Abstract
An optimal assay for high-throughput screening for new antituberculosis agents would combine the microplate format and low cost of firefly luciferase reporter assays and redox dyes with the ease of kinetic monitoring inherent in the BACTEC system. The green fluorescent protein (GFP) of the jellyfish Aequorea victoria is a useful reporter molecule which requires neither substrates nor cofactors due to the intrinsically fluorescent nature of the protein. The gene encoding a red-shifted, higher-intensity GFP variant was introduced by electroporation into Mycobacterium tuberculosis H37Ra and M. tuberculosis H37Rv on expression vector pFPV2. A microplate-based fluorescence assay (GFP microplate assay [GFPMA]) was developed and evaluated by determining the MICs of existing antimycobacterial agents. The MICs of isoniazid, rifampin, ethambutol, streptomycin, amikacin, ofloxacin, ethionamide, thiacetazone, and capreomycin, but not cycloserine, determined by GFPMA were within 1 log2 dilution of those determined with the BACTEC 460 system and were available in 7 days. Equivalent MICs of antituberculosis agents in the BACTEC 460 system for both the reporter and parent strains suggested that introduction of pFPV2 did not influence drug susceptibility, in general. GFPMA provides a unique tool with which the dynamic response of M. tuberculosis to the existing and potential antituberculosis agents can easily, rapidly, and inexpensively be monitored.
High-throughput screening for new antituberculosis agents requires the development of rapid, inexpensive microplate-based assays. Most of the newer methods for clinical drug susceptibility testing of Mycobacterium tuberculosis are either expensive and/or not amenable to high-throughput screening; these include the BACTEC 460 system (10), the BACTEC 9000MB system (13), E-test (16), the Mycobacterium Growth Indicator Tube (17), and the tube-based Alamar Blue colorimetric assay (19). Microplate-based methods used with M. tuberculosis have included the microplate Alamar Blue assay (MABA) (4) and the luciferase reporter gene assay (1, 5). The latter is the only reporter gene that has been described for use in the determination of the MICs for mycobacteria.
The green fluorescent protein (GFP) of the jellyfish Aequorea victoria is an unusual protein which is intrinsically fluorescent (i.e., it does not require substrates or cofactors). The chromophore p-hydroxybenzylidine-imidazolidinone is produced posttranslationally in the presence of oxygen from serine-tyrosine-glycine and is fluorescent only when it is embedded within the complete 238-amino-acid GFP. GFP has several properties which are favorable for use as a reporter for bacterial viability and growth, including cytoplasmic location, low toxicity, continuous production during replication, and easy imaging and quantitation (3, 18). Wild-type GFP has been used as a reporter for a variety of organisms including Mycobacterium smegmatis (8) and Mycobacterium bovis BCG (8, 11), with GFP expression detected microscopically (8, 11), by flow cytometry (8), or by fluorometry (11). In M. bovis, GFP expression was shown to be reduced in the presence of four antimycobacterial drugs, but no attempt was made to determine MICs (11). Valdivia et al. (15) used the mycobacterial expression vector pMV261 (14) to introduce into Mycobacterium marinum a mutant GFP (7) with a red-shifted excitation peak (488 nm versus 395 nm for the wild type) and fluorescence that was enhanced at least 20-fold.
In this study we describe the expression of this improved GFP in M. tuberculosis and assess the potential of a microplate-based fluorometric assay (the GFP microplate assay [GFPMA]) for high-throughput screening and kinetic monitoring by comparing the MICs of 12 antimycobacterial agents obtained by GFPMA with those obtained with the BACTEC 460 system.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. tuberculosis H37Ra (ATCC 25177) and M. tuberculosis H37Rv (ATCC 27294) were obtained from the American Type Culture Collection (ATCC; Rockville, Md.). pFPV2 (mycobacterial expression vector pMV261 [14] containing red-shifted, high-expression mutant gfp [7]) was obtained in Escherichia coli DH12S from Raphael Valdivia, Stanford University, and was cultured overnight in Luria-Bertani broth (12) with 30 μg of kanamycin per ml.
Electroporation, plasmid transformation, and clone selection.
Plasmid DNA from E. coli DH12S gfp was isolated by miniprep extraction and was cut with KpnI (one site for pFPV2). The presence of the linear plasmid was confirmed on a 1.0% agarose gel.
H37Ra and H37Rv were cultivated in 7H9GC broth containing 0.05% (vol/vol) Tween 80 as described previously (4), pelleted, and then suspended in 10% sterile glycerol.
Electroporation and selection of transformants were performed essentially as described by Cooksey et al. (5). The transformants (H37Rv gfp and H37Ra gfp) were cultured in 7H9GC broth with Tween 80 (30 μg of kanamycin per ml) and were incubated until turbidity was observed. The cultures were screened for fluorescence in a Cytofluor II microplate fluorometer (PerSeptive Biosystems, Framingham, Mass.) in the bottom-reading mode with excitation at 485 nm and emission at 508 nm. The transformants with the highest fluorescent output were cultured in 100 ml of 7H9GC with Tween 80 and 30 μg of kanamycin per ml. Suspensions were washed, suspended in 20 ml of phosphate-buffered saline, and passaged through an 8-μm-pore-size filter, and aliquots were stored at −80°C.
Antimicrobial agents.
Amikacin sulfate, capreomycin, cycloserine, ethambutol HCl, ethionamide, isoniazid, kanamycin sulfate, rifampin, streptomycin sulfate, and thiacetazone were purchased from Sigma Chemical Company. Clarithromycin and ofloxacin were gifts from Abbott Laboratories, North Chicago, Ill., and R. W. Johnson Pharmaceutical Research, Raritan, N.J., respectively. Stock solutions of antimicrobial agents were prepared in either dimethyl sulfoxide, distilled water, or 0.1 N NaOH, filter sterilized, and stored at −70°C for not more than 30 days.
GFPMA.
Antimicrobial susceptibility testing was performed in black, clear-bottom, 96-well microplates (black viewplates; Packard Instrument Company, Meriden, Conn.) in order to minimize background fluorescence. Outer-perimeter wells were filled with sterile water to prevent dehydration in the experimental wells. Initial drug dilutions were prepared in either dimethyl sulfoxide or distilled deionized water, and subsequent twofold dilutions were prepared in 0.1 ml of 7H9GC broth (minus Tween 80) in the microplates. Frozen H37Ra gfp and H37Rv gfp were thawed, sonicated for 15 s, and cultured at 37°C with shaking in 100 ml of 7H9GC with Tween 80 and 30 μg of kanamycin per ml until the optical density at 550 nm reached 0.4 to 0.5. Cultures were diluted in 7H9GC, and 105 CFU was added to each test well in a volume of 0.1 ml. The final medium volume was 200 μl, and the final bacterial density was 5 × 105 CFU/ml. Wells containing drug only were used to detect autofluorescence of the compounds. Additional control wells consisted of bacteria only (B wells) and medium only (M wells). Plates were incubated at 37°C. Fluorescence was measured daily for 8 days in a Cytofluor II microplate fluorometer in the bottom-reading mode with excitation at 485 nm and emission at 508 nm. The mean for triplicate M wells was used as a background subtraction for all test wells and B wells. Percent inhibition was defined as 1 − (test well fluorescence units/mean fluorescence units of triplicate B wells) × 100 on day 7 of incubation. The lowest drug concentration effecting inhibition of 90% was considered the MIC.
Determination of MBCs.
At day 7 of incubation of the plates used for MIC determination, 20 μl from each well was dropped onto complete 7H11 agar plates, and the plates were incubated until countable colonies appeared (approximately 14 days). The minimal bactericidal concentration (MBC) was considered to be the lowest concentration of drug which resulted in ≤1% of the CFU in the B wells on day zero.
Assay with the BACTEC 460 system.
Antimicrobial susceptibilities were determined in the BACTEC 460 system as described previously (4). In brief, twofold dilutions of each antimicrobial agent were made in the applicable solvent, and 50 μl was delivered to individual BACTEC vials. The inoculum was prepared as described above and diluted in BACTEC 12B medium, and 0.1 ml containing 2 × 106 CFU was delivered to 4 ml of BACTEC 12B medium (final bacterial density, 5 × 105 CFU/ml). Some control vials received an inoculum which was further diluted 1:100. The vials were incubated at 37°C, and the growth index (GI) for each vial was determined in a BACTEC 460 instrument (Becton Dickinson, Sparks, Md.) until the GI of the 1:100 controls reached at least 30. The GI was then read on the following day, and the GI and daily changes in GI (ΔGI) were recorded for each drug dilution. The MIC was defined as the lowest drug concentration for which the ΔGI was less than the ΔGI of the 1:100 control.
RESULTS
Electroporation, transformation, and colony screening.
H37Ra gfp and H37Rv gfp transformants growing on kanamycin-supplemented medium were identified by epifluorescence microscopy and spectrofluorometry. All kanamycin-resistant transformants which were examined demonstrated at least a low level of fluorescence over background levels. Those colonies demonstrating the most intense fluorescence were selected for antimicrobial susceptibility studies.
GFP expression during growth.
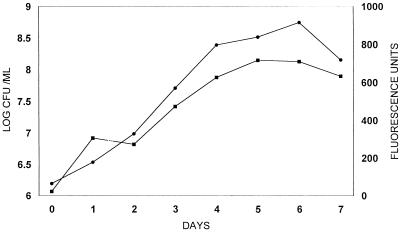
A linear increase in fluorescence units of H37Ra gfp growing in the absence of kanamycin closely paralleled the logarithmic increase in the numbers of CFU for approximately 1 week before reaching a growth plateau (Fig. 1). Significant fluorescence (over background levels) was not observed with nontransformed H37Ra even at a high cell density.
FIG. 1.
GFP expression (fluorescence units; •) and CFU (▪) during growth of M. tuberculosis H37Ra gfp.
Susceptibility testing.
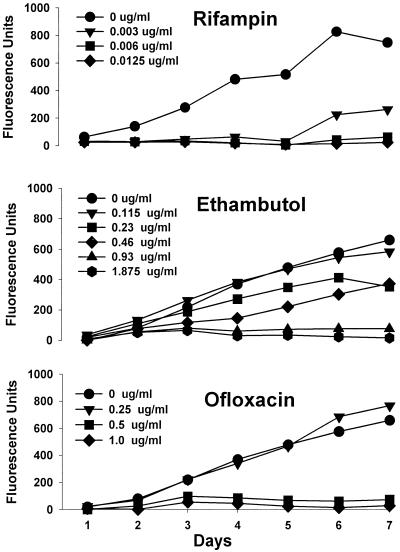
The kinetics of GFP expression by H37Ra gfp in the presence of twofold dilutions of antimicrobial agents was easily monitored by reading the plates daily. Examples of this are demonstrated for rifampin, ethambutol, and ofloxacin (Fig. 2). With rifampin (and also with isoniazid and clarithromycin [data not shown]) at least one drug concentration could be observed to suppress GFP expression for several days, after which expression resumed at a rate similar to that for drug-free controls. With ofloxacin (and capreomycin [data not shown]), completely inhibitory and relatively noninhibitory concentrations differed by as little as twofold. In general, dose-responses were evident for all drugs with the exception of thiacetazone (for which the lowest dose was completely inhibitory) and kanamycin (which failed to inhibit GFP expression due to the presence of the aph gene on pFPV2).
FIG. 2.
Kinetics of inhibition of GFP expression by M. tuberculosis H37Ra gfp during incubation with antimycobacterial agents. Data are from one representative experiment of three replicate experiments.
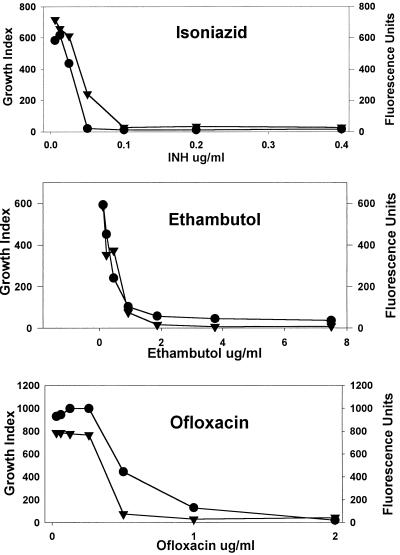
A comparison of the dose-response determined by GFPMA with that determined with the BACTEC 460 system was accomplished by comparing on day 7 of incubation the net fluorescence units with the GI. Examples of the results obtained with isoniazid, ethambutol, and ofloxacin are presented in Fig. 3. The shapes of most of the dose-response curves were very similar for the two assays, except for streptomycin, cycloserine, and ethionamide (data not shown).
FIG. 3.
Dose-responses of M. tuberculosis H37Ra gfp to antimycobacterial agents determined with the BACTEC 460 system (GI; •) and by measuring GFP expression (fluorescence units; ▾) on day 7 of incubation. Data are from one representative experiment of three replicate experiments.
The MICs for H37Ra gfp were determined in three replicate experiments with the BACTEC system and by GFPMA (Table 1). Essentially identical results were obtained in a single experiment with H37Rv gfp (data not shown). The overall correlation between the two assays with H37Ra gfp (Spearman correlation using ranked data) was 0.89346. The MICs obtained by GFPMA were not significantly different from those obtained with the BACTEC 460 system for 10 of 12 drugs (by the Kruskal-Wallis test, P > 0.05). The cycloserine MIC was significantly lower by GFPMA than with the BACTEC system (P < 0.05). Although the MICs of ethionamide for H37Ra gfp differed in the two assays by only one twofold dilution, the difference was statistically significant (P < 0.05). Clarithromycin MICs were variable in both assays, but they were more variable in the microplate assay. For 11 of the 12 drugs tested, the MICs were reproducible between experiments. With the exception of kanamycin, the MICs for H37Ra gfp and the H37Ra parent strain obtained with the BACTEC 460 system differed by no more than one twofold dilution, and overall, there was not a significant difference between the MICs for the strains (by the Kruskal-Wallis test, P > 0.05), indicating a lack of effect of the plasmid on drug susceptibility.
TABLE 1.
Activities of 12 antimicrobial agents against M. tuberculosis H37Ra and H37Ra gfp determined with the BACTEC 460 system and by GFPMA
| Drug | MIC (μg/ml)a
|
MBC (μg/ml)b for H37Ra gfp | ||
|---|---|---|---|---|
| BACTEC 460 system
|
GFPMA with H37Ra gfp | |||
| H37Ra | H37Ra gfp | |||
| Rifampin | 0.025 | 0.0125 | 0.003–0.006 | 0.0125 |
| Isoniazid | 0.05 | 0.0125–0.05 | 0.0125–0.05 | 0.0125 |
| Capreomycin | 2.5 | 2.5 | 2.5 | 5.0 |
| Ofloxacin | 1.0 | 1.0–2.0 | 0.5–1.0 | 0.5 |
| Cycloserine | 50 | 50 | 1.56–3.25c | >50 |
| Clarithromycin | 1.0 | 0.5–2.0 | 0.5–32 | 16 |
| Amikacin | 2.5 | 2.5 | 2.5 | >5.0 |
| Ethambutol | 1.88 | 1.88 | 1.88 | 7.5 |
| Thiacetazone | <0.01 | <0.01 | <0.01–0.03 | >2.0 |
| Ethionamide | 1.25 | 1.25 | 0.313–0.63c | 0.63 |
| Streptomycin | 1.5 | 1.5 | 0.75–1.5 | 3.0 |
| Kanamycin | 2.5 | >5.0 | >5.0 | >5.0 |
Results are ranges for three experiments.
Results are those of a single representative experiment in which the numbers of CFU of H37Ra gfp were determined.
Significantly different (P < 0.05) from the MIC for H37Ra gfp determined with the BACTEC 460 system.
The MBCs, calculated on the basis of the numbers of CFU from pre- and postincubation microplate wells as described above, of all 12 antimicrobial agents were obtained for H37Ra gfp (Table 1). The MBC/fluorometric MIC ratios were 1 to 2 for isoniazid, ofloxacin, capreomycin, and ethionamide, 2 to 4 for rifampin, streptomycin, and ethambutol, 16 to 32 for cycloserine, 0.5 to 32 for clarithromycin, >2 for amikacin, and >67 for thiacetazone.
DISCUSSION
The fact that GFP, despite its general popularity as a reporter, has yet to be exploited in high-throughput antimicrobial screening assays may be due in part to a relatively low signal output. Kremer et al. (11) used a high M. bovis BCG inoculum of 107 CFU/well for the fluorometric measurement of decreases in wild-type GFP during incubation with antimycobacterial agents. Since GFP is relatively stable, this approach risks falsely equating fluorescence with viability, and thus, some active compounds could be overlooked. Our initial attempts at detecting expression of wild-type GFP in M. tuberculosis by microfluorometry were unsuccessful. In the current study, use of a high-intensity, red-shifted, mutant gfp together with an emission filter of 508 nm made possible fluorescence measurement of GFP expression in viable M. tuberculosis at a relatively low bacterial density.
Most metabolism-based estimations of bacterial viability, including luciferase reporter assays, require the addition of substrates at each desired time point, necessitating the preparation of numerous replicate samples. Notable exceptions to this are the BACTEC 460 radiorespirometric system (10) and BACTEC 9000MB (13) and the Mycobacterium Growth Indicator Tube (17) fluorometric assays; however, these are not amenable to high-throughput screening. GFPMA, in contrast, allows repeated measurements of GFP expression in the same culture wells, resulting in a dynamic picture of the bacterial response to various concentrations of antimicrobial agents (Fig. 2). Since the organisms in the cultures are not killed during the assay, MBCs can be determined from the same wells by determining the numbers of CFU on solid medium.
The pMV261 vector used in GFPMA (pFPV2) is essentially identical to that used in the luciferase assay of Cooksey et al. (5, 6) except for the gfp insertion. In both the luciferase system and GFPMA, the vector does not appear to affect susceptibility to antibacterial agents other than the selection marker kanamycin. pFPV2 appears to be maintained, at least temporarily, in the absence of kanamycin pressure, as evidenced by continued GFP expression in M. tuberculosis for several days in 7H9GC. This is consistent with the maintenance of GFP expression in Salmonella typhimurium with the same vector (15). The selection of 7H9GC culture medium was based on both previous results with MABA (4) and the lack of effect of medium composition on GFP expression in M. bovis BCG (11).
To date, the luciferase expression assay has represented the sole example of an assay in which reporter genes are used for antimycobacterial drug screening. With clinical antimycobacterial agents, a good correlation of luminescence-based MICs with those obtained by established assays have been demonstrated for M. tuberculosis (1, 5), Mycobacterium avium (6), M. bovis BCG, and Mycobacterium intracellulare (1). The advantages of the luciferase assays include the ability to measure light output in either a luminometer or a scintillation counter as well as exquisite sensitivity, enabling the detection of as few as 104 CFU.
Luciferase reporter gene assays have also been used to detect antimycobacterial drug activity in macrophages (2) and mice (9). GFP has been demonstrated by epifluorescence microscopy in macrophages infected with gfp-recombinant M. bovis BCG (8), M. marinum (15), and M. tuberculosis (authors’ unpublished data), and frogs infected with M. marinum (15), but attempts to measure GFP expression by fluorometry in intracellular mycobacteria or tissue homogenates have yet to be reported. Assuming sufficient sensitivity, GFP measurement in intracellular environments might preclude the need for host cell lysis (since a substrate is not required), allowing direct and repeated measurements of cell viability.
While assays such as the microplate Alamar Blue assay (4) would appear to be optimal for high-throughput screening with nonengineered M. tuberculosis strains, GFPMA offers several advantages over luciferase detection for in vitro-based reporter assays. The intrinsically fluorescent nature of GFP precludes the need for a substrate; thus, GFPMA offers greater simplicity and easier kinetic monitoring and has a lower cost than the luciferase reporter assays, and GFPMA also has enhanced biosafety since (if MBCs are not also being determined) the microplate need not be reopened following inoculation.
ACKNOWLEDGMENTS
We thank Sandra Oby-Robinson, Carrie McCoy, Angelia Young, Anita Biswas, and Melissa McGuire for technical assistance with inoculum preparation and data management.
This study was supported by intra-agency agreement Y1-AI-50016 with the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Arain T M, Resconi A E, Hickey M J, Stover C K. Bioluminescence screening in vitro (Bio-Siv) assays for high-volume antimycobacterial drug discovery. Antimicrob Agents Chemother. 1996;40:1536–1541. doi: 10.1128/aac.40.6.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arain T M, Resconi A E, Singh D C, Stover C K. Reporter gene technology to assess activity of antimycobacterial agents in macrophages. Antimicrob Agents Chemother. 1996;40:1542–1544. doi: 10.1128/aac.40.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 4.Collins L A, Franzblau S G. Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooksey R C, Crawford J T, Jacobs W R, Shinnick T M. A rapid method for screening antimicrobial agents for activities against a strain of Mycobacterium tuberculosis expressing firefly luciferase. Antimicrob Agents Chemother. 1993;37:1348–1352. doi: 10.1128/aac.37.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooksey R C, Morlock G P, Beggs M, Crawford J T. Bioluminescence method to evaluate antimicrobial agents against Mycobacterium avium. Antimicrob Agents Chemother. 1995;39:754–756. doi: 10.1128/AAC.39.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 8.Dhandayuthapani S L, Via E, Thomas C A, Horowitz P M, Deretic D, Deretic V. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol Microbiol. 1995;17:901–912. doi: 10.1111/j.1365-2958.1995.mmi_17050901.x. [DOI] [PubMed] [Google Scholar]
- 9.Hickey M J, Arain T M, Shawar R M, Humble D J, Langhorne M H, Morgenroth J N, Stover C K. Luciferase in vivo expression technology: use of recombinant mycobacterial reporter strains to evaluate antimycobacterial activity in mice. Antimicrob Agents Chemother. 1996;40:400–407. doi: 10.1128/aac.40.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inderleid C B, Salfinger M. Antimicrobial agents and susceptibility tests: mycobacteria. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 1385–1404. [Google Scholar]
- 11.Kremer L, Baulard A, Estaquier J, Poulain-Godefroy O, Locht C. Green fluorescent protein as a new expression marker in mycobacteria. Mol Microbiol. 1995;17:913–922. doi: 10.1111/j.1365-2958.1995.mmi_17050913.x. [DOI] [PubMed] [Google Scholar]
- 12.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 13.Sorondo G, Rendo A, Rankin I, Boyle J F. Abstracts of the 97th General Meeting of the American Society for Microbiology, 1997. Washington, D.C: American Society for Microbiology; 1997. Comparison of the BACTEC 9000MB, BACTEC 460 and conventional culture in the detection of Mycobacteria from non-pulmonary specimens, poster U-154; p. 570. [Google Scholar]
- 14.Stover C K, de la Cruzz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, Snapper S B, Barletta R G, Jacobs W R, Bloom B R. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 15.Valdivia R H, Hromockyj A E, Monack D, Ramakrishnan L, Falkow S. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene. 1996;173:47–52. doi: 10.1016/0378-1119(95)00706-7. [DOI] [PubMed] [Google Scholar]
- 16.Wagner A, Mills K. Testing of Mycobacterium tuberculosis susceptibility to ethambutol, isoniazid, rifampin, and streptomycin by using E test. J Clin Microbiol. 1996;34:1672–1676. doi: 10.1128/jcm.34.7.1672-1676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters S B, Hanna B A. Testing of susceptibility of Mycobacterium tuberculosis to isoniazid and rifampin by Mycobacterium Growth Indicator Tube method. J Clin Microbiol. 1996;34:1565–1567. doi: 10.1128/jcm.34.6.1565-1567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Hazelrigg T. Implications for bcd mRNA localization from spacial distribution of exu protein in Drosophila oogenesis. Nature. 1994;369:400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- 19.Yajko D M, Madej J J, Lancaster M V, Sanders C A, Cawthon V L, Gee B, Babst A, Hadley K. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol. 1995;33:2324–2327. doi: 10.1128/jcm.33.9.2324-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]