
Randomization was performed by a random computer process. Sample-size calculations were based on the improvement of 25(OH)D levels after vitamin D3 intervention. A minimum sample size of 49 students per group was necessary in order to provide a statistical power of about 80% for a defect effect size of 0.25. The final sample was at least 62 students in each arm, with a projected dropout rate of 20%.
A repeated measure analysis of variance (ANCOVA) was used to evaluate the interaction effect for group by time. Differences in outcomes over time between groups were measured using the Mann–Whitney U-test. Pearson correlation coefficients were computed to assess relationships of variables. Multiple linear regression was performed to analyze the association between 25(OH)D levels or VO2max and outcomes. The regression model for estimating VO2max was evaluated with the coefficients of determination (adjusted R2) and absolute SE of the estimate (SEE). All data were presented as mean ± SD. The statistical significance level was set at p < 0.05.
3. Results
3.1. Baseline characteristics of subjects
One hundred and seventeen subjects (56 interventions and 61 controls) with a mean 25(OH)D of 19.2 ± 7.8 ng/mL were analyzed in the study (Table 1). There was no significant difference in age, height, weight, BMI, bone turnover indicators, physical performance indicators, hemoglobin-related parameters, fatigue-related indicators, and lipid metabolism-related parameters at baseline (p > 0.05).
Table 1.
Baseline characteristics of the participants.
| Variables | Vitamin D(n = 56) | Control(n = 61) |
|---|---|---|
| Age, year | 19.0(19.0—20.0) | 19.0(19.0—20.0) |
| Male, n (%) | 39.0(69.6%) | 40.0(65.6%) |
| Height, cm | 173.36 ± 9.19 | 173.08 ± 9.66 |
| Weight, kg | 66.77 ± 14.46 | 68.53 ± 14.54 |
| BMI, kg/m2 | 22.01 ± 3.29 | 22.64 ± 3.01 |
| Bone turnover | ||
| 25(OH)D, ng/ml | 18.85 ± 7.04 | 19.52 ± 8.65 |
| N-MID, ng/ml | 33.61 ± 13.96 | 30.58 ± 10.85 |
| PTH, pg/ml | 45.76 ± 18.65 | 50.92 ± 20.64 |
| Calcium, mmol/L | 2.37 ± 0.08 | 2.38 ± 0.08 |
| Lipid profiles | ||
| TC, mmol/L | 4.38 ± 0.74 | 4.45 ± 0.77 |
| HDL-C, mmol/L | 1.38 ± 0.31 | 1.37 ± 0.32 |
| LDL-C, mmol/L | 2.59 ± 0.67 | 2.58 ± 0.59 |
| TG, mmol/L | 0.91 ± 0.46 | 1.09 ± 0.74 |
| FFA, mmol/L | 0.39 ± 0.19 | 0.38 ± 0.17 |
| Erythrocytes and hemoglobin-related parameters | ||
| Hb, g/L | 152.80 ± 15.64 | 153.95 ± 14.41 |
| HCT, % | 46.23 ± 4.10 | 46.23 ± 3.67 |
| RBC, 10^12/L | 5.05 ± 0.46 | 5.05 ± 0.40 |
| RDW, % | 40.98 ± 4.85 | 41.60 ± 2.27 |
| Fatigue-related indicators | ||
| CK, U/L | 276.18 ± 559.42 | 180.38 ± 121.51 |
| LDH, U/L | 187.80 ± 37.77 | 186.70 ± 30.95 |
| T, ng/ml | 4.29 ± 2.66 | 3.87 ± 2.40 |
| Physical performance | ||
| VJH, cm | 39.86 ± 9.84 | 37.44 ± 11.19 |
| RHS, kg | 413.13 ± 114.60 | 401.15 ± 111.30 |
| FVC, L | 4.33 ± 0.92 | 4.18 ± 1.04 |
| FEV1, L | 3.84 ± 0.77 | 3.78 ± 0.86 |
| VO2max, ml/min | 3181.86 ± 1139.70a | 3073.13 ± 1048.32b |
| Physical activity level | ||
| Physical activity level, MET, h/day | 1071.07 ± 255.16 | 1011.43 ± 242.25 |
Data are presented as mean ± SD or median (IQR) values for continuous variables and n (%) for categorical variables.
an = 50. bn = 54.
3.2. Vitamin D status
As shown in Table 2, after intervention with 1000 IU of vitamin D3 for 1 month during wintertime, mean serum 25(OH)D levels of students were increased from 18.85 ± 7.04 to 26.98 ± 5.88 ng/mL, and a significant group × time interaction was observed for serum 25(OH)D concentrations in the intervention group (p < 0.01). Correspondingly, vitamin D3 supplementation resulted in a significant reduction in the incidence of vitamin D deficiency (<20 ng/mL) in the vitamin D group (from 64.3% at the baseline to 8.9% at end point), while no significant difference was observed in the placebo group.
Table 2.
Changes of between baseline and follow-up in the intervention and the control group.
| Variables | Vitamin D |
|
Control |
|
P valued | P valuee | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month 0 |
Month 1 |
Changec | Month 0 |
Month 1 |
Changec | |||||||
| n | Mean ±SD | n | Mean ±SD | Mean ±SD | n | Mean ±SD | n | Mean ±SD | Mean ±SD | |||
| Weight and BMI | ||||||||||||
| Weight, kg | 56 | 66.77 ± 14.46 | 56 | 66.98 ± 14.63 | 0.21 ± 2.51 | 61 | 68.53 ± 14.54 | 61 | 68.44 ± 14.76 | −0.09 ± 1.48 | NS. | NS. |
| BMI, kg/m2 | 56 | 22.01 ± 3.29 | 56 | 22.09 ± 3.41 | 0.08 ± 0.78 | 61 | 22.64 ± 3.01 | 61 | 22.60 ± 3.16 | −0.03 ± 0.51 | NS. | NS. |
| Bone turnover | ||||||||||||
| 25(OH)D, ng/ml | 56 | 18.85 ± 7.04 | 56 | 26.98 ± 5.88 | 8.14 ± 3.85 | 61 | 19.52 ± 8.65 | 61 | 17.61 ± 8.19 | −1.91 ± 2.58 | 0.000 | 0.000 |
| N-MID, ng/ml | 56 | 33.61 ± 13.96 | 56 | 32.05 ± 11.60 | −1.57 ± 5.57 | 61 | 30.58 ± 10.85 | 61 | 30.64 ± 10.52 | 0.06 ± 6.31 | NS. | NS. |
| PTH, pg/ml | 56 | 45.76 ± 18.65 | 56 | 41.99 ± 20.92 | −3.77 ± 15.71 | 61 | 50.92 ± 20.64 | 61 | 49.91 ± 19.52 | −1.01 ± 23.20 | 0.043 | NS. |
| Calcium, mmol/L | 56 | 2.37 ± 0.08 | 56 | 2.37 ± 0.09 | 0 ± 0.11 | 61 | 2.38 ± 0.08 | 61 | 2.38 ± 0.08 | −0.01 ± 0.1 | NS. | NS. |
| Erythrocytes and hemoglobin-related parameters | ||||||||||||
| Hb, g/L | 56 | 152.80 ± 15.64 | 56 | 153.88 ± 15.29 | 1.07 ± 11.73 | 61 | 153.95 ± 14.41 | 61 | 153.15 ± 16.49 | −0.8 ± 11.68 | NS. | NS. |
| HCT, % | 56 | 46.23 ± 4.10 | 56 | 46.56 ± 4.57 | 0.33 ± 3.25 | 61 | 46.23 ± 3.67 | 61 | 46.74 ± 4.56 | 0.51 ± 3.26 | NS. | NS. |
| RBC,10^12/L | 56 | 5.05 ± 0.46 | 56 | 5.12 ± 0.48 | 0.07 ± 0.30 | 61 | 5.05 ± 0.40 | 61 | 5.14 ± 0.52 | 0.09 ± 0.30 | NS. | NS. |
| RDW, % | 56 | 40.98 ± 4.85 | 56 | 40.80 ± 3.48 | −0.18 ± 5.42 | 61 | 41.60 ± 2.27 | 61 | 40.94 ± 3.64 | −0.67 ± 3.21 | NS. | NS. |
| Lipid metabolism | ||||||||||||
| TC, mmol/L | 56 | 4.38 ± 0.74 | 56 | 4.50 ± 0.86 | 0.13 ± 0.47 | 61 | 4.45 ± 0.77 | 61 | 4.68 ± 0.79 | 0.23 ± 0.45 | NS. | NS. |
| HDL-C, mmol/L | 56 | 1.38 ± 0.31 | 56 | 1.37 ± 0.29 | −0.01 ± 0.17 | 61 | 1.37 ± 0.32 | 61 | 1.42 ± 0.31 | 0.05 ± 0.18 | NS. | NS. |
| LDL-C, mmol/L | 56 | 2.59 ± 0.67 | 56 | 2.71 ± 0.81 | 0.13 ± 0.43 | 61 | 2.58 ± 0.59 | 61 | 2.80 ± 0.67 | 0.22 ± 0.45 | NS. | NS. |
| TG, mmol/L | 56 | 0.91 ± 0.46 | 56 | 0.93 ± 0.39 | 0.02 ± 0.38 | 61 | 1.09 ± 0.74 | 61 | 1.03 ± 0.45 | −0.06 ± 0.70 | NS. | NS. |
| FFA, mmol/L | 56 | 0.39 ± 0.19 | 56 | 0.44 ± 0.23 | 0.05 ± 0.26 | 61 | 0.38 ± 0.17 | 61 | 0.42 ± 0.27 | 0.05 ± 0.28 | NS. | NS. |
| Fatigue-related indicators | ||||||||||||
| CK, U/L | 56 | 276.18 ± 559.42 | 56 | 184.05 ± 195.59 | −92.13 ± 558.77 | 61 | 180.38 ± 121.51 | 61 | 154.79 ± 129.25 | −25.59 ± 120.76 | NS. | NS. |
| LDH, U/L | 56 | 187.80 ± 37.77 | 56 | 173.91 ± 28.62 | −13.89 ± 33.46 | 61 | 186.70 ± 30.95 | 61 | 171.44 ± 29.29 | −15.26 ± 19.16 | NS. | NS. |
| T, ng/ml | 56 | 4.29 ± 2.66 | 56 | 4.26 ± 2.67 | −0.03 ± 0.95 | 61 | 3.87 ± 2.40 | 61 | 3.85 ± 2.49 | −0.01 ± 0.87 | NS. | NS. |
| Physical performance | ||||||||||||
| VJH, cm | 56 | 39.86 ± 9.84 | 56 | 40.71 ± 10.39 | 0.85 ± 2.96 | 61 | 37.44 ± 11.19 | 61 | 37.82 ± 11.13 | 0.38 ± 3.21 | NS. | NS. |
| RHS, kg | 56 | 413.13 ± 114.60 | 56 | 416.07 ± 118.33 | 2.95 ± 27.99 | 61 | 401.15 ± 111.30 | 61 | 400.98 ± 114.26 | −0.16 ± 29.72 | NS. | NS. |
| FVC, L | 56 | 4.33 ± 0.92 | 56 | 4.35 ± 0.98 | 0.02 ± 0.26 | 61 | 4.18 ± 1.04 | 61 | 4.17 ± 1.06 | −0.01 ± 0.32 | NS. | NS. |
| FEV1, L | 56 | 3.84 ± 0.77 | 56 | 3.85 ± 0.81 | 0.01 ± 0.24 | 61 | 3.78 ± 0.86 | 61 | 3.77 ± 0.89 | −0.01 ± 0.28 | NS. | NS. |
Month 0: measurements at baseline; Month 1: measurements after 1 month of intervention.
cChange = Month 1 minus Month 0.
dA repeated measure analysis of variance (ANCOVA) was used to evaluated the interaction effect for group by time.
eBetween group differences were compared using the Mann–Whitney U-test.
NS: No significant.
3.3. Effect of vitamin D supplementation on PTH, hemoglobin-related parameters, and physical performance
The baseline and end of study values of PTH, hemoglobin-related parameters, fatigue-related indicators, and athletic performance are shown in Table 2. It was found that vitamin D supplementation could significantly decrease PTH (from 45.76 ± 18.65 to 41.99 ± 20.92 pg/ml) compared with the placebo group (p < 0.05), while Hb, HCT, RBC, CK, LDH, T, VJH, RHS, FVC, and FEV1 values were not significantly changed (p > 0.05). The same results were obtained after stratifying the analysis by gender (Table S1 and Table S2). At baseline and after 1 month of intervention, serum 25(OH)D levels were found to be negatively correlated with PTH values (p < 0.05) and positively correlated with the VJH values adjusted for confounding variables (p < 0.05) (Table 3).
Table 3.
Association of subjects’ characteristics with 25(OH)D by multiple regression analyses at baseline and after 1 month of intervention (n = 117).
| Variables | Month 0 |
Month 1 |
||
|---|---|---|---|---|
| ß (95% CI) | p-value | ß (95% CI) | p-value | |
| Bone turnover | ||||
| N-MID, ng/ml | −14.238 (−27.436 −1.041) | 0.035 | −0.132 (−0.338 − 0.074) | 0.206 |
| PTH, pg/ml | −24.866 (−49.424 −0.308) | 0.047 | −0.580 (−1.041 − 0.118) | 0.014 |
| Calcium, mmol/L | −0.0360 (−0.137 − 0.065) | 0.484 | −0.001 (−0.003 − 0.001) | 0.271 |
| Erythrocytes and hemoglobin-related parameters | ||||
| Hb, g/L | −3.473 (−18.088 − 11.141) | 0.639 | −0.162 (−0.393 − 0.069) | 0.166 |
| HCT, % | −2.028 (−5.656 − 1.601) | 0.271 | −0.078 (−0.145 − 0.011) | 0.022 |
| RBC,10^12/L | −0.326 (−0.703 − 0.051) | 0.089 | −0.009 (−0.016 − 0.002) | 0.016 |
| RDW, % | −1.906 (−6.504 − 2.692) | 0.413 | −0.001 (−0.078 − 0.075) | 0.977 |
| Lipid profiles | ||||
| TC, mmol/L | −0.959 (−1.904 − −0.014) | 0.047 | −0.023 (−0.042 − −0.004) | 0.016 |
| HDL-C, mmol/L | −0.154 (−0.496 − 0.187) | 0.372 | −0.005 (−0.010 − 0.001) | 0.121 |
| LDL-C, mmol/L | −0.681 (−1.470 − 0.108) | 0.090 | −0.012 (−0.029 − 0.005) | 0.151 |
| TG, mmol/L | −0.27 (−1.040 − 0.500) | 0.489 | −0.013 (−0.022 − 0.004) | 0.005 |
| FFA, mmol/L | −0.255 (−0.479 − −0.030) | 0.027 | −0.001 (−0.007 − 0.004) | 0.696 |
| Physical performance | ||||
| VJH, cm | 11.473 (2.979 − 19.966) | 0.009 | 0.245 (0.094 − 0.397) | 0.002 |
| RHS, kg | 8.766 (−63.598 − 81.131) | 0.811 | 0.787 (−0.525 − 2.100) | 0.237 |
| FVC, L | 0.337 (−0.469 − 1.143) | 0.410 | 0.014 (0.000 − 0.029) | 0.052 |
| FEV1, L | 0.236 (−0.421 − 0.893) | 0.479 | 0.007 (−0.005 − 0.019) | 0.273 |
Statistically significant variables were adjusted for age, gender, and BMI; ß, unstandardized coefficient; 95%CI, confidence interval.
The 25(OH)D levels at baseline was log transformed for the analysis.
3.4. Vitamin D status predicted VO2max
The relationships between the VO2max levels and the subjects’ characteristics were detailed in Table S4. At baseline, VO2max level was found to be positively associated with 25(OH)D, height, weight, Hb, RHS, FVC, and FEV1 values (p < 0.05). In order to predict VO2max, the multiple linear regression analysis was performed (Table 4). The multiple linear regression model 3 showed that 25(OH)D combined with athletic, gender, height, weight, Hb, and FVC could account for 84.0% of the VO2max (ml/min) value according to the following equation: VO2max(ml/min) = −726.065 × Athletic −638.730 × Gender + 28.210 × Height + 15.884 × Weight + 667.603 × 25(OH)D − 9.158 × Hb + 405.912 × FVC.
Table 4.
Multiple linear regression analysis of VO2max as the dependent variable (n = 104).
| Prediction models | Coefficients | β | p-value | R2 | Adjusted R2 |
|---|---|---|---|---|---|
| Model 1 | 0.818 | 0.809 | |||
| Athletic | −796.288 | −0.367 | .000 | ||
| Gender | −386.803 | −0.160 | .025 | ||
| Height, cm | 34.228 | 0.286 | .002 | ||
| Weight, kg | 15.992 | 0.213 | .002 | ||
| 25(OH)D, ng/ml | 678.148 | 0.099 | .041 | ||
| Model 2 | 0.830 | 0.820 | |||
| Athletic | −744.219 | −0.343 | .000 | ||
| Gender | −632.902 | −0.262 | .001 | ||
| Height, cm | 32.576 | 0.272 | .002 | ||
| Weight, kg | 17.282 | 0.230 | .001 | ||
| 25(OH)D, ng/ml | 664.428 | 0.097 | .039 | ||
| Hb, g/L | −11.065 | −0.141 | .011 | ||
| Model 3 | 0.840 | 0.825 | |||
| Athletic | −726.065 | −0.335 | .000 | ||
| Gender | −638.730 | −0.264 | .005 | ||
| Height, cm | 28.210 | 0.235 | .009 | ||
| Weight, kg | 15.884 | 0.211 | .008 | ||
| 25(OH)D, ng/ml | 667.603 | 0.097 | .036 | ||
| Hb, g/L | −9.158 | −0.117 | .040 | ||
| RHS, kg | −0.398 | −0.039 | .628 | ||
| FVC, L | 405.912 | 0.356 | .028 | ||
| FEV1, L | −355.659 | −0.258 | .115 |
B, unstandardized regression coefficients; β, standardized regression coefficients.
The 25(OH)D levels was log transformed for the analysis.
Athletic (athletes = 1, non-athletes = 2); Gender (Man = 1, Woman = 2).
Model 2: as in model 1 plus Hb.
Model 3: as in model 2 plus RHS、FVC、FEV1.
VO2max(ml/min) = −726.065 × Athletic − 638.730 × Gender + 28.210 × Height + 15.884 × Weight + 667.603 × 25(OH)D −9.158 × Hb + 405.912 × FVC.
3.5. Effect of vitamin D supplementation on serum lipid profiles
The baseline and end of study values of serum lipid profiles are shown in Table 2. No significant differences were noted for TG, TC, HDL-C or LDL-C levels among groups (p > 0.05). The same results were obtained after stratifying the analysis by gender (Table S1 and Table S2). After adjustment for age, gender and BMI, as shown in Table 3, there was no significant relationships between 25(OH)D and TG, HDL-C or LDL-C (p > 0.05). However, serum 25(OH)D levels remained negatively correlated with plasma TC values at baseline and after intervention (p < 0.05).
4. Discussion
The present data demonstrated that after one-month vitamin D3 supplementation with 1000 IU/d, 25(OH)D levels were increased by 43%, which was accompanied by a significant decrease in PTH. Even so, short-term vitamin D3 intervention was not sufficient to improve physical performance, bone turnover, and serum lipids. Furthermore, 25(OH)D levels combined with athletic, FVC, and Hb could be a predictor of VO2max. To the best of our knowledge, this was the first study to provide a potential model for VO2max predictor using vitamin D status. These results could provide a hint for vitamin D as an activator of aerobic capacity, and support the evidence for large dose supplementation in winter as a preventative strategy for vitamin D deficiency.
Vitamin D is essential to musculoskeletal health and exercise performance. Evidence from observational studies and meta-analyses of RCTs suggested that deficient or inadequate vitamin D status was associated with adverse muscle function, including muscle weakness [37–39], elevated markers of oxidative stress, and reduced mitochondrial function [39,40]. Additionally, a mechanism by which vitamin D3 supplementation could enhance muscle function is due to its synergy with testosterone [35]. And some trials have found that muscle strength has been improved with testosterone supplementation [3,18,41]. In our study, although 25(OH)D level was positively associated with vertical jump height at the baseline and end of the study, supplementation did not significantly increase vertical jump height or right handgrip strength when compared to the control group, which was consistent with findings from a recent RCT conducted in college-age soccer players [33]. One reason might be that vitamin D levels in the intervention group were less than 30 ng/mL at end. Some studies suggested that physically active individuals should maintain a higher vitamin D status, possibly as elevated as 50 ng/mL to achieve an optimal and performance health [42]. Future research will focus on comparing vitamin D and placebo groups for muscle strength changes as they move from deficient or inadequate to adequate vitamin D status in a wider population.
Our study did not observe a positive effect of vitamin D3 supplementation on FEV1 or FVC, corroborating findings from a recent RCT in healthy students [33]. Some observational studies have revealed that 25(OH)D levels may be linked to lung function, particularly in individuals with airway disease, contradicting our findings [4,43]. One reason for this may be that the subjects of our survey were healthy individuals. The response to vitamin D3 supplementation, in terms of benefits on lung function parameters, may vary between healthy individuals and those with disease.
Mechanistic studies support the concept that vitamin D could enhance VO2max through improving hematological levels [20]. Established primary determinants of VO2max are cardiac output and oxygen diffusion capacity [44], which is a function in which blood cells and Hb actively participate. An observational study from the United States found that a higher concentration of 25(OH)D was independently associated with better VO2max in adults [31,45]. It has been demonstrated that tissue oxygenation is improved on a cellar level, where higher 25(OH)D concentrations are present in blood [46]. Nevertheless, no effect of vitamin D supplementation on erythropoiesis and hemoglobin-related parameters was observed in our study. One possible reason was that reported associations between 25(OH)D levels and hematological levels were currently confirmed in subjects with diseases instead of healthy individuals [47,48]. Recently, additional data also revealed that vitamin D supplementation has no effect on VO2max in healthy individuals [17,32,33,49]. Therefore, the association between vitamin D and VO2max in collegiate students cannot be concluded based on our limited study.
The present study demonstrated 25(OH)D levels combined with athletic, FVC, and Hb could be a predictor of VO2max. Traditionally, direct measurement of VO2max is limited by the expensive and complex equipment, the qualification of the assessor, and the long test time. At the same time, the physical and mental states of the subjects also directly affect the absolute value of VO2max, making it difficult to promote the use of direct measurement of VO2max in many situations. Some indirect methods that are relatively safe, effective, and suitable for predicting VO2max have been proposed [50–53]. Among them, the submaximal model obtains exercise-related data through a specified motion protocol, such as shuttle runs, and constructs an estimation model along with other anthropometric features. Although submaximal models have overcome some of the limitations of cardiopulmonary function testing, they still require trained personnel to perform Submaximal testing [53]. Compared with other indirect measurement methods, our method for VO2max prediction is simple and easy to implement, and the results are relatively more accurate.
Vitamin D and PTH are two major regulators of mineral metabolism. They play critical roles in maintaining calcium and phosphate homeostasis as well as the development and maintenance of bone [54]. Low vitamin D status can be an independent contribution to high PTH concentrations. The negative association between serum 25(OH)D and PTH has been previously demonstrated, but mostly in elderly, women and/or osteoporotic populations [55,56]. In this study, it was observed that vitamin D3 supplementation decreased the level of PTH. Our study further validated the relationships between 25(OH)D and PTH in healthy collegiate students.
Previous studies that investigated the association between vitamin D status and serum lipid profiles showed different results. A recent systematic review and meta-analysis found that vitamin D supplementation has a beneficial effect on serum TC, LDL-C, and TG, but not HDL-C [57], whereas others found the serum 25(OH)D concentrations were inversely related to the LDL-C, HDL-C, and TG values in healthy adults [30]. Intriguingly, mechanistic studies support that vitamin D is inextricably linked to cholesterol metabolism with both metabolisms sharing an extensive common biosynthesis pathway [58]. In the present study, we found that TC was negatively correlated with 25(OH)D levels at the begin and end of the study after adjusting for potential confounders, which supports the findings of previous studies [29,59].
Several limitations should also be taken into account in interpreting our results. Firstly, we surmised that receiving vitamin D3 for a short term (1 month) may obscure its beneficial effects and the mean post-intervention 25(OH)D level (26.98 ng/mL) was not sufficient to improve other outcome variables in our study. Hence, longer intervention durations are needed to explore the effects of vitamin D3 intervention on bone turnover parameters, physical performance, and lipid parameters. Besides, due to the limitations in testing conditions, we did not complete post-intervention VO2max measurements for all subjects, but the 25(OH)D levels was identified as a predictor of VO2max in these young collegiate students highlighting the impact of 25(OH)D on physical performance. Finally, an appropriate placebo was not available in the control group, which would cause research bias. Since individuals in both intervention and control group realized they were being observed, participant reactivity or Hawthorne effect dose still occur. However, it may be hard to determine exactly how participant awareness impacts study results.
5. Conclusion
In summary, we provided data demonstrating a high prevalence of vitamin D insufficiency and deficiency among collegiate students during wintertime. Supplementation with a 1000IU/day vitamin D3 had beneficial effects on 25(OH)D status and PTH. However, vitamin D3 intervention, at the dose provided here for 1 month, did not improve physical performance. 25(OH)D levels were found to be positively correlated with vertical jump height and negatively correlated with PTH and total cholesterol. Furthermore, 25(OH)D levels combined with athletic, Hb and FVC could be a potential predictor of VO2max.
Supplementary Material
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This work was supported by the National Natural Science Foundation of China under Grant [number 12275191, 81703209, and 82192883]; a sub-project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); and the Suzhou Science and technology project under Grant SKY2021035.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
XLZ, QZ, WJW, and BYL participated in the design, conducted the statistical analyses, interpreted the data, and drafted the manuscript. GMZ, WJW, and BYL supervised the study, assisted in data interpretation, and critically reviewed the manuscript. JFL, XZ, QWG, LJD, HMZ, HMD, and FJ helped in conducting the study and revising the manuscript. YFP and ZLZ helped to manage and analyze the data. All authors read and approved the final manuscript.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15502783.2023.2258850.
References
- 1.Ksiazek, A, Zagrodna, A, Slowinska-Lisowska, M, et al. Relationship between metabolites of vitamin D, free 25-(OH)D, and physical performance in indoor and outdoor athletes. Front Physiol. 2022;13:909086. doi: 10.3389/fphys.2022.909086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byers, AW, Connolly, G, Campbell, WW.. Vitamin D status and supplementation impacts on skeletal muscle function: comparisons between young athletes and older adults. Curr Opin Clin Nutr Metab Care. 2020. Nov;23(6):421–804. doi: 10.1097/MCO.0000000000000692 [DOI] [PubMed] [Google Scholar]
- 3.Wicinski, M, Adamkiewicz, D, Adamkiewicz, M, et al. Impact of vitamin D on physical efficiency and exercise performance—A review. Nutrients. 2019 Nov 19;11(11):2826. doi: 10.3390/nu11112826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung, JY, Kim, YS, Kim, SK, et al. Relationship of vitamin D status with lung function and exercise capacity in COPD. Respirology. 2015. Jul;20(5):782–789. doi: 10.1111/resp.12538 [DOI] [PubMed] [Google Scholar]
- 5.Marawan, A, Kurbanova, N, Qayyum, R. Association between serum vitamin D levels and cardiorespiratory fitness in the adult population of the USA. Eur J Prev Cardiolog. 2019;26(7):750–755. doi: 10.1177/2047487318807279 [DOI] [PubMed] [Google Scholar]
- 6.Carson, EL, Pourshahidi, LK, Hill, TR, et al. Vitamin D, muscle function, and cardiorespiratory fitness in adolescents from the young hearts study. J Clin Endocrinol Metab. 2015. Dec;100(12):4621–4628. doi: 10.1210/jc.2015-2956 [DOI] [PubMed] [Google Scholar]
- 7.Zittermann, A, Trummer, C, Theiler-Schwetz, V, et al. Vitamin D and cardiovascular disease: an updated narrative review. Int J Mol Sci. 2021 Mar 12;22(6):2896. doi: 10.3390/ijms22062896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross, AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011. May;14(5):938–939. doi: 10.1017/S1368980011000565 [DOI] [PubMed] [Google Scholar]
- 9.Cashman, KD. 100 YEARS of VITAMIN D: global differences in vitamin D status and dietary intake: a review of the data. Endocr Connect. 2022 Jan 11;11(1). doi: 10.1530/EC-21-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charoenngam, N, Holick, MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020 Jul 15;12(7):2097. doi: 10.3390/nu12072097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou, M, Zhuang, W, Yuan, Y, et al. Investigation on vitamin D knowledge, attitude and practice of university students in Nanjing, China. Public Health Nutr. 2016. Jan;19(1):78–82. doi: 10.1017/S1368980015000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, W, Hu, J, Fang, Y, et al. Vitamin D status in mainland of China: a systematic review and meta-analysis. EClinicalMedicine. 2021. Aug;38:101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun, X, Tanisawa, K, Zhang, Y, et al. Effect of vitamin D supplementation on body composition and physical fitness in healthy adults: a double-blind, randomized controlled trial. Ann Nutr Metab. 2019;75(4):231–237. doi: 10.1159/000504873 [DOI] [PubMed] [Google Scholar]
- 14.Neal, S, Sykes, J, Rigby, M, et al. A review and clinical summary of vitamin D in regard to bone health and athletic performance. Phys Sportsmed. 2015. May;43(2):161–168. doi: 10.1080/00913847.2015.1020248 [DOI] [PubMed] [Google Scholar]
- 15.Ashcroft, SP, Fletcher, G, Philp, AM, et al. Diet-induced vitamin D deficiency reduces skeletal muscle mitochondrial respiration. J Endocrinol. 2021. May;249(2):113–124. doi: 10.1530/JOE-20-0233 [DOI] [PubMed] [Google Scholar]
- 16.Ramezani Ahmadi, A, Mohammadshahi, M, Alizadeh, A, et al. Effects of vitamin D3 supplementation for 12 weeks on serum levels of anabolic hormones, anaerobic power, and aerobic performance in active male subjects: a randomized, double-blind, placebo-controlled trial. Eur J Sport Sci. 2020. Nov;20(10):1355–1367. doi: 10.1080/17461391.2020.1713218 [DOI] [PubMed] [Google Scholar]
- 17.Montenegro, KR, Cruzat, V, Carlessi, R, et al. Mechanisms of vitamin D action in skeletal muscle. Nutr Res Rev. 2019. Dec;32(2):192–204. doi: 10.1017/S0954422419000064 [DOI] [PubMed] [Google Scholar]
- 18.Michalczyk, MM, Golas, A, Maszczyk, A, et al. Influence of sunlight and oral D(3) supplementation on serum 25(OH)D concentration and exercise performance in elite soccer players. Nutrients. 2020 May 4;12(5):1311. doi: 10.3390/nu12051311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsson, K, Saini, A, Stromberg, A, et al. Evidence for vitamin D receptor expression and direct effects of 1alpha,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology. 2016. Jan;157(1):98–111. doi: 10.1210/en.2015-1685 [DOI] [PubMed] [Google Scholar]
- 20.Mielgo-Ayuso, J, Calleja-Gonzalez, J, Urdampilleta, A, et al. Effects of vitamin D supplementation on haematological values and muscle recovery in elite Male traditional rowers. Nutrients. 2018 Dec 12;10(12):1968. doi: 10.3390/nu10121968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha, A, Hollingsworth, KG, Ball, S, et al. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab. 2013. Mar;98(3):E509–13. doi: 10.1210/jc.2012-3592 [DOI] [PubMed] [Google Scholar]
- 22.Owens, DJ, Allison, R, Close, GL. Vitamin D and the athlete: current perspectives and new challenges. Sports Med. 2018. Mar;48(Suppl 1):3–16. doi: 10.1007/s40279-017-0841-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyon, MA, Koutedakis, Y, Wolman, R, et al. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: a controlled study. J Sci Med Sport. 2014. Jan;17(1):8–12. doi: 10.1016/j.jsams.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 24.Close, GL, Russell, J, Cobley, JN, et al. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci. 2013;31(4):344–353. doi: 10.1080/02640414.2012.733822 [DOI] [PubMed] [Google Scholar]
- 25.Kujach, S, Lyzwinski, D, Chroboczek, M, et al. The effect of vitamin D(3) supplementation on physical capacity among active College-aged males. Nutrients. 2020 Jun 30;12(7):1936. doi: 10.3390/nu12071936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jastrzebska, M, Kaczmarczyk, M, Michalczyk, M, et al. Can supplementation of vitamin D improve aerobic capacity in well trained youth soccer players? J Hum Kinet. 2018. Mar;61(1):63–72. doi: 10.2478/hukin-2018-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Most, A, Dorr, O, Nef, H, et al. Influence of 25-hydroxy-vitamin D insufficiency on maximal aerobic power in elite indoor athletes: a cross-sectional study. Sports Med - Open. 2021 Oct 14;7(1):74. doi: 10.1186/s40798-021-00363-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jastrzębski, Z. Effect of vitamin D supplementation on the level of physical fitness and blood parameters of rowers during the 8-week high intensity training. Facicula Educ Fiz Şi Sport. 2014;2:57–67. [Google Scholar]
- 29.Song, K, Park, G, Choi, Y, et al. Association of vitamin D status and physical activity with lipid profile in Korean children and adolescents: a population-based study. Children (Basel). 2020 Nov 19;7(11):241. doi: 10.3390/children7110241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang, Z, Huang, S, Ma, R, et al. Low vitamin D status is associated with obesity but no other cardiovascular risk factors in Chinese children and adolescents. Nutr Metab Cardiovasc Dis. 2020 Aug 28;30(9):1573–1581. doi: 10.1016/j.numecd.2020.05.019 [DOI] [PubMed] [Google Scholar]
- 31.Jung, HC, Seo, MW, Lee, S, et al. Correcting vitamin D insufficiency improves some but not all aspects of physical performance during Winter training in taekwondo athletes. Int J Sport Nutr Exerc Metab. 2018 Nov 1;28(6):635–643. doi: 10.1123/ijsnem.2017-0412 [DOI] [PubMed] [Google Scholar]
- 32.Karefylakis, C, Sarnblad, S, Ariander, A, et al. Effect of vitamin D supplementation on body composition and cardiorespiratory fitness in overweight men-a randomized controlled trial. Endocrine. 2018. Sep;61(3):388–397. doi: 10.1007/s12020-018-1665-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todd, JJ, McSorley, EM, Pourshahidi, LK, et al. Vitamin D(3) supplementation using an oral spray solution resolves deficiency but has no effect on VO(2) max in Gaelic footballers: results from a randomised, double-blind, placebo-controlled trial. Eur J Nutr. 2017. Jun;56(4):1577–1587. doi: 10.1007/s00394-016-1202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amarasekera, AT, Assadi-Khansari, B, Liu, S, et al. Vitamin D supplementation lowers thrombospondin-1 levels and blood pressure in healthy adults. PLoS One. 2017;12(5):e0174435. doi: 10.1371/journal.pone.0174435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canguven, O, Talib, RA, El Ansari, W, et al. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male. 2017. Mar;20(1):9–16. doi: 10.1080/13685538.2016.1271783 [DOI] [PubMed] [Google Scholar]
- 36.Craig, CL, Marshall, AL, Sjostrom, M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exercise. 2003. Aug;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 37.Girgis, CM. Vitamin D and skeletal muscle: emerging roles in development, anabolism and repair. Calcif Tissue Int. 2020. Jan;106(1):47–57. doi: 10.1007/s00223-019-00583-4 [DOI] [PubMed] [Google Scholar]
- 38.Remelli, F, Vitali, A, Zurlo, A, et al. Vitamin D deficiency and sarcopenia in older persons. Nutrients. 2019 Nov 21;11(12):2861. doi: 10.3390/nu11122861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dzik, KP, Kaczor, JJ. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. Eur J Appl Physiol. 2019. Apr;119(4):825–839. doi: 10.1007/s00421-019-04104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dzik, KP, Skrobot, W, Kaczor, KB, et al. Vitamin D deficiency is associated with muscle atrophy and reduced mitochondrial function in patients with chronic low back pain. Oxid Med Cell Longev. 2019;2019:1–11. doi: 10.1155/2019/6835341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maghsoumi-Norouzabad, L, Zare Javid, A, Mansoori, A, et al. The effects of vitamin D3 supplementation on spermatogram and endocrine factors in asthenozoospermia infertile men: a randomized, triple blind, placebo-controlled clinical trial. Reprod Biol Endocrinol. 2021 Jul 5;19(1):102. doi: 10.1186/s12958-021-00789-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chun, RF, Liu, PT, Modlin, RL, et al. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5:151. doi: 10.3389/fphys.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park, Y, Kim, YS, Kang, YA, et al. Relationship between vitamin D-binding protein polymorphisms and blood vitamin D level in Korean patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:731–738. doi: 10.2147/COPD.S96985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz-Canestro, C, Pentz, B, Sehgal, A, et al. Differences in cardiac output and aerobic capacity between sexes are explained by blood volume and oxygen carrying capacity. Front Physiol. 2022;13:747903. doi: 10.3389/fphys.2022.747903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han, SS, Kim, M, Lee, SM, et al. Association between body fat and vitamin D status in Korean adults. Asia Pac J Clin Nutr. 2014;23(1):65–75. doi: 10.6133/apjcn.2014.23.1.10 [DOI] [PubMed] [Google Scholar]
- 46.Smith, EM, Alvarez, JA, Kearns, MD, et al. High-dose vitamin D(3) reduces circulating hepcidin concentrations: a pilot, randomized, double-blind, placebo-controlled trial in healthy adults. Clin Nutr. 2017. Aug;36(4):980–985. doi: 10.1016/j.clnu.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ernst, JB, Tomaschitz, A, Grubler, MR, et al. Vitamin D supplementation and hemoglobin levels in hypertensive patients: a randomized controlled trial. Int J Endocrinol. 2016;2016:6836402. doi: 10.1155/2016/6836402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad Fuzi, SF, Mushtaq, S. Vitamin D3 supplementation for 8 weeks leads to improved haematological status following the consumption of an iron-fortified breakfast cereal: a double-blind randomised controlled trial in iron-deficient women. Br J Nutr. 2019. May;121(10):1146–1157. doi: 10.1017/S0007114519000412 [DOI] [PubMed] [Google Scholar]
- 49.Bischoff-Ferrari, HA, Vellas, B, Rizzoli, R, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020 Nov 10;324(18):1855–1868. doi: 10.1001/jama.2020.16909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chenivesse, C, Boulanger, S, Langlois, C, et al. Oxygen desaturation during a 6-minute walk test as a predictor of maximal exercise-induced gas exchange abnormalities in sarcoidosis. J Thorac Dis. 2016. Aug;8(8):1995–2003. doi: 10.21037/jtd.2016.06.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ducharme, JB, Gibson, AL. Efficacy of estimating VO(2)max with the heart rate ratio method in middle-aged and older adults. Eur J Appl Physiol. 2021. Dec;121(12):3431–3436. doi: 10.1007/s00421-021-04808-z [DOI] [PubMed] [Google Scholar]
- 52.Kwon, SB, Ahn, JW, Lee, SM, et al. Estimating maximal oxygen uptake from daily activity data measured by a watch-type fitness tracker: cross-sectional study. JMIR mHealth uHealth. 2019 Jun 13;7(6):e13327. doi: 10.2196/13327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helgerud, J, Oiestad, BE, Wang, E, et al. Prediction of upper extremity peak oxygen consumption from heart rate during submaximal arm cycling in young and middle-aged adults. Eur J Appl Physiol. 2019. Dec;119(11–12):2589–2598. doi: 10.1007/s00421-019-04225-3 [DOI] [PubMed] [Google Scholar]
- 54.Jacquillet, G, Unwin, RJ. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (pi). Pflugers Arch - Eur J Physiol. 2019. Jan;471(1):83–98. doi: 10.1007/s00424-018-2231-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi, SW, Kweon, SS, Choi, JS, et al. The association between vitamin D and parathyroid hormone and bone mineral density: the dong-gu study. J Bone Miner Metab. 2016. Sep;34(5):555–563. doi: 10.1007/s00774-015-0696-9 [DOI] [PubMed] [Google Scholar]
- 56.Mendes, MM, Hart, KH, Lanham-New, SA, et al. Association between 25-hydroxyvitamin D, parathyroid hormone, vitamin D and calcium intake, and bone density in healthy adult women: a cross-sectional analysis from the D-SOL study. Nutrients. 2019 Jun 4;11(6):1267. doi: 10.3390/nu11061267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dibaba, DT. Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev. 2019 Dec 1;77(12):890–902. doi: 10.1093/nutrit/nuz037 [DOI] [PubMed] [Google Scholar]
- 58.Warren, T, McAllister, R, Morgan, A, et al. The interdependency and Co-regulation of the vitamin D and cholesterol metabolism. Cells. 2021 Aug 6;10(8):2007. doi: 10.3390/cells10082007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim, CH, Wheatley, CM, Behnia, M, et al. The effect of aging on relationships between lean body mass and VO2max in rowers. PLoS One. 2016;11(8):e0160275. doi: 10.1371/journal.pone.0160275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.


