Abstract
Hepatitis D virus (HDV), also referred to as hepatitis delta virus, is the smallest virus capable of causing human disease. It is unable to replicate on its own and can only propagate in the presence of hepatitis B virus (HBV). Infection with both HBV and HDV frequently results in more severe disease than HBV alone, with higher instances of cirrhosis, liver failure and hepatocellular carcinoma (HCC). Thus, there is a need for effective treatment for HDV; however, currently approved treatment options are very limited both in terms of their efficacy and availability. This makes the management of HDV a challenge for physicians. In this review, we look at the background, diagnosis and treatment of HDV, informed by our hospital data, to set out the optimal management of HDV; we also explore novel treatment options for this disease.
KEYWORDS: Hepatitis D virus, bulevirtide, lonafarnib, nucleic acid polymer REP 2139, pegylated interferon
Background
Hepatitis D virus (HDV) is a small, single-stranded RNA virus enveloped in the surface antigen of the hepatitis B virus (HBV).1 It only replicates in hepatocytes and requires the presence of HBV to enter a hepatocyte via the liver-specific bile acid transporter, sodium taurocholate co-transporting polypeptide (NTCP), for replication and further spread.1,2 Upon entering the cell, the HDV genome translocates into the nucleus and utilises host cellular RNA polymerases for replication.1 Further hepatocytes can be infected through not only the NTCP receptor, but also cell division-mediated RNA spread from already-infected cells.2 The damage to hepatocytes caused by HDV is via a combination of direct cytopathic effects and immune-mediated damage.1 The impact of this damage and the course of HDV infection can vary and can range from asymptomatic to fulminant liver failure.3
During the acute phase, HDV has a similar presentation to other forms of viral hepatitis, with the initial development of flu-like symptoms, fatigue, anorexia and nausea, accompanied by an increase in liver enzymes.4 This can then be followed by jaundice, dark urine, and pale stools as bilirubin increases before symptoms often resolve.4 This can occur in HDV co-infection, where the transmission of HDV occurs simultaneously with HBV.3
HDV co-infection can result in an otherwise unremarkable acute hepatitis with clearance of both viruses, but it can also cause severe hepatitis associated with a high fatality rate.2,3 It is thought that this severe presentation is due, in part, to an exaggerated immune response, leading to massive hepatocyte necrosis and, thus, hepatic failure.1 In rare cases, there is such catastrophic liver damage that it results in acute liver failure, accompanied by coagulopathy and hepatic encephalopathy, which invariably requires liver transplantation if available.4
The acute phase of the infection also occurs with superinfection, which is defined as de novo HDV acquisition in an individual already infected with HBV.3 In this case, the acute phase is often more severe and can also lead to severe hepatitis and liver failure.4 Where superinfection occurs, it can be transient but unfortunately, around 90% of cases will lead to a chronic infection.4 This failure to clear the virus is thought to result from a delayed and insufficient immune response, partly because cytotoxic T lymphocytes are unable to recognise the HDV-infected cells effectively.1
Chronic HDV infection can lead to the complications of liver cirrhosis, liver failure with decompensation and hepatocellular carcinoma (HCC).5 In fact, it is estimated that around 70% of cases will progress to liver cirrhosis within 5–10 years, although true longitudinal case series from divergent geographical areas are lacking.4 However, it has been demonstrated that the presence of HDV results in more rapid progression to liver cirrhosis and an increased risk of developing HCC compared with HBV infection alone.3
The clinical course of the infection is also affected by the HDV genotype. Eight genotypes of HDV have been identified so far, with two–four subtypes per genome.6 The precise genotype exerts an influence on clinical outcome. Genotype 1 is the most common worldwide. It has a lower rate of remission compared with other genotypes and also shows an aggressive disease.3,6 Additionally, genotype 3 has been found to be most pathogenic,6 but there is ongoing research to identify HDV genotypes, their associated phenotypes and their geographical distribution.
The worldwide prevalence of HDV has proven difficult to ascertain reliably because of differences in populations and testing, which has resulted in a wide variation in the figures published.6 Most of the figures are based on antibody positivity rates, whereas true viraemic prevalence rates are important to ascertain because only those individuals are likely to experience HDV-related morbidity and mortality.3 With this in mind, a recent systematic review estimated that 12 million people worldwide have experienced HDV infection, and that one in 22 people with HBV also have HDV.3 HDV is transmitted by all parenteral routes demonstrated for HBV, including sexual transmission.3 Vertical transmission from mother to child appears to be rare, but research is lacking.7 Epidemiological research has also been able to identify those most at risk of having HDV. It appears to be more common in injecting drug users, those with hepatitis C virus or HIV infection (possibly because of shared risk factors), recipients of haemodialysis, men who have sex with men, and sex workers.3,8 In addition, differences in geographical distribution have been identified, with Mongolia, the Republic of Moldova and Western and Middle Africa showing a high prevalence rate.3 These geographical differences have complex reasons but are partly because of differences in the prevalence of HBV.3
Diagnosis
Testing for HDV antibody positivity is the recommended screening test and should be done for all individuals known to be HBV S Ag positive.9–11 If the individual is antibody positive, then measurement of HDV RNA is mandatory to confirm the presence or absence of active infection.10 Periodic screening should then be carried out of those who continue to be at risk of HDV acquisition through ongoing risk factors, or those who are found to be anti-HDV positive but were HDV RNA negative initially.10
Current treatment
The aim of current treatment is to prevent complications associated with HDV.5 Treatment that reduces HDV RNA levels has been shown to improve outcomes even when undetectable levels could not be achieved.5 Treatment should aim to suppress HDV replication (suggested to be a decline of at least ≥2 log at the end of treatment) and normalise liver enzymes.5,10
Pegylated interferon-alpha (Peg-IFN-alpha) is the primary drug of choice for the treatment of HDV.9,10 IFN-alpha is a type I interferon, widely used to treat many viral infections because of its beneficial antiviral and immunomodulatory actions.12,13 Peg-IFN-alpha has shown useful on-treatment virological response rates, although low rates of HDV RNA negativity at 24 weeks after cessation have been shown.9,14 Additionally, late relapses are very common, at more than 50% of responder patients and there is a lack of data to confirm optimal duration of therapy.9 Given the lack of data on the optimal length of treatment, it is advised that Peg-IFN-alpha is continued for 48 weeks irrespective of response as long as it is well tolerated.9 One of the limitations of Peg-IFN-alpha are the significant adverse effects, including flu-like symptoms, depression and cytopenias.13 Additionally, Peg-IFN-alpha should be avoided in decompensated liver disease, leaving consideration for liver transplantation as one of the few remaining options.9 We assessed basic data from our local cohort of patients with HDV and also identified, similar to the literature, intolerability to Peg-IFN-alpha. The basic data of our cohort are provided in Table 1.
Table 1.
Basic characteristics of local patients with HDV diagnosed between July 2009 and December 2021
| Parameters (n=61) | Number of patients, n (%) |
|---|---|
| Mean age in years, (range) | 44.8 (23–76) |
| Sex | |
| Male | 26 (42%) |
| Female | 35 (58%) |
| Ethnicity | |
| Asian | 13 (21%) |
| Black | 8 (13%) |
| Mixed | 9 (15%) |
| White | 13 (21%) |
| Unknown | 18 (30%) |
| Virology | |
| HBS Ag | 61 (100%) |
| HBeAg +ve | 14 (23%) |
| HBeAg –ve | 47 (77%) |
| Hep e Ag Ab +ve | 46 (75%) |
| Hep e Ag Ab –ve | 15 (25%) |
| HBV core Ab +ve | 59 (89%) |
| HCV Ab +ve | 2 (3%) |
| HIV Ag/Ab | 0 (0%) |
| HDV virology | |
| HDV IgG Ab | 61 (100%) |
| HDV RNA +ve | 22 (36%) |
| Staging of liver disease | |
| Cirrhosis on imaging | 30 (49%) |
| Mean transient elastography at diagnosis in kPa (range) | 6.44 (2.7–23.2) |
| Chronic HBV therapy | |
| Tenofovir | 20 (33%) |
| Entecavir | 14 (23%) |
| Lamivudine | 7 (12%) |
| HDV-specific therapy | |
| IFN-α | 17 (28%) received treatment; 13 (21%) did not continue because of adverse effects |
| Liver transplantation | 13 (21%) |
| Death | 3 (5%) |
| Blood tests at diagnosis | Mean value (range) |
| AST (U/L) | 54.64 (14–199) |
| ALT (U/L) | 98.18 (9–2236) |
| ALP (U/L) | 72 (28–201) |
| Bilirubin (μmol/L) | 18.93 (2–319) |
| Albumin (g/L) | 43 (23–56) |
| Sodium (mmol/L) | 140.26 (128–146) |
| Creatinine (mmol/L) | 68.95 (36–164) |
| INR | 1.18 (0.9–3.7) |
| Hb (g/L) | 131.63 (79–169) |
| MCV (fL) | 86.2 (65–103.1) |
| Neutrophils (109/L) | 2.82 (0.4–7.7) |
| Lymphocytes count (109/L) | 1.53 (0.4–3.3) |
| Total white cell count (109/L) | 4.84 (1.7–11.2) |
| Platelet count (109/L) | 157.1 (29–383) |
Ab = antibody; Ag = antigen; ALP= alkaline phosphatase; ALT = alanine transaminase; AST = aspartate transaminase; HCV = hepatitis C virus; HDV = hepatitis D virus; HB = hepatitis B virus; IFN = inteferon; INR = international normalised ratio.
Bulevirtide is an entry inhibitor that competitively inhibits HDV binding to human (h)NTCP, an important step needed for virus entry into cells.13 It has been studied as both a monotherapy and in combination with older agents, such as Peg-IFN-alpha and tenofovir, in trials so far.13,15 As a monotherapy, patients have shown good responses to bulevirtide, with significant reductions in HDV RNA levels. However, most patients do not achieve undetectable HDV RNA levels with bulevirtide monotherapy, and some experience HDV RNA level rebounds after the end of treatment, with limited off-treatment sustained responses.13,16,17 Bulevirtide has also shown promise when used in combination with Peg-IFN-alpha, with 50% of patients having undetectable HDV RNA at 24 weeks follow-up.15 Bulevirtide has been given conditional approval in the EU as a monotherapy for patients with chronic HDV infection and compensated liver disease.18 Real-world cohort studies from Germany, Italy and France presented at both the main European and American liver society meetings in 2022 have shown on-going efficacy in terms of both viral suppression and alanine transaminase (ALT) normalisation in patients treated beyond 48 weeks with bulevirtide, with preliminary data demonstrating that there are early signs of improvements in liver stiffness (as assessed by liver elastography).19–23 It is licensed in the UK and has been provided to a very small number of patients with Child–Pugh Score 5 and 6 cirrhosis as part of a compassionate access programme. At the time of writing, the drug is being evaluated by the National Institute for Healthcare and Clinical Excellence (NICE). It is important to note the difference in price for bulevirtide compared with Peg-IFN-alpha (£6500 versus £76.51); this would need to be addressed further in practice, especially in low-resource settings.24,25
In the future, it is likely that the optimal treatment modalities for HDV will not only involve HDV-targeted therapies, but also the eradication of HBV; if HBV infection resolves, then HDV will also be cleared because of its reliance on HBV.15 This is why clinical trials evaluating the efficacy of agents being tested in functional cure programmes for HBV are now being extended into the HDV field.
New HDV-targeting therapies
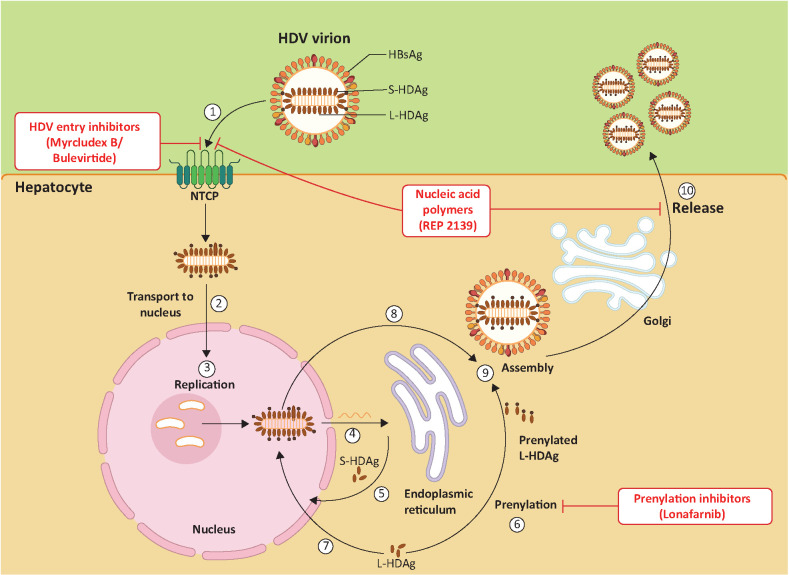
Novel therapies have been developed to target different steps in the life cycle of HDV and to activate innate immunity. These include novel interferons, prenylation inhibitors, and nucleic acid polymers,5,15 as summarised in Table 2. Figure 1 details the mechanism of action involved in relation to the HDV life cycle.
Table 2.
Emerging novel therapies for HDV
| Drug | Type | Actions | Delivery | Phase of development | Comments |
|---|---|---|---|---|---|
| Peg-IFN-lambda | Type III Interferon | Immune modulation | Subcutaneous injection | Phase II | Virological response at 24 weeks post treatment = 36% Better tolerated than Peg-IFN-alpha |
| Lonafarnib | Farnesyl transferase inhibitor | Inhibits HDV genome replication and induces innate immunity | Oral | Phase III | HDV RNA levels decline with monotherapy Combining with ritonavir allowed lower doses to be used and had fewer GI side effects Combining with Peg-IFN-lambda resulted in 50% of patients achieving undetectable HDV RNA levels at 24 weeks' post treatment Most adverse effects are mild to moderate and include GI disturbances, weight loss, hyperbilirubinaemia, anaemia and transient ALT increases |
| REP 2139-Ca | Nucleic acid polymer | Inhibits viral entry Interaction with amphipathic protein domains interrupts viral replication |
Intravenous infusion | Phase II | When administered as part of a regime including Peg-IFN-alpha, 75% of patients were HDV RNA negative at end of treatment Tolerable adverse effects |
ALT = alanine transaminase; GI = gastrointestinal; HCV = hepatitis D virus; IFN = inteferon; PEG = pegylated.
Fig 1.
Hepatitis D viral life cycle and sites of potential therapies. (1) Hepatitis D virus (HDV) attaches to hepatocytes via interactions between hepatitis D surface antigen protein and sodium taurocholate cotransporting polypeptide (NTCP). (2) HDV ribonucleoprotein (RNP) is translocated to the nucleus by the hepatitis D antigen (HDAg) and (3) HDV replication occurs. (4) The HDV antigenome is transported to the endoplasmic reticulum (ER), where (5) it is translated into small HDAg (S-HDAg) and large HDAg (L-HDAg). (6) L-HDAg is prenylated before assembly. (7) S-HDAg supports HDV replication and is recycled into the nucleus. (8) New RNPs via new HDAg molecules are formed and exported into the cytoplasm. (9) New HDV RNP associates with hepatitis B virus (HBV) envelope proteins and is assembled into HDV virions. (10) HDV virions are released from the hepatocyte via the Golgi apparatus. Figure created with BioRender (biorender.com).
Peg-IFN-lambda
Peg-IFN-lambda is a type III interferon that is associated with fewer adverse effects compared with Peg-IFN-alpha and, thus, is better tolerated and has better patient compliance.13,15 The improved adverse effect profile results from the receptors for type III interferons only being present on epithelial cells mostly in the liver and lungs, compared with the receptors for type I interferons, which are present on most cells in the body.13 Peg-IFN-lambda has shown a better virological response at 24 weeks' post treatment compared with Peg-IFN-alpha (36% versus 28%) and, although there is only limited histological evidence, this demonstrated fibrosis regression following therapy.13,15 It is currently being tested in larger phase III trials.21–23
Lonafarnib
Lonafarnib is a prenylation inhibitor that prevents the release of HDV particles by inhibiting HDV genome replication and inducing innate immunity.13,15 It prevents the prenylation of the HDV large antigen, a step that is crucial to the HDV life cycle.15,26 Initial trials looked at lonafarnib monotherapy and found that HDV RNA level declines were dose dependent and correlated with lonafarnib serum concentrations.13 Lonafarnib was then further studied as both a monotherapy and in combination with ritonavir and/or Peg-IFN-alpha, with the latter being found to work synergistically with lonafarnib.13,15,26,27 Additionally, low doses of oral lonafarnib achieved improved antiviral responses with better tolerated adverse effects compared with high-dose regimens.13 Most adverse effects have been mild to moderate, and have included gastrointestinal disturbances, weight loss, hyperbilirubinaemia and anaemia.15,26,27 Well-tolerated transient increases in ALT post treatment have been seen, with subsequent normalisation.13 Importantly, these studies identified regimens to advance into phase III trials; lonafarnib with ritonavir alone and lonafarnib, ritonavir and Peg-IFN-alpha combined.13 At the time of writing, this phase III trial is ongoing. Of note, phase II trials have also combined lonafarnib with Peg-IFN-lambda.13 The LIFT-1 study found that over 75% of patients achieved a significant decline in HDV RNA, with 50% achieving undetectable levels at 24 weeks.28 Adverse effects were mostly mild to moderate.13,28 Further phase II trials are being conducted.13
REP 2139
Nucleic acid polymers show antiviral activity, which is thought to result from entry inhibition and their interaction with amphipathic protein domains vital to viral replication.13 At the time of writing, only one trial had been conducted into the effects of nucleic acid polymers on HDV.29 This followed 12 patients for 3.5years following a regimen of weekly intravenous infusions of REP 2139-Ca for 30 weeks augmented with Peg-IFN-alpha.29 At the end of treatment, 75% of patients were HDV RNA negative, and follow-up showed sustained virological responses.29 Additionally, the regime had a good safety profile and tolerable adverse effects.29 Further studies with REP2139-Mg in combination with Peg-IFN-alpha and tenofovir are planned, with the added benefit of allowing transition to subcutaneous dosing in future trials.29–31 Compassionate use data from Bourliere et al. similarly found REP 2139 to be safe, tolerable and effective in three patients over a 48-week period, with HDV RNA becoming undetectable at 4–5 weeks of treatment.32
Conclusion
The presence of HDV often results in a more severe disease profile, with higher incidences of cirrhosis, liver failure and HCC. Although there is a lack of approved treatments, there are many promising targets for future antiviral therapies. Although treatment has previously relied exclusively on PEG-IFN-alpha, the provisional approval of bulevirtide heralds an era in which alternative therapeutic strategies should be available.
Therefore, early diagnosis and risk stratification of patients with HBV/HDV is increasingly important to prevent the development of more advanced liver disease. Future research will continue to identify different stages of the life cycle of HDV that could be targets for new treatments. Additionally, further evaluation of combination therapies will be important, looking at both their efficacy and safety.
Key practice implications
Until recently, there were no specific treatments for hepatitis D virus (HDV) apart from pegylated interferon-α (Peg-IFN-alpha). Most patients with HDV discontinue Peg-IFN-alpha treatment because of intolerability and it is also contraindicated in many patients. Therefore, there is a need for new agents to treat HDV.
Bulevirtide is a new HDV treatment, recently given approval by the European Medicine Agency and being evaluated by the National Institute for Health and Care Excellence. It shows some promise as a monotherapy and in combination with Peg-IFN-alpha for the treatment of HDV.
There is also ongoing research into new agents, such as lonafarnib and the nucleic acid polymer REP2139. Therefore, it will be important to ensure patients have access to novel therapies as they become available.
References
- 1.Abbas Z, Afzal R. Life cycle and pathogenesis of hepatitis D virus: a review. World J Hepatol 2013;5:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urban S, Neumann-Haefelin C, Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut 2021;70:1782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockdale AJ, Kreuels B, Henrion MYR, et al. The global prevalence of hepatitis D virus infection: systematic review and meta-analysis. J Hepatol 2020;73:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farci P, Niro GA. Clinical features of hepatitis D. Semin Liver Dis 2012;32:228–36. [DOI] [PubMed] [Google Scholar]
- 5.Sandmann L, Cornberg M. Experimental drugs for the treatment of hepatitis D. J Exp Pharmacol 2021;13:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da BL, Heller T, Koh C. Hepatitis D infection: from initial discovery to current investigational therapies. Gastroenterol Rep (Oxf) 2019;7:231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellier PO, Maylin S, Brichler S, et al. Hepatitis B Virus-Hepatitis D Virus mother-to-child co-transmission: a retrospective study in a developed country. Liver Int 2018;38:611–8. [DOI] [PubMed] [Google Scholar]
- 8.Chen HY, Shen DT, Ji DZ, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut 2019;68:512–21. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- 10.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivero A, Smedile A. Hepatitis delta virus diagnosis. Semin Liver Dis 2012;32:220–7. [DOI] [PubMed] [Google Scholar]
- 12.Lunemann S, Malone DF, Grabowski J, et al. Effects of HDV infection and pegylated interferon α treatment on the natural killer cell compartment in chronically infected individuals. Gut 2015;64:469–82. [DOI] [PubMed] [Google Scholar]
- 13.Elazar M, Glenn JS. Combination of novel therapies for HDV. Viruses 2022;14:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alavian SM, Tabatabaei SV, Behnava B, et al. Standard and pegylated interferon therapy of HDV infection: a systematic review and meta- analysis. J Res Med Sci 2012;17:967–74. [PMC free article] [PubMed] [Google Scholar]
- 15.Khan IW, Dad Ullah MU, Choudhry M, et al. Novel Therapies of Hepatitis B and D. Microorganisms 2021;9:2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J Hepatol 2016;65:490–8. [DOI] [PubMed] [Google Scholar]
- 17.Pietro L, Roulot D, Wedemeyer H. Bulevirtide with or without pegIFNα for patients with compensated chronic hepatitis delta: From clinical trials to real-world studies. J Hepatol 2022;77:1422–30. [DOI] [PubMed] [Google Scholar]
- 18.Kang C, Syed YY. Bulevirtide: first approval. Drugs 2020;80:1601–5. [DOI] [PubMed] [Google Scholar]
- 19.Fontaine H, Fougerou-Leurent C, Gordien E, et al. OS093 Real life study of bulevirtide in chronic hepatitis delta: preliminary results of the ANRS HD EP01 Bule Delta prospective cohort. J Hepatol 2022;77:S72. [Google Scholar]
- 20.Degasperi E, Anolli MP, Uceda Renteria SC, et al. Bulevirtide monotherapy for 48 weeks in patients with HDV-related compensated cirrhosis and clinically significant portal hypertension. J Hepatol 2022;77:1525–31. [DOI] [PubMed] [Google Scholar]
- 21.Loglio A, Ferenci P, Uceda Renteria SC, et al. Safety and effectiveness of up to 3 years' bulevirtide monotherapy in patients with HDV-related cirrhosis. J Hepatol 2022;76:464–9. [DOI] [PubMed] [Google Scholar]
- 22.Wedemeyer H, Schöneweis K, Bogomolov P, et al. Safety and efficacy of bulevirtide in combination with tenofovir disoproxil fumarate in patients with hepatitis B virus and hepatitis D virus coinfection (MYR202): a multicentre, randomised, parallel-group, open-label, phase 2 trial. Lancet Infect Dis 2022;23:117–29. [DOI] [PubMed] [Google Scholar]
- 23.Herta T, Hahn M, Maier M, et al. Efficacy and safety of bulevirtide plus tenofovir disoproxil fumarate in real-world patients with chronic hepatitis B and D co-infection. Pathogens. 2022;11:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joint Formulary Committee . Bulevirtide. https://bnf.nice.org.uk/drugs/Bulevirtide [Accessed: 24 May 2023].
- 25.Joint Formulary Committee . Peg-IFN-alpha. https://bnf.nice.org.uk/drugs/Peg-IFN-alpha [Accessed: 24 May 2023].
- 26.Yurdaydin C, Keskin O, Yurdcu E, et al. A phase 2 dose-finding study of lonafarnib and ritonavir with or without interferon alpha for chronic delta hepatitis. Hepatology 2022;75:1551–65. [DOI] [PubMed] [Google Scholar]
- 27.Yurdaydin C, Keskin O, Kalkan Ç, et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology 2018;67:1224–36. [DOI] [PubMed] [Google Scholar]
- 28.Koh C, Hercun J, Rahman F, et al. A Phase 2 study of peginterferon lambda, lonafarnib and ritonavir for 24 weeks: end-of-treatment results from the LIFT HDV study. J Hepatol 2020;73:S130. [Google Scholar]
- 29.Bazinet M, Pântea V, Cebotarescu V, et al. Persistent control of hepatitis B virus and hepatitis delta virus infection following REP 2139-Ca and pegylated interferon therapy in chronic hepatitis B virus/hepatitis delta virus coinfection. Hepatol Commun 2021;5:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaillant A. REP 2139: antiviral mechanisms and applications in achieving functional control of HBV and HDV infection. ACS Infect Dis 2019;5:675–87. [DOI] [PubMed] [Google Scholar]
- 31.Bazinet M, Pântea V, Cebotarescu V, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2017;2:877–889. [DOI] [PubMed] [Google Scholar]
- 32.Bourliere M, Loustaud-Ratti V, Stern C, et al. Compassionate use of subcutaneously administered REP2139-MG in cirrhotic HBV/HDV co-infection. Hepatology 2022:76:Poster 1223. [Google Scholar]