ABSTRACT
Elderly patients have a high risk of perioperative morbidity and mortality. Pluri-morbidities, polypharmacy, and functional dependence may have a great impact on intraoperative management and request specific cautions. In addition to surgical stress, several perioperative noxious stimuli such as fasting, blood loss, postoperative pain, nausea and vomiting, drug adverse reactions, and immobility may trigger a derangement leading to perioperative complications. Older patients have a high risk of major hemodynamic derangement due to aging of the cardiovascular system and associated comorbidities. The hemodynamic monitoring as well as fluid therapy should be the most accurate as possible. Aging is accompanied by decreased renal function, which is related to a reduction in renal blood flow, renal mass, and the number and size of functioning nephrons. Drugs eliminated predominantly by the renal route need dosage adjustments based on residual renal function. Liver mass, hepatic blood flow, and intrinsic metabolic activity are decreased in the elderly, and all drugs metabolized by the liver have a variable half-life, thus requiring dose reduction. Decreased neural plasticity contributes to a high risk for postoperative delirium. Monitoring of anesthesia depth should be mandatory to avoid overdosage of hypnotic drugs. Prevention of postoperative pulmonary complications requires both protective ventilation strategies and adequate recovery of neuromuscular function at the end of surgery. Avoidance of hypothermia cannot be missed. The aim of this review is to describe comprehensive strategies for intraoperative management plans tailored to meet the unique needs of elderly surgical patients, thus improving outcomes in this vulnerable population.
Keywords: Anesthesia, Elderly, Management, Patient-centered care
Introduction
Elderly patients have a higher risk of perioperative morbidity and mortality.[1] Pluri-morbidities, polypharmacy, and functional dependence may have a great impact on intraoperative management and request specific cautions. In addition to the surgery itself, several perioperative noxious stimuli such as fasting, blood loss, pain, nausea and vomiting, drug adverse reactions (ADRs), and immobility may trigger a derangement increasing the risk of perioperative complications.
Anesthesia management is strongly affected by age-related changes. Intraoperative care should be based on careful preoperative assessment and tailored to patients’ clinical requirements.
The aim of this review was to describe comprehensive strategies for intraoperative management plans tailored to meet the unique needs of elderly surgical patients, thus improving outcomes in this vulnerable population.
Literature search
A comprehensive literature search was performed in PubMed/MEDLINE, The Cochrane Library, EMBASE, and Scopus, Google Scholar to identify the relevant articles (published up to May 1, 2023) relating to the implication of age-related changes on anesthesia management. Reviews, meta-analyses, and randomized clinical trials were included. A total of 32 articles were included based on relevance.
Physiological changes in the elderly and general implication on anesthesia
In the elderly, pathophysiological changes determined by aging, comorbidities, polypharmacy, cognitive impairment, malnutrition, sensory deficits, and psychological problems may contribute to an impaired preoperative functional state that can significantly increase the risk of negative outcomes.[2,3,4,5,6]
At the cardiovascular level, the increase in peripheral vascular resistance due to the stiffness of the arterial wall, the alteration of the adrenergic tone with an increase in vagal tone and reduced sensitivity of the peripheral beta-adrenergic receptors, and the reduction in the number of myocytes with residual cell hypertrophy are responsible for ventricular hypertrophy leading to reduced cardiac compliance and reduction of stroke volume.[6] It also increases cardiac interstitial fibrosis which, together with the reduction of sinus node cells, determines a greater predisposition to arrhythmias. Reduced elasticity and stiffening of the arteries may result in altered regulation of blood pressure. All these alterations are the cause of the decreased ability to cope with acute changes in circulating blood volume. At the pulmonary level, both the elasticity of the lung parenchyma and that of the chest wall are reduced with a decrease in residual functional capacity. Moreover, there is an alteration of the exchanges due to the reduction of the alveolar surface. This mandates protective lung ventilation during and after surgery.
At the renal level, the progressive loss of nephrons and the reduction in blood flow contribute to the decrease of the glomerular filtration rate. Clearly, the kidney of the elderly patient is more exposed to damage in case of hypotension and/or hypovolemia.[6]
At the neurological level, the alterations concern both the central and the peripheral nervous systems. This essentially translates into a progressive decline in cognition, sensory perceptions, and motor components.[6]
Pharmacokinetics and pharmacodynamics are altered to varying degrees as a result of aging processes and associated conditions affecting metabolic processes. Drug absorption can be modified by decreased gastric acid secretion (which is accentuated by the administration of antacids and proton pump inhibitors), decreased gastric emptying rate, decreased splanchnic blood flow, and decreased absorption surface of the mucosa. The changes can significantly alter drug absorption and consequently change the onset time. The absorption of drugs undergoing first-pass metabolism (nitrates, propranolol) may also increase. Changes in body composition (increased fat mass and decreased body water) significantly change drug distribution. Fat-soluble drugs (diazepam) have a larger volume of distribution and may require careful dosage adjustment until the desired result is obtained. A reduced amount of body water can affect the volume of distribution of water-soluble drugs and lead to a higher plasma concentration. Reduced protein binding can lead to increased plasma concentrations, resulting in higher amounts of circulating drug and more pronounced effects.
Aging is accompanied by decreased renal function to varying degrees, which is related to reductions in renal blood flow, renal mass, and the number and size of functioning nephrons. Drug dosage adjustments based on residual renal function (creatinine clearance value) should be made, mainly for drugs eliminated predominantly by the renal route. The slowdown of any kind in the metabolic processes prolongs the half-life of the drugs and maximizes the risk of ADRs. An impaired nutritional status can affect metabolic capacity. Liver mass, hepatic blood flow, and intrinsic metabolic activity (including the CYP450 enzyme system) are decreased in the elderly, and all drugs metabolized by the liver have a variable half-life; consequently, their dosage should be reduced.[6]
The choice of anesthetic technique depends on both the surgical requirements and the patient’s needs, taking into consideration that there is no evidence establishing the superiority of regional anesthesia (RA) compared to general anesthesia (GA) when used as a primary procedure. The type of surgery or the expected duration may sometime prevent the use, at least exclusively, of RA. The association of RA and GA leads to better analgesia and metabolic balance.
Regarding premedication with midazolam, pharmacodynamic changes (increased brain sensitivity) and pharmacokinetic changes (reduced clearance due to reduced hepatic perfusion and an increase in the volume of distribution due to increased body fat) require dose reductions in the elderly. The intravenous administration of midazolam, when needed to reduce anxiety, should be decreased (0.25–1 mg) in the elderly, as well as the interval between doses, whereas long-acting benzodiazepines (lorazepam and diazepam) are not recommended. Dexmedetomidine may be used to prevent postoperative delirium (POD),[7,8] likely thanks to anti-inflammatory action caused by downregulation in tumor necrosis factor and interleukin-1β.[9,10] However, the anticipated benefits should be balanced against the repercussion on the elderly vulnerability of the most relevant adverse effects of alpha 2 agonists, that is, bradycardia and hypotension.
Certainly, in elderly patient, it is necessary to adjust the dosage of anesthetic drugs as their potency is increased due to central neurophysiological changes and greater receptor sensitivity.
Propofol reduces systemic vascular resistance (inhibits sympathetic vasoconstriction), decreases preload, and affects myocardial contractility. Significant hypotension may occur in elderly patient with impaired baroreflexes, particularly in the presence of hypovolemia or underlying ventricular dysfunction. The induction dose of propofol should be reduced by 40–50% and should be administered slowly. The maintenance dose should be reduced to avoid longer recovery (due to reduced clearance).[11] The minimum alveolar concentration (MAC) of all inhaled anesthetics decreases by about 6% per decade after age 40. At age 90, MAC is reduced by 30%. Although the reasons for MAC decline are unknown, a combination of age-related effects on synaptic activity and neurotransmitter function in the brain, brain atrophy, and changes in cerebral circulation are thought to be responsible for it. Therefore, anesthetic concentrations need to be titrated based on individual patient responses determined via anesthesia depth monitoring (see below). The adequate dose according to age-adjusted MAC values should be calculated using iso-MAC charts, in case of unavailability of depth anesthesia monitoring.[12,13]
Before positioning an elderly patient on the operating table, the musculoskeletal and skin conditions should be carefully assessed for the possible presence of muscle wasting, skin lesions, and joint stiffness. To avoid peripheral nerve damage and pressure sores, it is necessary to ensure that adequate padding is positioned near bony protrusions and to take special care during the transfer of the patient between the ward bed and the operating table and when removing adherent items such as surgical dressings.[3,4,5]
Hemodynamic management in the elderly
Hypotension is exacerbated by most anesthetics which cause systemic vasodilatation. Spinal anesthesia or epidural infusion with local anesthetics can also reduce blood pressure (BP) due to vasodilatation. In the elderly, hypotension could be particularly dangerous, due to the impaired compensatory mechanisms, and may lead to myocardial injury, acute kidney injury (AKI), stroke, and death. The perioperative quality initiative consensus statement on intraoperative hypotension recommends avoiding a mean arterial pressure (MAP) <65 mmHg and systolic blood pressure (SBP) <100 mmHg.[14]
Best practice guidelines for the perioperative care of the elderly from the Association of Anaesthetists recommend that intraoperative hypotension should be avoided in patients aged ≥65 y and define intraoperative hypotension as a 20% decrease in SBP.[15]
The incidence of intraoperative hypotension in older patients was investigated in a recent study, describing both the intended BP treatment threshold and the clinically applied treatment threshold for intraoperative hypotension in UK anesthetic practice in a cohort of 4750 elderly patients.
Thresholds at which anesthetists give vasopressor treatments [60.6 (9.7) mmHg] were lower than their intended treatment thresholds [64.2 (11.6)] and most patients (57.9%) spent >30 min >20% below a pre-induction baseline blood pressure.
At univariable analysis, there was no association between an isolated MAP <65 mmHg and any outcome. There was an association between cumulative time spent with SBP >20% below pre-induction value with death and a creatinine rise.
On multivariable analysis, there was an association between cumulative duration of systolic hypotension >20% below baseline and creatinine rise within the first seven postoperative days.
In this representative sample of UK perioperative practice, most older patients experienced intraoperative hypotension and treatment was delivered below suggested thresholds. This highlights both the potential for intraoperative organ injury and the substantial opportunity for improving the treatment of intraoperative hypotension.[16]
However, blood pressure may be only the tip of the iceberg. Only advanced monitoring permits to understand and treat the causes of hypotension. For example, if we do not have any cardiac output (CO) change, the hypotension could be caused by a too deep anesthesia that may decrease systemic vascular resistances (SVR). After decreasing the depth of anesthesia, if hypotension persists, we can use vasopressors to increase SVR. However, if we detect a decreased CO, we should determine if we can counteract it by using a volume load or if the patient needs a positive inotrope drug. It seems a complex diagram, but it summarizes the daily practice of anesthesiologists.[17]
We can better understand hemodynamic monitoring if we think about its aim. The real goal is the optimization of the delivery of oxygen to the tissues. To optimize CO, we can increase preload with fluids, improve contractility with inotropes, or increase afterloads with the use of vasopressors.[1]
All these actions need to be guided by advanced hemodynamic monitoring.[18]
Historically, goal directed fluid therapy (GDTF) was performed by pulmonary artery catheter,[19] but recently less invasive technologies have become available.[20,21]
Among minimally invasive hemodynamic monitoring systems used in major surgery, the most accredited in non-cardiac major surgery are those based on pulse contour analysis with special reference to the subgroup of devices that do not require external calibration.[18]
These systems are advantageous because they are mini-invasive and easy to use; they provide hemodynamic variables in real time, and the measures are independent of the operator. Nowadays, these systems incorporate the so-called Acumen software that—thanks to a machine learning-derived early warning system—is able to predict hypotension, allowing for a significant reduction in hypotension compared to the standard of care, obviously when combined with a treatment protocol also based on SVV, contractility, and afterload.[22] However, they suffer from low reliability in case of variation in vascular compliance (possible in unstable patients) or in case of arrhythmias and reduced precision in case of arterial pressure curve over or underdamped.
These monitors help anesthesiologists in performing the so-called GDFT. Without any doubt, we can state that anesthesiologists in the past were used to give so much fluids during surgery.[23,24]
After having established that aggressive fluid strategies adversely affect every system organ, for many years, restrictive fluid therapy was the new target to follow since an international trial enrolling 3000 patients undergoing major abdominal surgery was published. The authors showed that the restrictive approach was associated with a higher rate of perioperative acute renal failure and, therefore, must be avoided.[25]
GDFT is the key to optimize the intravascular volume and allow fluid administration only to fluid responders patients. In particular, GDFT with the uncalibrated pulse contour analyses methods is correlated with a significant decrease in postoperative morbidity, helping anesthesiologists to drive fluid therapy and vasopressors/inotropes supports.[26] GDFT, compared with standard care, showed reduced mortality in 95 RCTs, avoiding 18 deaths for every 1000 patients.[27]
Interestingly, in the elderly population, GDFT was useful in reducing POD incidence when guided by cerebral perfusion via regional cerebral oxygen saturation,[28] leading to a shorter recovery time and lower production of pro-inflammatory factors.[29]
The most benefits on mortality, complications, and length of stay arise from GDFT based on both hemodynamic goals and metabolic homeostasis.[30]
This means that GDFT ameliorates microperfusion and reduces healthcare costs, avoiding postoperative complications and kidney dysfunction, but there is not always a clear association between reduced length of stay (LOS) and hospital readmission.[31,32,33,34,35,36] Larger trials are necessary to better define the effects of GDFT on mortality and LOS, especially in elderly population.
In the meantime, GDFT can be used in high-risk patients or those undergoing complex surgery, whereas the intraoperative goal should always be to achieve a “zero” fluid balance. Optimizing hemoglobin levels allows the enhancement of oxygen delivery via patient blood management that includes careful surgical hemostasis, use of cell salvage use, and point-of-care testing.[37]
Monitoring of anesthesia depth
Another key strategy to optimize intraoperative anesthesiologic management in the elderly is maintaining an adequate anesthesia depth with the main aim to prevent postoperative delirium.[5]
As it is not possible to titrate anesthetics based on row electroencephalography (EEG) trace, due to technical difficulties, EEG-derived monitors, such as the bispectral index (BIS), have been developed employing the Fourier transformation method.[38,39] The introduction of these systems has completely moved the target of anesthesia depth from clinical indices (including pupils, movements, blood pressure and heart rate, drug concentration) to goals based on cerebral response to anesthetic drugs. It has been demonstrated that monitoring the depth of anesthesia can improve postoperative recovery promoting quicker awakening and significantly reducing POD and postoperative cognitive decline (pCD) in elderly patients undergoing major non-cardiac surgery.[40,41,42,43]
A meta-analysis of eight observational studies demonstrated that BIS values under 45 are associated with higher long-term mortality (≥1 year).[44] Not measuring anesthesia depth is correlated with anesthetics overdoses and worse outcomes for several reasons, among which organ hypoperfusion and dysfunction are the most important ones.[45] There is a subtle link between anesthesia depth and hemodynamics, which means that hypotension can reflect low BIS values due to low blood perfusion in the cerebral district, vice versa overdoses in hypnotics may result in low BIS and low blood pressure.
The advantages of multiparameter-guided anesthesia (based on index, burst suppression activity, density spectral array) versus index-guided anesthesia should be further investigated as index values may be less consistent in the elderly with neurodegeneration implying reduced cortical electrical activity, that is, low EEG power.[46] In any case, monitoring of the depth of anesthesia is recommended for patients at higher risk of adverse outcomes,[5] irrespective of the device used, as far as it is a brain monitoring system using processed EEG.[47,48,49,50,51,52,53]
Minor EEG changes may also indicate borderline cerebral perfusion in patients who develop pCD not always related to anesthesia depth and should not be missed.[54] In addition to the monitoring of anesthesia depth, transcranial Doppler could be used as an intraoperative tool to prevent the occurrence of unfavorable cognitive outcomes in elderly patients undergoing robotic-assisted laparoscopic prostatectomy. This supports that a great vulnerability of the cerebral circulation due to the combination of many factors, including anesthesia, age, positioning, and CO2 insufflation during robotic-assisted surgery, could predispose elderly patients to postoperative cognitive disorders.[55]
It has been demonstrated that cortisol response to surgical stress is efficiently counteracted by blood remifentanil infusion guided by cardiovascular parameters on the same anesthesia depth.[56] In a recent study, it has been demonstrated that elderly patients undergoing laparoscopic colorectal cancer surgery under GA may benefit from SPI-guided analgesia using remifentanil in terms of lower pain scores, reduced intraoperative hypertension and tachycardia, and improved postoperative outcomes, including postoperative pain and delirium in the post-anesthesia care unit.[57] This finding is also confirmed by preclinical studies on human microglia due to the lack of any toxic effect or neuroinflammatory action on the central nervous system (no effect on inflammatory mediators) but, rather, a neuroprotective action throughout the induced rise in basal BDNF production.[58,59]
A moderate–severe postoperative pain could be a relevant risk factor for cognitive impairment after surgery, and non-pharmacological strategies should be always considered.[60]
Ventilation strategy
Respiratory complications are more common in elderly patients and can lead to prolonged hospital stay and mortality.[61] The implementation of high-flow nasal cannula or noninvasive ventilation in the perioperative period in patients with chronic obstructive pulmonary disease, frequently present in the elderly, may reduce the risk of atelectasis and re-intubation after surgery.[62] During GA, a protective ventilation strategy is recommended to minimize stress and strain and reduce postoperative respiratory complications. This implies the use of low tidal volumes (6–7 ml/kg based on ideal body weight) that allow increasing functional residual capacity. The use of positive end-expiratory pressure (PEEP) may increase dynamic compliance, preventing damage from alveolar opening and closing and reducing the incidence of atelectasis, even if, in patients with cardiac diseases, these advantages should be balanced against its negative effect on cardiac output.[62,63] Recruitment maneuvers may be required to improve oxygenation, especially when patients are placed in the Trendelenburg position.[64]
In laparoscopic/robotic surgical approaches, mechanical ventilation may require multiple adjustments as systemic carbon dioxide (CO2) absorption can be relevant, leading to hypertension, dysrhythmias, and respiratory acidosis.[63] The latter may be more pronounced when difficult ventilation and increased dead space occur. To avoid hypercarbia, often deriving from reabsorption of CO2, also the use of deep neuromuscular block can be useful.[62]
Prevention of residual paralysis
The use of deep neuromuscular block (dNMB) is often required in modern mini-invasive surgery (laparoscopic and robotic) also to avoid the use of high pneumoperitoneum pressure, thus preventing the consequent hemodynamic (falls in both CO and MAP) and respiratory (decrease of pulmonary compliance and functional residual capacity) repercussions. However, as older patients are more sensitive to neuromuscular blocking agents (NMBAs), due to a slower hepatic and renal metabolism,[57] monitoring the depth of neuromuscular blockade is essential to ensure appropriate dosing during anesthesia and to avoid incomplete recovery from neuromuscular blockade after surgery (defined by a train of four ratio <0.9).[64,65]
The use of dNMB requires both neuromuscular monitoring and adequate reversal, by direct or indirect antagonists, to prevent postoperative residual curarization (PORC). Elderly patients are more prone to adverse postoperative respiratory events, including pneumoniae, due to frequently impaired airway protection predisposing them to aspiration.[5] Recovery of neuromuscular function, regardless of the type of antagonists used, is slower in the elderly and needs to be accurately monitored. In case of persistence of the dNMB at the end of surgery, the use of a direct antagonist is advisable taking into consideration that the dose can be adjusted based on neuromuscular monitoring.[65]
Prevention of hypothermia
Both GA and RA inhibit the control of thermoregulation through direct vasodilatory action. During anesthesia, there is a redistribution of blood and a thermoregulation impairment that causes a temperature drop of 1–1,5°C only in the first hour. Surgical site evaporation, decreased metabolism, and reduced tissue perfusion contribute to intraoperative hypothermia, leading to organ dysfunction, coagulopathy, impaired immunity, surgical site infection, altered drug metabolism, and shivering resulting in a prolonged recovery. In the elderly, there are several factors concurring to thermoregulation impairment, such as reduction of muscle mass, metabolic and hormonal dysfunction, and vascular reactivity.[66,67] In the context of geriatric anesthesia, it is recommended to monitor the core temperature together with the active heating of the patient (preferably with forced air systems) and infused liquids, in the intra- and perioperative periods.[5] It has been demonstrated that skin surface warming before induction of anesthesia does not significantly increase the core temperature but increases peripheral tissue temperature and total body heat content.[68] Therefore, skin surface warming for 30 min before induction of anesthesia prevents redistribution of hypothermia. During surgery, patient core temperature should be measured continuously or every 15 min (preferably with an esophageal probe) and operating room temperature should be at least 21°C for adult patients (the optimal temperature for the elderly is 23°C). Any fluid, colloid, or blood that runs greater than 500 ml/h should be warmed using a fluid-warming device.
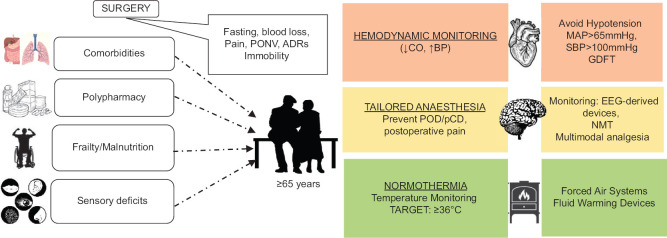
In the postoperative period, the patient’s temperature should be measured and documented on admission to the recovery room and then every 15 min. Ward transfer should not be arranged unless the patient’s temperature is 36.0°C or above.[68] A comprehensive strategy for anesthesiological intraoperative management is given in Figure 1.
Figure 1.
Comprehensive strategy for anesthesiological intraoperative management. ADRs, drug adverse reactions; CO, cardiac output; BP, blood pressure; EEG, electroencephalogram; NMT, neuromuscular monitoring; POD, postoperative delirium; pCD, postoperative cognitive decline; SAP, systolic arterial pressure; MAP, mean arterial pressure
In conclusion, geriatric anesthesia and perioperative geriatric care are demanding tasks, and today’s anesthesiologists are required to deepen and widen their knowledge about the pathophysiological specificities, vulnerabilities, and needs of older patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. Mortality after surgery in Europe: A 7-day cohort study. Lancet. 2012;380:1059–65. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shem Tov L, Matot I. Frailty and anesthesia. Curr Opin Anaesthesiol. 2017;30:409–17. doi: 10.1097/ACO.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 3.Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal perioperative management of the geriatric patient: A best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg. 2016;222:930–47. doi: 10.1016/j.jamcollsurg.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Parlow JL, Duceppe E, Devereaux PJ. Frailty, the elderly, and the guidelines on perioperative cardiac risk assessment and management in noncardiac surgery. Can J Cardiol. 2018;34:343.e13. doi: 10.1016/j.cjca.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Aceto P, Antonelli Incalzi R, Bettelli G, Carron M, Chiumiento F, Corcione A, et al. Perioperative Management of Elderly patients (PriME): Recommendations from an Italian intersociety consensus. Aging Clin Exp Res. 2020;32:1647–73. doi: 10.1007/s40520-020-01624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olotu C. Anesthesia for the elderly: A narrative review. Minerva Anestesiol. 2021;87:1128–38. doi: 10.23736/S0375-9393.21.15388-X. [DOI] [PubMed] [Google Scholar]
- 7.Tang Y, Wang Y, Kong G, Zhao Y, Wei L, Liu J. Prevention of dexmedetomidine on postoperative delirium and early postoperative cognitive dysfunction in elderly patients undergoing hepatic lobectomy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:219–25. doi: 10.11817/j.issn.1672-7347.2022.210280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Du X, Wu F, Hu Y, Xv Z, Mi W. Dexmedetomidine improves early postoperative neurocognitive disorder in elderly male patients undergoing thoracoscopic lobectomy. Exp Ther Med. 2020;20:3868–77. doi: 10.3892/etm.2020.9113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Turan A, Duncan A, Leung S, Karimi N, Fang J, Mao G, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): A randomised placebo-controlled trial. Lancet. 2020;396:177–85. doi: 10.1016/S0140-6736(20)30631-0. [DOI] [PubMed] [Google Scholar]
- 10.Likhvantsev VV, Landoni G, Grebenchikov OA, Ovezov AM, Skripkin YV, Lembo R, et al. Perioperative dexmedetomidine supplement decreases delirium incidence after adult cardiac surgery: A randomized, double-blind, controlled study. J Cardiothorac Vasc Anesth. 2021;35:449–57. doi: 10.1053/j.jvca.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 11.De Cosmo G, Congedo E, Clemente A, Aceto P. Sedation in PACU: The role of propofol. Curr Drug Targets. 2005;6:741–4. doi: 10.2174/138945005774574425. [DOI] [PubMed] [Google Scholar]
- 12.Aceto P, Perilli V, Lai C, Sacco T, Modesti C, Luca E, et al. Minimum alveolar concentration threshold of sevoflurane for postoperative dream recall. Minerva Anestesiol. 2015;81:1201–9. [PubMed] [Google Scholar]
- 13.Aceto P, Lai C, Perilli V, Dello Russo C, Federico B, Navarra P, et al. Stress-related biomarkers of dream recall and implicit memory under anaesthesia. Anaesthesia. 2013;68:1141–7. doi: 10.1111/anae.12386. [DOI] [PubMed] [Google Scholar]
- 14.Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–74. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths R, Beech F, Brown A, Dhesi J, Foo I, Goodall J, et al. Peri-operative care of the elderly 2014: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2014;69((Suppl 1)):81–98. doi: 10.1111/anae.12524. [DOI] [PubMed] [Google Scholar]
- 16.Wickham AJ, Highton DT, Clark S, Fallaha D, Wong DJN, Martin DS, et al. Treatment threshold for intra-operative hypotension in clinical practice-a prospective cohort study in older patients in the UK. Anaesthesia. 2022;77:153–63. doi: 10.1111/anae.15535. [DOI] [PubMed] [Google Scholar]
- 17.Meng L, Yu W, Wang T, Zhang L, Heerdt PM, Gelb AW. Blood pressure targets in perioperative care. Hypertension. 2018;72:806–17. doi: 10.1161/HYPERTENSIONAHA.118.11688. [DOI] [PubMed] [Google Scholar]
- 18.Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, et al. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209. doi: 10.1186/cc11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–86. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 20.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993 to 2004. JAMA. 2007;298:423–9. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 21.Ikuta K, Wang Y, Robinson A, Ahmad T, Krumholz HM, Desai NR. National trends in use and outcomes of pulmonary artery catheters among medicare beneficiaries, 1999-2013. JAMA Cardiol. 2017;2:908–13. doi: 10.1001/jamacardio.2017.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wijnberge M, Geerts BF, Hol L, Lemmers N, Mulder MP, Berge P, et al. Effect of a machine learning-derived early warning system for intraoperative hypotension vs standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: The HYPE randomized clinical trial. JAMA. 2020;323:1052–60. doi: 10.1001/jama.2020.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: It is time to consider the four D's and the four phases of fluid therapy. Ann Intensive Care. 2018;8:66. doi: 10.1186/s13613-018-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bundgaard-Nielsen M, Secher NH, Kehlet H. “Liberal” vs. “restrictive” perioperative fluid therapy-A critical assessment of the evidence: Review article. Acta Anaesthesiol Scand. 2009;53:843–51. doi: 10.1111/j.1399-6576.2009.02029.x. [DOI] [PubMed] [Google Scholar]
- 25.Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263–74. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 26.Saugel B, Reuter DA. Perioperative goal-directed therapy using invasive uncalibrated pulse contour analysis. Front Med. 2018;5:12. doi: 10.3389/fmed.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong MA, Wang Y, Berbenetz NM, McConachie I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes? A systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35:469–83. doi: 10.1097/EJA.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 28.Zhang N, Liang M, Zhang DD, Xiao YR, Li YZ, Gao YG, et al. Effect of goal-directed fluid therapy on early cognitive function in elderly patients with spinal stenosis: A case-control study. Int J Surg. 2018;54:201–5. doi: 10.1016/j.ijsu.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Wang J yu, Li M, Wang P, Fang P. Goal-directed therapy based on rScO2 monitoring in elderly patients with one-lung ventilation: A randomized trial on perioperative inflammation and postoperative delirium. Trials. 2022;23:687. doi: 10.1186/s13063-022-06654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng QW, Tan WC, Zhao BC, Wen SH, Shen JT, Xu M. Is goal-directed fluid therapy based on dynamic variables alone sufficient to improve clinical outcomes among patients undergoing surgery? A meta-analysis. Crit Care. 2018;22:298. doi: 10.1186/s13054-018-2251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jessen MK, Vallentin MF, Holmberg MJ, Bolther M, Hansen FB, Holst JM, et al. Goal-directed haemodynamic therapy during general anaesthesia for noncardiac surgery: A systematic review and meta-analysis. Br J Anaesth. 2022;128:416–33. doi: 10.1016/j.bja.2021.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao GL, Peng PH, Zhou YY, Li JJ, Jiang HY, Shao JL. The accuracy and effectiveness of goal directed fluid therapy in plateau-elderly gastrointestinal cancer patients: A prospective randomized controlled trial. Int J Clin Exp Med. 2018;11:8516–22. [Google Scholar]
- 33.Liu TJ, Zhang JC, Gao XZ, Tan ZB, Wang JJ, Zhang PP, et al. Clinical research of goal-directed fluid therapy in elderly patients with radical resection of bladder cancer. J Cancer Res Ther. 2018;14((Suppl)):S173–9. doi: 10.4103/0973-1482.183206. [DOI] [PubMed] [Google Scholar]
- 34.Ghoreifi A, Basin MF, Ghodoussipour S, Bazargani ST, Amini E, Aslzare M, et al. Perioperative outcomes of goal-directed versus conventional fluid therapy in radical cystectomy with enhanced recovery protocol. Int Urol Nephrol. 2021;53:1827–33. doi: 10.1007/s11255-021-02903-w. [DOI] [PubMed] [Google Scholar]
- 35.Yin K, Ding J, Wu Y, Peng M. Goal-directed fluid therapy based on noninvasive cardiac output monitor reduces postoperative complications in elderly patients after gastrointestinal surgery: A randomized controlled trial. Pak J Med Sci. 2018;34:1320–5. doi: 10.12669/pjms.346.15854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Zhang P, Liu MX, Ma JL, Wei XC, Fan D. Preoperative carbohydrate loading and intraoperative goal-directed fluid therapy for elderly patients undergoing open gastrointestinal surgery: A prospective randomized controlled trial. BMC Anesthesiol. 2021;21:157. doi: 10.1186/s12871-021-01377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perilli V, Aceto P, Sacco T, Modesti C, Ciocchetti P, Vitale F, et al. Anaesthesiological strategies to improve outcome in liver transplantation recipients. Eur Rev Med Pharmacol Sci. 2016;20:3172–7. [PubMed] [Google Scholar]
- 38.Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192–214. doi: 10.1097/EJA.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 39.Mashour GA, Woodrum DT, Avidan MS. Neurological complications of surgery and anaesthesia. Br J Anaesth. 2015;114:194–203. doi: 10.1093/bja/aeu296. [DOI] [PubMed] [Google Scholar]
- 40.Steiner LA. Postoperative delirium. Part 2: Detection, prevention and treatment. Eur J Anaesthesiol. 2011;28:723–32. doi: 10.1097/EJA.0b013e328349b7db. [DOI] [PubMed] [Google Scholar]
- 41.Eger EI, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: A standard of anesthetic potency. Anesthesiology. 1965;26:756–63. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Aceto P, Perilli V, Lai C, Ciocchetti P, Vitale F, Sollazzi L. Postoperative cognitive dysfunction after liver transplantation. Gen Hosp Psychiatry. 2015;37:109–15. doi: 10.1016/j.genhosppsych.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Hajat Z, Ahmad N, Andrzejowski J. The role and limitations of EEG-based depth of anaesthesia monitoring in theatres and intensive care. Anaesthesia. 2017;72((Suppl 1)):38–47. doi: 10.1111/anae.13739. [DOI] [PubMed] [Google Scholar]
- 44.Chan MT, Cheng BC, Lee TM, Gin T. CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 45.Leslie K, Short TG. Low bispectral index values and death: The unresolved causality dilemma. Anesth Analg. 2011;113:660–3. doi: 10.1213/ANE.0b013e31822401cc. [DOI] [PubMed] [Google Scholar]
- 46.Xu N, Li LX, Wang TL, Jiao LQ, Hua Y, Yao DX, et al. Processed multiparameter electroencephalogram-guided general anesthesia management can reduce postoperative delirium following carotid endarterectomy: A randomized clinical trial. Front Neurol. 2021;12:666814. doi: 10.3389/fneur.2021.666814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ling L, Yang TX, Lee SWK. Effect of anaesthesia depth on postoperative delirium and postoperative cognitive dysfunction in high-risk patients: A systematic review and meta-analysis. Cureus. 2022;14:e30120. doi: 10.7759/cureus.30120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chew WZ, Teoh WY, Sivanesan N, Loh PS, Shariffuddin II, Ti LK, et al. Bispectral Index (BIS) monitoring and postoperative delirium in elderly patients undergoing surgery: A systematic review and meta-analysis with trial sequential analysis. J Cardiothorac Vasc Anesth. 2022;36:4449–59. doi: 10.1053/j.jvca.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Shan W, Chen B, Huang L, Zhou Y. The effects of bispectral index–guided anesthesia on postoperative delirium in elderly patients: A systematic review and meta-analysis. World Neurosurg. 2021;47:e57–62. doi: 10.1016/j.wneu.2020.11.110. [DOI] [PubMed] [Google Scholar]
- 50.Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2014;2014:CD003843. doi: 10.1002/14651858.CD003843.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi: 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shepherd J, Jones J, Frampton G, Bryant J, Baxter L, Cooper K. Clinical effectiveness and cost-effectiveness of depth of anaesthesia monitoring (E-Entropy, Bispectral Index and Narcotrend): A systematic review and economic evaluation. Health Technol Assess. 2013;17:1–264. doi: 10.3310/hta17340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zorrilla-Vaca A, Healy RJ, Wu CL, Grant MC. Relation between bispectral index measurements of anesthetic depth and postoperative mortality: A meta-analysis of observational studies. Can J Anaesth. 2017;64:597–607. doi: 10.1007/s12630-017-0872-6. [DOI] [PubMed] [Google Scholar]
- 54.Aceto P, Lai C, De Crescenzo F, Crea MA, Di Franco V, Pellicano GR, et al. Cognitive decline after carotid endarterectomy: Systematic review and meta-analysis. Eur J Anaesthesiol. 2020;37:1066–74. doi: 10.1097/EJA.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 55.Aceto P, Russo A, Galletta C, Schipa C, Romanò B, Luca E, et al. Relationship between middle cerebral artery pulsatility index and delayed neurocognitive recovery in patients undergoing robot-assisted laparoscopic prostatectomy. J Clin Med. 2023;12:1070. doi: 10.3390/jcm12031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aceto P, Dello Russo C, Lai C, Perilli V, Fucci N, De Giovanni N, et al. Relationship between blood remifentanil concentration and stress hormone levels during pneumoperitoneum in patients undergoing laparoscopic cholecystectomy. Eur Rev Med Pharmacol Sci. 2017;21:4419–22. [PubMed] [Google Scholar]
- 57.Won YJ, Oh SK, Lim BG, Kim YS, Lee DY, Lee JH. Effect of surgical pleth index-guided remifentanil administration on perioperative outcomes in elderly patients: A prospective randomized controlled trial. BMC Anesthesiol. 2023;23:57. doi: 10.1186/s12871-023-02011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cappoli N, Aceto P, Tabolacci E, Mezzogori D, Sollazzi L, Navarra P, et al. Effects of remifentanil on human C20 microglial pro-inflammatory activation. Eur Rev Med Pharmacol Sci. 2021;25:5268–74. doi: 10.26355/eurrev_202108_26547. [DOI] [PubMed] [Google Scholar]
- 59.Dello Russo C, Cappoli N, Tabolacci E, Sollazzi L, Navarra P, Aceto P. Remifentanil does not affect human microglial immune activation in response to pro-inflammatory cytokines. EXCLI J. 2023;22:295–309. doi: 10.17179/excli2022-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aceto P, Lai C, Perilli V, Sacco T, Modesti C, Raffaelli M, et al. Factors affecting acute pain perception and analgesics consumption in patients undergoing bariatric surgery. Physiol Behav. 2016;163:1–6. doi: 10.1016/j.physbeh.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 61.Corcione A, Angelini P, Bencini L, Bertellini E, Borghi F, Buccelli C, et al. Joint consensus on abdominal robotic surgery and anesthesia from a task force of the siaarti and sic. Minerva Anestesiol. 2018;84:1189–208. doi: 10.23736/S0375-9393.18.12241-3. [DOI] [PubMed] [Google Scholar]
- 62.Aceto P, Beretta L, Cariello C, Claroni C, Esposito C, Forastiere EM, et al. Joint consensus on anesthesia in urologic and gynecologic robotic surgery: Specific issues in management from a task force of the SIAARTI, SIGO, and SIU. Minerva Anestesiol. 2019;85:871–85. doi: 10.23736/S0375-9393.19.13360-3. [DOI] [PubMed] [Google Scholar]
- 63.Aceto P, Galletta C, Cambise C, Punzo G, Luca E, Schipa C, et al. Challenges for anaesthesia for robotic-assisted surgery in the elderly: A narrative review. Eur J Anaesthesiol Intensive Care. 2023;2:e0019. [Google Scholar]
- 64.Lee LA, Athanassoglou V, Pandit JJ. Neuromuscular blockade in the elderly patient. J Pain Res. 2016;9:437–44. doi: 10.2147/JPR.S85183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biro P, Paul G, Dahan A, Brull SJ. Proposal for a revised classification of the depth of neuromuscular block and suggestions for further development in neuromuscular monitoring. Anesth Analg. 2019;128:1361–3. doi: 10.1213/ANE.0000000000004065. [DOI] [PubMed] [Google Scholar]
- 66.Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387:2655–64. doi: 10.1016/S0140-6736(15)00981-2. [DOI] [PubMed] [Google Scholar]
- 67.Bindu B, Bindra A, Rath G. Temperature management under general anesthesia: Compulsion or option. J Anaesthesiol Clin Pharmacol. 2017;33:306–16. doi: 10.4103/joacp.JOACP_334_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simegn GD, Bayable SD, Fetene MB. Prevention and management of perioperative hypothermia in adult elective surgical patients: A systematic review. Ann Med Surg. 2021;72:103059. doi: 10.1016/j.amsu.2021.103059. [DOI] [PMC free article] [PubMed] [Google Scholar]