Abstract
Interest in liquid, or nonsurgical rhinoplasty, has increased in demand as patients pursue less invasive techniques to achieve their aesthetic goals. Improved filler technology and refinement in injection techniques have made liquid rhinoplasty a reasonable choice for well‐selected patients in both primary and revision rhinoplasty cases. This article reviews nasal anatomy, injection techniques, selected applications, and safety measures pertinent to performing nonsurgical rhinoplasty.
Keywords: hyaluronic acid, liquid rhinoplasty, minimally invasive rhinoplasty, nonsurgical rhinoplasty, revision rhinoplasty
Key points
Liquid, or nonsurgical rhinoplasty, is an important minimally invasive technique that has many practical applications in both primary and revision rhinoplasty cases.
Significant and potentially devastating complications demand that injectors have an in‐depth knowledge of nasal anatomy, and that proper injection technique and strict adherence to safety precautions are followed when performing nonsurgical rhinoplasty.
INTRODUCTION
Liquid, or nonsurgical, rhinoplasty (NSR), describes the use of injectable filler in the nose to achieve aesthetic and, in some cases, functional results. NSR can be performed in lieu of, or as an adjunct, to surgical rhinoplasty. Despite its steady increase in popularity since the mid‐2000s, “liquid” rhinoplasty is not new. The use of injectable materials to alter the nose was initially described in the late 1800s, when paraffin became the filler of choice for nasal augmentation. This was followed by the use of other injectable liquids including vegetable oil, mineral oil, lanolin, beeswax, and silicone, all of which were abandoned due to complications ranging from granulomatous foreign‐body infections, emboli‐associated phenomena, and severe disfigurement. 1 In 2003, the US Federal Drug Administration (FDA) approved Restylane (Galderma), a nonanimal stabilized hyaluronic acid (HA) filler, for cosmetic use. The ready availability of a safe and reliable product, combined with the advent of the social media age, ushered in a resurgence of demand for minimally invasive filler procedures, not least among them, NSR. Refinements in filler material and technique have made NSR a useful addition to the rhinoplasty surgeon's armamentarium. Despite these technological advancements, potentially catastrophic risks associated with NSR require an extensive knowledge of nasal anatomy, appropriate patient selection, and strict adherence to safety measures.
REVIEW OF ANATOMY
From superficial to deep, the anatomic layers of the nose overlying bone and cartilage are the skin, subcutaneous tissue, superficial fatty layer, superficial musculoaponeurotic system (SMAS), deep areolar plane, and periosteum/perichondrium. 2 The skin of the nose is thicker at the nasion, thinner over the rhinion, and thicker at the nasal tip.
The vascular supply to the nose is located in either the nasal SMAS or the layers superficial to the SMAS. Arterial supply to the nose is derived from the internal carotid artery and the external carotid artery. The internal carotid artery gives rise to the ophthalmic artery. The primary terminal branch of the ophthalmic artery supplying the skin and soft tissue of the nose is the dorsal nasal artery, with contributions from the external nasal branch of the anterior ethmoid artery. The primary external carotid artery branch that supplies the nose is the facial artery, with a contribution from the maxillary artery. As the facial artery courses superiorly, it gives off the superior labial artery, which supplies the columellar artery; the anterior lateral nasal artery; and the angular artery. The anterior lateral nasal artery supplies the nasal tip, and anastomoses with the columellar artery, the infraorbital artery (a branch of the maxillary artery), and the dorsal nasal artery to supply the remainder of the skin and soft tissue of the external nose.
There is significant variation in vascular anatomy with varied patterns of anastomosis. In most patients, the paired lateral nasal arteries and dorsal nasal arteries are found lateral to the midline (Figure 1). In some patients, the vessels anastomose into a complex subdermal plexus. In others, a single larger artery can be identified crossing the midline.
Figure 1.
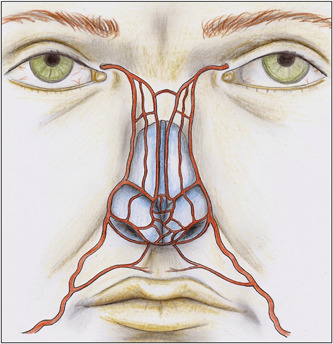
Nasal arterial vasculature from branches of the external carotid artery system (from Saban Y, Amodeo CA, Bouaziz D, et al. Nasal arterial vasculature: medical and surgical applications. Arch Facial Plast Surg. 2012;14:429‐436; with permission).
In a study of 50 cadavers evaluating the presence of the dorsal nasal artery in the nose, Tansatit et al. 3 demonstrated that the dorsal nasal artery arose as a pair in 34% of specimens. In 38% of specimens, the dorsal nasal arteries became small and formed a subcutaneous plexus distributed superficially in the upper two‐thirds of the nose. In the remaining specimens, a single large dorsal nasal artery was present. In 14% of the specimens, the dorsal nasal artery crossed the midline to anastomose with the contralateral lateral nasal artery at the middle third of the nose. In 8% of specimens, a single large dorsal nasal artery descended as a midline artery and formed the nasal tip plexus. In 6% of specimens, the dorsal nasal artery descended and anastomosed with the ipsilateral lateral nasal artery.
Accordingly, the safest location to place filler in the nose is in the deep areolar plane just superficial to the periosteum or perichondrium, and deep to the SMAS and the nasal arteries. The injection should be placed in the midline, and the injector must be mindful that a vessel may be present. Therefore, aspiration before injection should be performed in every case. 4
FILLER CHOICE
FDA‐approved injectable fillers currently in use in facial aesthetics include HAs, calcium hydroxyapatite (CaHA), poly‐l‐lactic acid, and polymethylmethacrylate (PMMA). Use of any dermal filler in the nose is off‐label and patients should be advised accordingly. Autologous fat is also favored by some injectors.
HAs (Restylane; Juvederm, AbbVie) are the most popular fillers used for facial and nasal injections. 5 HA is a polysaccharide that occurs naturally in human tissues. The ability to reverse HA products with hyaluronidase provides an additional layer of safety in cases of patient dissatisfaction or an adverse event.
A variety of HA products exist, each with varied rheological properties that dictate the deformation and flow of the product. G’ is the rheological property that represents a filler's ability to recover its shape after shear deformation is applied to its surface. High G’ products are more firm and provide more lift. Cohesivity describes the internal adhesion forces among individual HA units within the gel. In the nose, a product with high G’ and cohesivity is required to overcome high compression and shearing forces. 6
Restylane is an excellent choice for treatment of both the nasal dorsum and the tip. Restylane has high G’, high cohesivity, and is less hydrophilic than its counterparts, and thus retains its shape after injection with less edema at the injection site.
Duration of effect for HA products is reported to range from 6 months to 18 years depending on the product and site of injection. However, in the nose, multiple authors report duration of treatment up to 8 years after a single injection. 7 , 8 , 9 , 10 Wang et al. 11 demonstrated that injection of cross‐linked HA stimulates de novo production of Type I collagen in the skin. This may account for longer‐term results observed after placement of dermal filler.
Nonreversible filler options include CaHA (Radiesse, Merz Aesthetics), PMMA (Bellafill, Suneva), and autologous fat. CaHA is composed of CaHA spheres suspended in a carboxymethylcellulose gel carrier which are degraded and metabolized by the body over time. CaHA stimulates fibroblast induction and neocollagenesis, with increased collagen production present for a year or longer after injection. 12 CaHA has higher G’ and viscosity than HA fillers, providing adequate definition for use in the nose.
PMMA (Bellafill, Suneva) is a permanent nonreversible filler that consists of PMMA spheres suspended in bovine collagen. Therefore, bovine allergy skin testing is required before use. Unlike CaHA, PMMA microspheres are not metabolized or eliminated by the body. The PMMA microspheres remain permanently in the tissue and stimulate neocollagenesis and fibroplasia, with results lasting 5 years or more. 13 In 2013, Rivkin 14 conducted a prospective study assessing safety and efficacy of NSR using PMMA. The study included 19 patients. 86% of patients were very satisfied or satisfied after injection. No infections, vascular occlusive events, hypersensitivity reactions, or granulomas were seen.
Autologous fat has a long history of use in NSR. Saadoun et al. 15 report that advantages of autologous fat include low cost, biocompatibility, enhanced soft tissue quality of the recipient site, and the regenerative effect of fat grafts on surrounding tissue. However, fat graft resorption, which has been reported to be up to 55%, should be considered.
INDICATIONS
NSR has applications in both primary and secondary rhinoplasty. Patients requiring reduction rhinoplasty are generally not good candidates, as placement of filler in the skin‐soft tissue envelope invariably adds volume to the nose. NSR is a reasonable choice for patients with mild‐to‐moderate nasal deformities, who seek changes to the appearance of the nose, but are not appropriate surgical candidates; who wish to avoid the operative risks, recovery time, and costs associated with surgical rhinoplasty; who seek a temporary solution while awaiting surgery; or who prefer a more immediate solution due to time constraints. Disadvantages of liquid rhinoplasty include its temporary duration and its risk of serious adverse events including necrosis of the nasal skin and irreversible blindness due to vascular occlusion.
Rhinoplasty is a highly challenging surgical procedure. Skin contracture over the bony and cartilaginous framework during the postoperative healing period can result in surface deformities not apparent in the operating room. Published rates for revision rhinoplasty range from 9% to 20%. 16 , 17 For these patients, correction of minor iatrogenic deformities can produce desirable results while avoiding a second surgical procedure. In some cases, the collagen stimulation triggered by filler can provide stable, long‐term results that obviate the need for both revision surgery and repeated injections (Figure 2).
Figure 2.
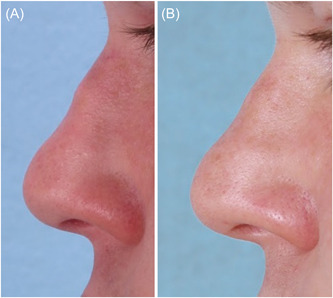
A 22‐year‐old patient who had been treated with three CaHA injections to a postrhinoplasty supratip depression (A). A 20‐year follow‐up after no injections to that region showed improvement of the deformity (B).
A multitude of aesthetic deformities can be adequately addressed during NSR. Dorsal deformities that are amenable to primary NSR include augmentation of the radix, dorsal hump correction, and treatment of mild saddle nose deformity. Correction of nasal sidewall deformities and asymmetries can be performed. In the lower third of the nose, filler can be used to alter tip rotation and projection, to define the supratip break, to improve the infratip lobule, and to address mild cases of alar and columellar retraction. Functionally, filler can be used as in injectable cartilage graft to strengthen the lower lateral cartilage and nasal sidewall and treat dynamic collapse. Fillers can also be used as an injectable spreader graft to widen a narrow internal nasal valve.
PATIENT EVALUATION
The patient most well‐suited for NSR is in good overall health with reasonable postprocedure expectations. This includes an explicit understanding that, in most cases, results obtained with NSR are temporary. Repeat injections may be required to maintain results, with associated costs that may approximate the cost of surgical rhinoplasty over time.
A thorough assessment of aesthetic nasal deformities is performed. Inspection and palpation of the skin of the nose is paramount, as adequate skin laxity is required to accommodate the placement of filler material in the correct plane. Extensive skin tightness of the nasal soft‐tissue envelope, often seen in postrhinoplasty patients, can lead to increased risk of improper filler placement and complications associated with vascular compression. NSR is therefore best avoided in these patients. 18
TECHNIQUE
Before proceeding with NSR, written consent detailing the potential risks, benefits, and alternatives associated with NSR is obtained. Preprocedure photos are taken. An anesthetic agent is not required, but a topical anesthetic such as benzocaine/lidocaine/tetracaine cream can be applied 15–30 min before injection. The skin is thoroughly cleansed with isopropyl alcohol or chlorhexidine gluconate 4.0%.
A 30‐gauge needle is used to deposit small boluses (<0.1 ml) of filler in the supraperiosteal/supraperichondrial plane. The needle should be placed at the level of the periosteum/perichondrium. The injection should be placed in the midline and should proceed from proximal to distal (radix to tip). Given the possible presence of a midline dorsal vessel, a reflux maneuver on the syringe is recommended before each injection. Absence of vascular regurgitation into the syringe does not guarantee against vascular occlusion. The injection should require only light pressure and should proceed slowly. The filler can then be gently massaged to achieve the desired effect. 19
SELECTED APPLICATIONS
Insufficient radix
The radix height should be level with the supratarsal crease or the upper eyelid margin. Filler is placed in small boluses of 0.05 ml or less to avoid blunting the nasofrontal angle. Injection proceeds caudally towards the tip, with care not to overfill the supratip region and create a pollybeak deformity.
Dorsal hump
Filler is first placed superiorly to the hump in the region of the radix. Small boluses of <0.05 ml are used. The filler material is molded into place and reassessed incrementally. Attention is then directed to the area inferior to the hump (Figure 3). Full correction of the hump may require increasing projection of the tip, described below.
Figure 3.
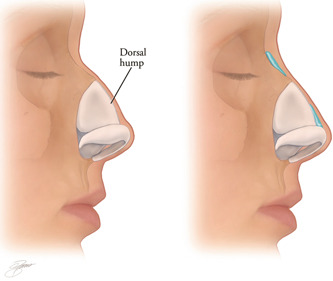
The nasal dorsum is straightened by injection of filler above and below the dorsal nasal hump (Kontis TC, Lacombe V. Cosmetic Injection techniques: A text and video guide to neurotoxins and fillers. 2nd Ed. New York, NY: Thieme Medical Publishers, Inc. 2019, with permission).
Saddle nose deformity
In patients with remaining septal cartilage, filler can be placed against the underlying septum to lift the dorsal soft tissues. This technique is not appropriate for patients with no remaining underlying support or with septal perforation.
Nasal sidewall
Sidewall deformities usually consist of areas of asymmetric concavities. Filler can be placed in the area of concavity in small increments (0.05–0.1 ml). The needle is placed through the skin at the midline and an oblique midline‐to‐lateral approach can be used to approach the concavity, if possible. Filler can also be placed laterally, using extreme caution and very small boluses (Figures 4 and 5).
Figure 4.
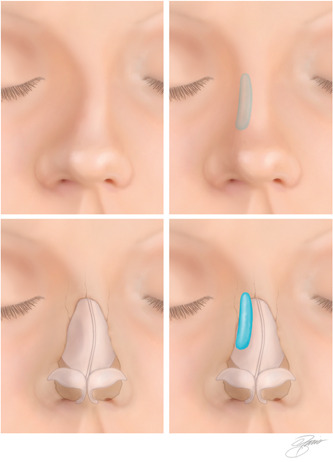
Correction of a saddle nose deformity with filler injection (Kontis TC, Lacombe V. Cosmetic injection techniques: A Text and video guide to neurotoxins and fillers. 2nd Ed. New York, NY: Thieme Medical Publishers, Inc. 2019, with permission).
Figure 5.
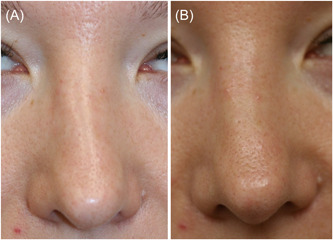
Nonsurgical correction of a crooked nose (A). Hyaluronic acid filler was placed along the left nasal sidewall to give the illusion of a straighter dorsum (B).
Tip refinement
Filler can be placed in the nasal tip to correct small depressions or irregularities. Increased tip projection can be achieved by placing a small amount of filler in the tip, similar to a cap graft or shield graft. A narrow nasal tip can be widened by injecting filler in the midline between the domes. Filler can be used as a plumping graft to make an acute nasolabial angle more obtuse and give the illusion of rotation of the tip. The illusion of increased rotation can also be achieved by injecting the area just below the domes. If no scar tissue is present, minor degrees of alar retraction and mild‐to‐moderate columellar retraction can be corrected. Tiny amounts of filler should be placed and incrementally molded and shaped until the desired correction is achieved.
Undercorrection should be the goal, as the hydrophilic nature of filler will lead to soft tissue edema that may result in a bulky appearance. Marked improvement in the tip can be achieved with as little as 0.1 ml of filler material (Figures 6 and 7).
Figure 6.
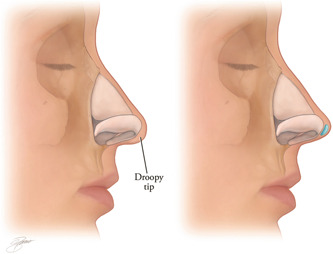
Filler can be placed to refine the tip and/or increase tip rotation. (Kontis TC, Lacombe V. Cosmetic injection techniques: A text and video guide to neurotoxins and fillers. 2nd Ed. New York, NY: Thieme Medical Publishers, Inc. 2019; with permission).
Figure 7.
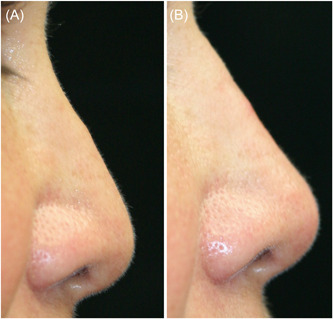
Postrhinoplasty patient complained of poor tip projection, dorsal irregularity, and tip ptosis (A). Hyaluronic acid injected to the dorsum and tip improved tip rotation and projection and the dorsal hump was camouflaged (B).
Internal and external valve treatment
Functional correction of a narrow nasal valve is achieved by using a nasal speculum to inject a small quantity of filler at the apex or just lateral to the internal nasal valve. The injection acts as a batten graft to stiffen the valve area and can be titrated until the patient experiences improvement in nasal airway during inspiration. Collapse of the external nasal valve can be addressed by placing a small amount of filler parallel to the alar rim (Figures 8 and 9).
Figure 8.
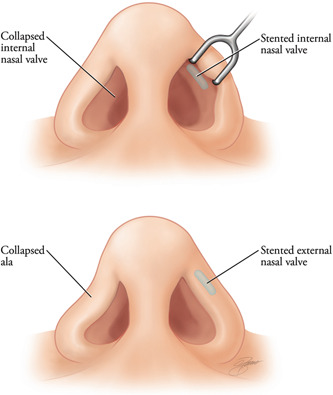
Filler can be placed intranasally into the internal valve region to act as a spreader graft. The external valve can be improved by placement of filler into the alar rim to act as a batten or rim graft (Kontis TC, Lacombe V. Cosmetic injection techniques: A text and video guide to neurotoxins and fillers. 2nd Ed. New York, NY: Thieme Medical Publishers, Inc. 2019:144‐151; with permission).
Figure 9.
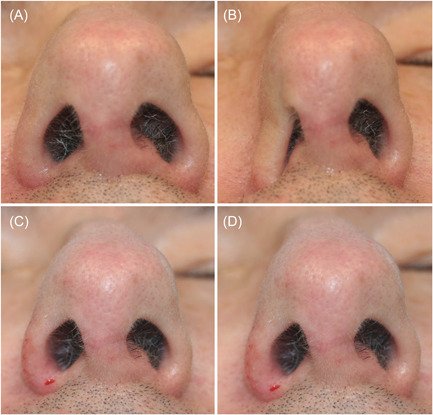
Base view of patient as rest (A). Collapse of the right nasal ala with sniff (B). Immediately after alar rim injection with hyaluronic acid at rest (C). Less collapse of ala seen after injection when patient sniffs (D).
REPORTED COMPLICATIONS
Mild bruising, ecchymosis, and edema can be seen after NSR. Other mild‐to‐moderate complications include hyperpigmentation, Tyndall effect, and nodule formation. Major complications include vascular compromise, skin ischemia and necrosis, and vision loss and stroke. 20
In a systematic review and meta‐analysis of 37 publications with 8604 patients undergoing NSR, DeVictor et al. 5 found that the overall complication rate was 2.52%. Bruising (1.58%) was the most common complication. Major complications included transient vessel occlusion (0.35%), vision loss (0.09%), and skin necrosis (0.08%). Infection and nodule formation were each reported at a rate of 0.07%. Granuloma and anaphylaxis were reported at a rate of <0.01% each.
Irreversible blindness and stroke are among the most dreaded complications that can be caused by filler injection, especially in the nose. The mechanism of filler‐associated blindness is retrograde flow of filler to the ophthalmic artery system via the dorsal nasal artery and its numerous anastomoses with the external carotid artery system.
A literature review by Beleznay et al. 21 found that the most common site of facial filler placement that led to vision changes was injection in the nasal region (56.3%). This was followed by the glabella (27.1%), the forehead (18.8%), and the nasolabial fold (14.6%). HA was the filler used in most cases that caused vision change (81.3%) followed by CaHA (10.4%).
TREATMENT OF VASCULAR COMPROMISE
Because injection into the nose is such a high‐risk procedure, NSR is not a technique for the novice injector. A thorough understanding of nasal anatomy, careful patient selection, and conservative treatment using small amounts of filler injected slowly in the correct anatomic plane are necessary practices. Due to the highly variable vascular anatomy of the nose, inadvertent vascular‐mediated injury is possible, even in the most experienced hands. Therefore, any injector must be prepared to manage a vascular complication.
Recognizing and treating a suspected vascular compromise early is key. Signs of vascular compromise leading to soft tissue ischemia include disproportionate pain that is either immediate or delayed in onset; soft tissue blanching in a reticulated pattern; and slow capillary refill. Patients experiencing filler‐associated vision changes report complete or partial loss of vision during or immediately after injection. Fifty percent of patients have skin involvement, ophthalmoplegia, or ptosis. Headache, nausea, and vomiting might also be present. 4 , 21 If any such symptoms are apparent, the injection must be immediately stopped.
In 2021, The American Society of Dermatologic Surgery convened a task force to issue recommendations for the treatment of filler‐associated vascular compromise. 4 In the event of vision changes after filler injection, the visual status of the patient should be assessed and documented before initiating treatment. An ophthalmologist or retina specialist should be contacted immediately. At least 150 IU of hyaluronidase should be injected into the area where dermal filler was placed and in areas that demonstrate signs of ischemia. Arteries along the path leading to the eye and the area adjacent to and in the supratrochlear and supraorbital foramina should be injected. Breathing into a paper bag, ocular massage, topical timolol, and oral acetazolamide 500 mg are adjunctive treatments that can be administered while the patient is being treated with hyaluronidase.
Tissue ischemia without blindness is treated by injecting hyaluronidase 150 IU into the area where filler was placed and surrounding ischemic tissue. Injections should be repeated hourly until reperfusion is achieved or doses nearing 1500 IU have been reached. 20 , 21 Warm compress, massage, and 2% nitroglycerin paste can promote vasodilation. 20
CONCLUSION
Since its first description in the late 1800s, NSR has become a useful tool in well‐selected primary and revision rhinoplasty cases. Advances in filler technology and technique have led to improved safety and results. The complex and variable vascular anatomy of the nose, and potential for devastating complications, demand that the injector possess a deep anatomic knowledge, and maintain a strict adherence to safety measures.
AUTHOR CONTRIBUTIONS
Suzel S. Hall and Theda Kontis both contributed significantly to the acquisition of data, drafting, editing, and submission of this article.
CONFLICT OF INTEREST STATEMENT
No federal or industry funding was received by the authors for this article. Theda Kontis is a Clinical Investigator for Allergan, a Clinical Investigator and Consultant for Revance, a Clinical Investigator for Galderma, and receives royalties from Thieme.
ETHICS STATEMENT
This study was a review and thus no ethics statement or IRB approval was needed.
ACKNOWLEDGMENTS
None.
Hall SS, Kontis TC. Nonsurgical rhinoplasty. World J Otorhinolaryngol Head Neck Surg. 2023;9:212‐219. 10.1002/wjo2.104
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Kontis T, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67‐72. [DOI] [PubMed] [Google Scholar]
- 2. Toriumi DM, Mueller RA, Grosch T, Bhattacharyya TK, Larrabee WF Jr.. Vascular anatomy of the nose and the external rhinoplasty approach. Arch Otolaryngol Head Neck Surg. 1996;122:24‐34. [DOI] [PubMed] [Google Scholar]
- 3. Tansatit T, Apinuntrum P, Phetudom T. Facing the worst risk: confronting the dorsal nasal artery, implication for non‐surgical procedures of nasal augmentation. Aesthetic Plast Surg. 2017;41:191‐198. [DOI] [PubMed] [Google Scholar]
- 4. Jones DH, Fitzgerald R, Cox SE, et al. Preventing and treating adverse events of injectable fillers: evidence‐based recommendations from the American Society for Dermatologic Surgery Multidisciplinary Task Force. Dermatol Surg. 2021;47:214‐226. [DOI] [PubMed] [Google Scholar]
- 5. DeVictor S, Ong AA, Sherris DA. Complications secondary to nonsurgical rhinoplasty: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2021;165:611‐616. [DOI] [PubMed] [Google Scholar]
- 6. Choi MS. Basic rheology of dermal filler. Arch Plast Surg. 2020;47:301‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson O 3rd, Kontis T. Nonsurgical rhinoplasty. Facial Plast Surg. 2016;32:500‐506. [DOI] [PubMed] [Google Scholar]
- 8. Kurkjian TJ, Ahmad J, Rohrich RJ. Soft‐tissue fillers in rhinoplasty. Plast Reconstr Surg. 2014;133:121e‐126e. [DOI] [PubMed] [Google Scholar]
- 9. Mehta U, Fridirici Z. Advanced techniques in nonsurgical rhinoplasty. Facial Plast Surg Clin North Am. 2019;27:355‐365. [DOI] [PubMed] [Google Scholar]
- 10. Hedén P. Nasal reshaping with hyaluronic acid: an alternative or complement to surgery. Plast Reconstr Surg Glob Open. 2016;4:e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross‐linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155‐163. [DOI] [PubMed] [Google Scholar]
- 12. Merz Aesthetics . Mechanism of Action ‐ Radiesse Injectables, 2022. https://radiesse.com/professionals/mechanism-of-action/ [Google Scholar]
- 13. Liu MH, Beynet DP, Gharavi NM. Overview of deep dermal fillers. Facial Plast Surg. 2019;35:224‐229. [DOI] [PubMed] [Google Scholar]
- 14. Rivkin A. PMMA‐collagen gel in nonsurgical rhinoplasty defects: a methodological overview and 15‐year experience. Plast Reconstr Surg Glob Open. 2022;10:e4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saadoun R, Solari MG, Rubin JP. The role of autologous fat grafting in rhinoplasty. Facial Plast Surg. 2023;39:185‐189. [DOI] [PubMed] [Google Scholar]
- 16. Neaman KC, Boettcher AK, Do VH, et al. Cosmetic rhinoplasty: revision rates revisited. Aesthet Surg J. 2013;33:31‐37. [DOI] [PubMed] [Google Scholar]
- 17. Kontis T. The art of camouflage: when can a revision rhinoplasty be nonsurgical. Facial Plast Surg. 2018;34:270‐277. [DOI] [PubMed] [Google Scholar]
- 18. Harb A, Brewster CT. The nonsurgical rhinoplasty: a retrospective review of 5000 treatments. Plast Reconstruct Surg. 2020;145:661‐667. [DOI] [PubMed] [Google Scholar]
- 19. Kontis TC, Lacombe V. Cosmetic Injection Techniques: A Text and Video Guide to Neurotoxins and Fillers. 2nd Ed. Thieme Medical Publishers, Inc.; 2019:144‐151. [Google Scholar]
- 20. Mella J, Oyer S. Nonsurgical rhinoplasty: prevention and management of associated complications. Curr Opin Otolaryngol Head Neck Surg. 2022;30:241‐248. [DOI] [PubMed] [Google Scholar]
- 21. Beleznay K, Carruthers JDA, Humphrey S, Carruthers A, Jones D. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39:662‐674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


