Abstract
The interaction of chemotactic peptide (e.g., fMet-Leu-Phe)-grafted liposomes with macrophages is noted to be rapid and specific. At a grafted peptide concentration of 100 nmol, internalization of the peptide-grafted liposomes by the macrophages is found to reach equilibrium in 30 min. The peptide alone and the peptide-grafted empty liposomes are found to show moderate antileishmanial activity in vitro. Primaquine, which is known to generate O2− in phagocytic cells, showed leishmanicidal properties when it was tested in vitro against parasite-infected macrophages over a certain range of concentrations. It showed much better efficacy against experimental leishmaniasis when it was used in the fMet-Leu-Phe-grafted liposomal form in comparison with its efficacy when it was either in the free form or encapsulated in ungrafted liposomes. The conventional toxicity parameters (e.g., blood pathology and tissue histology-specific enzyme levels related to normal liver function) are found to be very close to normal when fMet-Leu-Phe-grafted liposomal primaquine is used. The biodegradabilities of both the drug and the delivery systems are also found to be very satisfactory. Thus, this delivery system may have possible applications for the treatment of leishmaniasis as well as other macrophage-associated disorders.
Macrophages are chemotactically responsive cells that are of central importance to both the recognition and the effector limbs of the immune response. Chemotaxis, by definition, is the directed movement of cells along a chemical gradient, and it appears to be an important mechanism by which inflammatory cells accumulate at local sites (18, 19). The chemotactic agonists stimulate the macrophages and induce the respiratory burst (2, 3), resulting in the activation of NADPH oxidase, which catalyzes the conversion of molecular oxygen to superoxide anion (O2−). This O2− is the precursor of a series of microbicidal products (6). This activation is initiated by the binding of a chemotactic agonist to its receptor. It is prevented by the antagonist and is interrupted by agonist displacement, indicating that the agonist receptor complex must persist (3). We have developed a delivery system using one such chemotactic agonist, i.e., one peptide (N-formyl-methionine-leucine-phenylalanine [fMLP]) that has the chemoattracting properties for macrophages. Here we report the feasibility of applying fMLP-grafted liposomes as a delivery system for primaquine against experimental leishmaniasis in animal models.
MATERIALS AND METHODS
Materials.
Phosphatidylethanolamine (PE), cholesterol, phosphatidic acid, fMLP, and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC1) were purchased from Sigma. RPMI 1640 medium and medium 199 were from Gibco. All other reagents were of analytical grade.
Peritoneal macrophages were isolated from Swiss albino mice 4 days after the intraperitoneal injection of 1 ml of sodium thioglycolate (4%). They were cultured in RPMI 1640 medium containing l-glutamine (4 mM), HEPES (25 mM), streptomycin (100 μg/ml), penicillin (100 U/ml), and 20% fetal bovine serum. The amastigotes of Leishmania donovani AG83 were maintained in susceptible golden hamsters. The promastigotes were obtained after 5 days of continuous culture of the amastigotes in medium 199 containing 20% fetal calf serum, HEPES (0.15 M), penicillin (100 U/ml), and streptomycin (100 μg/ml). The bovine serum albumin (BSA) was radioiodinated with chloramine T by the standard method (10) and was purified from unreacted Na125I by Sephadex G-50 column chromatography.
Preparation of liposomes.
The liposomes were prepared by following the standard procedure (8). In brief, PE, cholesterol, and phosphatidic acid were placed in a round-bottom flask at a molar ratio of 7:2:1 and were dissolved in CHCl3-methanol (85:15; vol/vol). The organic solvents were evaporated to dryness in a rotary evaporator under an N2 atmosphere. The dry lipid film was swelled with 125I-labeled BSA or with the drug for 1 h at 37°C followed by sonication for 30 s. This suspension was centrifuged in a Beckman ultracentrifuge (100,000 × g) for 30 min. The pellet was similarly washed twice and was finally suspended in 2 ml of phosphate-buffered saline (PBS; pH 7.4).
Grafting of fMLP onto the liposomal surface.
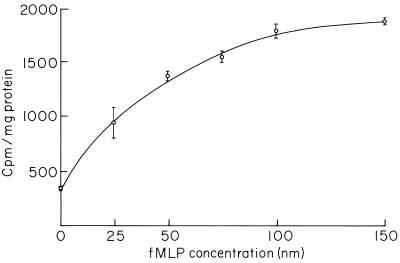
The coupling between the —NH2 group of PE liposomes and the —COOH group of fMLP was done by using EDC1 by following the published procedure (5). In brief, the required amount of fMLP was added dropwise to the liposomal suspension, followed by the dropwise addition of EDC1. The pH of the solution was maintained at 4.3 to 4.5 with 0.1 N HCl. The reaction was allowed to proceed for 2 h at room temperature. The suspension was then layered over 7 ml of Ficoll-Paque and was centrifuged at 750 × g for 20 min. The liposome suspension floating on top was taken out and was washed twice by centrifugation at (100,000 × g) for 30 min. The pellet finally obtained was suspended in PBS containing 0.1 N CaCl2. The grafting efficiency was about 50 to 60% with the various amounts of fMLP (see Fig. 1).
FIG. 1.
Uptake of peptide-grafted liposomes by macrophages as a function of peptide concentration. Results are expressed as means ± standard deviations (n = 3).
In vitro uptake of peptide-grafted liposomes by macrophages as a function of both peptide concentration and time.
For the uptake study, the fMLP-grafted liposomes containing 125I-labeled BSA were used. A total of 200 μl of each liposomal suspension (2 mg of phospholipid) in which various amounts of fMLP (0 to 150 nmol) were grafted onto the liposomal surface was incubated with the macrophages (105 cells in 300 μl of RPMI 1640 medium) at 37°C. After 1 h the suspension was centrifuged (500 × g for 2 min) and washed twice and the pellet was taken for counting of the radioactivity.
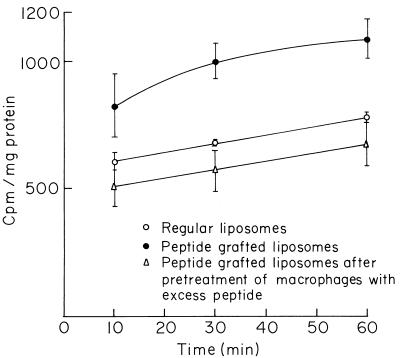
For studying the dependence of uptake with time, 200 μl of liposomal suspension, both regular and modified with 100 nmol of fMLP grafted onto the liposomal surface, was incubated with the cells (105 in 300 μl of RPMI 1640 medium) at 37°C for various time periods. At different time intervals, the suspension was centrifuged as described above and washed and the radioactivity was counted. The nonspecific uptake study was done by preincubating the macrophages (105) with a large excess of free fMLP (1 μmol) for 30 min at room temperature. After 30 min, the cell suspension was centrifuged and washed. The cell pellet was suspended in 300 μl of RPMI 1640 medium. A total of 200 μl of the liposomal suspension with 100 nmol of fMLP on the liposomal surface was added to the cell suspension, and the mixture was incubated at 37°C for various time periods. At various time intervals the suspension was centrifuged and washed and the radioactivity was counted. The ungrafted liposomes were used as controls. The cell protein in all the cases was assayed by the method of Lowry et al. (11).
In vitro parasite killing by the liposomes, free peptide, and primaquine.
The macrophages (105 cells) were incubated with L. donovani promastigotes at a ratio of 1:20 for 2 h at 37°C on a coverslip. After 2 h the nonadhering cells were removed by washing with PBS. These infected macrophages were then incubated with either 200 μl of free peptide (100 nmol), 200 μl of empty liposomes, or 200 μl of peptide (100 nmol)-grafted empty liposomes in RPMI 1640 medium at 37°C for various time periods. At various time periods, the cover glasses were removed and their contents were washed with PBS, air dried, and stained with Giemsa. For each glass the total number of parasites per 300 cells was counted. The untreated infected macrophages were used as controls. The infected macrophages on the cover glass were also incubated with 200 μl of free primaquine in RPMI 1640 medium over a certain range of concentrations, washed, stained with Giemsa, and examined for morphology and internal parasites.
Encapsulation of primaquine within the liposomes.
The liposomes were prepared as described above. A certain amount of primaquine was given during swelling of the lipid film. After 1 h, the suspension was sonicated briefly, centrifuged at (100,000 × g for 30 min), and washed twice. All the washings were collected, and the amount of primaquine present in the supernatant was determined by measuring the optical density at 360 nm (ɛ = 3,100 M−1 cm−1). The level of encapsulation was found to be 14%.
Animal experiment.
Our colony of golden hamsters (Mesocricatus auratus), originally from the Haffkine Research Institute, Bombay, India, was used to maintain L. donovani AG83 from an Indian patient with kala-azar by intracardial passage every 6 weeks. Amastigotes were isolated from spleen by the standard method (12). Each animal was injected with 2 × 106 amastigotes intracardially. A group of 24 hamsters of average body weight (100 g) was infected at a time and was ready for drug testing after 30 days. The hamsters (four in each group) were distributed for drug treatment in the following manner: (i) free primaquine (100 mg/kg of body weight), (ii) empty normal liposomes, (iii) empty peptide-grafted liposomes, (iv) primaquine encapsulated in ordinary liposomes, (v) primaquine encapsulated in peptide-grafted liposomes, and (vi) untreated infected controls.
The amount of primaquine given to each animal was 100 mg/kg of body weight. For determination of the 50% lethal dose, the single dose subcutaneous treatment was followed by treatment with various amounts of primaquine (40 to 500 mg/kg). The optimum dose was found to be about 100 mg/kg of body weight. For chemotherapy, a multiple-dose treatment was used. In practice, 10 mg of primaquine in 0.5 ml of liposomal suspension containing 6 mg of lipid was injected subcutaneously into each hamster every 3 days for a total of four doses over 10 days. Free drug (10 mg/0.5 ml of PBS) was also injected similarly. The animals were killed 3 days after the last injection. Parasite burdens were assessed from stained impression smears by using the formula of Stauber et al. (20). The peptide concentration was kept constant (10 μmol/ml) for all liposomal preparations used for the in vivo experiments.
Investigation of drug toxicity.
A few parameters like blood pathology, tissue histology, and specific enzyme levels related to normal liver function were chosen to determine the toxic effects of the drugs delivered in both the free and the liposomal forms. The animals were killed after the drug treatment. The spleens of the animals were removed for histological examination by eosin and hematoxylin staining (9). Blood samples from the animals were assayed for (i) levels of specific enzymes (e.g., serum glutamate pyruvate transaminase [SGPT] and alkaline phosphatase [4]), (ii) the serum bilirubin concentration (17), (iii) blood urea concentration (14), and (iv) hemoglobin concentration by established procedures.
RESULTS
Uptake of peptide-grafted liposomes as a function of peptide concentration and time.
The saturability of the peptide receptors on the macrophage surface is shown by incubating the macrophages with increasing amounts of peptide grafted onto the liposomal surface at 37°C. The saturation occurred with about 100 nmol of peptide grafted onto the liposome surface (Fig. 1). The phagocytosis of fMLP-grafted liposomes by macrophages was time dependent and reached equilibrium at about 30 min. The uptake at 4°C is very low and is independent of time (data not shown). The process of binding between the peptide receptors and peptide-grafted liposomes was found to be specific in nature. Preincubation of the macrophages with excess free fMLP inhibited further binding, followed by the internalization of fMLP-grafted liposomes through the peptide receptors. A nonsignificant decrease in uptake was noticed for fMLP-grafted liposomes in comparison to that for the regular liposomes (Fig. 2). The microbicidal activities of fMLP itself and fMLP-grafted empty liposomes were determined by incubating them separately with L. donovani-infected macrophages for various time periods. The number of parasites within the macrophages was markedly reduced within 3 h of incubation compared to the number of parasites within untreated cells. The almost similar extent of parasite killing for both the peptide and the peptide-grafted liposomes indicated unaltered microbicidal activity even after chemical modification. Parasite killing to the extent of 5 to 8% was also noticed for empty ungrafted liposomes (Table 1).
FIG. 2.
Specific uptake of peptide-grafted liposomes by macrophages. Results are expressed as means ± standard deviations (n = 3).
TABLE 1.
Microbicidal effect of free peptide, empty liposomes, and peptide-grafted empty liposomes on L. donovani-infected macrophages as a function of time (in vitro)
| Group | No. of parasites/100 macrophages (% reduction) at the following timesa:
|
||
|---|---|---|---|
| 1 h | 2 h | 3 h | |
| Control | 266 ± 12 | 287 ± 20 | 351 ± 59 |
| Free peptide | ND | ND | 218 ± 32 (37.6) |
| Empty liposomes | 251 ± 6 (5.6) | 266 ± 7 (7.3) | 320 ± 20 (8.5) |
| Peptide-grafted empty liposomes | 231 ± 9 (13.5) | 207 ± 36 (28.4) | 201 ± 42 (42.7) |
Results are expressed as means ± standard deviations (n = 3). For each case, at least 300 cells were counted. ND, not determined.
Microbicidal activity and toxicity of peptide-grafted liposomal primaquine.
The microbicidal activity of fMLP-grafted liposomal primaquine in the hamster model of a 30-day L. donovani infection is shown in Table 2. About 14% of the drug used for swelling was encapsulated within the liposomes λmax = 360 nm; ɛ = 3,100 M−1 cm−1). With multiple treatments with free drug at a dose of 100 mg/kg of body weight, the reduction in the spleen parasite load was 17.2%, whereas with the same type of treatment but with the liposomal drug the reduction in the spleen parasite load was 50%. The peptide-grafted empty liposomes and the peptide-grafted liposomal primaquine reduced the spleen parasite load by 31 and 75%, respectively. The reduction in the spleen parasite load (10 to 15%) brought about by empty liposomes was probably the result of an adjuvant effect.
TABLE 2.
Effect of peptide-grafted liposomal primaquine on hamster model of 30-day L. donovani infectiona
| Treatment | Spleen parasite load (107)b | % Suppression of spleen parasite load |
|---|---|---|
| Infected control | 9.6 ± 4.0 | |
| Free drug | 7.9 ± 0.8 | 17.0 |
| Liposomal drug | 4.9 ± 0.5c | 49.0 |
| Peptide-grafted empty liposomes | 6.9 ± 0.8 | 31.0 |
| Peptide-grafted liposomal drug | 2.3 ± 0.2c | 75.4 |
The reduction in the parasite load by empty liposomes was about 10 to 15%.
Spleen parasite load = number of amastigotes per host cell × weight of spleen (in milligrams) × (2 × 107). Results are expressed as means ± standard deviations (n = 4).
P < 0.001 compared to infected control.
In an attempt to determine the toxicity of the drug and/or the delivery system itself, the levels of two specific enzymes (alkaline phosphatase and SGPT), serum bilirubin levels, and blood urea levels were determined. The results are presented in Table 3. The activities of both enzymes increased with free drug treatment but were reduced and became close to the normal level with treatment with peptide-grafted liposomal drug. The serum bilirubin level was maximum for the infected animal, indicating liver blockage by the parasites, but the level decreased with the reduction in the parasite load with treatment with the peptide-grafted liposomal drug. The almost constant level of urea in blood indicated the absence of any nephrotoxicity. The other blood parameters like the erythrocyte level, the leukocyte level, and the hemoglobin content were practically unchanged (data not shown). Histological examination of spleen was also performed after the various treatments. The sizes or shapes of the white pulp, red pulp, and arteries after the treatment of the fMLP-grafted liposomal drug were very close to those of a normal spleen (data not shown), again indicating reduced toxicity under the given conditions.
TABLE 3.
Effect of peptide-grafted liposomal primaquine on specific toxicity parametersa
| Group (treatment) | Alkaline phosphatase level (μmol of p-nitrophenol released/min/liter of serum) | SGPT level (μmol of sodium pyruvate released/min/dl of serum) | Serum bilirubin concn (μmol/liter) | Blood urea concn (mg/100 ml of blood) |
|---|---|---|---|---|
| Normal | 8.2 ± 2.5 | 42.0 ± 6.0 | 5.6 ± 1.4 | 14.9 ± 3.1 |
| Infected control | 16.2 ± 2.3 | 56.5 ± 5.6 | 71.2 ± 2.3 | 11.6 ± 2.9 |
| Free drug | 23.0 ± 3.0 | 94.8 ± 5.8 | 55.0 ± 9.6 | 13.9 ± 1.3 |
| Liposomal drug | 19.3 ± 2.6 | 74.9 ± 3.6 | 31.4 ± 4.5 | 11.5 ± 1.0 |
| fMLP-grafted empty liposomes | 14.2 ± 2.2 | 57.1 ± 4.1 | 40.4 ± 4.2 | ND |
| fMLP-grafted liposomal drug | 17.3 ± 2.7 | 60.0 ± 6.9 | 7.9 ± 3.1 | 15.3 ± 2.0 |
Results are expressed as means ± standard deviations (n = 4). ND, not determined.
DISCUSSION
The reversibility of the binding (at 4°C) between the ligand and the fMLP receptor on the cell surface has already been reported (19), but the internalization of the receptor-ligand complex leads to occasional irreversibility at 37°C. It has been reported that these receptors are not recycled on the cell surface during 2 h of incubation (16). The uptake in vitro of the fMLP-grafted liposomes at 37°C is rapid and highly specific. The uptake attained equilibrium in about 30 min. This in vitro specificity of the receptor-ligand interaction was also noticed when it was tested in vivo. The peptide-grafted liposomes are found to be rapidly cleared from the blood circulation (half-life, ∼2 min) and taken up by the cells of the reticuloendothelial system. It is known that neutrophils possess the receptor for the peptides (2, 3, 16), but due to the short circulation time, the level of uptake of these liposomes by neutrophils is very low (∼1%) compared to that of cells of the reticuloendothelial system.
The macrophage-activating property of fMLP is well known, resulting in the production of a series of reactive toxic oxygen metabolites like OH·, O2−, and H2O2 (2, 3). These metabolites are the causative agent of nonspecific killing of various pathogens. The in vitro time-dependent killing of the leishmania parasite within the macrophages demonstrates that the activating property of fMLP is not affected after the chemical grafting with the —NH2 group of PE liposomes (Table 1).
The applicability of this delivery system has been tested in vivo against experimental leishmaniasis in the hamster model by using primaquine. The mechanism of action of primaquine is through the production of O2− (13, 15). It has been found that with equivalent drug concentrations, fMLP-grafted liposomes are much more effective than either regular liposomes or free drug. Interestingly, the empty fMLP-grafted liposomes reduced the spleen parasite load by 31%, supporting the supposition that the peptide-triggered production of O2− kills the microorganisms. Therefore, both the drug alone and the delivery system alone are capable of killing parasites through O2− production. Enhanced drug activity (Table 2) or reduced drug toxicity (Table 3) is evidenced in the liposomal or the peptide-grafted liposomal form.
This study demonstrates that the efficacy of a drug against reversible leishmaniasis is increased if the drug is used in the fMLP-grafted liposomal form. Unlike other delivery systems, the fMLP-grafted liposome is not only capable of delivering drugs to the macrophages but it also activates the macrophage’s respiratory burst, leading to nonspecific killing of pathogens. It appears that fMLP-grafted or other chemoattractant-grafted (1, 7) liposomes would possibly have useful applications against other macrophage-associated disorders in the near future.
ACKNOWLEDGMENTS
Financial assistance from the Council of Scientific & Industrial Research, New Delhi, India, and the Department of Science & Technology, New Delhi, India, in the form of Senior Research Fellowship (to G.B.) and Research Associateship (to S.M.) is gratefully acknowledged.
REFERENCES
- 1.Babcock G F, Amoscold A A, Nishioka P K. Effect of tuftsin on the migration, chemotaxis and differentiation of macrophages and granulocytes. Ann N Y Acad Sci. 1983;419:64–74. doi: 10.1111/j.1749-6632.1983.tb37092.x. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Kerran K P. Neutrophil activation-control of shape changes exocytosis and the respiratory burst. News Physiol Sci. 1992;7:215–219. [Google Scholar]
- 3.Baggiolini M, Bouley F, Badwey J A, Cournutte J T. Activation of neutrophil leukocytes: chemoattractant receptor and respiratory burst. FASEB J. 1993;7:1004–1010. doi: 10.1096/fasebj.7.11.8396540. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee G, Bhaduri A N, Basu M K. Mannose-coated liposomal hemycin in the treatment of experimental leishmaniasis in hamster. Biochem Med Metab Biol. 1994;53:1–7. doi: 10.1006/bmmb.1994.1050. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty P, Bhaduri A N, Das P K. Sugar receptor mediated drug delivery to macrophages in the therapy of experimental visceral leishmaniasis. Biochem Biophys Res Commun. 1990;166:404–410. doi: 10.1016/0006-291x(90)91959-v. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty R, Mukherjee S, Basu M K. Oxygen-dependent leishmanicidal activity of stimulated macrophages. Mol Cell Biochem. 1996;154:23–29. doi: 10.1007/BF00248457. [DOI] [PubMed] [Google Scholar]
- 7.Cilliari E, Arcollo F, Dieli M, D’Agonisto R, Gromo G, Leoni F, Mileno S. The macrophage-activating tetrapeptide tuftsin induces nitric oxide synthesis and stimulates murine macrophages to kill Leishmania parasites in vitro. Infect Immun. 1994;62:2649–2652. doi: 10.1128/iai.62.6.2649-2652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregoriadis G, Ryman B E. Lysosomal localization of β-fructofuranosidase-coating liposomes injected into rats. Biochem J. 1972;129:123–133. doi: 10.1042/bj1290123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurr E. Staining: animal tissues, practical and theoretical. London, United Kingdom: Leonard Hill Ltd.; 1962. [Google Scholar]
- 10.Hunter W M. Radioimmunoassay. In: Weir D M, editor. Handbook of experimental immunology. Oxford, United Kingdom: Blackwell Scientific Publication; 1978. pp. 14.1–14.4. [Google Scholar]
- 11.Lowry O H, Rosebrough N Y, Farr A L, Randall R J. Protein measurement with the Folin phenol method. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Looker D L, Bercus R L, Marr J J. Purine metabolism in Leishmania donovani amastigotes and promastigotes. Mol Biochem Parasitol. 1983;9:15–28. doi: 10.1016/0166-6851(83)90053-1. [DOI] [PubMed] [Google Scholar]
- 13.Marr J J. In: Parasitic diseases. Mansfield J M, editor. New York, N.Y: Marcel Dekker; 1984. pp. 201–227. [Google Scholar]
- 14.Natelson S. Microtechniques of clinical chemistry for the routine laboratory. Springfield, Ill: Charles C Thomas, Publisher; 1957. p. 381. [Google Scholar]
- 15.New R R C, Chance M L, Heath S. Liposome therapy for experimental cutaneous and visceral leishmaniasis. Biol Cell. 1983;47:59–64. [Google Scholar]
- 16.Niedel J, Wilkinson S, Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979;264:10700–10706. [PubMed] [Google Scholar]
- 17.O’Brien D, Ibbott F A. Laboratory manual of pediatric micro and ultramicro biochemical techniques. 3rd ed. New York, N.Y: Harper & Row Publishers, Inc.; 1962. p. 54. [Google Scholar]
- 18.Schiffman E, Corcoron A, Wahl S M. N-Formylmethionyl peptides as chemoattractant for leucocytes. Proc Natl Acad Sci USA. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyderman R, Fudman E J. Demonstration of a chemotactic factor receptor on macrophages. J Immunol. 1980;124:2754–2757. [PubMed] [Google Scholar]
- 20.Stauber L A, Franchino E, Grun J. An eight day method for screening compounds against Leishmania donovani in the golden hamster. J Protozool. 1958;5:269–273. [Google Scholar]