Abstract
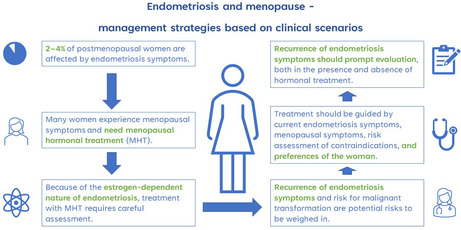
Endometriosis is largely considered a premenopausal disease with symptoms often improving during menopausal transition. However, 2%–4% of postmenopausal women are affected by endometriosis symptoms. At the same time, many peri‐ and postmenopausal women experience menopausal symptoms and inquire about treatment. Because of the estrogen‐dependent nature of endometriosis, treatment with menopausal hormone therapy requires careful assessment of the patient but should nevertheless be considered. Recurrence of endometriosis symptoms and risk for malignant transformation are potential risks to weigh when prescribing menopausal hormonal therapy. Choice of treatment should be guided by the presence and severity of current endometriosis symptoms, nature of menopausal symptoms, risk assessment of potential contraindications for treatment in patient history, and preferences of the woman after an informative discussion. Recurrence of endometriosis symptoms in a postmenopausal patient should always prompt rigorous evaluation, both in the presence and absence of hormonal treatment. Many recommendations on the topic are based on expert opinion and new studies are urgently needed to obtain evidence for optimal patient care.
Keywords: endometriosis, menopausal hormone therapy, menopausal symptoms, menopause, perimenopause
Women with menopausal symptoms and a history of endometriosis need careful evaluation and informed consent when menopausal hormone therapy is considered. Symptoms of postmenopausal endometriosis always require further investigation.
Abbreviations
- BSOE
bilateral salpingo‐oophorectomy
- MHT
menopausal hormone therapy
- POI
premature ovarian insufficiency
1. INTRODUCTION
Endometriosis is a chronic disease classically characterized by dysmenorrhea, painful intercourse and infertility. 1 The exact pathogenesis is unknown, but the hallmark of the disease is chronic inflammation caused by the presence of estrogen‐dependent endometrial‐like tissue in locations other than the uterine lining. 2 Although lesions have been found in many anatomical areas, the pelvis is the most affected location. Because of the estrogen‐dependent nature of the disease, menopausal transition usually alleviates or eliminates symptoms of endometriosis making it mostly a premenopausal condition. 3 Nevertheless, 2%–4% of postmenopausal women are affected by endometriosis. 4 Whether it is possible to develop postmenopausal endometriosis de novo is unclear because of the possibility of asymptomatic premenopausal disease. Endometriosis may have a deceptive clinical presentation where the severity of symptoms not always correlates with the extent of the disease on surgical exploration. 5
The average age for menopause is 51 years but individual variation is wide. 6 The cessation of menstruations and the low estrogen levels it implies often entail considerable symptoms, such as sweating, hot flushes, sleep disturbances, mood changes and joint pain. 7 Approximately 75% of all women experience at least some degree of vasomotor symptoms. Menopausal hormone therapy (MHT) is the most effective treatment for menopausal symptoms. MHT consists of estradiol alone or in combination with progestins. The progestin component protects the endometrial lining of the uterus from hyperplasia and is therefore recommended to all women with a uterus. Alternatively, tibolone, a synthetic steroid with estrogenic, progestogenic and androgenic effects can be prescribed. MHT is available in many administration routes, formulations, and doses to improve user satisfaction and suit patients with different risk profiles. 6 The decision about whether to offer MHT to a specific patient should be individualized and consider the expected risk–benefit balance.
When prescribing MHT for women with a history of endometriosis, clinicians must acknowledge the potential risk of increasing or recurring symptoms and even the theoretical risk for malignant transformation of endometriotic lesions. The most common anatomic location for postmenopausal endometriosis appears to be the ovaries. 8 Malignant transformation is the progression of benign endometriotic lesions to cancer and is a rare but possible complication to consider in postmenopausal endometriosis. The rate of postmenopausal recurrence of endometriosis in patients with a clinically moderate to severe disease is approximately 4%. 9 Recurrence may appear after several decades and be affected by the use and type of MHT. 9
Specific guidelines on how to manage endometriosis in peri‐ and postmenopausal women are based on scarce data consisting mainly of case reports, retrospective studies and women with surgical menopause. 10 Here, we present five hypothetical clinical scenarios to discuss the challenges faced by clinicians managing patients with endometriosis in the peri‐ and postmenopause.
2. CLINICAL SCENARIOS
2.1. Scenario 1: Prescribing MHT to a hysterectomized woman with endometriosis
A 50‐year‐old woman who has previously undergone a hysterectomy without bilateral salpingo‐oophorectomy (BSOE) for a benign indication (menorrhagia caused by numerous submucosal and intramural leiomyomas) is experiencing debilitating vasomotor symptoms and vaginal dryness. She has a history of endometriosis with dysmenorrhea as the main symptom.
All peri‐and postmenopausal women with bothersome vasomotor symptoms that interfere with everyday life should be offered MHT after careful consideration of the risk–benefit balance. There are several administration routes and regimes to choose from that enable finding the optimal treatment for a specific patient. Current literature and guidelines do not recommend against MHT for symptomatic peri‐ and postmenopausal women with a history of endometriosis. 10 , 11 However, as with all other patient groups, an informed decision by the patient requires discussing the current state of knowledge about the possible risks and benefits.
Regarding the choice of MHT, there are several aspects to be considered. Data about the effect of different combinations of MHT on symptoms of endometriosis and malignant transformation are sparse. Current recommendations support the use of combined estrogen‐progestin regimes or tibolone, a synthetic steroid exerting progestogenic effect on the endometrium, also in hysterectomized women based on small retrospective observational studies and case reports. 10 , 11 There are no large studies comparing the effect of sequential and continuous progestin regimes on recurrence of endometriosis or malignant transformation. The European Society of Human Reproduction and Embryology (ESHRE) guidelines suggest considering MHT with continuous as opposed to sequential progestin administration in women with endometriosis. 11
Tanmahasamut et al. recently published a paper describing the use of MHT in young women after definitive surgery for endometriosis. Definitive surgery includes both hysterectomy and BSOE. They found no significant association between the use of MHT and the recurrence of endometriosis or malignant transformation, regardless of the type of hormone substitution used. The patients had received estrogen only, combined estrogen and progesterone (either continuously or sequentially) or tibolone for a median of 66 months. 3 However, in the absence of larger randomized studies, common knowledge about estrogen sensitivity of endometriosis makes MHT with continuous progestin administration a reasonable suggestion, but the lack of strong evidence should be kept in mind.
Whereas MHT containing continuous progestin might be appropriate to reduce endometriosis‐associated risks, the potential for increased breast cancer risk must be considered when choosing a suitable therapy. Combined MHT containing both progestins and estradiol has been associated with higher breast cancer risk compared to MHT containing estrogen alone. 12 Furthermore, synthetic progestins and continuous as opposed to sequential administration of progestins have been shown to entail greater breast cancer risk. 13 Therefore, the risk of endometriosis recurrence and malignant transformation must be balanced against the increased risk of breast cancer.
In this specific scenario of a hysterectomized woman with a history of endometriosis requiring MHT, it is recommended to prescribe estrogen in a continuous regimen with progestins, or tibolone. However, shared decision‐making with the patient, considering different aspects of the treatment, including the increased breast cancer risk, is appropriate. The lowest effective estrogen dose and shortest therapy duration should be targeted. It is not known whether transdermal or oral administration of estrogen affects the risk for endometriosis symptoms, and therefore other considerations than endometriosis should decide whether oral or transdermal estrogen is offered. Although studies have shown suspected signs of hypercoagulability in patients with endometriosis, there is no data to suggest it increasing the risk of deep vein thrombosis. 14 , 15 , 16 Thus, although transdermal estrogen is associated with lower or no increased risk of thrombosis compared to the oral route, there are no studies to suggest that transdermal route should be recommended to endometriosis patients based on their diagnosis only. 17
2.2. Scenario 2: Management of surgical premature ovarian insufficiency (POI) in a woman with endometriosis
A 35‐year‐old woman is scheduled for risk‐reducing BSOE because of positive BRCA1 mutation. She has a previous history of suspected endometriosis responding moderately to treatment with 52 mg levonorgestrel intrauterine device. During surgery pelvic peritoneal endometriotic lesions are found. A biopsy from one lesion is taken; however, no excision of other lesions is performed.
POI is defined as spontaneous or surgical menopause before 40 years of age. Like women who cease to menstruate after the age of 45, many women with POI experience menopausal symptoms requiring treatment, such as sweating, hot flashes and sleep problems. The symptoms tend to be more severe and acute after surgical intervention compared to natural menopause, possibly due to the sudden decline in hormone production. 3 Estrogen deficiency, characteristic of POI, increases the long‐term risk of cardiovascular disease, decreased bone mineral density, dementia and sexual dysfunction. 18 Because of this, MHT is recommended at least up to the age of natural menopause (approximately 50 years), regardless of menopausal symptoms. 19
The diagnosis of POI seems to be associated with decreased risk of breast cancer. 20 When discussing breast cancer risk and MHT use in patients with POI, it should be stressed that MHT use until the age of natural menopause does not increase their risk of breast cancer compared to healthy age‐matched controls. 19 , 21
A recent systemic review and meta‐analysis showed a very small positive association between endometriosis and breast cancer. 22 The patient discussed in scenario 2 had tested positive for BRCA1 mutation which indicates a breast cancer risk of 72% and ovarian cancer risk of 44% by the age of 80. Risk‐reducing BSOE is recommended to these women between 35–40 years of age or after completed childbearing. 21 According to available literature, MHT can be recommended in this patient group after risk reducing BSOE until the age of natural menopause. 23 , 24
The negative effect of BSOE on the quality of life of young women with endometriosis despite improved pain is an important aspect to consider. 25 A study by Mu et al. 26 showed that endometriosis is generally associated with an increased risk of coronary heart disease and this association was only partially dependent on previous hysterectomy and/or BSOE. Both hysterectomy alone, and with BSOE before the age of 50 have been associated with an increased risk of coronary heart disease due to the potential effect of surgery on the production of ovarian hormones. 26 , 27 , 28 This suggests that for women with concomitant endometriosis and POI, MHT might be particularly important for reducing the risk of cardiovascular disease. Treatment should not be denied based solely on the presence of endometriosis in patient history in the fear of recurring symptoms.
POI is a known risk factor for decreased bone mineral density. In 1992, Barbieri published an article about estrogen threshold hypothesis which suggests that because different tissues have various sensitivity to estradiol, a circulating estradiol concentration of 30–45 pg/mL (110–165 pmoL/L) might be sufficient to prevent bone loss while not aggravating endometriosis symptoms. 29 There are no relevant studies on young women with POI and endometriosis that evaluate suitable evidence‐based MHT doses in this patient group. Estrogen substitution with at least 2 mg oral 17β‐estradiol or 100 μg transdermal estradiol to achieve serum levels of 50–100 pg/mL (180–370 pmoL/L) is usually recommended to young women with POI to substitute physiological levels of estrogen. 19 , 30 There are no studies to evaluate the potential for aggravating endometriosis‐related risks that this suggestion might imply.
Young women with endometriosis and POI should be carefully counseled about the necessity of estrogen substitution. As for this patient, addition of oral or transdermal estrogen in the presence of hormonal intrauterine device would be a good alternative. There are no studies comparing the effect of hormonal intrauterine device, sequential and continuous progestins on the recurrence or worsening of endometriosis symptoms or malignant transformation. 19 , 30 Furthermore, knowledge is lacking about the use of combined oral contraceptives in order to reduce the risk of recurring symptoms in this patient group. In summary, the counseling should include the risk–benefit estimation weighing the unclear risk of persistent or increasing endometriosis symptoms in the presence of MHT against the risk of negative long term health consequences without MHT.
2.3. Scenario 3: Prescribing MHT to a perimenopausal woman with endometriosis who is still menstruating
A 47‐year‐old woman with endometriosis and vasomotor symptoms, inquires about possible treatment options for her menopausal symptoms. The patient was shown to have peritoneal lesions and bilateral endometriomas on diagnostic laparoscopy 10 years ago; however, she only experienced mild cyclic pelvic pain which subsided in her forties. She is still menstruating, albeit somewhat irregularly. She currently has no hormonal treatment for endometriosis.
At present, there is no published data about the management of menopausal symptoms in women with known endometriosis undergoing normal menopausal transition. All published data is based on women with presumably more severe endometriosis in need of surgical intervention including oophorectomy due to failed symptom relief with medical treatment. Therefore, no evidence‐based recommendations can be made for women with endometriosis suffering from symptoms in association with perimenopause. However, it is reasonable to believe that a balanced discussion with the patient leads to an informed decision based on individual needs.
The individualized recommendation should consider the need for contraception, type of menopausal symptoms (vasomotor symptoms, irregular bleeding, mood disorders), activity of endometriosis‐related symptoms and patient preferences. As for women without endometriosis, MHT should be offered in the presence of bothersome menopausal symptoms. A sequential regime might provide a more regular bleeding pattern in a perimenopausal woman who is still menstruating; however, expert opinion suggests changing to continuous therapy might be warranted in case of a change in endometriosis‐related symptoms. Whereas progestins are often suggested for endometriosis‐associated pain relief, they have generally little effect on vasomotor symptoms. Combined oral contraceptives, effective treatment for endometriosis‐related symptoms that can ease menopausal symptoms, are often not recommended to perimenopausal women due to high prevalence of various risk factors for venous thrombosis. 31 , 32 Women with vasomotor symptoms without contradictions can be prescribed combined oral contraceptives if contraception is a priority or the patient needs treatment for endometriosis‐associated pelvic pain.
In addition to hormonal medications, there are nonhormonal alternatives that can be considered and have shown variable efficacy in randomized trials. Cognitive behavioral therapy, hypnotherapy, selected antidepressants (paroxetine, venlafaxine, escitalopram, citalopram) and gabapentin are some of the therapies shown to reduce vasomotor symptoms in menopausal women. 6 Exercise might also be effective in treating vasomotor symptoms, however more studies are needed on the topic. 33
For the woman presented in scenario 3, MHT can be considered. A sequential regime with combined estrogen‐progestin therapy or estradiol in combination with hormonal intrauterine device are both reasonable alternatives, depending on patient preference, need for contraception and presence of endometriosis‐related complaints. Even combined oral contraceptives are an option in case MHT aggravates symptoms of pelvic pain and no contraindications can be identified.
2.4. Scenario 4: Management of suspected endometriosis recurrence in a postmenopausal woman with previous endometriosis
A 58‐year‐old woman with previously symptomatic premenopausal endometriosis characterized by dysmenorrhea and deep dyspareunia, presents with pelvic pain five years after menopause. She has not had any endometriosis‐related symptoms the last six years and has not needed MHT.
Endometriosis can be the first step in tumorigenesis and has been associated with increased risk of gynecological malignancies. A meta‐analysis from 2019 demonstrated a risk ratio of 1.96 (95% CI: 1.69 to 2.29) for patients with endometriosis developing ovarian cancer. 34 The risk of ovarian endometriosis progressing into ovarian cancer appears to be about 1%. 35 The strongest association has been observed for clear cell and endometrioid ovarian cancer.22 At the same time, no statistically significant increased risk for endometrial cancer and potentially lower prevalence of cervical cancer has been observed. 22 , 34 A recent study showed a possible protective effect of hysterectomy on ovarian cancer risk in women with endometriosis. 36 The mechanisms behind these phenomena are still unclear. Both endogenous and exogenous estrogen in association with obesity and MHT, respectively, are considered to possibly increase the risk of malignant transformation and recurrence of endometriosis. 4 Local conversion of cholesterol to estradiol has been described in endometriotic lesions contributing to the synthesis of estrogen in women with endometriosis. 37 Endometrioid adenocarcinoma appears to be the most common type of MHT‐associated malignancy in this patient group. 4 Case reports indicate that the most frequent symptoms of malignant transformation include pelvic mass, pelvic pain and abnormal uterine bleeding. Many patients, however, remain asymptomatic. A meta‐analysis from 2021 listed known previous endometriosis, premenopausal BSOE/hysterectomy, and long‐term use of estrogen‐only MHT as potential risk factors for malignant transformation. 4
Malignant transformation of endometriosis is rare and management strategies are based on low‐quality data. However, suspected postmenopausal recurrence of endometriosis, as presented in scenario 4, should always warrant thorough evaluation. 38 High index of suspicion and the need to exclude malignancy are necessary in the management of postmenopausal women presenting with symptoms and signs characteristic of endometriosis.
2.5. Scenario 5: Management of suspected symptoms of endometriosis in a postmenopausal woman currently using MHT
A 55‐year‐old woman with previous endometriosis, where symptoms resolved during menopausal transition, and current sequential MHT complaining of constant pelvic pain for the last 3 months.
Symptoms of recurring endometriosis in a postmenopausal woman can include pain (in the abdomen, iliac fossae, genitals), abnormal bleeding (vaginal, rectal, hematuria) and ovarian cysts. 3 , 8 , 10 Immunohistochemical examination of pre‐ and postmenopausal endometriotic lesions show similar characteristics. However, postmenopausal endometriosis seems to be less widespread and more inactive compared to the premenopausal type. 39 In general, data regarding the more specific characteristics of postmenopausal endometriosis are rare and mostly based on case reports. 10 , 40 Deep infiltrating endometriosis and ovarian masses appear to be more common than superficial disease in women with postmenopausal occurrence of endometriosis. Symptoms may appear many years after cessation of menstruations. 40 Because of the hormone‐dependent nature of endometriosis, symptoms usually regress or disappear after menopause. Nevertheless, endometriosis after menopause has been described in the literature both in the presence and absence of MHT. 41
Regarding the management of pelvic pain in a postmenopausal woman using MHT, there are no clear guidelines. In one study evaluating safety of postoperative MHT after endometriosis surgery, all cases of recurrence (3%) were successfully treated conservatively with either discontinuing the MHT or switching the type or dose of the treatment. 3 Therefore, depending on the specific clinical situation, these two options should be considered. However, a systematic literature review from 2017 indicated that surgical intervention and excision is usually necessary with only one case resolving using conservative measures. 10 The management of the woman presented in scenario 5 depends on the results of the physical examination. If no signs of pelvic mass, free fluid or other suspicious signs are found on imaging, stopping treatment with exogenous hormones, switching to continuous MHT, or lowering the estrogen dose can be considered, depending on patient preference. Because of the possibility of endometriosis‐associated sensitization of the peripheral and central nervous system contributing to the clinical symptomatology, multimodal approach should be considered. 42 If the patient history or physical examination reveals a potential risk for malignancy or the symptoms do not resolve with conservative therapy, further investigations and surgical intervention are warranted.
3. CONCLUSION
As an estrogen‐dependent disease, endometriosis symptoms can potentially be aggravated by treatment with exogenous estrogen. The aim should be to balance the need for treating menopausal symptoms against the risk of inducing endometriosis‐related symptoms. If MHT is considered necessary, combined estrogen‐progestin preparations should be considered independent of hysterectomy instead of unopposed estrogens; however, the evidence for this regimen in hysterectomized women is weak. Suspected postmenopausal endometriosis often requires surgical intervention because of unspecific symptoms and risk for malignant disease, especially if conservative measures are not effective or considered inappropriate.
The management of peri‐ and postmenopausal women with endometriosis is not well studied, and further research is therefore urgently needed. Individualized counseling based on available literature and clinical experience is vital. The effect of sequential vs continuous MHT, or estrogen only MHT vs combined treatment with estrogen and progestins on malignant transformation and symptom recurrence in hysterectomized and nonhysterectomized women with endometriosis are some of the important questions that need to be addressed in larger carefully designed trials. Other potential topics of interest for further research include the effect of MHT on menopausal symptoms and sexuality in women with endometriosis.
AUTHOR CONTRIBUTIONS
IJ drafted the article and critically revised it together with the co‐authors. ALH critically revised the first draft and contributed to the concept of the article.SBG critically revised the first draft and contributed to the concept of the article. All authors have approved the manuscript to be published.
CONFLICT OF INTEREST STATEMENT
The authors declare there are no conflicts of interest.
Jakson I, Hirschberg AL, Gidlöf SB. Endometriosis and menopause—management strategies based on clinical scenarios. Acta Obstet Gynecol Scand. 2023;102:1323‐1328. doi: 10.1111/aogs.14583
REFERENCES
- 1. Habib N, Buzzaccarini G, Centini G, et al. Impact of lifestyle and diet on endometriosis: a fresh look to a busy corner. Prz Menopauzalny. 2022;21:124‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horne AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ. 2022;379:e070750. [DOI] [PubMed] [Google Scholar]
- 3. Tanmahasamut P, Rattanachaiyanont M, Techatraisak K, Indhavivadhana S, Wongwananuruk T, Chantrapanichkul P. Menopausal hormonal therapy in surgically menopausal women with underlying endometriosis. Climacteric. 2022;25:388‐394. [DOI] [PubMed] [Google Scholar]
- 4. Giannella L, Marconi C, di Giuseppe J, et al. Malignant transformation of postmenopausal endometriosis: a systematic review of the literature. Cancers. 2021;13:4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Streuli I, Gaitzsch H, Wenger JM, Petignat P. Endometriosis after menopause: physiopathology and management of an uncommon condition. Climacteric. 2017;20:138‐143. [DOI] [PubMed] [Google Scholar]
- 6. Davis SR, Baber RJ. Treating menopause—MHT and beyond. Nat Rev Endocrinol. 2022;18:490‐502. [DOI] [PubMed] [Google Scholar]
- 7. Monteleone P, Mascagni G, Giannini A, Genazzani AR, Simoncini T. Symptoms of menopause—global prevalence, physiology and implications. Nat Rev Endocrinol. 2018;14:199‐215. [DOI] [PubMed] [Google Scholar]
- 8. Secosan C, Balulescu L, Brasoveanu S, et al. Endometriosis in menopause—renewed attention on a controversial disease. Diagnostics. 2020;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lobo RA, Quaresma F, de Moura K. The use of hormone therapy after surgery for endometriosis: an analysis. J Endometr Pelvic Pain Disord. 2016;8:152‐156. [Google Scholar]
- 10. Gemmell LC, Webster KE, Kirtley S, Vincent K, Zondervan KT, Becker CM. The management of menopause in women with a history of endometriosis: a systematic review. Hum Reprod Update. 2017;23:481‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Becker CM, Bokor A, Heikinheimo O, et al. ESHRE guideline: endometriosis. Hum Reprod Open. 2022;2022:hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collaborative Group on Hormonal Factors in Breast Cancer . Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta‐analysis of the worldwide epidemiological evidence. Lancet. 2019;394:1159‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vinogradova Y, Coupland C, Hippisley‐Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case‐control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ding D, Liu X, Guo SW. Further evidence for hypercoagulability in women with ovarian Endometriomas. Reprod Sci. 2018;25:1540‐1548. [DOI] [PubMed] [Google Scholar]
- 15. Wu Q, Ding D, Liu X, Guo SW. Evidence for a Hypercoagulable state in women with ovarian Endometriomas. Reprod Sci. 2015;22:1107‐1114. [DOI] [PubMed] [Google Scholar]
- 16. Viganò P, Ottolina J, Sarais V, Rebonato G, Somigliana E, Candiani M. Coagulation status in women with endometriosis. Reprod Sci. 2018;25:559‐565. [DOI] [PubMed] [Google Scholar]
- 17. Oliver‐Williams C, Glisic M, Shahzad S, et al. The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes in women: a systematic review. Hum Reprod Update. 2019;25:257‐271. [DOI] [PubMed] [Google Scholar]
- 18. Podfigurna‐Stopa A, Czyzyk A, Grymowicz M, et al. Premature ovarian insufficiency: the context of long‐term effects. J Endocrinol Invest. 2016;39:983‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Webber L, Anderson RA, Davies M, Janse F, Vermeulen N. HRT for women with premature ovarian insufficiency: a comprehensive review. Hum Reprod Open. 2017;2017:hox007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu X, Cai H, Kallianpur A, et al. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PLoS One. 2014;9:e89597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rozenberg S, di Pietrantonio V, Vandromme J, Gilles C. Menopausal hormone therapy and breast cancer risk. Best Pract Res Clin Endocrinol Metab. 2021;35:101577. [DOI] [PubMed] [Google Scholar]
- 22. Kvaskoff M, Mahamat‐Sale Y, Farland LV, et al. Endometriosis and cancer: a systematic review and meta‐analysis. Hum Reprod Update. 2021;27:393‐420. [DOI] [PubMed] [Google Scholar]
- 23. Huber D, Seitz S, Kast K, Emons G, Ortmann O. Hormone replacement therapy in BRCA mutation carriers and risk of ovarian, endometrial, and breast cancer: a systematic review. J Cancer Res Clin Oncol. 2021;147:2035‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loizzi V, Dellino M, Cerbone M, et al. Hormone replacement therapy in BRCA mutation carriers: how shall we do no harm? Hormones. 2023;22:19‐23. [DOI] [PubMed] [Google Scholar]
- 25. Gosset A, Susini M, Vidal F, et al. Quality of life of patients with bilateral oophorectomy before the age of 45 for the treatment of endometriosis. Maturitas. 2022;162:52‐57. [DOI] [PubMed] [Google Scholar]
- 26. Mu F, Rich‐Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9:257‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ingelsson E, Lundholm C, Johansson ALV, Altman D. Hysterectomy and risk of cardiovascular disease: a population‐based cohort study. Eur Heart J. 2011;32:745‐750. [DOI] [PubMed] [Google Scholar]
- 28. Howard B v, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women's Health Initiative observational study. Circulation. 2005;111:1462‐1470. [DOI] [PubMed] [Google Scholar]
- 29. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740‐745. [DOI] [PubMed] [Google Scholar]
- 30. Costa GPO, Ferreira‐Filho ES, Simoes R, et al. Impact of hormone therapy on the bone density of women with premature ovarian insufficiency: a systematic review. Maturitas. 2023;167:105‐112. [DOI] [PubMed] [Google Scholar]
- 31. Linton A, Golobof A, Shulman LP. Contraception for the perimenopausal woman. Climacteric. 2016;19:526‐534. [DOI] [PubMed] [Google Scholar]
- 32. Troìa L, Martone S, Morgante G, Luisi S. Management of perimenopause disorders: hormonal treatment. Gynecol Endocrinol. 2021;37:195‐200. [DOI] [PubMed] [Google Scholar]
- 33. Liu T, Chen S, Mielke GI, McCarthy AL, Bailey TG. Effects of exercise on vasomotor symptoms in menopausal women: a systematic review and meta‐analysis. Climacteric. 2022;25:552‐561. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Liu R, Tang S, et al. Impact of endometriosis on risk of ovarian, endometrial and cervical cancers: a meta‐analysis. Arch Gynecol Obstet. 2019;299:35‐46. [DOI] [PubMed] [Google Scholar]
- 35. Volpi E, Peano E, Ferrero A, Mosso L, Daniele A, Sismondi P. Association between ovarian endometriosis and malignancy in the peri‐menopausal period: report of two cases and review of the literature. Gynecol Surg. 2010;7:13‐17. [Google Scholar]
- 36. Khoja L, Weber RP, Webb PM, et al. Endometriosis and menopausal hormone therapy impact the hysterectomy‐ovarian cancer association. Gynecol Oncol. 2022;164:195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bulun SE. Endometriosis. Mechanisms of disease. N Engl J Med. 2009;360(3):305. [DOI] [PubMed] [Google Scholar]
- 38. Tan DA, Almaria MJG. Postmenopausal endometriosis: drawing a clearer clinical picture. Climacteric. 2018;21:249‐255. [DOI] [PubMed] [Google Scholar]
- 39. Cumiskey J, Whyte P, Kelehan P, Gibbons D. A detailed morphologic and immunohistochemical comparison of pre‐ and postmenopausal endometriosis. J Clin Pathol. 2008;61:455‐459. [DOI] [PubMed] [Google Scholar]
- 40. de Almeida AF, Ribeiro HA, Ayrosa Ribeiro P, et al. Symptomatic endometriosis developing several years after menopause in the absence of increased circulating estrogen concentrations: a systematic review and seven case reports. Gynecol Surg. 2019;16:3. [Google Scholar]
- 41. Bhat RA, Teo M, Bhat AK. Endometriosis after surgical menopause mimicking pelvic malignancy: Surgeons' predicament. Oman Med J. 2014;29:226‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McNamara HC, Frawley HC, Donoghue JF, et al. Peripheral, central, and cross sensitization in endometriosis‐associated pain and comorbid pain syndromes. Front Reprod Health. 2021;3:729642. [DOI] [PMC free article] [PubMed] [Google Scholar]


