Abstract
Objective
The aim of this review article is to discuss the currently available facial fillers, their differences and indications, relevant anatomy, injection techniques, and avoidance and management of complications.
Data Sources
Clinical experience and scientific papers.
Conclusions
Reversal of facial aging via filler injection has been around since the late 1800s with the initial use of detrimental products. Today, many safe and effective products exist and can be tailored to the individual patient's desired effect. With the evolution of both products and injection techniques, the rate of complications with facial filler use is low. Nonetheless, providers offering facial filler injections should have detailed knowledge of facial anatomy, including facial planes and soft tissue compartments. Multiple injection techniques exist. Different techniques should be used, depending on the anatomic target. Providers should also know how to avoid and manage complications.
Keywords: cosmetic, facial, filler, injection, plastic surgery
Key Points
Facial filler is a widely popular procedure today. Various products and injection techniques can be tailored to each individual patient to achieve ideal results. A detailed knowledge of facial anatomy is required to properly treat patients and to manage complications.
INTRODUCTION
Key hallmarks of facial aging include loss of facial volume and descent of facial structures. Volume loss occurs within the subcutaneous fat compartments of the face, leading to a deflated appearance. Weakening of facial retaining ligaments, thinning of the epidermis, and dermal atrophy with loss of collagen results in descent of facial fat compartments. This can accentuate facial features such as the nasolabial folds and jowls. This leads to the characteristic “inverted triangle” appearance associated with aging.
Facial fillers are used to counteract age‐related facial volume loss as well as to reduce the appearance of fine lines and wrinkles associated with aging. Facial filler injection is the second most common nonsurgical cosmetic procedure in the United States and accounts for 3.4 million procedures annually. 1 Facial filler injections are popular in large part due to their ease of administration and minimal recovery time. Nonetheless, filler injections are not without risk. Injection techniques and knowledge of facial anatomy are vital for safe administration and avoidance of complications. The ideal filler is biocompatible, immunologically inert, retains volume as injected, lasts many years but is easily reversible, looks and feels natural, and is economical in use as well as storage. Few fillers meet all of these criteria. Therefore, filler selection depends on a combination of patient and injector‐centric factors. In this article, we review the available fillers as well as the indications for use, relevant anatomy, and injection techniques.
DISCUSSION
Anatomy
Detailed knowledge of facial anatomy is essential to treat patients safely and properly. The superficial musculoaponeurotic system (SMAS) is an anatomic structure through which important facial vessels and nerves travel. Surek suggests thinking of facial anatomy as a 2‐tiered cake sitting on a platter (the facial skeleton). 2 The middle layer of icing of the cake is the SMAS, the lower layer is the sub‐SMAS and associated structures (deep fat, potential spaces, and preperiosteal layer), and the upper layer is the supra‐SMAS and associated structures (subcutaneous layer). 2 The supra‐SMAS and sub‐SMAS layers are the most common targets for injection. To inject facial fillers correctly, it is paramount to understand the appropriate injection depth. Knowledge of facial fat compartments is also important when performing facial filler injections. Facial fat compartments are discussed below and displayed in Figure 1.
Figure 1.
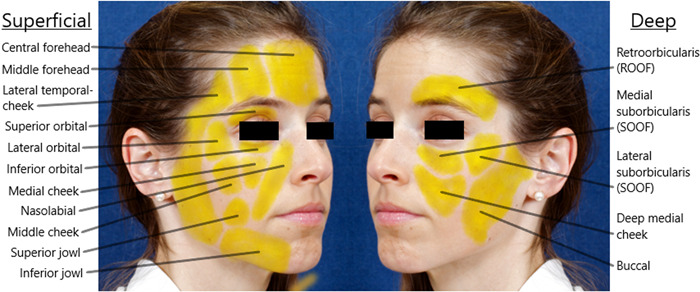
Facial fat compartments.
Upper face
In the upper face, there are five forehead and temporal fat compartments. Starting medially, the central compartment abuts the middle compartment on either side. The lateral compartments are contiguous with the temporal cheek fat which extends to the cervical fat. 3 The orbital fat compartments are made up of three distinct compartments: superior, inferior, and lateral. The superiormost compartment lies around the superior orbit from the medial canthus to the lateral canthus. 3 The inferior fat compartment is similar in that it also extends from medial canthus to lateral canthus. 3 The lateral orbital fat compartment is contained between the temporal septum and superior cheek septum and this fat pad is adherent to the zygomaticus major muscle. 3
Midface
The sub‐SMAS region of the midface is divided into upper and lower regions, demarcated by the malar equator which divides the cheek in half horizontally. The upper region includes the suborbicularis oculi and preperiosteal fat as well as the prezygomatic space. Injections in the tear trough target the prezygomatic space. The angular artery in this area has been reported to be approximately 17 mm from midline and 13 mm inferior to the medial canthus. However, the angular artery can be aberrant in up to 30% of patients. 2 , 4 The lower region of the midface includes deep medial cheek fat. Deep medial cheek fat lies deep to muscles that elevate the lip. This fat compartment can soften the nasolabial fold prominence, and is typically accessed within 15 mm of the alar base. 2 The angular artery in this region is located laterally and superficially to the deep medial cheek fat compartment and is within 5 mm of the nasolabial fold. 2 , 4 The location of the angular artery is shown in Figure 2.
Figure 2.
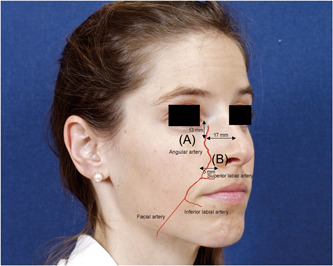
Landmarks for course of angular artery. Angular artery can be found 13 mm from medial canthus and 17 mm from midline (A). Angular artery can be found within 5 mm of the nasolabial fold (B).
The supra‐SMAS region of the midface is comprised of multiple fat compartments (nasolabial, medial and middle superficial, and lateral temporal cheek compartments) through which the orbital retaining ligament passes superiorly and the zygomaticocutaneous ligaments pass inferiorly. Injection between these two ligaments can disrupt lymphatics and subsequently cause iatrogenic malar mounds. 2 Therefore, careful attention to midface anatomy is necessary. The cheek fat compartments include the medial, middle, and lateral temporal cheek fat. 3 The medial cheek fat is located lateral to the nasolabial fat and superior to the jowl fat. 3 The middle cheek fat lies anterior and superficial to the parotid gland. 3 The lateral temporal cheek fat is superficial to the parotid gland and connects to the cervical subcutaneous fat. 3
Lower face
This region contains the retroorbicularis oris fat pad, a common target for volumization. The sub‐SMAS region of the perioral face contains the facial artery. Facial artery branches include the superior and inferior labial arteries. In most patients, these arteries remain sub‐SMAS. Therefore, in this region, injections should remain in the supra‐SMAS plane. 2 The sub‐SMAS region of the jawline contains the mandibular osteocutaneous retaining ligament and the platysma mandibular ligament. These ligaments are the injection boundaries used when addressing the prejowl area. The supra‐SMAS region of the jawline is made up of superior and inferior jowl compartments separated by the platysma mandibular ligament. The jowl fat compartment underlies the nasolabial fat compartment. The jowl fat compartment is a distinct entity which is adherent to the depressor anguli oris muscle. 3
Types of facial filler
Reversal of facial aging via the injection of facial fillers was first trialed in the late 1800s. Paraffin, created from beech wood tar, is the first described cosmetic facial filler. 5 Although the concept of reversing age‐related facial volume loss was sound, complications and side effects were so negative in the public eye that historians attributed paraffin injections to slowing advancements in the cosmetic field for the first half of the 20th century. Silicone liquid injection was popularized in the 1960s. However, it was banned for use as a cosmetic injectable by the Food and Drug Administration (FDA) in 1979 due to foreign body granuloma reaction, migration, fistula formation, and even death. 5 Today, injectable silicone is available for treatment of retinal detachment, but is considered illegal for cosmetic use in some states. 5 Despite setbacks with some filler materials, newer and safer products are now currently available. These products are categorized by length of action, intended use, advantages, and disadvantages in Tables 1 and 2.
Table 1.
Filler length of action.
| Filler length of action | Time |
|---|---|
| Temporary | Up to 6 months |
| Long lasting | 6 months to 2 years |
| Semipermanent | 2–5 Years |
| Permanent | Greater than 5 years |
Table 2.
Summary of facial fillers.
| Filler | Trade name | Length of action | Purpose | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Hyaluronic acid (HA) | Multiple including: Restylane (Galderma), Juvederm (Allergan), Belotero (Merz), Revanesse (Prollenium), RHA (Teoxane) | Temporary | Wide range of indications including fine wrinkles to deep wrinkles, nasolabial folds, periocular and perioral lines, malar and lip augmentation | Available in different G′ formulas which remain easy to inject, reversible with hyaluronidase | Temporary, may require repeat injections to maintain desired effect |
| Collagen | Human: CosmoDerm and CosmoPlast (Allergan); Bovine: Zyderm (Allergan); Porcine: Evolence (Ortho Dermatologies) | Temporary | Fine wrinkles up to deep wrinkles | Naturally occurring material with 20+ year history of safe use | Requires double skin testing (if nonhuman derived) before injection, chance of allergic reaction. Most require refrigerated storage |
| Poly‐l‐lactic acid (PLLA) | Sculptra (Sanofi‐aventis) | Long lasting | Stimulates collagen production, higher viscosity, moderate to severe rhytids, facial volumization | Can last up to 2 years. FDA‐approved for facial adipose loss associated with HIV/AIDS | Requires time to see effects and repeated injections initially, not recommended for periocular or perioral injection |
| Calcium hydroxyapatite (CaH) | Radiesse (Bioform) | Semipermanent | Stimulates collagen production. Moderate to severe wrinkles, nasolabial folds, marionette lines, prejowl sulcus, facial volumization | FDA‐approved for facial adipose loss associated with HIV/AIDS | Highly viscous and predisposed to nodule formation, not recommended for fine lines, lips, or periocular injection |
| Autologous Fat | Not applicable | Permanent (varies) | Wide range of facial filler applications | Autologous, relatively easy to harvest, may serve regenerative capacity given presence of multipotent stem cells | Typically needs repeat injections as some fat is resorbed |
| Polymethylmethacrylate (PMMA) |
Artefil (Suneva) Aquamid (Contura) |
Permanent | Medium to deep wrinkles, nasolabial folds, facial acne scars | Can last up to 5 years | Contains bovine collagen, needs skin testing. Can take months to see final effects, not recommended for perioral or periocular injection |
Hyaluronic acid (HA) gel is the most popular facial filler. 6 HA is available in various forms with differing viscoelastic properties. Restylane (Galderma) was the first FDA‐approved HA filler in 2003. 7 HA is a naturally occurring substance in the body. It, therefore, has a very low immunogenic reaction and may be stored at room temperature. 7 When used as a facial filler, HA acts to stabilize, lubricate, hydrate, and increase the viscoelastic properties of the extracellular matrix. 8 The viscoelastic properties vary depending on crosslinking of the gel and substrate used to make the gel. These properties are detailed most relevantly by the G′ (elastic modulus), which incorporates the sum of multiple factors which determine the strength of the gel (including HA concentration and degree of chemical crosslinking). 9 Swelling factor refers to the gel's ability to uptake water from its surroundings after injection. As a general principle, as G′ increases, swelling factor decreases. 9 As G′ increases, the product has greater resistance to compressive forces and therefore provides more correction. Lower G′ values are injected in more superficial planes with fewer degrees of correction needed. Higher G′ values are indicated for deeper plane injections with more degrees of correction needed. 9 Resistant HA (RHA) fillers are a newly FDA‐approved type of HA filler. RHA is crosslinked using technology that reduces degradation of HA chains during production. 10 This leads to a product with preserved natural HA polymer. The preserved HA polymer results in fewer covalent bonds for stabilization, culminating in a product that is less rigidly crosslinked. 10 These properties allow RHA gels to respect and accompany facial dynamics while maintaining resiliency. 10
Calcium hydroxyapatite (CaH) is a semi‐permanent filler made up of small spheres suspended in a carboxymethylcellulose carrier. CaH stimulates collagen synthesis through an inflammatory mediated mechanism whereby collagen replaces the injectate over the course of 3 months while maintaining the volume initially injected. 11 CaH is thicker and has a higher elastic modulus than HA. CaH tends to last longer than HA. 8 , 11
Poly‐l‐lactic acid (PLLA) is a biodegradable inactive material made from corn starch. PLLA is comprised of small particles (40–63 µm) which cause an inflammatory response and subsequent fibroblast activation. 12 , 13 The particles are slowly degraded over time and replaced with collagen. The effects from PLLA injection can be seen for up to 2 years. 13
Collagen, a temporary facial filler, is available in human, bovine, or porcine‐derived formulas. Nonhuman‐derived formulas require skin testing, as allergic reactions can occur. 13 The FDA approved bovine collagen as the first cosmetic injectable filler in 1981. 5 , 7 Collagen has a short duration of effect, typically 3–6 months, and requires refrigerated storage. 7 , 13 Polymethylmethacrylate (PMMA) is a synthetic permanent filler made of microspheres suspended in bovine collagen. The microspheres in PMMA promote collagen production in the body. PMMA effects can last up to 5 years. Fillers such as HA, CaH, and PLLA are all FDA‐approved and each promotes production of the body's own collagen. 12
Autologous fat, an avascular‐free tissue graft, can also be used for facial fillers. Autologous fat was first described for cosmetic use in the late 1800s and early 1900s. Although widely used today, it did not gain wide popularity until high vacuum suction was introduced in 1892. 5 Fat is typically harvested from the abdomen or thigh with liposuction cannula. Harvested fat is then treated either via centrifuge or filtration before it is injected into the patient. Advantages of autologous fat include its biocompatibility, easy harvesting, and ability to achieve a natural appearance. Disadvantages of autologous fat include variable length of effects due to loss of injected fat. 12 Various factors play a role in long‐term retention of adipose tissue such as patient age, technique of harvesting, processing, and injection of adipose tissue. 14 Gerth and colleagues showed fat processed via filtration had significantly higher long‐term mean retention compared to centrifuged fat (31.8% vs. 41.2%) but retention was quite variable. 14 Gerth and colleagues showed that those younger than 55 years old and those who did not undergo concurrent rhytidectomy both had significantly higher fat retention at follow‐up. 14 Adipose tissue has been shown to contain multipotent stem cell populations. 15 Therefore, autologous fat grafts may serve some regenerative effects when used as a facial filler.
Injection techniques
Before any injection, the patient's skin should be cleaned and sterilized. This is commonly done with alcohol. Topical anesthetic or nerve blocks may be used depending on the provider and patient preference. Many filler products are available with lidocaine premixed. Injections can be performed with either a needle or cannula. Injection performed with needle has higher risk of vascular occlusion compared to cannula but other factors such as experience play a role in vascular occlusion rates. 16 Needle injection allows faster acquisition of desired injection depth in the tissue with a higher risk of vascular injury. Cannula requires a large bore needle or similar skin incision to introduce the cannula but carries a lower risk of vascular occlusion.
Linear threading technique is performed by injecting filler as the needle is either advanced or withdrawn within the tissue. This technique is commonly employed in lips and the nasolabial fold regions. 7 , 13 Serial puncture technique involves small aliquot of filler to be delivered to an area via multiple separate punctures. This technique is ideal for depositing filler in the superficial levels of the dermis. It is commonly used in the glabellar region to address fine wrinkles in a bolus fashion. 7
Cross‐hatching and fanning techniques are two techniques employed to distribute filler over larger areas. 7 Fanning is the process of making multiple linear passes along the same plane over an area without withdrawing the needle or cannula from the tissue. This technique can be useful for the deep malar region and nasolabial fold. 13 Cross‐hatching is the process of injecting filler in a grid‐like pattern. Cross‐hatching is commonly employed around the oral commissure. 13 Figure 3 shows examples of each injection technique.
Figure 3.
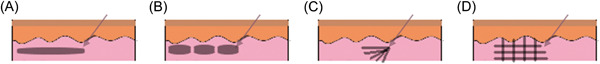
Filler injection techniques. (A) Linear threading, (B) depot/serial puncture, (C) fanning, (D) cross‐hatching.
Injection zones
Different injection techniques should be employed in different areas based on the anatomic location. These areas are discussed below and graphically depicted in Figure 4.
Figure 4.
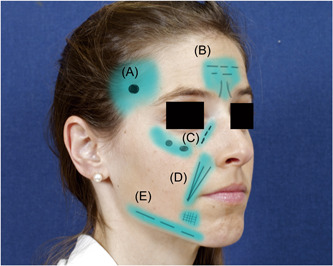
Anatomic zones and techniques for filler injection. Temporal region (A), glabellar and brow region (B), orbital and cheek region (C), nasolabial fold (D), prejowl sulcus and mandibular line (E).
Temporal region
In the temporal region, the superficial temporal artery and middle temporal vein travel in the fat pad between superficial and deep temporal fascia. 17 Multiple injection techniques can be used in this area based on injector preference. Linear threading injection to avoid vasculature can be employed. In this area, cannula technique is recommended to reduce the risk of vascular injury as well as inadvertent deep injection preperiosteally. 18 One useful technique is to place a single depot of filler at the depth of the hollow in the sub‐SMAS plane just deep to the superficial layer of the deep temporal fascia. This fascial layer is a natural glide plane that allows the injector to massage the filler within the hollow and to smooth out any irregularities. A single depot site at the depth of the hollow avoids injection near the superficial temporal artery, sentinel vein, and perforating vessels.
Brow region
The supraorbital, supratrochlear, dorsal nasal, and angular arteries anastomose in the glabellar region are relatively superficial. Thus, this region incurs an increased risk of vascular injury. The supratrochlear artery is closely related to glabellar frown lines. 17 , 19 Injection techniques directed medially in this area should consist of superficial, intradermal serial punctures along the facial wrinkles as well as digital pressure to occlude the supratrochlear and supraorbital arteries to prevent backflow. 18 Supraorbital injections are directed near the junction of the lateral and superior orbital fat compartments at the tail of the lateral brow. A slow supraperiosteal bolus along the orbital rim in this location can be massaged upward to both support and lift the tail of the lateral brow.
Cheek region
The infraorbital foramen can be approximated by visualizing a vertical plane along the medial limbus that is one fingerbreadth below the orbital rim. 18 Injections should be administered lateral to the infraorbital foramen. Injections medial to the foramen should be avoided. If filler is needed in this area, it should be injected laterally and massaged medially. 18 Injection in this area can be performed either with serial punctures, with larger volumes of filler in a supraperiosteal plane, or with a fanning technique in a more superficial but sub‐SMAS plane.
Tear trough
The angular artery and infraorbital artery are at risk in this region. Injection can be accomplished via needle or cannula injection. The most common technique is retrograde linear threading. 20 The ideal injection depth is deep to the orbicularis oculi muscle and superficial to the periosteum. In a review by Rao et al. 20 the authors could not definitively determine the safest technique when comparing use of needle versus cannula.
Nasolabial fold
The facial artery closely follows the nasolabial fold. Injections in this region should use a linear threading technique or fanning injection techniques. Use of cannula should be considered in this region to lessen vascular injury risk. 18
Prejowl sulcus and mandibular line
The submental artery and facial artery are the two main vessels in this region. The facial artery crosses over the mandible just anterior to the masseter and can be palpated at the antegonial notch. The submental artery branches off the facial artery and is typically encountered in the paramedian chin. 17 Multiple injection techniques may be employed for the prejowl area. These techniques include fanning or crosshatching in either deep dermal or dermal‐subcutaneous depths. Avoidance of the facial artery is important during jawline injections. Depth of injection should be kept subdermal along the jawline. 17 Chin injections should be placed deep in the midline to avoid submental artery branches. 17
Complications of facial fillers
Complications from injection of facial fillers are relatively rare but can have devastating consequences. Systematic review of nonpermanent facial fillers by Oranges et al. 8 found the nose and nasolabial fold areas to have the highest complication rates. This is likely related to complex and dense vascular branching in these areas. Helpful techniques to avoid complications in this area include aspiration before injection as well as serial injections into the periosteal layer. 8 Trinh and Gupta 6 performed a systematic review of adverse events related to HA injection. They found that higher injection volume, delayed hyaluronidase administration, and use of fanlike injection techniques all contributed to adverse events. 6 One specific complication, named the Tyndall effect, results in a bluish discoloration of the overlying skin. This is caused by very superficial injections of filler which causes light to scatter differently over areas containing the superficial injected material. 7 , 13 The Tyndall effect is most likely to occur in areas of thin skin such as the lower eyelid. To avoid this complication in the tear trough area it is recommended to avoid superficial injection, not overfill (typically no more than 1 mL), and use a product suitable for this area. 21
Associated complication rates based on needle vs cannula technique have been studied in the literature. A retrospective cohort study of board‐certified dermatologists evaluated complications of filler injected with needle or cannula. This study found 77.1% lower odds of vascular occlusion with cannula compared to needle injection. 16 This study also showed those with more than 5 years of injecting experience had 70.7% lower odds of occlusion than those with less experience. 16
One major advantage of HA use is its reversibility. Hyaluronidase dissolves HA and can be used to reverse HA injection. Hyaluronidase effectively reduces edema and improves blood flow from vascular occlusion. 22 Dosages of hyaluronidase vary depending on the product used. Doses range from 50 to 150 units to remove nodules and up to 1500 units in divided injections for intravascular injection. 17 , 23 King and colleagues suggest selecting hyaluronidase dosage based on anatomic location or by complication. 23 To correct HA filler injection, they suggest small doses in the lower lid (1.5 units) and larger doses in the nose or perioral region (15–30 units). 23 In the event of vascular occlusion, high doses of up to 1500 units should be used. 23 In the setting of impending blindness, retrobulbar injection of 150–200 units is recommended in 2–4 mL of diluent. 22
Protocols to prevent skin necrosis after vascular occlusion vary. Common recommendations consist of warm compresses to the affected area, nitroglycerin paste to promote local vasodilation, administration of filler reversal agent (hyaluronidase), systemic steroids to reduce swelling and inflammation, acetylsalicylic acid to reduce plate aggregation, and antibiotics to prevent infection. 24 Some protocols even recommend hyperbaric oxygen therapy. 24 Complications and management of filler injections are summarized in Table 3.
Table 3.
Complications of filler injection and management strategies.
| Complication | Incidence 25 , 26 | Management |
|---|---|---|
| Mild/temporary | ||
| Local edema | 0.26–0.44 | Skin massage, 8 hyaluronidase, 8 , 27 , 28 ice packs, 27 , 28 , 29 intralesional steroids, 28 oral steroids 8 , 28 , 29 |
| Injection site pain | 0.20–0.38 | Ice packs 29 |
| Ecchymosis | 0.23–0.35 | Ice packs, 29 firm pressure, 27 vascular lasers, 27 , 28 , 29 topical arnica 30 |
| Erythema | 0.19–0.33 | Hyaluronidase, 8 , 27 ice packs, 27 topical steroids 29 |
| Tyndall effect | 0.03–0.11 | Hyaluronidase, 27 , 28 , 29 minimal stab incision with evacuation of residual filler if persistent 28 |
| Skin paresthesia | 0.01–0.35 | Hyaluronidase, 29 intralesional steroids 29 |
| Filler migration | 0.01–0.17 | Removal of filler material 27 |
| Nodules or granulomas | 0.01–0.10 | Skin massage, 27 , 28 hyaluronidase, 29 intralesional steroids +/− 5‐FU, 27 , 28 , 29 oral steroids, 27 , 28 , 29 oral antibiotics, 28 , 29 minimal stab incision with evacuation, 28 excision 8 , 28 , 29 |
| Local infection | 0.01–0.03 | Oral antibiotics, 8 , 27 , 28 , 29 acyclovir if herpetic vesicles, 8 , 28 , 29 avoid hyaluronidase until infection cleared 27 |
| Severe/permanent | ||
| Vascular occlusion | 0–0.02 | Hyaluronidase, 8 , 27 aspirin, 8 , 27 skin massage, 27 warm compresses, 8 , 27 topical nitroglycerin paste 8 , 27 |
| Skin necrosis | 0–0.02 | Antibacterial ointment, 8 , 31 daily local wound care regimen with debridement, 8 , 31 hyperbaric oxygen 8 |
| Ophthalmoplegia | Case reports | Retrobulbar space injection of hyaluronidase and corticosteroids, 8 , 32 oral steroids 8 , 31 |
| Decreased visual acuity or vision loss | Case reports | Ophthalmic arterial injection of hyaluronidase and urokinase, 8 , 27 , 32 retrobulbar space injection of corticosteroids and tobramycin, 8 , 27 , 32 ocular massage, 32 intravenous mannitol, 32 acetazolomide, 32 anterior chamber paracentesis, 8 , 31 , 32 hyperbaric oxygen 8 , 31 |
CONCLUSION
Facial filler injections are commonly utilized as volumizing agents in cosmetic procedures to reduce the effects of aging. Over time, advancements have been made that enhance the safety profile and utility of different filler substances as well as injection techniques. While individual expectations and provider experience introduce variability to the injection approach, a thorough understanding of facial anatomy and evidence‐based techniques such as distribution, dose, and depth can reduce complication rates. Successful management of complications also relies on a current knowledge of available mitigation strategies.
AUTHOR CONTRIBUTIONS
Nicholas Clark performed literature searches, compiled, and edited the manuscript, and created tables and figures. Debbie Pan was involved with literature search, edited manuscript, and creation of figures. Dane Barrett was involved with the editing, creation of the manuscript, and creation of figures.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
None.
ACKNOWLEDGMENTS
The authors have nothing to report.
Clark NW, Pan DR, Barrett DM. Facial fillers: relevant anatomy, injection techniques, and complications. World J Otorhinolaryngol Head Neck Surg. 2023;9:227‐235. 10.1002/wjo2.126
DATA AVAILABILITY STATEMENT
The data is publicly available as this is the review article. Any deidentified data can be made available upon request to the corresponding author.
REFERENCES
- 1. Plastic Surgery Statistics Report 2020 . American Society of Plastic Surgeons National Clearinghouse of Plastic Surgery Procedural Statistics. 2020. Accessed October 20, 2022. https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf
- 2. Surek CC. Facial anatomy for filler injection. Clin Plast Surg. 2019;46:603‐612. [DOI] [PubMed] [Google Scholar]
- 3. Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219‐2227. [DOI] [PubMed] [Google Scholar]
- 4. Lee HJ, Won SY, O J, et al. The facial artery: a comprehensive anatomical review. Clin Anat (New York, N.Y.). 2018;31:99‐108. [DOI] [PubMed] [Google Scholar]
- 5. Kontis T, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67‐72. [DOI] [PubMed] [Google Scholar]
- 6. Trinh LN, Gupta A. Hyaluronic acid fillers for midface augmentation: a systematic review. Facial Plast Surg. 2021;37:576‐584. [DOI] [PubMed] [Google Scholar]
- 7. Kim JE, Sykes J. Hyaluronic acid fillers: history and overview. Facial Plast Surg. 2011;27:523‐528. [DOI] [PubMed] [Google Scholar]
- 8. Oranges CM, Brucato D, Schaefer DJ, Kalbermatten DF, Harder Y. Complications of nonpermanent facial fillers: a systematic review. Plast Reconstr Surg Global Open. 2021;9:e3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fagien S, Bertucci V, von Grote E, Mashburn JH. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast Reconstr Surg. 2019;143:707e‐720e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaufman‐Janette J, Taylor SC, Cox SE, Weinkle SH, Smith S, Kinney BM. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate‐to‐severe nasolabial folds: a 64‐week, prospective, multicenter, controlled, randomized, double‐blind and within‐subject study. J Cosmet Dermatol. 2019;18:1244‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bass LS. Injectable filler techniques for facial rejuvenation, volumization, and augmentation. Facial Plast Surg Clin North Am. 2015;23:479‐488. [DOI] [PubMed] [Google Scholar]
- 12. Crowley JS, Kream E, Fabi S, Cohen SR. Facial rejuvenation with fat grafting and fillers. Aesthet Surg J. 2021;41:S31‐S38. [DOI] [PubMed] [Google Scholar]
- 13. Rohrich RJ, Nguyen AT, Kenkel JM. Lexicon for soft tissue implants. Dermatol Surg. 2009;35(suppl 2):1605‐1611. [DOI] [PubMed] [Google Scholar]
- 14. Gerth DJ, King B, Rabach L, Glasgold RA, Glasgold MJ. Long‐term volumetric retention of autologous fat grafting processed with closed‐membrane filtration. Aesthet Surg J. 2014;34:985‐994. [DOI] [PubMed] [Google Scholar]
- 15. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng. 2001;7:211‐228. [DOI] [PubMed] [Google Scholar]
- 16. Alam M, Kakar R, Dover JS, et al. Rates of vascular occlusion associated with using needles vs cannulas for filler injection. JAMA Dermatol. 2021;157:174‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wollina U, Goldman A. Facial vascular danger zones for filler injections. Dermatol Ther. 2020;33:e14285. [DOI] [PubMed] [Google Scholar]
- 18. Scheuer JF 3rd, Sieber DA, Pezeshk RA, Gassman AA, Campbell CF, Rohrich RJ. Facial danger zones: techniques to maximize safety during soft‐tissue filler injections. Plast Reconstr Surg. 2017;139:1103‐1108. [DOI] [PubMed] [Google Scholar]
- 19. Vural E, Batay F, Key JM. Glabellar frown lines as a reliable landmark for the supratrochlear artery. Otolaryngol Head Neck Surg. 2000;123:543‐546. [DOI] [PubMed] [Google Scholar]
- 20. Rao BK, Berger LE, Reilly C, Alamgir M, Galadari H. Tear trough filler techniques utilizing hyaluronic acid: a systematic review. Plast Reconst Surg. 2022;149:1079‐1087. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Massry G, Holds JB. Complications of periocular dermal fillers. Facial Plast Surg Clin North Am. 2021;29:349‐357. [DOI] [PubMed] [Google Scholar]
- 22. Loghem J, Funt D, Pavicic T, et al. Managing intravascular complications following treatment with calcium hydroxylapatite: an expert consensus. J Cosmet Dermatol. 2020;19:2845‐2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. King M, Convery C, Davies E. This month's guideline: the use of hyaluronidase in aesthetic practice (v2.4). J Clin Aesthet Dermatol. 2018;11:61. [PMC free article] [PubMed] [Google Scholar]
- 24. Halepas S, Peters SM, Goldsmith JL, Ferneini EM. Vascular compromise after soft tissue facial fillers: case report and review of current treatment protocols. J Oral Maxillofac Surg. 2020;78:440‐445. [DOI] [PubMed] [Google Scholar]
- 25. Stefura T, Kacprzyk A, Droś J, et al. Tissue fillers for the nasolabial fold area: a systematic review and meta‐analysis of randomized clinical trials. Aesthetic Plast Surg. 2021;45:2300‐2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee W, Koh IS, Oh W, Yang EJ. Ocular complications of soft tissue filler injections: a review of literature. J Cosmet Dermatol. 2020;19:772‐781. [DOI] [PubMed] [Google Scholar]
- 27. Chiang YZ, Pierone G, Al‐Niaimi F. Dermal fillers: pathophysiology, prevention and treatment of complications. J Eur Acad Dermatol Venereol. 2017;31:405‐413. [DOI] [PubMed] [Google Scholar]
- 28. Gupta A, Miller PJ. Management of lip complications. Facial Plast Surg Clin North Am. 2019;27:565‐570. [DOI] [PubMed] [Google Scholar]
- 29. Singh K, Nooreyezdan S. Nonvascular complications of injectable fillers‐prevention and management. Indian J Plast Surg. 2020;53:335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sherban A, Wang JV, Geronemus RG. Growing role for arnica in cosmetic dermatology: lose the bruise. J Cosmet Dermatol. 2021;20:2062‐2068. [DOI] [PubMed] [Google Scholar]
- 31. Sito G, Manzoni V, Sommariva R. Vascular complications after facial filler injection: a literature review and meta‐analysis. J Clin Aesthet Dermatol. 2019;12:65. [PMC free article] [PubMed] [Google Scholar]
- 32. Carruthers JDA, Fagien S, Rohrich RJ, Weinkle S, Carruthers A. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197‐1201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is publicly available as this is the review article. Any deidentified data can be made available upon request to the corresponding author.


