Abstract
Indinavir sulfate is a human immunodeficiency virus type 1 (HIV-1) protease inhibitor indicated for treatment of HIV infection and AIDS in adults. The purpose of this report is to summarize single-dose studies which characterized the pharmacokinetics of the drug and the effect of food in healthy volunteers. Indinavir concentrations in plasma and urine were obtained by high-pressure liquid chromatography and UV detection assay methods. The results indicate that indinavir was rapidly absorbed in the fasting state, with the time to the maximum concentration in plasma occurring at ∼0.8 h for all doses studied. Over the 40- to 1,000-mg dose range studied, concentrations in plasma and urinary excretion of unchanged drug increased greater than dose proportionally. The nonlinear pharmacokinetics were attributed to the dose-dependent oxidative metabolism of first-pass metabolism as well as to metabolism in the systemic circulation. Renal clearance slightly exceeded the glomerular filtration rate, suggesting a net tubular secretion component. At high concentrations in plasma, tubular secretion appeared to be lowered because there was a trend for a decreased renal clearance. Administration of 400 mg of indinavir sulfate following a high-fat breakfast resulted in a blunted and decreased absorption (areas under the concentration-time curves [AUCs], 6.86 μM·h in the fasted state versus 1.54 μM·h in the fed state; n = 10). However, two types of low-fat meals were found to have no significant effect on the absorption of 800 mg of indinavir sulfate (AUCs, 23.15 μM·h in the fasted state versus 22.71 and 21.36 μM·h, respectively, in the fed state; n = 11). Immediately following dosing, the concentrations of indinavir in urine often exceeded its intrinsic solubility. To reduce the risk of nephrolithiasis, it is recommended that indinavir sulfate be administered with water.
Protease inhibitors are a new class of antiretroviral agents which inhibit the human immunodeficiency virus (HIV) protease in the cleavage of the gag and pol regions of the viral polyprotein (7, 10, 22). HIV protease is a member of the aspartyl protease family. However, it is structurally dissimilar to human aspartyl proteases such as renin and pepsin. The processing of the polyproteins is essential for the production of individual functional proteins by the virus, including nucleocapsid protein p24, reverse transcriptase, integrase, and the protease itself. Inhibition of the cleavage results in the production of immature noninfectious viral particles.
Indinavir sulfate (MK-639, L-735,524, CRIXIVAN) is a specific and potent HIV protease inhibitor which, by binding to the protease active site, inhibits the activity of the enzyme (11, 31). The concentration for 95% inhibition of the spread of virus in cell culture is ∼50 to 100 nM (31). The drug has been approved for treatment of AIDS and HIV infection in adults in numerous countries. Treatment of HIV-infected patients with indinavir in combination with nucleoside analogs results in a marked decline in HIV type 1 (HIV-1) RNA levels in blood, and significantly delays disease progression (8, 9). Initial reports of the clinical pharmacokinetics of indinavir have been presented elsewhere (3, 25–27, 29, 32). The purpose of this report is to provide a more detailed summary of the pharmacokinetic characteristics and safety of indinavir following single-dose studies with healthy volunteers. Additionally, this report also summarizes the effects of different types of meals on the absorption of indinavir.
MATERIALS AND METHODS
Introductory single-dose study.
This was a two-part, double-blind, placebo-controlled, single-rising-dose study for the investigation of the safety, tolerability, and pharmacokinetics of indinavir in 28 healthy male volunteers (age range, 19 to 44 years; mean age, 27.6 years; weight range, 65 to 92 kg; mean weight, 73.8 kg). This and the two ensuing studies were approved by the Institutional Review Board of Thomas Jefferson University Hospital. Subjects enrolled in the study were nonsmokers, did not take prescription medication for 14 days or any nonprescription medication for 7 days, and were not regular users of any illicit drugs and had no history of drug or alcohol abuse. This was the first human study for the indinavir clinical program. In part 1, rising single doses of 20, 40, 100, 200, 400, 700, and 1,000 mg of indinavir free base in dry-filled capsules (DFCs) were administered to two alternating panels of subjects. For each dose, subjects (n = 12 per panel) were randomized such that eight subjects received active drug and four subjects received the placebo. The investigator was blinded with respect to treatment (active drug or placebo) but not dose. There was a minimum interval of 48 h before the alternate panel received the next higher dose (72 h between receipt of the 200- and the 400-mg doses and thereafter). There was a minimum washout interval of 7 days between the administration of doses within each panel. Each dose was administered in the fasting state with 250 ml of water. All indinavir doses were expressed as the milligram equivalent of the anhydrous free base.
Part 2 of the study examined the following: (i) in the eight subjects who received the active 100-mg dose in part 1, an alternate formulation consisting of a 100-mg dose dissolved in 0.05% citric acid solution (six subjects received the active solution with one dropout, and two subjects received placebo solution); (ii) in the eight subjects who received the active 200-mg dose in part 1, an alternate formulation of 200 mg administered as the sulfate salt in the DFC formulation (six subjects received the active sulfate salt formulation and two subjects received the placebo sulfate formulation).
In part 2, the effect of a high-fat, high-calorie breakfast (meal A) on the absorption of a 200-mg free-base dose was also examined in the eight subjects who received the 200-mg dose in part 1. The meal was consumed in 20 min, and the dose was administered immediately after the completion of the meal. The nutritional analyses of meal A are presented in Table 1.
TABLE 1.
Nutritional analyses of the meals
| Meal | Food item | Amt of protein (g) | Amt of carbohydrate (g) | Amt of fat (g) | Calorie (kcal) |
|---|---|---|---|---|---|
| Meal A | 2 scrambled eggs | 13.0 | 1.2 | 11.6 | 164 |
| 2 slices of toast | 4.0 | 23.0 | 1.8 | 128 | |
| 2 pats of butter | 0 | 0 | 12.0 | 100 | |
| 2 strips of bacon | 3.8 | 0.06 | 6.2 | 72 | |
| 8 oz. of whole milk | 8.0 | 11.0 | 8.0 | 150 | |
| 4 oz. of hash brown potato | 2.5 | 21.9 | 9.0 | 170 | |
| Total | 31.3 | 57.16 | 48.6 | 784 | |
| Meal B | 2 slices of toast | 4.0 | 23.0 | 1.8 | 128 |
| 2 tsp. of jelly | 0 | 8.4 | 0.0 | 32 | |
| 6 oz. of apple juice | 0.15 | 21.75 | 0.23 | 87 | |
| 1 cup of coffee | 0.1 | 0.8 | 0 | 4 | |
| 2 tbs. of skim milk | 1.1 | 1.5 | 0.1 | 11 | |
| 2 tsp. of sugar | 0.0 | 8.0 | 0 | 30 | |
| Total | 5.35 | 63.45 | 2.13 | 292 | |
| Meal C | 3/4 oz. of cornflakes | 1.5 | 18.8 | 0.8 | 83 |
| 1 tsp. of sugar | 0 | 4.0 | 0 | 15 | |
| 1/2 cup of skim milk | 4.2 | 6.0 | 0.2 | 43 | |
| Total | 5.7 | 28.8 | 1.0 | 141 |
For assay of indinavir concentrations, plasma samples were collected predosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 32, and 48 h postdosing (sampling stopped at 12 h following the administration of the two lowest doses), and urine was collected predosing and over the following intervals: 0 to 3, 3 to 6, 6 to 12, 12 to 24, and 24 to 48 h postdosing.
Rising-single-dose sulfate study.
The rising-single-dose sulfate study was a four-period, placebo-controlled, crossover, single-rising-dose study with 12 nonsmoking healthy male subjects (age range, 22 to 39 years; mean age, 27.6 years; weight range, 60 to 85 kg; mean weight, 76.1 kg) to investigate the safety, tolerability, and concentration profiles of the sulfate salt formulation in plasma. The four treatments were as follows: (i) 400 mg in the fasted state, (ii) 400 mg following the consumption of meal A, (iii) 700 mg in the fasted state, and (iv) 1,000 mg in the fasted state. For each treatment, the same 10 subjects received active drug and the same two subjects received placebo. Following an overnight fast, each subject received the assigned treatment with 250 ml of water. Meal A was consumed in 20 min, and the 400-mg dose was administered within 5 min thereafter. The first two treatments (400-mg dose with and without a meal) were randomized according to a two-period crossover design and were administered in periods 1 and 2. The last two treatments (700- and 1,000-mg doses in the fasted state) were administered in periods 3 and 4, respectively. The treatments were separated by at least 7 days. The plasma sampling schedule for assay of indinavir concentrations was identical to that for the introductory study. Urine for assay of indinavir concentrations was collected predosing and over the intervals 0 to 4, 4 to 8, 8 to 12, 12 to 24, and 24 to 48 h postdosing.
Low-fat meals study.
The low-fat meals study was an open-label, four-period, crossover study with 12 healthy volunteers (8 males and 4 females; age range, 18 to 34 years; mean age, 28.3 years; weight range, 59 to 102 kg; mean weight, 77.5 kg). The study was designed to investigate the safety, tolerability, and concentration profile in plasma of single 800-mg doses of indinavir administered to fasted subjects versus those for subjects who had consumed two low-fat, low-calorie meals (meals B and C). The study also included a treatment to examine the profile in plasma following the administration of a liquid free-base suspension formulation. Subjects received, in random order, the following four treatments following an overnight fast: (i) 800 mg of indinavir in the fasted state, (ii) 800 mg of indinavir following the consumption of meal B, (iii) 800 mg of indinavir following the consumption of meal C, and (iv) 800 mg of indinavir free-base oral suspension in the fasted state. Except for the 800-mg free-base suspension, the indinavir doses were administered in four 200-mg sulfate salt DFCs. The meals were consumed in 15 min, and the indinavir dose was given within 5 min thereafter. The nutritional analyses of the meals are presented in Table 1. All treatments were administered with 250 ml of water. Serial plasma samples for assay of indinavir concentrations were collected predosing and at 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 14, and 24 h postdosing.
Assay for indinavir in plasma and urine.
All plasma and urine samples were stored at −20°C until they were analyzed. Indinavir concentrations were determined by a high-pressure liquid chromatography and UV detection method with column switching (35), which had a quantification limit of 5 ng/ml (8.1 nM). Samples from the low-fat meals study were analyzed by a modified version of the assay, with a standard curve range of 25 to 5,000 ng/ml, as opposed to the 5 to 500 ng/ml range in the published procedure. The modification was made so that the range of concentrations detectable by the assay would better reflect the concentrations of indinavir in plasma anticipated following the administration of the dose of the compound given during the study. In order to use the higher standard curve range, working standard concentrations were increased to 100, 80, 40, 20, 10, 4, 2, 1, and 0.5 μg/ml. The addition of 50 μl of each working standard to 1 ml of control plasma resulted in plasma standards that covered the range for the modified procedure. Additionally, the concentration of the internal standard spiking solution was increased from 10 to 100 μg/ml, and the sample injection volume was decreased from 125 to 20 μl. All other methods and conditions specified in the published procedure were used for the modified assay. The intra- and interday variabilities, as well as the accuracy of the modified assay, were comparable to those of the published procedure.
Pharmacokinetic analysis.
The peak concentration in plasma (Cmax) and the time to reach Cmax (Tmax) were obtained by inspection. The area under the plasma concentration-time curve (AUC) was calculated by the modified trapezoidal rule with stable piecewise cubic polynomials (36). Half-life in plasma was computed as the quotient of the natural log of 2 and the terminal slope for plasma. The terminal slope was obtained by unweighted nonlinear regression of the terminal data for plasma by an empirical biexponential or monoexponential decay function. For the latter, the onset of the log-linear phase was determined visually for each data set, which usually occurred at concentrations below 100 to 500 nM and at 6 to 8 h postdosing. Renal clearance was computed as the quotient of urinary excretion and the corresponding AUC over each urine collection interval.
Statistical analysis.
For the introductory single-dose study, the geometric means (GMs) for AUC and Cmax were obtained from analysis of variance (ANOVA) models used to compare the various dose groups of interest and to explore dose proportionality. The AUC and Cmax data were log transformed prior to analysis. The means on the log scale were exponentiated to provide the GM. For the rising-single-dose sulfate study, the effect of food was analyzed by an ANOVA with subject and treatment as factors, with data from all periods being included after confirming that there were no period or sequence effects from the two-period crossover part of the study. The mean squared error from this model was used to obtain 90% confidence intervals (CIs) for the differences in the log-transformed measurements between subjects receiving 400-mg doses in the fasting and fed states. CIs were exponentiated to obtain the 90% CIs for the GM ratios. For the low-fat meals study, an ANOVA appropriate for a four-period crossover was used with the log-transformed measurements of AUC, Cmax, and the concentration of drug in plasma at 8 h postdosing (C8). Carryover effect was removed from the model since it was not significant. The mean squared error from this model was used to obtain 90% CIs for the differences in mean log values. CIs were exponentiated to obtain the 90% CIs for the GM ratios.
RESULTS
Safety and tolerability.
Safety and tolerability were carefully monitored in these initial phase I trials of indinavir. In the introductory study, doses of the free-base capsule of between 20 and 1,000 mg were evaluated, as was a 100-mg dose of citric acid solution and a 200-mg dose of sulfate salt capsule. Seven of 28 subjects had clinical adverse experiences, but none was judged to be drug related and none required hospitalization. Infections including upper respiratory infection, flu-like illness, and gastroenteritis were among the frequently observed adverse experiences following the administration of either indinavir or placebo. Five subjects had abnormalities in laboratory test results judged to be adverse events. Four subjects had elevations in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels judged to be possibly related to drug, but after unblinding it was observed that several of these elevations followed the administration of placebo. The greatest elevation was an ALT level 2.2-fold times the upper limit of normal 3 days following the administration of placebo. One subject had markedly elevated creatine phosphokinase levels both prior to taking any study drug (the level was available after drug was administered) and following receipt of a 40-mg dose of indinavir. The etiology of his event was unclear, but it was judged by the investigator to be definitely not drug related. The local tolerability of a citric acid solution was also evaluated in the introductory study. In all five subjects who received the indinavir solution, as well as the two subjects who received placebo vehicle alone (which contained a bitter taste-masking agent), severe aftertaste was a prominent complaint.
In the second clinical trial, the rising-dose study, doses of the sulfate salt form of between 400 g and 1,000 mg were administered. Two subjects experienced diarrhea, one following receipt of a 400-mg dose and one while off of the drug. Two subjects experienced microscopic hematuria (3 to 20 erythrocytes per high-power field). One subject had hematuria both immediately preceding and following receipt of a 1,000 mg dose of indinavir. One subject had hematuria following receipt of a 400-mg dose of indinavir. In this study no subject had an elevated AST or ALT levels deemed to be an adverse event. The safety and tolerability profile of indinavir in these two single-dose studies were thought to be adequate to permit investigation of multiple doses.
In the low-fat meals study, six subjects had clinical adverse experiences, the most common of which was nausea. One episode each of nausea, taste perversion, and headache was judged to be possibly drug related. No adverse event was serious, and no subject had an adverse experience on the basis of laboratory data. No subject was discontinued from the study due to a clinical adverse experience.
Concentration profiles in plasma.
Immediately after the completion of the clinical phase of the initial introductory study and the initial assay for a subset of samples (from the 200-mg treatments), a lower intersubject variation was observed with the sulfate salt than with the free base (Table 2) (the coefficient of variation [CV] decreased from 75 to 31% for AUC and from 69 to 28% for Cmax), although the two preparations appeared to have given comparable mean concentrations in plasma for the six subjects who completed both treatments (the ratio of the sulfate salt AUC to the free base AUC was 1.19, with a 90% CI of 0.72 and 1.99). On the basis of these preliminary results, the development of the free-base formulation was terminated, and the indinavir sulfate formulation was investigated further in the rising-dose study.
TABLE 2.
Summary pharmacokinetics of indinavir in the introductory studya
| Free-base dose (mg) | No. of subjects | AUC (μM·h) | Cmax (μM) | Tmax (h) | Half-life in plasma (h) | Urinary recovery (% of dose) | Renal clearance (ml/min) |
|---|---|---|---|---|---|---|---|
| 40 | 8 | 0.04 (67) | 0.5 ± 0.0 | —b | |||
| 100 | 5 | 0.31 (65) | 0.29 (69) | 0.6 ± 0.2 | — | ||
| 100c | 5 | 0.36 (47) | 0.31 (48) | 0.5 ± 0.0 | — | ||
| 200 | 8 | 1.04 (75) | 0.80 (69) | 0.9 ± 0.5 | 1.9 | 4.4 ± 2.8 | 164 |
| 200 (fed) | 6 | 0.68 (66) | 0.27 (80) | 2.8 ± 1.9 | 1.6 | — | |
| 200 (sulfate) | 6 | 1.83 (31) | 1.30 (28) | 0.8 ± 0.3 | 2.4 | 5.0 ± 2.0 | 143 |
| 400 | 8 | 2.90 (67) | 2.05 (56) | 0.7 ± 0.3 | 2.0 | — | |
| 700 | 8 | — | — | 8.6 ± 6.5 | |||
| All doses | 1.9 | 154 |
Values are GMs except for Tmax and urinary recoveries, which are arithmetic means ± standard deviations; values in parentheses are CVs (in percent).
—, no assays.
In citric acid solution.
Summary values of the pharmacokinetic parameters from the first, introductory study are presented in Table 2. Indinavir concentrations in many samples following administration of the low doses were below the analytical quantification limit. Summary values of the pharmacokinetic parameters for the indinavir sulfate capsule formulation from the rising-dose study and the low-fat meals study are presented in Tables 3 and 4., respectively. Profiles of the mean concentrations in plasma following the three studies are presented in Fig. 1 and 2.
TABLE 3.
Summary pharmacokinetics of indinavir in the rising-dose studya
| Sulfate salt dose (mg) | AUC (μM·h) | AUC ratio (90% CI)b | Cmax (μM) | Tmax (h) | C8 (nM) | Half-life in plasma (h) | Urinary recovery (% of dose) | Renal clearance (ml/min) |
|---|---|---|---|---|---|---|---|---|
| 400 | 6.86 (48) | 4.48 (47) | 0.7 ± 0.3 | 35 (62) | 1.9 | 8.9 ± 3.7 | ||
| 400 (fed state) | 1.54 (46) | 0.22 (0.19, 0.27) | 0.62 (55) | 2.0 ± 1.1 | 29 (31) | 2.4 ± 1.0 | ||
| 700 | 17.94 (41) | 2.6 (2.2, 3.2) | 9.85 (44) | 0.8 ± 0.4 | 69 (62) | 1.8 | 10.4 ± 4.9 | |
| 1,000 | 33.50 (36) | 4.9 (4.1, 5.9) | 16.38 (29) | 0.8 ± 0.3 | 144 (76) | 1.7 | 12.0 ± 4.9 | |
| All doses | 1.8 | 116 |
Values are GMs for 10 subjects except for Tmax and urinary recoveries, which are arithmetic means ± standard deviations; values in parentheses are CVs (in percent) unless indicated otherwise.
Relative to the 400-mg dose.
TABLE 4.
Summary pharmacokinetics of indinavir following the administration of single doses of 800 mg of indinavir in the study with low-fat mealsa
| Treatment | No. of subjectsb | AUC (μM·h) | AUC ratio (90% CI)c | Tmax (h) | Cmax (μM) | Cmax ratio (90% CI) | C8 (nM) | C8 ratio (90% CI) |
|---|---|---|---|---|---|---|---|---|
| Fasted | 11 | 23.15 (43) | 0.77 ± 0.26 | 11.68 (33) | 123 (71) | |||
| Meal B | 11 | 22.71 (34) | 0.98 (0.75, 1.28) | 1.41 ± 0.83 | 9.37 (26) | 0.80 (0.59, 1.08) | 138 (61) | 1.12 (0.87, 1.45) |
| Meal C | 11 | 21.36 (43) | 0.92 (0.71, 1.20) | 1.36 ± 0.98 | 8.85 (53) | 0.76 (0.56, 1.02) | 152 (94) | 1.24 (0.96, 1.60) |
| Oral liquid | 10 | 7.75 (69) | 0.33 (0.25, 0.44) | 0.73 ± 0.80 | 4.72 (65) | 0.40 (0.33, 0.55) | 52 (64) | 0.43 (0.33, 0.55) |
Values shown are GMs except for Tmax values, which are arithmetic means ± standard deviations; values in parentheses are CVs (in percent) unless indicated otherwise.
One subject did not complete the study, and another subject in the oral liquid treatment was excluded from analysis due to undetectable concentrations in plasma for all samples from that treatment.
Relative to the fasted treatment; values in parentheses are 90% CI.
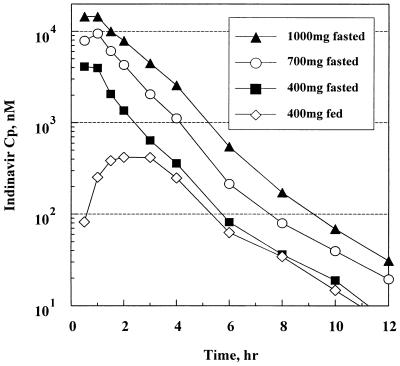
FIG. 1.
Mean plasma concentration (Cp)-time profiles of indinavir following the administration of single oral doses in the rising-dose study.
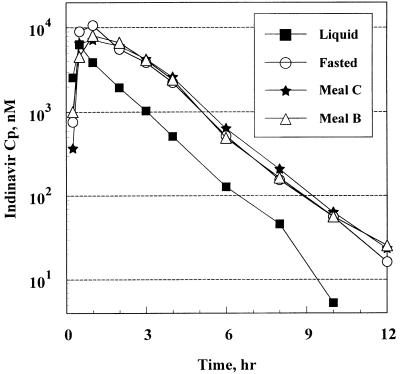
FIG. 2.
Mean plasma concentration (Cp)-time profiles of indinavir following the administration of 800-mg doses in the low-fat meals study.
The data from the introductory study suggested a greater than proportional increase in the concentration in plasma with the dose. Two- and fourfold increases in the free-base dose from 100 mg resulted in a 3.4- and 9.4-fold increases in AUC values, respectively, although the lack of subject crossover among the doses limited this interpretation. This disproportionate increase in the concentration in plasma was further confirmed with the sulfate salt formulation in the rising-dose study, in which the AUC increased 4.9-fold (90% CI = 4.1 and 5.9) when the dose was increased 2.5-fold from 400 to 1,000 mg. The Cmax also showed the same greater-than-dose-proportional trend. As the dose was increased, the concentrations in plasma after Cmax was reached dropped less precipitously, resulting in a slightly more sustained shoulder. However, Tmax was relatively constant, with its value averaging approximately 0.8 h across all doses, indicating rapid drug absorption in the fasting state.
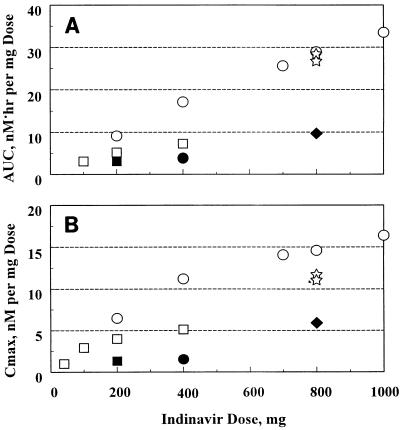
Figure 3 provides an overall summary of the nonlinear increase in the dose-normalized concentrations in plasma in relation to the administered doses.
FIG. 3.
Dose-normalized AUC (A) and Cmax (B) of indinavir as a function of the administered single-dose; AUC and Cmax values were normalized to a 1-mg dose. ○, sulfate salt, fasted state; •, sulfate salt, following a high-fat meal; ⋆, sulfate salt, following a low-fat meal; □, free-base capsules, fasted state; ▪, free-base capsules, following a high-fat meal; ⧫, free-base oral suspension, fasted state.
Sulfate salt versus free base.
In the introductory study, the ratio (n = 5) for the GM AUC for the citric acid solution treatment to the GM AUC for the free-base capsule treatment at the 100-mg dose was 1.16 (90% CI = 0.66 and 2.03). These data, in addition to the comparable ratio for the GM AUC for the sulfate salt treatment to the GM AUC for the free-base treatment at the 200-mg dose, suggest that at these low doses absorption of the free base, which has extremely low aqueous solubility (∼19 μg/ml at pH 6.9), and absorption of the citric acid solution preparation or the sulfate salt, which has an aqueous solubility in excess of 100 mg/ml, are comparable. However, as the dose was escalated to 800 mg in the study with low-fat meals, the indinavir free base administered in an oral suspension was absorbed to a significantly lesser extent than an equivalent dose of the sulfate salt.
Urinary excretion.
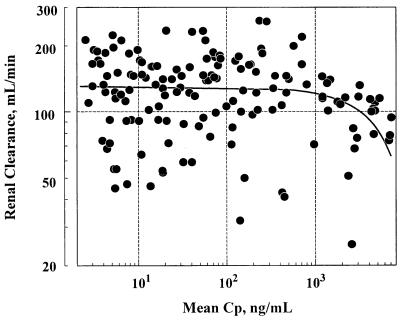
Similar to the concentrations in plasma, urinary excretion of intact indinavir also increased more than dose proportionally. The recovery of unchanged drug as a percentage of the dose increased from 4.4% ± 2.8% at the 200-mg dose to 12.0% ± 4.9% at the 1,000-mg dose. Figure 4 displays the renal clearance of indinavir as a function of the concentration in plasma observed in the rising-dose study. These data suggest that the renal clearance remained relatively constant in the majority of urine samples collected and over a wide concentration range. However, there was a trend of decreasing renal clearance with increasing concentration in plasma, especially when the concentration was above 1 μg/ml (∼1.6 μM). As a first approximation, the overall renal clearance was estimated to be 154 ml/min in the introductory study and 116 ml/min in the rising-dose study, both of which slightly exceeded the glomerular filtration rate.
FIG. 4.
Individual renal clearance of indinavir as a function of mean concentration in plasma (Cp) over each urine collection period in the rising-dose study. The solid line represents the average (n = 10) of the least-squares linear relationship between renal clearance and the concentration in plasma for each subject.
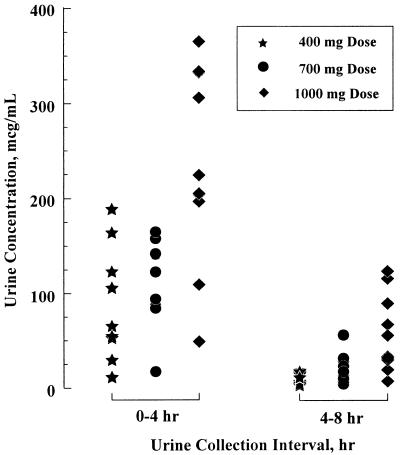
Figure 5 displays the indinavir concentrations in urine during the first two collection intervals following the administration of doses of 400, 700, and 1,000 mg in the fasting state in the rising-dose study. As a basic drug with pKas of 3.7 and 6.0 (28), indinavir exhibits a pH dependency in aqueous solubility. At a typical urine pH of 6.3 (23), the solubility of the indinavir free base is less than 40 μg/ml (14). The concentrations in urine in the present study significantly exceeded the intrinsic solubility of the indinavir free base in water.
FIG. 5.
Concentrations of indinavir in urine following the administration of single doses of 400, 700, and 1,000 mg of indinavir sulfate to fasting subjects.
Half-life in plasma.
The terminal half-life of indinavir was calculated for subjects receiving the 200- and 400-mg doses in the introductory study and for all subjects receiving treatments in the fasting state in the rising-dose study. For the lower-dose treatments, the half-life in plasma was not calculated because the indinavir concentration in the plasma samples was nonquantifiable. Although there was a slightly higher variability of the half-life among doses from the introductory study (Table 2), the half-life was not dose dependent and was similar for all treatments, averaging 1.8 to 1.9 h.
Effect of high-fat meal.
As indicated in Tables 2 and 3, the administration of either the free base or the sulfate salt of indinavir with the high-fat meal (meal A) resulted in decreases in AUC and Cmax and an increase in Tmax, indicating a reduced and delayed absorption of the drug following the consumption of the high-fat meal. For the six subjects who completed the treatments in the introductory study in both the fasting and the fed states, AUC decreased 56%, from 1.53 to 0.68 μM·h, while Cmax decreased 73%, from 1.14 to 0.27 μM. In the rising-dose study, there was a 78% reduction in AUC (i.e., ratio of the GM AUC for the fasted state versus the GM AUC for the fed fasted state was equal to 0.22 [90% CI = 0.19 and 0.27]) and an 86% reduction in Cmax when the sulfate salt formulation was administered after the subjects had consumed the high-fat meal. Significantly lower steady-state levels would be expected if multiple doses of indinavir were administered following the ingestion of high-fat meals.
Effects of low-fat meals.
Table 4 displays summary values of the pharmacokinetic parameters following the administration of 800-mg single doses in the fasting state as well as immediately following the consumption of two low-fat meals. The corresponding profiles of the concentration in plasma are presented in Fig. 2.
These data indicate that the consumption of low-fat meal B or C did not significantly alter the profile of the concentration of indinavir in plasma Cmax and AUC were not significantly reduced, and Tmax was slightly but not significantly delayed from those measured under fasted conditions. The ratios (90% CIs) of the GM AUCs for meals B and C versus the GM AUC for the fasted state were 0.98 (0.75 and 1.28) and 0.92 (0.71 and 1.2), respectively. The ratios (90% CI) of the GM Cmax for meals B and C versus the GM Cmax for the fasted state were 0.80 (0.59 and 1.08) and 0.76 (0.56 and 1.02), respectively. C8s were slightly but not significantly elevated with the low-fat meals.
DISCUSSION
The investigations presented in this paper indicate that indinavir sulfate is rapidly absorbed following oral administration, achieving peak concentrations in plasma in ∼0.8 h. Indinavir has a relatively short terminal half-life which is not dose dependent. The concentrations in plasma and urinary excretion increased greater than proportionally to the dose, indicating nonlinear pharmacokinetics. Protein binding of indinavir in plasma is constant over the range of 0.1 to 50 μg/ml (16). Thus, taking into account the protein binding (39% unbound), the present data suggest renal clearance of free drug of approximately 300 to 400 ml/min, which exceeded the creatinine clearance. This suggests a net tubular secretion component to the indinavir renal clearance. The trend toward decreased renal clearance at high concentrations in plasma suggests decreased tubular secretion or increased reabsorption.
Despite the slight decrease in renal clearance at high concentrations in plasma, the concentrations of indinavir in urine immediately following ingestion of doses above 400 mg generally were supersaturated with respect to the aqueous solubility of the drug. However, as expected, the concentrations of indinavir in urine were dependent on dose, time postdosing, and urine volume. The highest concentrations in urine occurred transiently around the peak concentrations in plasma for the high doses immediately following administration. Because the concentrations in plasma decreased with time after dosing, there was a corresponding decrease in the drug concentrations in urine. Nevertheless, the potential exists for the drug to precipitate or coprecipitate with other urinary components following the administration of indinavir.
Urine is often supersaturated with many components which ordinarily do not form insoluble crystals without other predisposing factors. Thus, the observation of supersaturating concentrations of indinavir in urine was not deemed to be of particular clinical significance at the time of the phase I studies. In retrospect, this presumption was incorrect. Subsequently, nephrolithiasis has been observed in other clinical trials (6, 13). The microscopic hematuria observed in two volunteers in the rising-dose study could have been reflective of crystallization of indinavir in the urine, although microscopic hematuria is also a common non-drug-related event in generally healthy subjects. In any event, there is a clear pharmacokinetic explanation for the potential for indinavir crystallization in the urine. Increasing the urinary flow rate by ingesting large quantities of water at the time of indinavir administration may reduce the precipitation potential as a result of the decrease in the high concentrations in urine associated with the peak concentrations in plasma (6, 13, 17).
A previous study with radiolabeled indinavir suggested that on the basis of the urinary and fecal recovery data, the absorption of indinavir was appreciable after oral administration (2). Indinavir has also been found to be extensively metabolized by the cytochrome P-450 system, and CYP3A4 has been identified to be the isoform responsible for the oxidative metabolism of indinavir (4), resulting in the recovery of a number of metabolites in the urine as well as the feces (1, 2). In previous animal studies, linear disposition pharmacokinetics over the 2- to 10-mg/kg doses were found following intravenous administration to rats and dogs, and extensive first-pass metabolism following oral administration has been noted in rats (15).
The wide dose range (40 to 1,000 mg) at which disproportionate increases in AUC are seen suggests that indinavir may be subject to a significant extent of first-pass metabolism when it is given at low doses and may exhibit nonlinear systemic disposition kinetics when it is given at high doses. The systemic clearance is most likely to be linear at low doses, and the observed nonlinearity could be attributed mainly to the dose-dependent first-pass metabolism during absorption of the drug. As the dose is increased, first-pass metabolism could gradually become more saturated and the fraction of the unchanged drug entering the systemic circulation could become less dose dependent, while the concentration-dependent systemic disposition, including the oxidative metabolism, would be expected to play a greater role.
The marketed formulation of indinavir is the sulfate salt, which was chosen over the free-base formulation primarily because of the lower intersubject variability observed in the introductory study with the sulfate salt relative to that observed with the free base. At higher doses, the sulfate salt also has higher bioavailability. Compared to the free-base AUC, the sulfate salt AUC was not substantially different at the 200-mg dose in the introductory study. However, the sulfate salt capsule exhibited a threefold higher AUC than a suspension of the free base in a subsequent study with a dose of 800 mg, suggesting a dose dependency in the relative bioavailability. These differences could be caused by a number of factors, such as the different dosage forms between the two free-base preparations; however, differences in the dissolution of the free base between the two doses in the two studies may also be important. Because of finite gastric acidity and gastric residence time, a large percentage of the 800-mg doses of indinavir free base may not be efficiently dissolved and could remain in a nonabsorbable solid form during its passage through the gut lumen. The dissolution of indinavir sulfate salt, due to its high aqueous solubility, does not appear to be dose limited.
The effect of food on the absorption of indinavir varied with the meal ingested. Meal A, with its high caloric content as well as its high fat, protein, and carbohydrate contents, resulted in an appreciable reduction of absorption of both the free base and the sulfate salt. Meals B and C contained considerably lower fat and protein contents and fewer calories than meal A. Except for a small but nonsignificant delay in the Tmax and a small increase in C8, these low-fat meals had no discernible effects. Thus, high fat and/or high protein levels but not high carbohydrate levels up to 63 g appear to affect the absorption of indinavir. Unpublished data (20) on the effect of meals typically consumed in Japan suggest that a high protein content may be less deleterious to indinavir absorption than a high fat content.
Many factors are known to contribute to drug-food interactions (12, 30, 33, 34), which can result in increased, decreased, delayed, or unaffected drug absorption. The reported effect of food on other protease inhibitors has also been found to depend specifically on the drug. While food has no definitive effect on the absorption of ritonavir (19), it significantly increases the absorption of saquinavir (18, 24) and nelfinavir (21). In the present study, low-fat meals B and C did not appear to interfere with indinavir absorption, and the pharmacokinetic profiles of indinavir following the consumption of either meal were comparable to that when indinavir was administered in the fasted state. The absorption of indinavir, in common with other basic drugs, probably occurs predominantly from the upper region of the small intestine (5, 23). In a fasting stomach, indinavir in its protonated solution form could be emptied rapidly and could be efficiently absorbed. Similarly, the residual gastric acidity appeared to be sufficient to dissolve the low-dose free base, resulting in efficient absorption. However, meals rich in fat contents may delay gastric emptying and have a buffering and neutralizing effect, resulting in a rise in the gastric pH and possibly some precipitation of the drug. This combination of effects could result in a decreased flux of drug reaching the absorption site, a decreased absorption rate, a net increase in first-pass metabolism, and a net reduction of the unchanged indinavir fraction reaching the general circulation.
Several abstracts (3, 25, 26, 32) and one publication (27) have described the multiple-dose pharmacokinetic characteristics of indinavir in volunteers and HIV-infected patients. When administered every 6 or every 8 h, there is only modest drug accumulation, consistent with the accumulation expected for dosing intervals which are considerably in excess of the 1.8-h terminal half-life. There is no evidence of decreasing concentrations in plasma upon extensive multiple dosing, which would be indicative of a P-450 enzyme inductive effect. The increases in the values of the pharmacokinetic parameters for indinavir are greater than dose proportional when the drug is administered as multiple doses, as described here for single doses. Thus, the single-dose pharmacokinetic characteristics of indinavir described here are predictive of the pharmacokinetic characteristics of this potent antiretroviral agent at steady state.
REFERENCES
- 1.Balani S K, Arison B H, Mathai L, Kaufman L R, Miller R R, Sterns R A, Chen I W, Lin J H. Metabolites of L-735,524, a potent HIV-1 protease inhibitor, in human urine. Drug Metab Dispos. 1995;23:266–270. [PubMed] [Google Scholar]
- 2.Balani S K, Woolf E J, Sturgill M, Deutsch P J, Yeh K C, Lin J H. Disposition of indinavir, a potent HIV-1 protease inhibitor, after oral dose in humans. Drug Metab Dispos. 1996;24:1389–1394. [PubMed] [Google Scholar]
- 3.Bjornsson T, Chiou R, Deutsch P, Haddix H, Hoagland V, Justice S, Nessly M, Pomerantz R, Saag M, Squires K, Teppler H, Waldman S, Woolf E, Yeh K C. Pharmacokinetics of indinavir. Pharm Res. 1996;13:S485. [Google Scholar]
- 4.Chiba M, Hensleigh M, Nishime J A, Balani K S, Lin J H. Role of CYP3A4 in human metabolism of MK-639, a potent HIV protease inhibitor. Drug Metab Dispos. 1996;24:307–314. [PubMed] [Google Scholar]
- 5.Chiou R, Deutsch P, Carides A, Fu I, Kwei G, Hoagland V, Lambert G, Sturgill M, Yeh K C. Indinavir absorption following extended-release formulations in man and in the dog. Pharm Res. 1996;13:S498. [Google Scholar]
- 6.Daudon M, Estepa L, Viard J P, Joly P, Jungers P. Urinary stones in HIV-1 positive patients treated with indinavir. Lancet. 1997;349:1294–1295. doi: 10.1016/s0140-6736(05)62506-8. [DOI] [PubMed] [Google Scholar]
- 7.Debouck C. The HIV-1 protease as a therapeutic target for AIDS. AIDS Res Hum Retroviruses. 1992;8:153–164. doi: 10.1089/aid.1992.8.153. [DOI] [PubMed] [Google Scholar]
- 8.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A, Deutsch P J, Holder D, Schleif W A, Condra J H. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 9.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Feinberg J E, Balfour H H, Deyton L R, Chodakewitz J A, Fischl M A, Phair J P, Spreen W, Pedneault L, Nguyen B Y, Cook J C ACTG 320 Study Team. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 10.Huff J R. HIV protease: a novel chemotherapeutic target for AIDS. J Med Chem. 1991;34:2305–2314. doi: 10.1021/jm00112a001. [DOI] [PubMed] [Google Scholar]
- 11.Huff J R, Vacca J R, Dorsey B D, Emini E A, Condra J H, Schleif W A, Massari F E, Deutsch P J, Chodakewitz J, Kuo L C, Chen Z. Abstracts of the 211th ACS National Meeting. 1996. Crixivan®: a potent inhibitor of HIV-1 protease in vivo, abstr. Medi-255. [Google Scholar]
- 12.Kirk J K. Significant drug-nutrient interactions. Am Fam Physician. 1995;51:1175–1182. [PubMed] [Google Scholar]
- 13.Kopp J B, Miller K D, Mican J A M, Feuerstein I M, Vaughan E, Baker C, Pannell L K, Falloon J. Crystalluria and urinary tract abnormalities associated with indinavir. Ann Intern Med. 1997;127:119–125. doi: 10.7326/0003-4819-127-2-199707150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kwei G Y, Novak L B, Hettrick L A, Reiss E R, Ostovic D, Loper A E, Lui C Y, Higgins R J, Chen I W, Lin J H. Regiospecific intestinal absorption of HIV protease inhibitor L-735,524 in beagle dogs. Pharm Res. 1995;12:884–888. doi: 10.1023/a:1016269206048. [DOI] [PubMed] [Google Scholar]
- 15.Lin J H, Chen I W, Vastag K J, Ostovic D. pH-dependent oral absorption of L-735,524, a potent HIV-protease inhibitor, in rats and dogs. Drug Metab Dispos. 1995;23:730–735. [PubMed] [Google Scholar]
- 16.Lin J H, Chiba M, Balani S K, Chen I W, Kwei G Y-S, Vastag K J, Nishime J A. Species differences in the pharmacokinetics and metabolism of indinavir, a potent human immunodeficiency virus protease inhibitor. Drug Metab Dispos. 1996;24:1111–1120. [PubMed] [Google Scholar]
- 17.Medical Economics Co. Physician’s desk reference. Vol. 51. Montvale, N.J: Medical Economics; 1997. pp. 1670–1673. [Google Scholar]
- 18.Medical Economics Co. Physician’s desk reference. Vol. 51. Montvale, N.J: Medical Economics Co.; 1997. pp. 2291–2294. [Google Scholar]
- 19.Medical Economics Co. Physician’s desk reference. Vol. 51. Montvale, N.J: Medical Economics Co.; 1997. pp. 447–451. [Google Scholar]
- 20.Merck Research Laboratories. Data on file. Merck Research Laboratories, West Point, Pa.
- 21.Quart B D, Chapman S K, Peterkin J, Webber S, Oliver S. Abstracts of the 2nd National Conference on Human Retrovirus and Related Infections. 1995. Phase I safety, tolerance, pharmacokinetics and food effect studies of AG1343, abstr. LB3. [Google Scholar]
- 22.Robert N A, Marin J A, Kinchington D, Broadhurst A V, Craig J C, Duncan I B, Galpin S A, Handa B K, Kay J, Krohn A, Lambert E W, Merrett J H, Mills J S, Parkes K E B, Redshaw S, Ritchie A J, Taylor D L, Thomas G J, Machin P J. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990;248:358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- 23.Rowland M, Tozer T N. Clinical pharmacokinetics. 3rd ed. Philadelphia, Pa: Lea & Febiger; 1995. p. 174. [Google Scholar]
- 24.Shaw T M, Williams P E O, Nuirhead G J, Harris S, Watson N, Nimmo W. Abstracts of the International Conference on AIDS. 1993. Effect of timing of food and gastric pH on exposure to Ro31-8959, HIV proteinase inhibitor in healthy subjects, abstr. PO-B30-2199; p. 502. [Google Scholar]
- 25.Squires K E, Saag M S, Teppler H, Pomerantz R, Waldman S, Bjornsson T, Woolf E, Yeh K, Emini E, Deutsch P. Phase I studies of L-735,524, an HIV protease inhibitor: pharmacokinetics, tolerability and short-term antiviral activity. Clin Res. 1994;42:280A. [Google Scholar]
- 26.Steigbigel R T, Berry P, Mellors J, McMahon D, Teppler H, Stein D, Drusano G, Deutsch P, Yeh K, Hildebrand C, Nessly M, Emini E, Chodakewitz J. Proceedings of the 3rd Conference on Retroviruses and Opportunistic Infections. 1996. Efficacy and safety of the HIV protease inhibitor indinavir sulfate (MK-639) at escalating dose. [Google Scholar]
- 27.Stein D S, Fish D G, Bilello J A, Preston S L, Martineau G L, Drusano G L. A 24-week open-label phase I/II evaluation of the HIV protease inhibitor MK-639 (indinavir) AIDS. 1996;10:485–492. doi: 10.1097/00002030-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Stelmach C, Ostovic D. Physical and chemical characterization of the HIV protease inhibitor Crixivan®. Pharm Res. 1996;13:S280. [Google Scholar]
- 29.Stone J A, Ju W D, Sterritt A, Woolf E J, Yeh K C, Deutsch P, Waldman S, Bjornsson T D. Effect of food on the pharmacokinetics of indinavir in man. Pharm Res. 1996;13:S414. [Google Scholar]
- 30.Thomas J A. Drug-nutrient interactions. Nutr Res. 1995;53:271–282. doi: 10.1111/j.1753-4887.1995.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 31.Vacca J P, Dorsey B D, Schleif W A, Levin R B, McDaniel S L, Darke P L, Zugay J, Quintero J C, Blahy P M, Roth E, Sardana V V, Schlabach A J, Graham P I, Condra J H, Gotlib L, Holloway M K, Lin J, Chen I W, Vastag K, Ostovic D, Anderson P S, Emini E A, Hoff J E. L-735,524, an orally bioavailable HIV-1 protease inhibitor. Proc Natl Acad Sci USA. 1994;91:4096–4100. doi: 10.1073/pnas.91.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldman S A, Teppler H, Osborne B, Bjornsson T D, Pomerantz R, Woolf E, Yeh K, Deutsch P, Squires K, Saag M. Pharmacokinetics of L-735,524, an HIV protease inhibitor. Clin Pharmacol Ther. 1994;55:195. [Google Scholar]
- 33.Welling P G. Effect of food on drug absorption. Pharmacol Ther. 1989;43:425–441. doi: 10.1016/0163-7258(89)90019-3. [DOI] [PubMed] [Google Scholar]
- 34.Winstanley P A, Orme M L E. The effect of food on drug bioavailability. Br J Clin Pharmacol. 1989;28:621–628. doi: 10.1111/j.1365-2125.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolf E, Au T, Haddix H, Matuszewski B. Determination of L-735,524, an human immunodeficiency virus protease inhibitor, in human plasma and urine via HPLC with column switching. J Chromatogr. 1995;692:45–52. doi: 10.1016/0021-9673(94)00608-c. [DOI] [PubMed] [Google Scholar]
- 36.Yeh K C, Small R D. Pharmacokinetic evaluation of stable piecewise cubic polynomials as numerical integration functions. J Pharmacokinet Biopharm. 1989;17:721–740. doi: 10.1007/BF01062126. [DOI] [PubMed] [Google Scholar]