Abstract
Background.
Chronic kidney disease is common after non-renal solid organ transplantation, mainly secondary to calcineurin inhibitors toxicity. Uterus transplantation (UTx) is an innovative treatment for women with absolute uterine factor infertility. UTx is exclusive because it is transient with the absence of lifelong immunosuppression and is performed in young healthy participants. Therefore, UTx provides a unique setting for evaluating the effect of time-limited calcineurin inhibitors treatment on recipients’ kidney function.
Methods.
In the first UTx cohort worldwide, we studied kidney function using estimated glomerular filtration rate (eGFR) in 7 women over a median follow-up of 121 (119–126) mo.
Results.
Median eGFR (mL/min/1.73 m2) of the cohort was 113 at UTx, which declined to 74 during month 3, 71 at months 10–12, 76 at hysterectomy (HE), and 83 at last follow-up. Median duration of tacrolimus exposure was 52 (22–83) mo, and median trough levels (µg/L) were 10 during month 3 and 5.8 at HE. Between UTx and month 3, decline in kidney function was observed in all 7 participants with a median eGFR slope for the whole cohort of −24 mL/min/1.73 m2, which declined further by −4 mL/min/1.73 m2 until months 10–12. Thereafter, eGFR slope improved in 3 participants, remained stable in 3, and worsened in 1 until HE/tacrolimus discontinuation, after which it improved in 2. Eventually, between UTx and last follow-up, 4 of 7 participants had a decline in their eGFR, the median annual eGFR slope being negative at −1.9 mL/min/1.73 m2/y for the whole group.
Conclusions.
Kidney function declined in all recipients early after UTx followed by a persistent long-term decrease in majority, despite transplantectomy and discontinuation of immunosuppression. Thus, UTx may incur an increased risk of chronic kidney disease even in this young and healthy population, highlighting the importance of close surveillance of kidney function and minimization of tacrolimus exposure.
Uterus transplantation (UTx) is an innovative treatment for infertility that has given women with absolute uterine factor infertility (AUFI) the possibility of carrying a pregnancy and giving birth to a child. AUFI is caused by either uterine absence (surgical/congenital) or uterine defects (anatomic/functional), which impede embryo implantation or pregnancy completion. It is estimated that more than 20 000 fertile-aged women in a total population of 100 million people are affected by AUFI.1 UTx is unique among all organ transplants because it is a transient transplantation, with the graft intended only for a restricted number of years until live childbirth(s) has been accomplished. Transplantectomy/hysterectomy (HE) typically occurs within 5–6 y after UTx and immunosuppression is completely discontinued. The first live birth after UTx occurred in 2014 at our center in Sweden, and this case is included in the present Swedish study of living donor (LD) UTx.2 Up until today, more than 80 UTx procedures have been performed in around 20 centers worldwide, resulting in more than 40 live births.3
Chronic kidney disease (CKD) is common after non-renal solid organ transplantation (NRSOT) such as the liver, heart, and lungs, with a cumulative incidence of approximately 20% at 3 y post-transplant.4–6 The etiology of CKD after NRSOT is multifactorial, and several risk factors such as exposure to calcineurin inhibitors (CNI), hemodynamic instability, postoperative acute kidney injury (AKI), nephrotoxic drugs, pre-existing primary kidney disease, ischemia-reperfusion injury, and viral infections have been described.7–9 The presence of CKD has significant clinical implications in NRSOT recipients and in its more advanced stages has been associated with a 4-fold increase in patient mortality.7 Moreover, it is also a significant risk factor for adverse maternal and fetal outcomes during pregnancy, including neonatal death.10,11 However, unlike other NRSOT, UTx is performed in rigorously screened young and healthy participants in whom one may not expect predisposing risk factors for CKD. Nonetheless, the need for CNI-based immunosuppression and congenital renal anomalies that coexist in approximately one-third of the women with congenital uterine agenesis may increase the risk of CKD in this population.
Given the exclusive situation of uterine graft removal after up to 2 successful pregnancies, this study provides a unique opportunity to evaluate the effect of total CNI discontinuation on recipients’ kidney function after transplantation. This is the first report on the long-term course of kidney function in 7 recipients after UTx and transplantectomy/CNI discontinuation.
MATERIALS AND METHODS
This prospective observational study was approved by the Regional Human Ethics Committee of the University of Gothenburg (Dnr: 088-12; NCT01844362). Nine women (participants No. 1–9) were included in the world’s first clinical UTx trial and underwent LD UTx at Sahlgrenska University Hospital, Gothenburg, in 2012 and 2013. Detailed reports from this trial on outcomes after surgery and during the first 12 mo post-transplant have been published previously.12,13 Two participants (Nos. 2 and 9) lost their grafts within the first 4 mo because of intrauterine infection and arterial thrombosis, respectively, and were not included in the present study. Detailed data on the reproductive, obstetric, and long-term outcomes of born children have been published separately.14
Surgical Procedure
The surgical procedure has been previously described in detail.12 Renal circulation or ureteral structures were not compromised during any stage of the transplantation procedure or postoperative follow-up (FU) period. The median operative time and estimated blood loss were 4.8 h (4.2–5.9) and 700 mL (250–800), respectively. The median intraoperative volume of intravenous (iv) crystalloid infusion was 2800 (2500–4000) mL. A perioperative median arterial pressure of 71 (65–74) mm Hg was maintained to obtain a measured urinary output of 1–3 mL/kg/h. HE was performed in all participants at 3–6 mo after a successful single pregnancy or directly following delivery after the second pregnancy.15
Immunosuppression
Induction therapy consisted of 2 doses of iv antithymocyte globulin 2.5 mg/kg body weight and iv methylprednisolone 250 mg, the first dose of both before reperfusion and the second dose within 12 h post-transplant.
Maintenance immunosuppression consisted of oral prednisolone for 4 postoperative days and tacrolimus with 12-h trough levels targeted between 10 and 15 μg/L during the first month and 5 and 10 μg/L from the second month onward. Additionally, 1 g of mycophenolate mofetil (MMF) was administered orally preoperatively and twice daily thereafter. The MMF dosage was adjusted according to the MMF area under curve monitoring to maintain serum levels at 40–60 mg/L·h measured on postoperative day 4 and after 2 mo. Owing to the potentially teratogenic effects of MMF, it was scheduled to be discontinued after 6 mo, and tacrolimus monotherapy was planned thereafter as maintenance treatment.
The standard treatment for biopsy-proven acute rejections (BPARs) consisted of iv methylprednisolone (500 mg daily for 3 consecutive days), followed by 20 mg oral prednisone daily, with subsequent tapering to 5 mg daily over a 5-wk period. Additionally, the tacrolimus dose was adjusted to increase the 12-h trough levels to 8–10 μg/L until the resolution of rejection was confirmed by cervical biopsy.
Clinical FU
Post-transplant FU with regular clinical visits, laboratory tests, and protocol cervical biopsies were performed at weeks 1, 2, 3, 4, and monthly for up to 6 mo and every other month thereafter. The estimated glomerular filtration rates (eGFRs) and tacrolimus trough levels are reported at the following time points/intervals: UTx, month 3, months 10–12, embryo transfer (ET) leading to first pregnancy, pregnancy, HE, and the last FU. Twelve-hour trough levels of tacrolimus in whole blood were measured using an automated chemiluminescent immunoassay developed for use with the ARCHITECT system (Abbott Scandinavia AB). Serum creatinine levels were determined using a standardized colorimetric enzymatic assay.
Kidney Function
Kidney function assessment was based on eGFR measurements using serum creatinine according to the CKD Epidemiology Collaboration formula.16 eGFR values at the individual level during the time intervals stated above were calculated as medians (min, max) of all measurements. The eGFR values at the cohort level were calculated as medians (min, max).
The eGFR slope, defined as the change in eGFR over time, was calculated between different time intervals, and the annual eGFR slope was calculated between UTx and the last FU. Because the observed course of post-transplant kidney function was nonlinear, the following nonlinear regression model was used by our statistician to calculate the eGFR slope: in which at each post-transplant time point/interval random variables were independent and normally distributed with zero mean and constant variance. Parameters K, A, B, and C are estimated using nonlinear regression and govern how the mean eGFR changes with time post-transplant. The regression method employs the Levenberg-Marquardt algorithm to find a set of parameters that minimizes the squared error between the observed eGFR and the proposed eGFR according to the model above. Parameter K describes eGFR a long time after transplantation (the asymptotic eGFR-value as time tends to infinity) and parameter A is the permanent loss in kidney function; a person who regains kidney function as it was at baseline has A = 0. Parameters A, B, and C together govern how rapidly kidney function deteriorates; kidney function reaches its lowest point at days post-transplantation:
Albuminuria was checked by urine dipstick at UTx and ET, and by urine albumin/creatinine ratio in all participants throughout pregnancy and at later FU time points. AKI was defined using the AKI Network criteria17; stage I AKI was defined as an increase in serum creatinine ≥26.2 μmol/L or an increase in creatinine >150% over baseline within 48 h.
RESULTS
In total, 7 women who underwent a surgically successful LD UTx at Sahlgrenska University Hospital in Gothenburg between September 15, 2012, and March 17, 2013, were included. None of the participants had primary kidney disease, cardiovascular disease, diabetes mellitus, or arterial hypertension at UTx. Serum creatinine levels, eGFR, and urine dipstick analysis results were normal before UTx. The median age at the time of UTx was 28 (27–34) y. Six participants had AUFI because of congenital uterine agenesis, Mayer-Rokitansky-Küster-Hauser syndrome (MRKH), type 1 or 2, and 1 had undergone HE because of cervical cancer (No. 1). Three participants (Nos. 4, 5, and 8) had congenital unilateral renal agenesis (URA) and only 1 kidney, whereas, in another participant (No. 3), 1 of the 2 kidneys was ectopic and located in the pelvis (Table 1). The median total time between UTx and the last FU was 121 (119–126) mo in the cohort, the median time between UTx and HE/tacrolimus discontinuation was 52 (22–83) mo, and between HE/tacrolimus discontinuation and last FU was 74 (38–99) mo.
TABLE 1.
Demographic and clinical characteristics of the study cohort
| Patient number | No. 1 | No. 3a | No. 4b | No. 5b | No. 6 | No. 7 | No. 8b | Median (range) |
|---|---|---|---|---|---|---|---|---|
| Age at UTx (y) | 32 | 28 | 27 | 34 | 27 | 27 | 32 | 28 (27–34) |
| Diagnosis | CxCa | MRKH | MRKH | MRKH | MRKH | MRKH | MRKH | n.a. |
| URA | No | No | Yes | Yes | No | No | Yes | n.a. |
| Race | White | White | White | White | White | White | White | White |
| LD | Mother | Maternal aunt | Mother | Friend | Mother | Mother | Sister | n.a. |
| Pregnancies (n) | 2 | 6 | 2 | 1 | 2 | 1 | 1 | 2 |
| PE | No | n.a. | Yes | Yes | No | No | Yes | n.a. |
| Other complications during pregnancy | No | Miscarriage x 6 | No | No | No | Hydronephrosis | No | n.a. |
| Number of live births | 2 | 0 | 2 | 1 | 2 | 1 | 1 | 1 |
| Gestational week + day | 35 + 035 + 6 | n.a. | 35 + 335 + 6 | 31 + 6 | 37 + 138 + 0 | 34 + 4 | 31 + 6 | n.a. |
| Number of graft rejections | 1 | 3 | 0 | 1 | 2 | 3 | 1 | 1 |
| Time of rejection | W2 | M28, 29c,43 | n.a. | M16d | M42, 48d | W3, M4, 10 | W2 | n.a. |
| Maintenance immunosuppression at ET | Tac | Tac, aza, pred | Tac | Tac, aza, pred | Tac, aza, pred | Tac, aza, pred | Tac, aza, pred | n.a. |
| Maintenance immunosuppression at HE | Tac, pred | Tac, aza, pred | Tac, pred | Tac, aza, pred | Tac, aza, pred | Tac, aza, pred | Tac, aza, pred | n.a. |
| Hypertension at any time point after UTx | No | No | Yes, before delivery | Yes, before delivery | No | No | Yes, before delivery | n.a. |
| Urine dipstick at UTx | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | n.a. |
| UACR during pregnancy | 0.10.7 | 1.4 | 00.15 | 1.1 | 0.40.45 | 1.0 | 0.9 | n.a. |
| UACR at delivery (±4 d) if PE | n.a. | n.a. | 54 | 45.5 | n.a. | n.a. | 34.5 | n.a. |
| UACR after HE | 0.15 | 3.3 | 0.7 | 0.3 | 0.3 | 2.0 | 0 | n.a. |
| AKI within first 48 h post-UTx | No | No | No | No | No | No | No | n.a. |
aA patient with an ectopic kidney in the pelvis.
bPatients with URA.
cSteroid-resistant, thymoglobulin-treated rejection.
dRejection during pregnancy.
Patients No. 2 and No. 9 were not included in this study because of graft loss within 4 mo post-UTx.
AKI, acute kidney injury (AKIN definition17); aza, azathioprine; CxCa, cervical cancer; ET, embryo transfer; HE, hysterectomy; LD, living donor; MRKH, Mayer-Rokitansky-Küster-Hauser syndrome; n.a., not applicable; PE, preeclampsia; pred, prednisolone; Tac, tacrolimus; UACR, urine albumin/creatinine ratio (mmol/mol); URA, unilateral renal agenesis; UTx, uterus transplantation.
Immunosuppression and Rejections
Only 2 participants remained on the scheduled maintenance regimen with tacrolimus monotherapy after MMF withdrawal (6 mo after UTx). When clinical pregnancy was evident on ultrasound examination at around gestational weeks 7–8, 5 mg prednisolone was added to prevent under-immunosuppression because of possible descending trough levels of tacrolimus. In the other 5 participants, MMF was replaced by azathioprine after 6 mo together with prednisolone because of BPAR/repeated borderline changes in the uterine graft within the first 6 mo. A total of 11 BPARs were diagnosed in 6 of the 7 participants. All BPARs were treated with steroids. One rejection (in patient No. 3) was steroid-resistant and treated with iv thymoglobulin. Two of these BPARs occurred during pregnancies (Table 1).
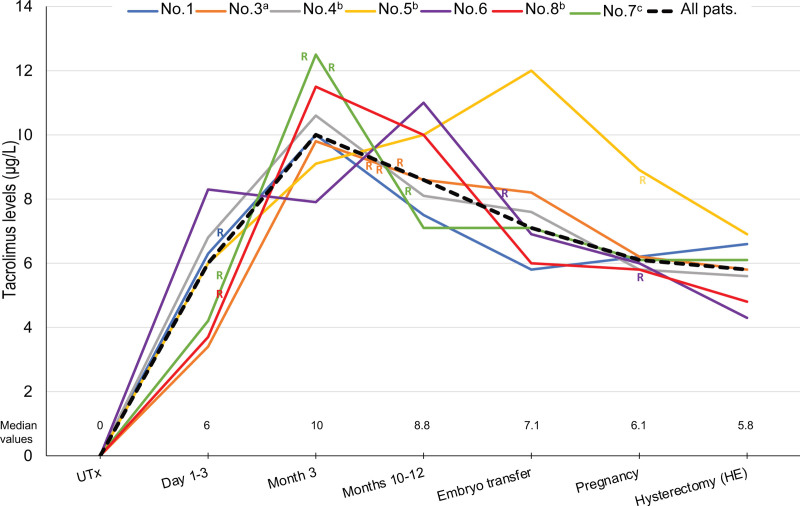
The median 12-h tacrolimus trough level was 6.0 (3.4–8.3) µg/L during days 1–3, 10.0 (7.9–12.5) µg/L during month 3 post-transplant, 8.6 (7.1–11) µg/L during months 10–12, 7.1 (5.8–12.0) µg/L at the time of ET leading to the first pregnancy, 6.1 (5.6–8.9) during first pregnancy, and 5.8 (4.3–6.9) µg/L at HE (Table 2, Figure 1). The association between the median tacrolimus trough levels and the median eGFR of the cohort is shown in Figure 2. The median exposure time of tacrolimus corresponding to the period from UTx to HE was 52 (22–83) mo; this wide range in time is attributed to the fact that participants became pregnant at different time intervals after UTx because of differences in the number of ETs required to achieve pregnancy as well as 3 participants who had 2 deliveries. The median time from UTx to the first pregnancy was 19 (12–45) mo. Three participants (Nos. 4, 1, and 6) with 2 pregnancies and live births had time spans between deliveries of 16.5, 26, and 30 mo, respectively. One participant (No. 3) had 6 miscarriages and no live births (Table 1).
TABLE 2.
Time of FU, eGFR, and tacrolimus trough levels after UTx
| Patient number | No. 1 | No. 3a | No. 4b | No. 5b | No. 6 | No. 7c | No. 8b | Median overall cohort (range) |
|---|---|---|---|---|---|---|---|---|
| Time: UTx−last FU | 126 | 125 | 125 | 121 | 121 | 120 | 119 | 121 (119–126) |
| UTx−HE (tacrolimus exposure) | 52 | 71 | 55 | 22 | 83 | 23 | 34 | 52 (22–83) |
| HE−last FU | 74 | 54 | 70 | 99 | 38 | 97 | 85 | 74 (38–99) |
| Tac. Level | ||||||||
| During D2–3 | 6.3 | 3.4 | 6.8 | 6.0 | 8.3 | 4.2 | 3.7 | 6 (3.4–8.3) |
| During M3 | 10.0 | 9.8 | 10.6 | 9.1 | 7.9 | 12.5 | 11.5 | 10 (7.9–12.5) |
| During M10–12 | 7.5 | 8.6 | 8.1 | 10.0 | 11.0 | 7.1 | 10.0 | 8.6 (7.1–11) |
| At ET | 5.8 | 8.2 | 7.6 | 12.0 | 6.9 | 7.1 | 6.0 | 7.1 (5.8–12) |
| During the first pregnancy | 6.2 | 6.2 | 5.8 | 8.9 | 6.0 | 6.1 | 5.6 | 6.1 (5.6–8.9) |
| At HE | 6.6 | 5.8 | 5.6 | 6.9 | 4.3 | 6.1 | 4.8 | 5.8 (4.3–6.9) |
| Serum creatinine at UTx (µg/L) | 64 | 65 | 84 | 83 | 65 | 62 | 85 | 65 (62–85) |
| eGFR | ||||||||
| At UTx | 113 | 114 | 85 | 82 | 115 | 121 | 80 | 113 (80–121) |
| During D1–3 after UTx | 109 | 124 | 101 | 80 | 105 | 123 | 84 | 105 (80–124) |
| During M3 | 85 | 80 | 71 | 74 | 103 | 65 | 66 | 74 (65–103) |
| During M10–12 | 74 | 79 | 71 | 63 | 94 | 65 | 67 | 71 (65–103) |
| At ET | 84 | 86 | 75 | 63 | 97 | 78 | 65 | 78 (63–97) |
| During the first pregnancy | 91 | 91 | 83 | 68 | 108 | 48 | 83 | 83 (48–108) |
| At HE | 80 | 70 | 83 | 69 | 117 | 60 | 76 | 76 (60–117) |
| At last FU | 93 | 88 | 80 | 83 | 100 | 76 | 80 | 83 (76–100) |
| Change in eGFR | ||||||||
| UTx−HE (%) | −33 (−29.2) | −44 (−38.6) | −2 (−2.4) | −13 (−15.9) | 2 (1.7) | −61 (−50.4) | −4 (−5) | −13 (−61 to 2)−15.9 |
| HE−last FU (%) | 13 (16.3) | 18 (25.7) | −3 (−3.6) | 14 (20.3) | −17 (−14.5) | 16 (26.7) | 4 (5.3) | 8 (−13 to 21)(16.3) |
| Change in eGFR: UTx−last FU (%) | −20 (−17.7) | −26 (−22.8) | −5 (−5.9) | 1 (1.2) | −15 (−13) | −45 (−37.2) | 0 | −15 (−45 to 1)(−9.5) |
aA patient with an ectopic kidney in the pelvis.
bPatients with URA.
cA patient with hydronephrosis during pregnancy.
Time in months, tacrolimus level in µ/L.
eGFR, estimated glomerular filtration rate in mL/min/1.73 m2; ET, embryo transfer; FU, follow-up; HE, hysterectomy; pred, prednisolone; Tac, tacrolimus; URA, unilateral renal agenesis; UTx, uterus transplantation.
FIGURE 1.
Tacrolimus levels in individual participants and median tacrolimus levels for the whole cohort. aPatient with an ectopic kidney in pelvis; bPatients with URA; cPatient with hydronephrosis during pregnancy. HE, hysterectomy; R, rejection; URA, unilateral renal agenesis; UTx, uterus transplantation.
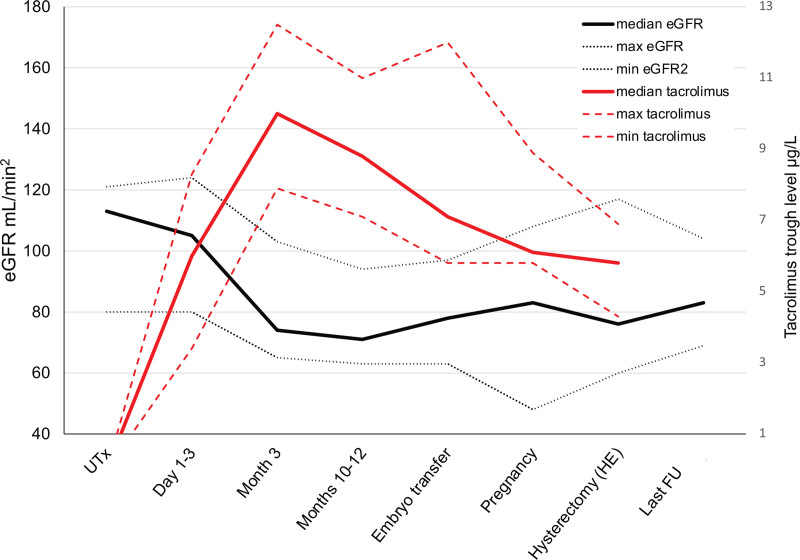
FIGURE 2.
Median eGFR (min, max) and median tacrolimus trough levels (min, max) of the whole cohort. eGFR, estimated glomerular filtration rate; FU, follow-up; HE, hysterectomy; UTx, uterus transplantation.
Clinical and eGFR Course
None of the participants developed post-transplantation diabetes mellitus after UTx. Three participants developed preeclampsia (PE) with transient hypertension and proteinuria before delivery (Table 1). None of the participants was treated with renin-angiotensin-aldosterone system blockers. One participant (No. 1) developed a serious postoperative infection requiring iv antibiotics.
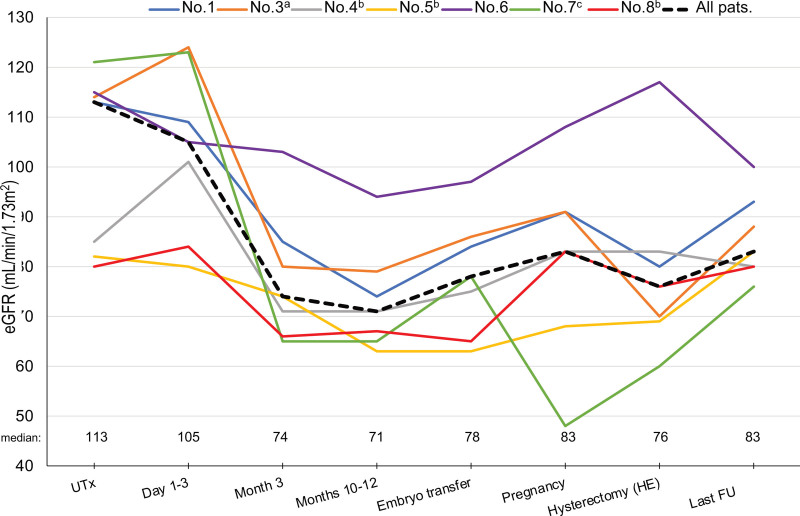
The individual and overall median eGFRs are presented in Table 2 and Figure 3. The overall median eGFR of the cohort at UTx was 113 (80–121) mL/min/1.73 m2. No signs of AKI were observed in any participant within the first 3 d after surgery. During month 3 post-UTx, the median eGFR of the whole cohort declined to 74 (65–103) mL/min/1.73 m2 and further to 71 (65–103) at months 10–12. Thereafter, eGFR levels remained stable throughout the pre-pregnancy period and pregnancy until HE/tacrolimus discontinuation, when the median eGFR was 76 (60–117) mL/min/1.73 m2. At the end of the FU, the median eGFR of the whole group was 83 (76–100) mL/min/1.73 m2.
FIGURE 3.
eGFR in individual participants and median eGFR for the whole cohort. aPatient with an ectopic kidney in pelvis; bPatient with unilateral kidney agenesis; cPatient with hydronephrosis during pregnancy. eGFR, estimated glomerular filtration rate; FU, follow-up; HE, hysterectomy; UTx, uterus transplantation.
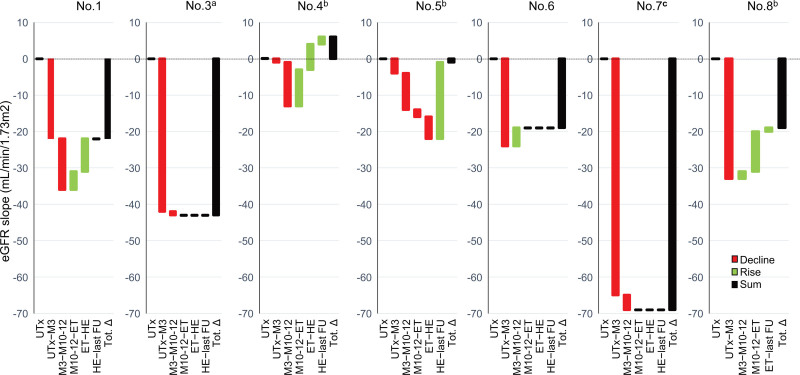
The eGFR slopes calculated using the nonlinear regression model described earlier are shown in Table 3 and Figure 4. Between UTx and month 3, all 7 participants had a decline in kidney function; the median eGFR slope for the whole cohort was −24 (−65 to −1) mL/min/1.73 m2, and it declined further by −4 (−14 to +5) mL/min/1.73 m2 between months 3 and 10–12. Thereafter, eGFR slopes improved in 3 participants (Nos. 1, 4, and ), remained stable in 3 (Nos. 3, 6, and 7), and worsened in 1 participant (No. 5) until HE/tacrolimus discontinuation. After HE/tacrolimus discontinuation, the eGFR slope improved in 2 participants (Nos. 4 and 5) and remained unchanged in the other 5 until the end of FU.
TABLE 3.
eGFR slope, annual eGFR slope/y between different time points/intervals
| Patient number | No. 1 | No. 3a | No. 4b | No. 5b | No. 6 | No. 7c | No. 8b | Median overall cohort (range) |
|---|---|---|---|---|---|---|---|---|
| eGFR slope | ||||||||
| UTx−M3 | −22 | −42 | −1 | −4 | −24 | −65 | −33 | −24 (−65 to −1) |
| M3−M10–12 | −14 | −1 | −12 | −10 | +5 | −4 | +2 | −4 (−14 to 5) |
| M10–12−ET | +5 | 0 | +10 | −2 | 0 | 0 | +11 | 0 (−2 to 11) |
| ET−HE | +9 | 0 | +7 | −6 | 0 | 0 | +1 | 0 (−6 to 1) |
| HE−last FU | 0 | 0 | +2 | +21 | 0 | 0 | 0 | 0 (0–21) |
| UTx−HE | −22 | −43 | +4 | −22 | −19 | −69 | −19 | −22 (−69 to 4) |
| UTx−last FU | −22 | −43 | +6 | −1 | −19 | −69 | −19 | −22 (−69 to 4) |
| Annual eGFR slope: UTx− HE | −5.1 | −7.3 | +0.9 | −11.6 | −2.8 | −36.3 | −6.8 | −22 (−69 to 4) |
| Annual eGFR slope: UTx−last FU | −2.1 | −4.1 | +0.6 | −0.1 | −1.9 | −6.8 | −1.9 | −1.9 (−6.8 to 0.6) |
aA patient with an ectopic kidney in the pelvis.
bPatients with unilateral renal agenesis.
cA patient with hydronephrosis during pregnancy.
eGFR slope calculated with a nonlinear regression model. The annual eGFR slope in mL/min/1.73 m2/y.
eGFR, estimated glomerular filtration rate in mL/min/1.73 m2; ET, embryo transfer; FU, follow-up; HE, hysterectomy; UTx, uterus transplantation.
FIGURE 4.
eGFR slopes calculated with the nonlinear regression model in individual patients between time periods of FU. aPatient with an ectopic kidney in pelvis; bPatients with unilateral renal agenesis; cPatient with hydronephrosis during pregnancy. eGFR, estimated glomerular filtration rate; ET, embryo transfer; FU, follow-up; HE, hysterectomy; M, month; Tot.∆, eGFR slope between UTx and last FU; UTx, uterus transplantation.
According to the model, between UTx and the last FU, 5 of the 7 participants (Nos. 1, 3, 6, 7, 8) had a decline of kidney function with an annual eGFR slope <−1 mL/min/1.73 m2/y (ie, a decline >1 mL/min/1.73 m2/y). The median eGFR slope was −22 mL/min/1.73 m2 for the whole group, representing a median annual eGFR slope of −1.9 mL/min/1.73 m2/y. However, the actual eGFR in participant No. 8 at the last FU was the same as that at the UTx and thus had stable kidney function. In this case, we observed a discrepancy between the eGFR slope calculated using the nonlinear model and the actual difference in the eGFR.
The greatest decline in eGFR, −65 mL/min/1.73 m2, was observed in participant No. 7 from UTx to month 3, despite having the highest eGFR at baseline in the cohort and 2 kidneys. Moreover, she developed hydronephrosis from the second trimester onward until delivery. A kidney biopsy performed perioperatively during HE did not reveal any pathological findings. She was strongly advised to avoid a second pregnancy and HE was performed 3 mo after childbirth. Although partial improvement occurred after HE/tacrolimus discontinuation, more than one-third of her kidney function was lost from UTx until the last FU (120 mo) with an annual eGFR slope calculated as −6.8 mL/min/1.73 m2/y.
Participant No. 3, with 1 normal kidney and 1 ectopic kidney, exhibited the second greatest decline in eGFR from UTx to month 3 (−42 mL/min/1.73 m2) as well as to the last FU (−43 mL/min/1.73 m2 representing an annual slope of −4.1 mL/min/1.73 m2). This participant had the second longest tacrolimus exposure (71 mo), and her clinical course was complicated by steroid-resistant rejection, 6 miscarriages, and no live births.
URA and PE
Three participants (Nos. 4, 5, and 8) in the present cohort had URA and eGFR at UTx of 85, 82, and 80 mL/min/1.73 m2, respectively. All 3 participants developed PE with hypertension, thrombocytopenia, and proteinuria, which caused acute delivery by cesarean section somewhat earlier than originally planned. Participant No. 4 had a second pregnancy with a live birth but did not develop PE. One participant (No. 4) improved, 1 (No. 5) completely recovered, and 1 (No. 8) had mild worsening of her kidney function from UTx to the last FU, with an annual eGFR slope +0.6, −0.1, and −1. 9 mL/min/1.73 m2/y, resp, when calculated using the model. However, the last participant (No. 8) had the same actual eGFR at UTx and at the end of FU. None of the 3 participants had persistent proteinuria or hypertension after delivery.
DISCUSSION
UTx is exclusive among all organ transplants because it is a temporary transplantation with graft removal after a few years, and CNI exposure is not lifelong in these participants.
In this first UTx cohort, we found a considerable decline in kidney function in all participants during the first 3 mo post-UTx. This early decline was coupled with a return to the baseline value after total discontinuation of tacrolimus in only 2 participants, whereas continued eGFR decline was observed in the remaining 5 participants at the last FU, despite transplantectomy with discontinued immunosuppression for more than 1 y and even beyond the last observation. Our finding of early eGFR decline is consistent with observations from a recent multicenter study in UTx participants.18 However, in this study by Sawinski et al,18 no significant decline in eGFR from baseline was observed in a subgroup of 4 participants who reached a 1-y FU after delivery/HE, in contrast to the decline observed in our cohort at this time point and beyond. The target tacrolimus levels in their study, as described in their methods, were in general between 6 and 10 ng/mL. It should be emphasized here that in the above-mentioned study, the number of participants in the subgroup analysis was smaller, the FU was much shorter, and the proportion of participants with renal malformations was markedly lower at 6% compared with 60% in the present study.
The use of CNI may play a major role in CKD development in NRSOT recipients. It is well established that CNI, in general, are associated with both acute and chronic nephrotoxicity.19–22 Acute nephrotoxic effects of CNI are considered reversible and dose-dependent and may be mediated by afferent arteriolar vasoconstriction, resulting in decreased kidney blood flow and, consequently lower GFR.19 In contrast, chronic CNI nephrotoxicity may be associated with glomerular changes such as afferent arteriolar hyalinosis, tubular atrophy, and interstitial fibrosis and may, therefore, have a lesser degree of reversibility.20–22 The critical exposure time allowing the recovery of affected kidney function has not yet been established.
In addition to CNI, other risk factors have been identified that can adversely affect kidney function after NRSOT.4–6 However, the absence of chronic liver, cardiopulmonary, or other comorbidities in our participants makes the possibility of concomitant primary or secondary glomerular diseases highly unlikely, especially considering the absence of proteinuria or other potential risk factors for kidney disease, such as hypertension or diabetes, at baseline and during FU. Additionally, there were no evident factors during surgery such as ureteral injury or hemodynamic instability, which may have caused injury to the kidneys. The possibility of ischemia-reperfusion injury as the underlying cause of subsequent decline in eGFR also seems unlikely because none of the participants experienced any AKI during the first 3 d post-UTx.
In our cohort, the median tacrolimus trough level in the early period after UTx was approximately 10 µg/L and was maintained at approximately 8 µg/L throughout the rest of the first post-transplant year. This was due to frequent histological findings of borderline changes and rejection episodes, together with simultaneous efforts to phase out MMF before the planned pregnancies. We speculate that our finding of an early pronounced decline in the young and overall healthy cohort may be related to high tacrolimus exposure during this period, and we expected this early decline to be transient. However, we did not observe any substantial improvement in eGFR even after tacrolimus was tapered to maintain lower trough levels of approximately 5–6 µg/L after the first year post-UTx, when ETs were initiated. Although a partial improvement in eGFR slope occurred after HE/tacrolimus discontinuation, a persistent decline in eGFR slope was observed in the majority of participants between baseline and the end of FU. Therefore, in our opinion, relatively high exposure to tacrolimus during the early period may have been an important risk factor for a decline in kidney function, even in the long term. Moreover, an increase in the circulating tacrolimus-free fraction because of altered pharmacokinetics during pregnancy (likely because of decreases in albumin concentration and hematocrit) may have also contributed to nephrotoxicity in these participants.23 Based on these findings, we have chosen in the subsequent study on robotic-assisted UTx, a dual immunosuppressive protocol with minimized tacrolimus and azathioprine.24
Of note, 3 participants in this cohort had URA, which is the most common upper urinary tract malformation in participants with MRKH.25–27 This malformation may be associated with an increased risk of developing proteinuria, hypertension, and CKD.28–30 The absence of 1 kidney increases the rate of PE about 3- to 4-fold in pregnancy, as shown in MRKH patients31 and kidney donors.32 Additionally, immunosuppression as such may contribute to an increased risk of PE in transplant patients.33 Hypertensive disorders of pregnancy including PE have also been described to be associated with a 2-fold increased risk of CKD in these patients.34 Because all our participants with URA had eGFR at lower normal range at baseline and PE during pregnancy, we expected them to have worse kidney function at the last FU as compared with the participants with 2 kidneys. Nevertheless, all 3 recovered their kidney function. Studies have shown that eGFR decline is strongly related to baseline eGFR, with a faster decline at a higher baseline eGFR and a slower decline at a lower baseline eGFR.35,36 The mechanism underlying these observations is not completely understood, but glomerular hyperfiltration has been suggested to play a role.
Natural age–related GFR decline over time is usually observed after 35 y of age in the general Caucasian population.37,38 Therefore, kidney function in healthy female recipients age 27–34 y at the UTx should not be affected by natural age–related changes. However, the possible influence of the physiological and age-related decrease in GFR over time should be taken into consideration because the study FU extended over more than 5 y despite the young age of the participants at the outset. The estimated physiological decrease ranging from 0.4 to 0.8 mL/min/y/1.73 m2 for this age group38–40 may be expected in 3 participants after they passed the age of 35 y during the FU but does not explain the magnitude of decline observed in these participants.
The findings of this study addressing the course of kidney function after UTx in humans are important because an annual eGFR slope of ≤−5 mL/min/1.73 m2/y in a normal healthy population has been found to be an independent risk factor for all-cause mortality41 and ≤−1 mL/min/1.73 m2 associated with an increased risk of kidney failure.42 Furthermore, progression to CKD may also increase the risk of morbidity and mortality as well as potentially detrimental effects on pregnancy outcomes,10,11 which is of significance in the context of UTx in which successful transplantation is defined as the birth of a healthy child with no harm to the mother’s health. Women with CKD may also develop a further decline in kidney function during pregnancy.43,44 Therefore, close surveillance of kidney function and CNI levels coupled with prompt interventions such as CNI minimization, especially in the early period by combining with azathioprine, reducing the interval from UTx to first ET, and avoiding a second pregnancy in participants showing a significant decline in their kidney function, are required to reduce the risk of CKD and, therewith, adverse maternal and fetal outcomes after UTx.
Our study has several strengths. This study is the first to investigate the longitudinal course of kidney function over a median period of approximately 8 y after innovative UTx. It is prospective in nature, with long-term FU, and a well-characterized population. Furthermore, this study provides a unique opportunity to study the course of kidney function after transplantectomy and total discontinuation of immunosuppression, specifically tacrolimus.
This study has some limitations. First and foremost is the small size of the study cohort, in which the number of participants was limited by ethical approval for this study. However, most ongoing trials are of similar size. Second, the immunosuppressive protocol was not completely uniform across the cohort, although the general maintenance immunosuppressive therapy was similar. Third, kidney biopsy was available for only 1 participant at 1 time point. Fourth, kidney function assessment by mGFR was not performed at baseline, only after the first pregnancy, which did not allow comparison with baseline. Fifth, we report eGFR measurement calculated using the CDK Epidemiology Collaboration formula at all time points and intervals, even during pregnancy, although this method is not sufficiently reliable to estimate kidney function in pregnant women. Other limitations include the lack of availability of proteinuria data at the UTx and ET, assessed only by dipstick and not by urine albumin/creatinine ratio. Last but not least, the findings are from a single center comprising only a Caucasian population and cannot be generalized to other populations or ethnicities.
In conclusion, this report of the first UTx cohort clearly identified an early decline in eGFR followed by a persistent long-term decrease in the majority of recipients. Thus, even this unique transplantation model, despite transplantectomy and the absence of lifelong immunosuppression in a young and healthy population, may increase the risk of CKD with detrimental effects on long-term outcomes, similar to other NRSOT. These results highlight the importance of close surveillance of kidney function coupled with minimization of tacrolimus in terms of both levels and total exposure time. Further prospective studies with larger numbers and longer FU comparing differing CNI exposures are essential to determine the risk factors and outcomes of eGFR decline in this vulnerable population.
ACKNOWLEDGMENTS
We are very grateful to all the women who participated in our study and to the dedicated colleagues and nurses who collaborated in the FU of the participants.
Footnotes
The authors declare no funding or conflicts of interest.
J.E. participated in the research design, research performance, data interpretation, and manuscript writing. M.H. participated in the research performance and manuscript writing. Å.N. participated in manuscript writing. M.B. participated in the research design and manuscript writing. G.H. participated in the research design, data interpretation, and manuscript writing. S.B.-A. participated in the research design, data interpretation, and manuscript writing.
REFERENCES
- 1.Nair A, Stega J, Smith JR, et al. Uterus transplant: evidence and ethics. Ann N Y Acad Sci. 2008;1127:83–91. [DOI] [PubMed] [Google Scholar]
- 2.Brannstrom M, Johannesson L, Bokstrom H, et al. Livebirth after uterus transplantation. Lancet. 2015;385:607–616. [DOI] [PubMed] [Google Scholar]
- 3.Brännström M, Belfort MA, Ayoubi JM. Uterus transplantation worldwide: clinical activities and outcomes. Curr Opin Organ Transplant. 2021;26:616–626. [DOI] [PubMed] [Google Scholar]
- 4.Herlenius G, Fistouris J, Olausson M, et al. Early renal function post-liver transplantation is predictive of progressive chronic kidney disease. Scand J Gastroenterol. 2008;43:344–349. [DOI] [PubMed] [Google Scholar]
- 5.Herlenius G, Fägerlind M, Krantz M, et al. Chronic kidney disease--a common and serious complication after intestinal transplantation. Transplantation. 2008;86:108–113. [DOI] [PubMed] [Google Scholar]
- 6.van Gelder T, Balk AH, Zietse R, et al. Renal insufficiency after heart transplantation: a case-control study. Nephrol Dial Transplant. 1998;13:2322–2326. [DOI] [PubMed] [Google Scholar]
- 7.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Akalin E, Dikman S, et al. The variable pathology of kidney disease after liver transplantation. Transplantation. 2010;89:215–221. [DOI] [PubMed] [Google Scholar]
- 9.Kubal C, Cockwell P, Gunson B, et al. Chronic kidney disease after nonrenal solid organ transplantation: a histological assessment and utility of chronic allograft damage index scoring. Transplantation. 2012;93:406–411. [DOI] [PubMed] [Google Scholar]
- 10.Fischer MJ, Lehnerz SD, Hebert JR, et al. Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis. 2004;43:415–423. [DOI] [PubMed] [Google Scholar]
- 11.Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brännström M, Johannesson L, Dahm-Kähler P, et al. First clinical uterus transplantation trial: a six-month report. Fertil Steril. 2014;101:1228–1236. [DOI] [PubMed] [Google Scholar]
- 13.Johannesson L, Kvarnström N, Mölne J, et al. Uterus transplantation trial: 1-year outcome. Fertil Steril. 2015;103:199–204. [DOI] [PubMed] [Google Scholar]
- 14.Brännström M, Dahm-Kähler P, Kvarnström N, et al. Reproductive, obstetric, and long-term health outcome after uterus transplantation: results of the first clinical trial. Fertil Steril. 2022;118:576–585. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson CC, Dahm-Kähler P, Kvarnström N, et al. Hysterectomy after uterus transplantation and detailed analyses of graft failures. Acta Obstet Gynecol Scand. 2022;101:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63:820–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawinski D, Johannesson L, Kristek J, et al. A multi-institutional study of renal outcomes and renal-related pregnancy outcomes in uterus transplant recipients. Am J Transplant. 2022;12:3101–3110. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama I, Hayakawa A, Kobayashi T, et al. Endothelin-1 and in vitro evidence of renal artery vasoconstriction after liver transplantation. Res Exp Med (Berl). 1995;195:381–387. [DOI] [PubMed] [Google Scholar]
- 20.Myers BD, Ross J, Newton L, et al. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699–705. [DOI] [PubMed] [Google Scholar]
- 21.Laine J, Krogerus L, Fyhrquist F, et al. Renal function and histopathologic changes in children after liver transplantation. J Pediatr. 1994;125(6 Pt 1):863–869. [DOI] [PubMed] [Google Scholar]
- 22.Campistol JM, Sacks SH. Mechanisms of nephrotoxicity. Transplantation. 2000;69(12 Suppl):SS5–S10. [DOI] [PubMed] [Google Scholar]
- 23.Zheng S, Easterling TR, Umans JG, et al. Pharmacokinetics of tacrolimus during pregnancy. Ther Drug Monit. 2012;34:660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brännström M, Dahm-Kähler P, Ekberg J, et al. Outcome of recipient surgery and 6-month follow-up of the Swedish live donor robotic uterus transplantation trial. J Clin Med. 2020;9:2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oppelt P, Renner SP, Kellermann A, et al. Clinical aspects of Mayer-Rokitansky-Kuester-Hauser syndrome: recommendations for clinical diagnosis and staging. Hum Reprod. 2006;21:792–797. [DOI] [PubMed] [Google Scholar]
- 26.Strübbe EH, Willemsen WN, Lemmens JA, et al. Mayer-Rokitansky-Küster-Hauser syndrome: distinction between two forms based on excretory urographic, sonographic, and laparoscopic findings. AJR Am J Roentgenol. 1993;160:331–334. [DOI] [PubMed] [Google Scholar]
- 27.Herlin MK, Petersen MB, Brännström M. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome: a comprehensive update. Orphanet J Rare Dis. 2020;15:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argueso LR, Ritchey ML, Boyle ET, Jr, et al. Prognosis of patients with unilateral renal agenesis. Pediatr Nephrol. 1992;6:412–416. [DOI] [PubMed] [Google Scholar]
- 29.Wikstad I, Celsi G, Larsson L, et al. Kidney function in adults born with unilateral renal agenesis or nephrectomized in childhood. Pediatr Nephrol. 1988;2:177–182. [DOI] [PubMed] [Google Scholar]
- 30.Hegde S, Coulthard MG. Renal agenesis and unilateral nephrectomy: what are the risks of living with a single kidney? Pediatr Nephrol. 2009;24:439–446. [DOI] [PubMed] [Google Scholar]
- 31.Heinonen PK. Gestational hypertension and preeclampsia associated with unilateral renal agenesis in women with uterine malformations. Eur J Obstet Gynecol Reprod Biol. 2004;114:39–43. [DOI] [PubMed] [Google Scholar]
- 32.Garg AX, Nevis IF, McArthur E, et al. ; DONOR Network. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKay DB, Josephson MA. Pregnancy in recipients of solid organs--effects on mother and child. N Engl J Med. 2006;354:1281–1293. [DOI] [PubMed] [Google Scholar]
- 34.Ayansina D, Black C, Hall SJ, et al. Long term effects of gestational hypertension and pre-eclampsia on kidney function: record linkage study. Pregnancy Hypertens. 2016;6:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baba M, Shimbo T, Horio M, et al. Longitudinal study of the decline in renal function in healthy subjects. PLoS One. 2015;10:e0129036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melsom T, Nair V, Schei J, et al. Correlation between baseline GFR and subsequent change in GFR in Norwegian adults without diabetes and in Pima Indians. Am J Kidney Dis. 2019;73:777–785. [DOI] [PubMed] [Google Scholar]
- 37.Peralta CA, Vittinghoff E, Bansal N, et al. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis. 2013;62:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenton A, Montgomery E, Nightingale P, et al. Glomerular filtration rate: new age- and gender-specific reference ranges and thresholds for living kidney donation. BMC Nephrol. 2018;19:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wetzels JF, Willems HL, den Heijer M. Age- and gender-specific reference values of estimated glomerular filtration rate in a Caucasian population: results of the Nijmegen Biomedical Study. Kidney Int. 2008;73:657–658. [DOI] [PubMed] [Google Scholar]
- 40.Cohen E, Nardi Y, Krause I, et al. A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol. 2014;27:635–641. [DOI] [PubMed] [Google Scholar]
- 41.Turin TC, Coresh J, Tonelli M, et al. Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney Int. 2013;83:684–691. [DOI] [PubMed] [Google Scholar]
- 42.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 43.Fischer MJ. Chronic kidney disease and pregnancy: maternal and fetal outcomes. Adv Chronic Kidney Dis. 2007;14:132–145. [DOI] [PubMed] [Google Scholar]
- 44.Ramin SM, Vidaeff AC, Yeomans ER, et al. Chronic renal disease in pregnancy. Obstet Gynecol. 2006;108:1531–1539. [DOI] [PubMed] [Google Scholar]