Abstract
Background
Asthma is a public health problem requiring focused attention. This study aimed to systematically evaluate the association between dietary structure and asthma or wheezing in children.
Methods
The study protocol of this meta-analysis has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration code CRD42023390191. A total of 8397 articles were retrieved, searching PubMed, Medline, Embase, Web of Science, and Scopus databases as of November 21, 2022. Two independent authors were responsible for independently conducting the literature screening process. Effect-size estimates were expressed as odds ratio (OR) in cross-sectional studies and risk ratio (RR) in cohort studies with a 95% confidence interval (CI). Summary effect estimates were evaluated with random-effect models. Meanwhile, subgroup and sensitivity analyses were performed to assess the potential sources of heterogeneity and the robustness of the pooled estimation.
Results
A total of 65 studies, including 567,426 subjects had been analyzed. Overall analyses of cross-sectional studies revealed that a healthy diet was protective against asthma (adjusted OR=0.85, 95% CI: 0.80–0.89, P <0.001, I2=69.8%, Tau2=0.026) and wheezing (adjusted OR=0.85, 95% CI: 0.81–0.89, P <0.001, I2=66.8%, Tau2=0.015) in children and adolescents. Conversely, unhealthy diets can exacerbate asthma (adjusted OR=1.28, 95% CI: 1.20–1.36, P <0.001, I2=64.9%, Tau2=0.019) and wheeze (adjusted OR=1.09, 95% CI: 1.02–1.16, P =0.006, I2=75.2%, Tau2=0.023) in children and adolescents. The same trend was found in cohort studies (adjusted RR=0.72, 95% CI: 0.58–0.90, P =0.003, I2=83.5%, Tau2=0.105). A clear trend was observed between high-frequency healthy diets (OR=0.80; 95% CI: 0.71–0.89; P <0.001) is more protective against asthma than low-frequency healthy diets (OR=0.81; 95% CI: 0.70–0.94; P =0.007).
Conclusion
Our findings highlight the protective effects of a healthy diet on asthma and wheezing in children, including fruit, seafood, cereals, and the Mediterranean diet.
Keywords: dietary habits, wheezing, asthma, children, meta-analysis
Introduction
Asthma is one of the most common chronic diseases in children.1 It is an allergic airway inflammatory disease involving several pathogenic factors.2 Asthma is a public health problem requiring focused attention. Research statistics have reported 300 million asthma patients worldwide, including about 30 million children.3 According to the guidelines of the Global Initiative for Asthma (GINA), the number of people with asthma worldwide will increase year by year and is expected to reach 400 million by 2025.4 In recent decades, the steep increase in asthma prevalence worldwide has created an urgent need to search for effective prevention methods.
The development of asthma cannot be separated from genetic and environmental factors. The increased prevalence of asthma is thought to be related to environmental exposures and lifestyle changes, especially diet.5,6 Foods can sometimes be allergens and trigger asthma, but proper nutrition could also be a protective factor.7 A study found that people who ate a diet rich in whole grains had fewer asthma symptoms and better overall asthma control.8 Currently, the benefits of whole wheat and whole grain foods rich in dietary fiber on children with asthma are mainly attributed to regulating the intestinal flora, promoting the production of metabolites such as butyrate that have anti-inflammatory effects on the intestinal flora, regulating the body’s sugar metabolism, and controlling body weight.9 Allergic airway diseases are often associated with gut inflammation, and dietary patterns regulate the composition of the microbiome, which affects the immune response.10,11 Studies have shown that changes in gut flora caused by diet can affect lung inflammation.12
Numerous studies have shown that eating fruits and vegetables daily may reduce the risk of asthma in children and adults, especially with apples and oranges.13,14 Moreover, fruits and vegetables can also make asthma symptoms more manageable. Extended periods of fruit consumption in asthmatic children have been associated with better symptom control and a lower risk of developing allergic symptoms.13 Alternatively, diets high in total and saturated fats increase airway inflammation in people with asthma.15 A 15-year interval study in the UK showed that Omega-6 fatty acid levels and Omega-3 fatty acid consumption increased as childhood asthma increased.16,17 In contrast, fat is an essential component of a healthy diet for children, but the proper sources and composition of fat for children should be further investigated.18 Moreover, a novel approach exploring the whole diet patterns and diet diversity has been proposed.19
Some Western dietary characteristics have been associated with asthma.20 These “Western” diets emphasize a high proportion of animal meats and a low proportion of fruits, vegetables, grains and legumes. As the consumption of high-calorie, high-fat foods increases, the incidence of allergic diseases and asthma in children increases.11,21
In contrast, the plant-based diet emphasizes a high intake of fruits, vegetables, and grains and a low intake of fatty meats and dairy products.22 Not only does a plant-based diet reduce the risk of developing asthma, but also reduces a child’s lifetime risk of being diagnosed with asthma. While animal products contain almost no fiber, vegan diets tend to emphasize the consumption of high-fiber fruits, vegetables, and whole grains. Therefore, a plant-based diet may potentially improve airway inflammation by promoting anti-inflammatory cytokines, improving blood sugar control, and regulating the intestinal immune response.15
Traditional nutritional epidemiology explores food combinations and the relationship between nutrition and health, but this approach only considers one food and overlooks the interaction between the nutrients it contains.19 However, the reduction and increase of the intake of different foods or nutrients go hand in hand, so distinguishing the role of a single food or nutrient is challenging.
Due to the interaction of nutrients contained in some foods, exploring the impact of a single food on health is difficult. Moreover, drawing effective conclusions might be limited by the small amount of nutrients contained in some foods, and the impact of a certain nutrient on the body might differ based on various dietary patterns and individuals. The dietary pattern refers to the composition of a variety of foods or nutrients.23 It encompasses many food items, evaluates the correlation between each food item, and then identifies the potential hidden variable. Exploring complete eating habits enables a more integrated study of the relationship between various foods, providing a more scientific and authoritative method than traditional research.24 Moreover, a novel approach considering the whole diet pattern and diet diversity has been proposed.
Certain food components can act as allergens to induce asthma in children,15,16 and others can act as protective factors to mitigate asthma.13,14 The majority of existing studies have focused on the effects of individual nutrients on asthma.14,25 However, children’s daily routine is more of a dietary pattern consisting of a combination of different food components. Our study aimed to systematically evaluate the association between dietary structure and asthma or wheezing in children. Understanding the dietary effects on wheezing and asthma may provide a theoretical basis for diet modifications in the prevention of asthma in children.
Methods
Registration and Reporting Format
The study protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration code CRD42023390191. The study protocol was also completed in accordance with the Preferred Reporting Items for Systematic Evaluation and Meta-Analysis (PRISMA) statement (Table S1).
Search Strategy and Selection Process
Relevant literature published before November 21, 2022, was searched in the PubMed, Medline, Embase, Web of Science, and Scopus databases. The search terms such as asthma and dietary patterns and how they were used can be found in Table S2.
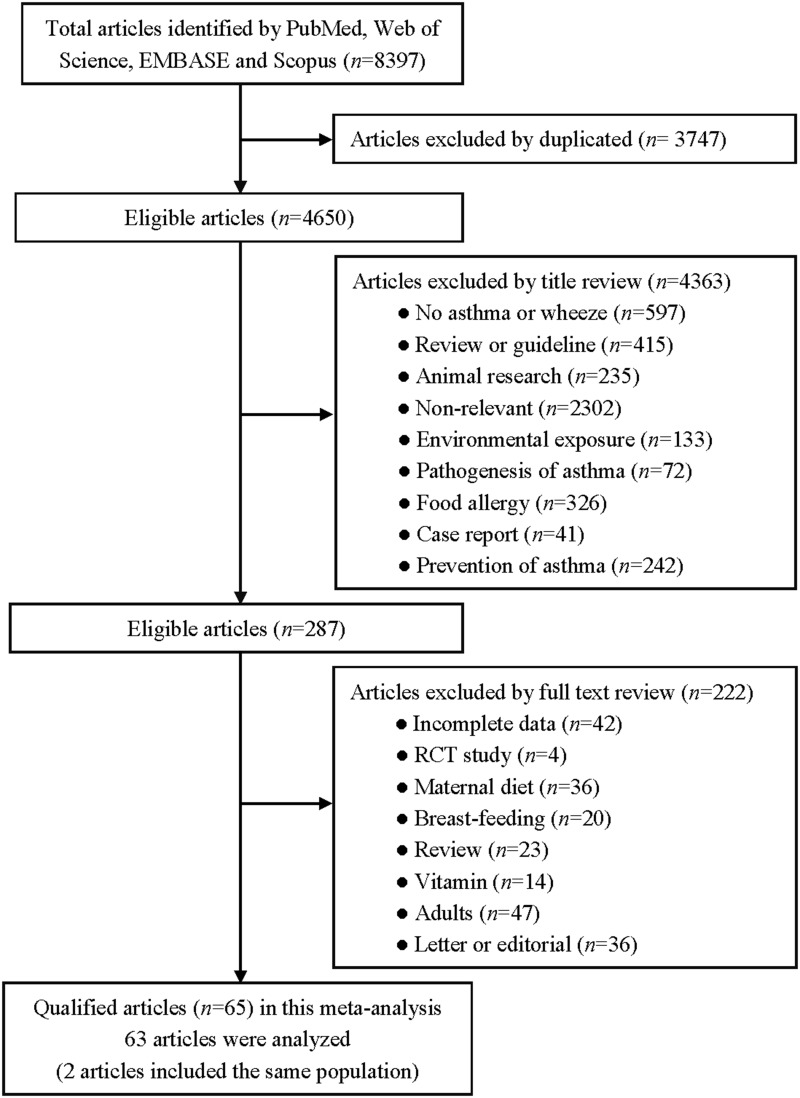
All the included literature was screened using Endnote 20 literature management software. In the first round of screening, duplicates were removed and the study titles were screened. Articles that corresponded to the study subject were selected for the second round of screening, which assessed the relevance of the article abstracts to the research topic. Finally, the full texts were read to determine whether the inclusion criteria were met. The screening details can be seen in Figure 1. Two authors (J.Z. and M.H.) each performed the screening process independently, and disagreements were resolved by a third author (Q.Y.).
Figure 1.
Flow chart of articles retrieved, screened and included in this meta-analysis.
Eligibility Criteria
Our analysis was limited to articles that met the following criteria: (1) study participants: children and adolescents; (2) study endpoint: asthma or wheeze; (3) study type: cohort or cross-sectional study; (4) follow-up rate: at least 70%; (5) study data including dietary patterns or specific dietary components. Case reports, animal studies, and narrative reviews were excluded.
Data Extraction
Statistics from the eligible studies were extracted independently by two authors (J.Z. and M.H.), including the name of the first author, year of publication, country, gender, sample size, age, type of study, duration of follow-up, method of diagnosis of asthma or wheeze, scales of food frequency, detailed dietary patterns, study outcomes, frequency of foods, and confounding risk factors. Disagreements were resolved by discussion with a third author (F.X.).
Risk of Bias of Individual Studies
The Newcastle-Ottawa Scale (NOS) and Agency for Healthcare Research and Quality (AHRQ) scales were used to assess publication bias in the included literature. The NOS scale was developed to assess the quality of cohort studies, incorporating quality assessment into the interpretation of meta-analysis results. In this scale, a study is evaluated from three broad perspectives: selection of the study group, comparability between groups, and separately determining the exposure or outcome of the cohort study. The NOS conducts literature quality evaluations on a full scale of 9 stars.
The AHRQ scale has recommended criteria for evaluating the quality of observational studies. The recommended criteria for evaluating cross-sectional studies include 11 items, which are answered with “yes”, “no” or “unclear”.
Statistical Analyses
Data were managed and analyzed using STATA software for Windows 14.1 (STATA Corp, College Station, TX, USA). In cohort studies, effect sizes were expressed as risk ratios (RR) with 95% confidence intervals (CI). In cross-sectional studies, effect sizes were expressed as odds ratios (ORs) and 95% CI. In addition, the Z-test proposed by Altman and Bland26 was used to compare the magnitude of the two statistics.
The degree of heterogeneity was quantified from the random effects Mantel-Haenszel model using the inconsistency index (I2) statistic, which represents the percentage of diversity due to heterogeneity (rather than chance). I2 > 50% indicates the presence of significant heterogeneity, with higher percentages suggesting a higher degree of heterogeneity. Another indicator, τ2 (Tau2), was used to evaluate the sensitivity of the results to the level of heterogeneity between studies. Taking into account possible sources of clinical and methodological heterogeneity, pre-defined subgroup analyses were performed based on geographical region, study design, age, type of food, and diet frequency. To avoid giving large weights to relatively small studies, a fixed-effect model was fitted using sensitivity analyses.
The probability of publication bias was assessed using Begg’s funnel plot and Egger regression asymmetry tests at a significance level of 10%. The number of theoretically missing studies was estimated using the trim-and-fill method.
Results
Eligible Studies
A total of 8379 articles were initially identified using predefined medical subject words to search the predefined public database, and 65 studies22,27–89 met the inclusion criteria, including 567,426 subjects. The detailed selection process is shown in Figure 1.
Study Characteristics
Table S3 shows the baseline characteristics of the cohort and cross-sectional studies recorded separately in this meta-analysis, while Table S4 presents the specific statistical values in each article. In total, 63 articles were included for data analysis, due to the inclusion of the same population in the four articles by Arvaniti et al57,58 and Antonogeorgos et al.86,87
Of the 63 articles included, 833,34,59–62,69,81 were cohort studies and 5522,27–32,35–57,63–68,70–80,82–86,88–90 were cross-sectional studies. Regarding the geographical area, one article83 originated from Africa, one article51 was international, 13 articles30–32,35,36,41,47,64,73,74,76,77,80 were published in Asia, 27 articles22,27,29,33,34,37–39,43–46,48,49,55–57,59,62,69,70,72,81,82,86,88,90 originated from Europe, 7 articles50,53,54,63,68,84,89 from North America, 4 articles28,40,60,61 from Oceania and 10 articles42,52,65–67,71,75,78,79,85 were published in South America.
All study participants were divided into four age groups:91 toddlers61 (1–2 years), preschool-age children33,34,38,59,60,69 (3–5 years), school-age children22,27,28,30,32,35–37,39–50,52–58,61,62,65,66,68,70,72,74,80–83,87,88,90 (6–13 years), and teenagers31,64,67,77,79,86 (14–17 years).
Of the included eligible literature, only four articles29,45,54,55 analyzed the relationship between dietary habits and asthma in boys and girls separately.
Depending on the type of dietary composition included in the literature, healthy dietary patterns were characterized by a high intake of fruit, vegetables, whole grains, and/or fish, while unhealthy dietary patterns tended to be high in refined grains, red meat, processed meat, fast food, and high-fat foods.92
In order to conduct a detailed subgroup analysis, all dietary components were divided according to the Dietary Guidelines for Americans.93 The categories included vegetables, fruits, starchy choices like potatoes and corn, grains, dairy, and protein foods. At least half of the grains were whole grain; dairy included fat-free or low-fat milk, yogurt, and cheese and yogurt as alternatives; protein foods included lean meats, poultry, eggs, seafood, beans, peas, lentils, nuts, seeds, and soy products.
Quality Assessment
Supplementary Materials show the quality assessment of all eligible articles by using the NOS scales for cohort studies and the AHRQ scales for cross-sectional studies. The average AHRQ score was 7.04 (range: 5 to 9), with a standard deviation of 0.98. The average NOS score was 7.88 (range: 7 to 9), with a standard deviation of 0.64 (Tables S5 and S6).
Overall Analyses
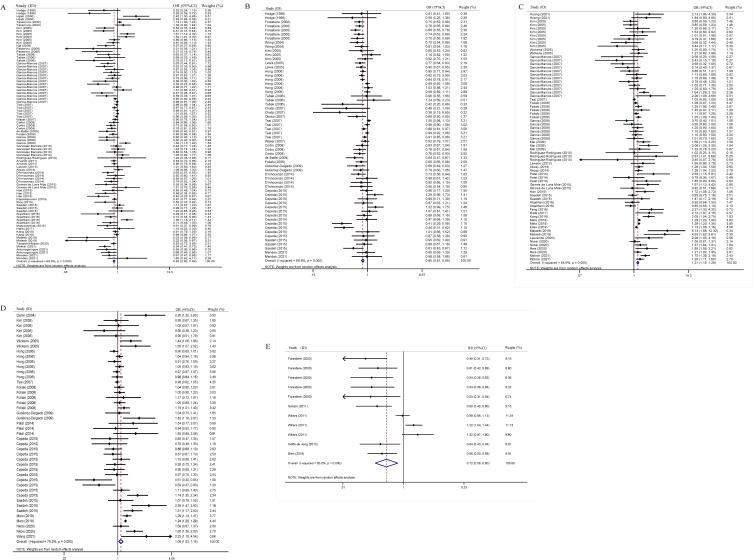
After pooling the statistical results of all the eligible cross-sectional studies, a healthy diet was found to be protective against asthma (adjusted OR=0.85, 95% CI: 0.80–0.89, P <0.001, I2=69.8%, Tau2=0.026) (Figure 2A) and wheeze (adjusted OR=0.85, 95% CI: 0.81–0.89, P <0.001, I2=66.8%, Tau2=0.015) (Figure 2B) in children and adolescents.
Figure 2.
(A) Overall analysis of a healthy diet and childhood asthma risk in cross-sectional studies. (B) a healthy diet and childhood wheeze risk in cross-sectional studies. (C) an unhealthy diet and childhood asthma risk in cross-sectional studies. (D) an unhealthy diet and childhood wheeze risk in cross-sectional studies. (E) a healthy diet and childhood asthma risk in cohort studies.
Conversely, unhealthy diets can exacerbate asthma (adjusted OR=1.21, 95% CI: 1.15–1.28, P <0.001, I2=64.9%, Tau2=0.019) (Figure 2C) and wheeze (adjusted OR=1.09, 95% CI: 1.02–1.16, P =0.006, I2=75.2%, Tau2=0.023) (Figure 2D) in children and adolescents (Table 1).
Table 1.
Overall and Subgroup Analyses of Diet Pattern with Asthma/Wheeze of Children and Adolescents in the Cross-Sectional Studies
| Group | Number of Qualified Observations | Asthma | Wheeze | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI); P | I2 | Tau2 | OR (95% CI); P | I2 | Tau2 | ||
| Overall analyses | |||||||
| Unhealthy diet (unadjusted) | 20/19 | 1.28 (1.20–1.36); <0.001 | 50.4% | 0.006 | 0.99 (0.88–1.13); 0.916 | 81.4% | 0.050 |
| Unhealthy diet (adjusted) | 70/44 | 1.21 (1.15–1.28); <0.001 | 64.9% | 0.019 | 1.09 (1.03–1.16);.006 | 75.2% | 0.023 |
| Healthy diet (unadjusted) | 20/20 | 0.70 (0.58–0.85); <0.001 | 72.2% | 0.113 | 0.83 (0.72–0.96); 0.010 | 72.4% | 0.071 |
| Healthy diet (adjusted) | 85/60 | 0.85 (0.80–0.89); <0.001 | 69.8% | 0.026 | 0.85 (0.81–0.89); <0.001 | 66.8% | 0.015 |
| Subgroup analyses based on adjusted healthy diet | |||||||
| By region | |||||||
| Europe | 44/25 | 0.83 (0.77–0.89); <0.001 | 62.3% | 0.025 | 0.79 (0.74–0.84); <0.001 | 25.0% | 0.005 |
| Oceania | 2/2 | 0.40 (0.21–0.77); 0.006 | 8.2% | 0.020 | 0.72 (0.42–1.22); 0.216 | 0.0% | 0.000 |
| Asian | 19/13 | 0.98 (0.91–1.06);.626 | 71.4% | 0.012 | 0.92 (0.87–0.97); 0.003 | 73.3% | 0.006 |
| South America | 14/16 | 0.75 (0.62–0.90);.002 | 68.4% | 0.075 | 0.87 (0.75–1.01); 0.069 | 72.6% | 0.069 |
| North America | 5/3 | 0.63 (0.47–0.83);.001 | 31.6% | 0.032 | 0.71 (0.59–0.84); <0.001 | 0.0% | 0.000 |
| By age | |||||||
| Preschool-age children | */2 | *;.* | * | * | 0.79 (0.69–0.89); <0.001 | 67.5% | 0.016 |
| School-age children | 63/57 | 0.84 (0.79–0.89); <0.001 | 70.2% | 0.023 | 0.85 (0.81–0.89); <0.001 | 0.0% | 0.000 |
| Teenagers | 12/* | 0.91 (0.78–1.05); 0.198 | 69.3% | 0.039 | *; * | * | * |
| Z-test | School-age VS Teenagers | P=0.163 | Preschool-age VS School-age | P=0.146 | |||
| By gender | |||||||
| Boys | 3/1 | 0.97 (0.91–1.05); 0.475 | 0.0% | 0.000 | 0.74 (0.62–0.89); 0.001 | * | 0.000 |
| Girls | 3/1 | 1.01 (0.82–1.25); 0.916 | 56.0% | 0.199 | 0.70 (0.56–0.88); 0.003 | * | 0.000 |
| By diet frequency | |||||||
| Low | 11/9 | 0.81 (0.70–0.94); 0.007 | 71.3% | 0.033 | 0.85 (0.72–1.01); 0.068 | 74.3% | 0.049 |
| High | 33/26 | 0.80 (0.71–0.89); <0.001 | 73.4% | 0.062 | 0.78 (0.72–0.85); <0.001 | 47.6% | 0.020 |
| Z-test | Low VS High | P=0.448 | Low VS High | P=0.186 | |||
| By type of food | |||||||
| Fruit | 21/21 | 0.86 (0.78–0.95); 0.003 | 58.4% | 0.023 | 0.80 (0.76–0.84); <0.001 | 32.3% | 0.004 |
| Vegetables | 19/13 | 0.91 (0.81–1.02); 0.110 | 68.9% | 0.032 | 0.93 (0.85–1.01); 0.096 | 53.9% | 0.010 |
| Dairy products | 24/15 | 0.90 (0.81–1.01); 0.065 | 79.4% | 0.044 | 0.94 (0.87–1.01); 0.104 | 60.6% | 0.011 |
| Sea food | 16/13 | 0.76 (0.65–0.89); 0.001 | 77.1% | 0.048 | 0.88 (0.78–0.98); 0.019 | 68.1% | 0.019 |
| Cereals | 6/4 | 0.63 (0.40–1.00); 0.049 | 87.9% | 0.283 | 0.98 (0.90–1.06); 0.566 | 0.0% | 0.000 |
| Nuts | 6/5 | 0.93 (0.82–1.06); 0.292 | 40.5% | 0.001 | 0.90 (0.83–0.98); 0.011 | 11.8% | 0.001 |
| Protein foods | 29/14 | 1.03 (0.93–1.13); 0.597 | 77.7% | 0.035 | 1.06 (0.96–1.17); 0.100 | 94.8% | 0.324 |
| Starchy foods | 8/13 | 0.85 (0.63–1.14); 0.285 | 73.4% | 0.123 | 0.86 (0.76–0.97); 0.015 | 79.1% | 0.032 |
| Mediterranean diet | 12/1 | 0.88 (0.79–0.97); 0.014 | 63.2% | 0.015 | 0.64 (0.47–0.87); 0.004 | * | 0.000 |
| Subgroup analyses based on adjusted unhealthy diet | |||||||
| By region | |||||||
| Europe | 31/10 | 1.09 (0.95–1.26); 0.207 | 59.9% | 0.072 | 1.20 (0.96–1.50); 0.100 | 52.3% | 0.061 |
| Oceania | 2/2 | 1.26 (0.97–1.63); 0.082 | 0.0% | 0.000 | 1.51 (1.18–1.94); 0.001 | 0.0% | 0.000 |
| Asian | 12/8 | 1.51 (1.22–1.88); <0.001 | 83.5% | 0.093 | 0.99 (0.93–1.05); 0.699 | 45.7% | 0.003 |
| South America | 11/14 | 1.18 (1.08–1.28); <0.001 | 65.9% | 0.006 | 1.00 (0.89–1.14); 0.961 | 77.2% | 0.034 |
| North America | 7/3 | 1.49 (1.26–1.76); <0.001 | 23.6% | 0.012 | 1.52 (0.95–2.44); 0.081 | 64.9% | 0.110 |
| Africa | 2/2 | 1.19 (0.92–1.54); 0.199 | 40.6% | 0.014 | 1.32 (0.91–1.92); 0.150 | 80.8% | 0.060 |
| International | 5/5 | 1.17 (1.09–1.26); <0.001 | 11.1% | 0.001 | 1.08 (1.00–1.17); 0.051 | 0.0% | 0.000 |
| By age | |||||||
| School-age children | 43/36 | 1.22 (1.09–1.36); <0.001 | 66.6% | 0.063 | 1.07 (0.99–1.15); 0.073 | 66.0% | 0.023 |
| Teenagers | 14/* | 1.21 (1.13–1.29); 0.001 | 60.6% | 0.023 | *; * | * | * |
| Z-test | School-age VS Teenagers | P=0.450 | * | * | |||
| By gender | |||||||
| Boys | 1/* | 2.06 (1.29–3.30); 0.003 | * | 0.000 | *; * | * | * |
| Girls | 1/* | 1.32 (0.78–2.23); 0.300 | * | 0.000 | *; * | * | * |
| By diet frequency | |||||||
| Low | 12/8 | 1.10 (0.92–1.31); 0.322 | 67.2% | 0.071 | 1.00 (0.85–1.17); 0.979 | 55.6% | 0.027 |
| High | 32/19 | 1.26 (1.17–1.36); <0.001 | 64.9% | 0.014 | 1.21 (1.09–1.35); <0.001 | 70.1% | 0.027 |
| Z-test | Low VS High | P=0.08 | Low VS High | P=0.03 | |||
| By type of food | |||||||
| Fast food | 26/11 | 1.38 (1.26–1.50); <0.001 | 66.4% | 0.018 | 1.33 (1.18–1.49); <0.001 | 71.2% | 0.020 |
| Margarine | 8/5 | 1.23 (1.13–1.35); <0.001 | 0.0% | 0.000 | 1.01 (0.87–1.17); 0.930 | 48.4% | 0.013 |
Note: *Data are not available.
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
In the cohort studies, a healthy diet was a protective factor for wheezing (adjusted RR=0.72, 95% CI: 0.58–0.90, P =0.003, I2=83.5%, Tau2=0.105) in children and adolescents (Figure 2E) (Table 2).
Table 2.
Overall and Subgroup Analyses of Diet Pattern with Asthma/Wheeze of Children and Adolescents in the Cohort Studies
| Group | Number of Qualified Observations | Asthma | Wheeze | ||||
|---|---|---|---|---|---|---|---|
| RR (95% CI); P | I2 | Tau2 | RR (95% CI); P | I2 | Tau2 | ||
| Overall analyses | |||||||
| Unhealthy diet (adjusted) | 7/7 | 1.00 (0.93–1.08); 0.971 | 7.6% | 0.001 | 1.03 (0.96–1.12);.385 | 0.0% | 0.000 |
| Healthy diet (adjusted) | 6/11 | 0.91 (0.76–1.09); 0.302 | 75.8% | 0.030 | 0.72 (0.58–0.90); 0.003 | 83.5% | 0.105 |
| Subgroup analyses based on adjusted healthy diet | |||||||
| By region | |||||||
| Europe | 3/7 | 0.68 (0.54–0.87); 0.002 | 0.0% | 0.000 | 0.58 (0.50–0.67); <0.001 | 0.0% | 0.000 |
| Oceania | 3/4 | 1.05 (0.88–1.25); 0.626 | 80.3% | 0.019 | 1.04 (0.83–1.30); 0.752 | 75.9% | 0.037 |
| By age | |||||||
| Preschool-age children | 4/4 | 1.02 (0.87–1.19); 0.842 | 72.3% | 0.017 | 1.01 (0.79–1.30); 0.944 | 81.7% | 0.050 |
| School-age children | 2/6 | 0.60 (0.44–0.81); 0.001 | 0.0% | 0.000 | 0.58 (0.49–0.68); <0.001 | 0.0% | 0.000 |
| Z-test | Preschool-age VS School-age | P=0.001 | Preschool-age VS School-age | P<0.001 | |||
| By gender | |||||||
| Boys | */1 | *; * | * | * | 0.54 (0.36–0.84); 0.004 | * | 0.000 |
| Girls | */1 | *; * | * | * | 0.53 (0.30–0.92); 0.025 | * | 0.000 |
| By diet frequency | |||||||
| Low | */1 | *; * | * | * | 0.48 (0.31–0.73); 0.068 | * | 0.000 |
| Moderate | */1 | *; * | * | * | 0.61 (0.42–0.89); <0.001 | * | 0.000 |
| High | */3 | *; * | * | * | 0.54 (0.41–0.70); <0.001 | 0.0% | 0.000 |
| By type of food | |||||||
| Sea food | 4/4 | 0.80 (0.55–1.18); 0.260 | 77.6% | 0.115 | 0.77 (0.53–1.11); 0.162 | 80.8% | 0.118 |
| Fruit | 1/6 | 0.90 (0.82–0.99); 0.028 | * | 0.000 | 0.61 (0.45–0.84); 0.003 | 79.9% | 0.119 |
| Vegetables | 1/1 | 1.10 (0.98–1.24); 0.112 | * | 0.000 | 1.22 (1.04–1.43); 0.017 | * | 0.000 |
| Starchy foods | 2/2 | 0.79 (0.51–1.23); 0.302 | 74.9% | 0.079 | 0.93 (0.81–1.07); 0.300 | 0.0% | 0.000 |
| Dairy products | 3/3 | 0.97 (0.88–1.08); 0.605 | 0.0% | 0.000 | 0.91 (0.66–1.27); 0.585 | 81.4% | 0.064 |
| Subgroup analyses based on adjusted unhealthy diet | |||||||
| By region | |||||||
| Europe | 5/5 | 0.97 (0.67–1.41); 0.876 | 36.5% | 0.066 | 1.09 (0.89–1.34); 0.418 | 0.0% | 0.000 |
| Oceania | 2/2 | 1.00 (0.94–1.07); 0.935 | 0.0% | 0.000 | 1.03 (0.95–1.11); 0.539 | 0.0% | 0.000 |
| By age | |||||||
| Preschool-age children | 4/4 | 1.00 (0.94–1.06); 0.934 | 0.0% | 0.000 | 1.02 (0.95–1.11); 551 | 0.0% | 0.000 |
| School-age children | 3/3 | 1.14 (0.61–2.14); 0.676 | 58.7% | 0.181 | 1.28 (0.90–1.82); 0.177 | 0.0% | 0.000 |
| By diet frequency | |||||||
| High | 5/5 | 0.97 (0.67–1.41); 0.876 | 36.5% | 0.066 | 1.09 (0.89–1.34); 0.418 | 0.0% | 0.000 |
| By type of food | |||||||
| Margarine | 2/2 | 1.00 (0.93–1.09); 0.919 | 0.0% | 0.000 | 1.00 (0.91–1.11); 0.931 | 0.0% | 0.000 |
| Butter | 2/2 | 0.99 (0.89–1.10); 0.785 | 0.0% | 0.000 | 1.06 (0.93–1.19); 0.397 | 0.0% | 0.000 |
Note: *Data are not available.
Abbreviations: RR, risk ratio; 95% CI, 95% confidence interval.
Cumulative and Influential Analyses
When exploring the effects of healthy and unhealthy diets on asthma and wheezing in cross-sectional studies, the cumulative analysis of the included studies yielded similar findings and trends converged. Sensitivity analyses showed no significant effect of any single study on the overall effect size estimates. However, the results of the cohort studies showed instability in the trend of the protective effect of a healthy diet on wheezing.
Publication Bias
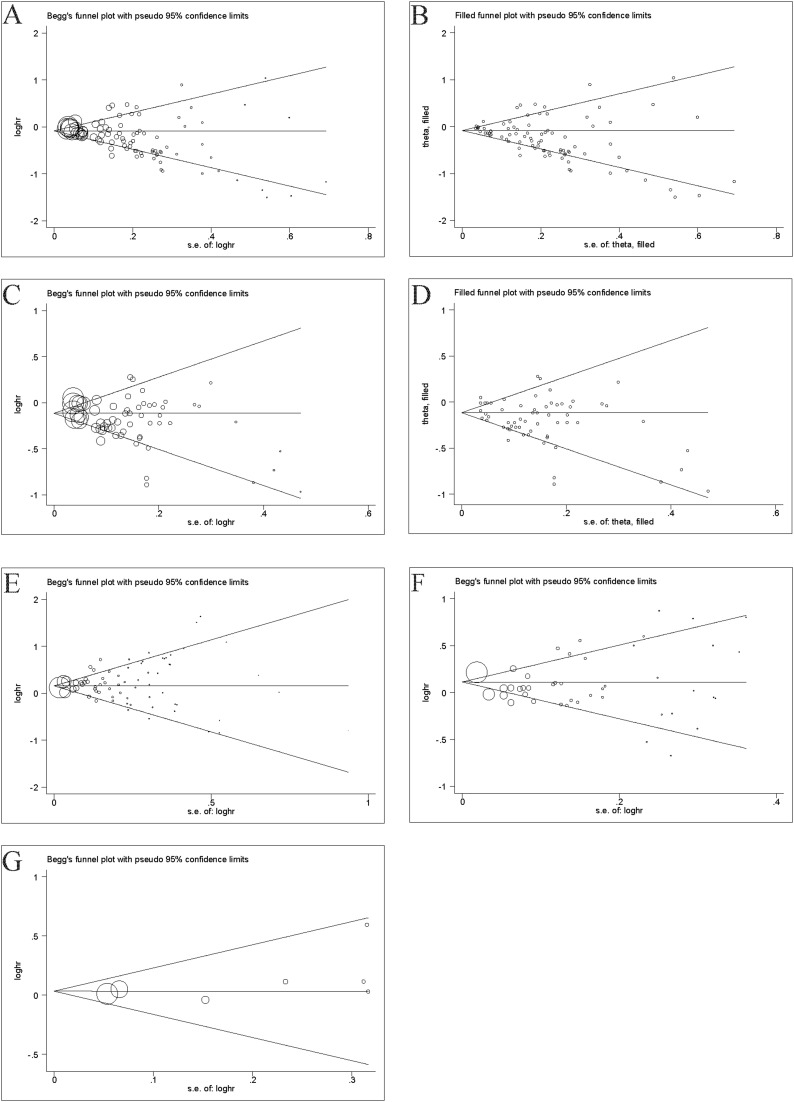
Figure 3 shows the Begg’s and filled funnel plots on the association between healthy/unhealthy diet with asthma/wheezing in children and adolescents. Heterogeneity was observed in the overall analysis of healthy diets for asthma and wheeze, and Begg’s and filled funnel plots suggested publication bias.
Figure 3.
(A) Begg’s funnel plot of healthy diet with asthma in cross-section studies. (B) Filled funnel plot of healthy diet with asthma in cross-sectional studies. (C) Begg’s funnel plot of healthy diet with wheeze in cross-sectional studies. (D) Filled funnel plot of healthy diet with wheeze in cross-sectional studies. (E) Begg’s funnel plot of unhealthy diet with asthma in cross-sectional studies. (F) Begg’s funnel plot of unhealthy diet with wheeze in cross-sectional studies. (G) Begg’s funnel plot of unhealthy diet with wheeze in cohort studies.
In the cross-sectional studies, the asymmetry of study effects was found in terms of healthy diets on asthma (P <0.001) (Figure 3A). Further analysis using the “trim and fill” method showed that the statistical results of healthy diet on asthma converged before and after trimming (Figure 3B), with no significant reversal. The asymmetry of study effects was found in terms of healthy diets on wheezing (P =0.002) (Figure 3C). Further analyzed using the “trim and fill” method, the statistical results of healthy diet on wheezing were consistent before and after trimming (Figure 3D), with no significant reversal.
In the cross-sectional studies, the overall analysis of the unhealthy diets with asthma (P =0.204) (Figure 3E) and wheezing (P =0.343) (Figure 3F) revealed no publication bias on Egger’s test.
In the cohort studies, the overall analysis of the unhealthy diets with wheeze (P =0.224) (Figure 3G) revealed no publication bias on Egger’s test.
Subgroup Analyses
Between-study heterogeneity was present in the overall analysis evaluating healthy/unhealthy diets with regard to children’s asthma/wheezing, which was indicated by I2 > 50.0%. A series of pre-specified subgroup analyses were done to explore possible sources of between-study heterogeneity (Table 1). The overall analysis showed the protective effect of a healthy diet against asthma and wheezing in children and adolescents, and the subgroup analysis results provided strong evidence.
A significant association between a healthy diet and asthma was observed based on geographic area, including Europe (OR=0.83; 95% CI: 0.77–0.89; P <0.001), Oceania (OR=0.40; 95% CI: 0.21–0.77; P =0.006), South America (OR=0.75; 95% CI: 0.62–0.90; P =0.002) and North America (OR=0.63; 95% CI: 0.47–0.83; P <0.001). However, there is no association between a healthy diet with asthma in Asia (OR=0.98; 95% CI: 0.91–1.06; P =0.626).
Furthermore, a significant association between a healthy diet with wheezing was observed, including Europe (OR=0.79; 95% CI: 0.74–0.84; P <0.001), Asia (OR=0.92; 95% CI: 0.87–0.99; P =0.003) and North America (OR=0.71; 95% CI: 0.59–0.84; P <0.001). However, no such association was found in South America (OR=0.87; 95% CI: 0.75–1.01; P =0.069) and Oceania (OR=0.72; 95% CI: 0.42–1.22; P =0.216).
Subgroup analysis based on age revealed that a healthy diet was significantly associated with lower rates of asthma in school-age children (OR=0.84; 95% CI: 0.79–0.89; P <0.001) and wheezing (OR=0.85; 95% CI: 0.81–0.89; P <0.001). A significant association was observed between a healthy diet and wheezing in preschool-age children (OR=0.79; 95% CI: 0.69–0.89; P <0.001) (Preschool-age vs School-age Z-test P =0.146). However, no association was found between a healthy diet and asthma in teenagers (OR=0.91; 95% CI: 0.78–1.05; P =0.198) (School-age VS Teenagers Z-test P =0.163).
The results of the subgroup analyses of gender show that healthy dietary patterns are a protective factor for childhood wheeze in both boys (OR=0.74; 95% CI: 0.62–0.89; P =0.001) and girls (OR=0.70; 95% CI: 0.56–0.88; P =0.003) (Boys VS Girls Z-test P =0.353). Unhealthy diets are a risk factor for childhood asthma for boys (OR=2.06; 95% CI: 1.29–3.30; P =0.003).
The frequency of foods included in the article was divided into low (<=1–2 weeks) and high (≥3 weeks). A clear trend was observed between high-frequency healthy diets (OR=0.80; 95% CI: 0.71–0.89; P <0.001) is more protective against asthma than low-frequency healthy diets (OR=0.81; 95% CI: 0.70–0.94; P =0.007) (two-sample Z-test P =0.163). This trend was also evident in the protective effect of the high-frequency healthy diet (OR=0.78; 95% CI: 0.72–0.85; P <0.001) on wheezing compared to the low-frequency healthy diet (OR=0.85; 95% CI: 0.72–1.01; P =0.068) (two-sample Z-test P =0.186).
In order to explore which dietary components of a healthy dietary pattern were more protective, a more detailed subgroup analysis was carried out. The results of the subgroup analysis showed that the protective effects of fruits (OR=0.86; 95% CI: 0.78–0.95; P=0.003), seafood (OR=0.76; 95% CI: 0.65–0.89; P=0.001), cereals (OR=0.63; 95% CI: 0.40–1.00; P=0.045) and the Mediterranean dietary pattern (OR=0.88; 95% CI: 0.79–0.97; P=0.014) on asthma were more prominent. The evidence for the protective effect of the same food components on wheezing was equally valid (Figure S1).
The overall analysis revealed that an unhealthy diet can exacerbate asthma and wheezing in children and adolescents, and the evidence from the subgroup analysis was consistent with the overall analysis.
A significant association was observed between a healthy diet and asthma based on geographic area, including Asia (OR=1.51; 95% CI: 1.22–1.88; P <0.001), South America (OR=1.18; 95% CI: 1.08–1.28; P <0.001), and North America (OR=1.49; 95% CI: 1.26–1.76; P <0.001). However, no association was observed between a healthy diet and asthma in Oceania (OR=1.26; 95% CI: 0.97–1.63; P =0.082), Europe (OR=1.09; 95% CI: 0.95–1.26; P =0.207), and Africa (OR=1.19; 95% CI: 0.92–1.54; P =0.199).
In addition, an unhealthy diet can aggravate asthma in school-age children (OR=1.22; 95% CI: 1.09–1.36; P <0.001) and teenagers (OR=1.21; 95% CI: 1.13–1.29; P =0.001) (School-age vs Teenagers Z-test P =0.450).
A clear trend was observed between high-frequency unhealthy diets (OR=1.26; 95% CI: 1.17–1.36; P <0.001) and poor asthma control compared to low-frequency healthy diets (OR=1.10; 95% CI: 0.92–1.31; P =0.322) (two-sample Z-test P =0.08). The Z-test results indicated that a high-frequency unhealthy diet (OR=1.21; 95% CI: 1.09–1.35; P <0.001) had stronger effects on wheezing than a low-frequency unhealthy diet (OR=1.00; 95% CI: 0.85–1.17; P =0.979) (two-sample Z-test P =0.03).
The results of the subgroup analysis showed a significant association between fast food and asthma (OR=1.38; 95% CI: 1.26–1.50; P <0.001) and wheezing (OR=1.33 95% CI: 1.18–1.49; P <0.001). Meanwhile, margarine is proven to be harmful to asthma in children and adolescents (OR=1.23; 95% CI: 1.13–1.35; P <0.001).
Discussion
This is the most comprehensive meta-analysis to date on the relationship between dietary factors and asthma or wheezing in children and adolescents. Our findings indicate that an unhealthy diet can worsen asthma or wheezing, while healthy eating habits can protect against these conditions. These results emphasize the importance of maintaining a nutritious diet for children and adolescents with asthma or wheeze, and provide valuable statistical evidence for preventing and treating asthma.
Among the dietary items in our study, fruits, seafood, cereals, and the Mediterranean dietary pattern were particularly effective in protecting against asthma or wheezing. Fruit is thought to be rich in antioxidants and other bioactive factors that help support lung health. Antioxidants are dietary components that motivate lung tissue in response to oxidative stress and reduce respiratory damage caused by reactive oxygen radicals.70 Therefore, fruits can reduce inflammatory reactions, asthma symptoms, and improve lung function. Reactive oxygen species released by eosinophils and neutrophils may play a key role in the development of asthma.94 Seafood, being high in long-chain n-3 polyunsaturated fatty acids, has been proven to effectively decrease inflammatory responses and alleviate asthma symptoms.95 The protective mechanisms of cereals against asthma are not fully understood and whole grains may protect against asthma through the antioxidant and anti-inflammatory effects of their contents (eg, vitamins, minerals, and phytonutrients).96 The Mediterranean diets, characterized by high fruit and whole grain intake and low consumption of meat and dairy products, has been linked to a lower risk of asthma in numerous epidemiological studies.23,57,97 Vegetables, like fruits, are commonly known for their antioxidant properties. However, contrary to previous studies that found a protective effect of regular vegetable consumption on asthma development,88,98,99 no such significant effect was observed in this study. This discrepancy could be due to variations in the selection and preparation methods of vegetables, which may have contributed to the heterogeneity in the studies.
In the subgroup analysis based on age, we found the protective effect of a healthy diet against asthma or wheeze was most pronounced in school-age children compared to teenagers. This could be attributed to the fact that school-age children’s dietary choices are more influenced by their parents.100 In contrast, adolescents have more freedom to select their preferred foods, and their peers are believed to have a significant impact on their eating habits.101 Meanwhile, in the present study, the frequency of healthy eating was further categorized into low (1–2/week), moderate (3–4/week), and high (>5/week) based on data from the included articles. Regardless of asthma and wheezing, there was a stronger protective effect observed with higher-frequency healthy eating habits compared to lower-frequency habits. This can be explained by the presence of bioactive factors in foods consumed more frequently, which enhance their ability to protect against asthma and wheezing.
On the contrary, our research indicates that an unhealthy diet can worsen asthma and wheezing in children and adolescents. Specifically, we found that both margarine and fast food significantly contribute to the exacerbation of these respiratory conditions. These findings align with the lipid hypothesis, which suggests that a higher intake of polyunsaturated fatty acids (PUFA) compared to saturated fats may increase the risk of developing asthma and allergies. It is important not to overlook the potential role of margarine as a significant source of PUFAs in relation to the development of asthma and allergies.102–105 Additionally, our analysis using Egger’s test did not reveal any publication bias regarding unhealthy dietary patterns’ impact on asthma/wheeze.
Similarly, the present study also found that fast food consumption as an unhealthy diet was negatively associated with the prevalence of asthma. A potential mechanism for this association is the high content of hydrogenated fats, saturated fatty acids, and n6 polyunsaturated fatty acids, which are major components of the body’s inflammatory cells. High consumption of these saturated fats may also lead to innate immune system activation through excessive production of pro-inflammatory cytokines and reduced production of anti-inflammatory cytokines.106 This situation must be taken seriously, as the increase in consumption of processed foods and changes in culinary practices have confirmed a gradual shift from traditional diets to diets high in saturated fats, refined carbohydrates, and foods of animal origin.107 The World Health Organization108 has also developed a global strategy on diet and health, aimed at reducing unhealthy diets and preventing different diseases, including asthma.
However, in both healthy and unhealthy dietary patterns, it is regrettable that we cannot clearly see the gender differences in the impact of dietary patterns on asthma from gender-based subgroup analysis, because there are few cases where gender was analyzed separately in the original studies. Apart from that, there are some other shortcomings that need to be pointed out. First, although the overall analysis of the cohort study showed a protective effect of healthy diet on wheezing in children and adolescents, publication bias and heterogeneity were observed due to the limited number of cohort study articles (8 cohort studies). Secondly, the included cross-sectional studies also involved challenges in quantifying dietary intake, reverse causality, and lack of temporal factors. Unfortunately, limited longitudinal data is available regarding dietary habits in asthma. Additional longitudinal studies are required to elucidate the association between healthy or unhealthy dietary habits and asthma. In addition, this analysis was restricted to self-reported or physician-diagnosed asthma and dietary intake data, which could lead to selective underreporting or misreporting. Despite these potential limitations, the results suggest that the dietary habits of the target population should be taken into account to explain the effect of specific foods on the development of asthma in clinical practice. Thirdly, the differences in the effect of dietary habits on asthma based on gender in children need to be supported by more data from future studies.
Funding Statement
This study was supported by both National High Level Hospital Clinical Research Funding (NO.2022-NHLHCRF-YS-04) and the Project of Disciplines Construction of State Key Clinical Department [grant (2011)873].
Data Sharing Statement
The datasets used and/or analyzed during the current meta-analysis are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Ethics approval and consent to participate were received by each involved study in this meta-analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Porsbjerg C, Melen E, Lehtimaki L, Shaw D. Asthma. Lancet. 2023;401(10379):858–873. doi: 10.1016/S0140-6736(22)02125-0 [DOI] [PubMed] [Google Scholar]
- 2.Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42(1):5–15. doi: 10.1007/s00281-020-00785-1 [DOI] [PubMed] [Google Scholar]
- 3.Mortimer K, Lesosky M, Garcia-Marcos L, et al. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN Phase I study. Eur Respir J. 2022;60(3):2102865. doi: 10.1183/13993003.02865-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS. WAO White Book on Allergy. Vol. 3. Milwaukee, WI: World Allergy Organization; 2011:156–157. [Google Scholar]
- 5.Melen E, Koppelman GH, Vicedo-Cabrera AM, Andersen ZJ, Bunyavanich S. Allergies to food and airborne allergens in children and adolescents: role of epigenetics in a changing environment. Lancet Child Adolesc Health. 2022;6(11):810–819. doi: 10.1016/S2352-4642(22)00215-2 [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Pacheco N, Kere M, Melen E. Gene-environment interactions in childhood asthma revisited; expanding the interaction concept. Pediatr Allergy Immunol. 2022;33(5):e13780. doi: 10.1111/pai.13780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530–2539. doi: 10.1056/NEJMoa1503734 [DOI] [PubMed] [Google Scholar]
- 8.Verstegen REM, Kostadinova AI, Merenciana Z, et al. Dietary fibers: effects, underlying mechanisms and possible role in allergic asthma management. Nutrients. 2021;13(11):4153. doi: 10.3390/nu13114153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthon BS, Macdonald-Wicks LK, Gibson PG, Wood LG. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology. 2013;18(3):447–454. doi: 10.1111/resp.12015 [DOI] [PubMed] [Google Scholar]
- 10.Vaccaro JA, Niego J, Huffman FG. Dietary factors, body weight, and screen time in U.S. children with and without asthma. Children’s Health Care. 2016;45(1):22–38. doi: 10.1080/02739615.2014.948165 [DOI] [Google Scholar]
- 11.Arshad SH, Bateman B, Matthews SM. Primary prevention of asthma and atopy during childhood by allergen avoidance in infancy: a randomised controlled study. Thorax. 2003;58(6):489–493. doi: 10.1136/thorax.58.6.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budden KF, Gellatly SL, Wood DL, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142 [DOI] [PubMed] [Google Scholar]
- 13.Chatzi L, Apostolaki G, Bibakis I, et al. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax. 2007;62(8):677–683. doi: 10.1136/thx.2006.069419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini B, Berthon BS, Wark P, Wood LG. Effects of fruit and vegetable consumption on risk of asthma, wheezing and immune responses: a systematic review and meta-analysis. Nutrients. 2017;9(4). doi: 10.3390/nu9040341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogenkamp A, Ehlers A, Garssen J, Willemsen LEM. Allergy modulation by N-3 Long chain polyunsaturated fatty acids and fat soluble nutrients of the mediterranean diet. Front Pharmacol. 2020;11:1244. doi: 10.3389/fphar.2020.01244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brigham EP, Woo H, McCormack M, et al. Omega-3 and Omega-6 intake modifies asthma severity and response to indoor air pollution in children. Am J Respir Crit Care Med. 2019;199(12):1478–1486. doi: 10.1164/rccm.201808-1474OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams EJ, Berthon BS, Stoodley I, Williams LM, Wood LG. Nutrition in Asthma. Semin Respir Crit Care Med. 2022;43(5):646–661. doi: 10.1055/s-0042-1742385 [DOI] [PubMed] [Google Scholar]
- 18.Radzikowska U, Rinaldi AO, Celebi Sozener Z, et al. The influence of dietary fatty acids on immune responses. Nutrients. 2019;11(12):2990. doi: 10.3390/nu11122990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venter C, Greenhawt M, Meyer RW, et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: novel concepts and implications for studies in allergy and asthma. Allergy. 2020;75(3):497–523. doi: 10.1111/all.14051 [DOI] [PubMed] [Google Scholar]
- 20.Li J, Xun P, Zamora D, et al. Intakes of long-chain omega-3 (n-3) PUFAs and fish in relation to incidence of asthma among American young adults: the CARDIA study. Am J Clin Nutr. 2013;97(1):173–178. doi: 10.3945/ajcn.112.041145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaheen SO, Northstone K, Newson RB, Emmett PM, Sherriff A, Henderson AJ. Dietary patterns in pregnancy and respiratory and atopic outcomes in childhood. Thorax. 2009;64(5):411–417. doi: 10.1136/thx.2008.104703 [DOI] [PubMed] [Google Scholar]
- 22.Patel S, Custovic A, Smith JA, Simpson A, Kerry G, Murray CS. Cross-sectional association of dietary patterns with asthma and atopic sensitization in childhood - in a cohort study. Pediatr Allergy Immunol. 2014;25(6):565–571. doi: 10.1111/pai.12276 [DOI] [PubMed] [Google Scholar]
- 23.Alwarith J, Kahleova H, Crosby L, et al. The role of nutrition in asthma prevention and treatment. Nutr Rev. 2020;78(11):928–938. doi: 10.1093/nutrit/nuaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilleminault L, Williams EJ, Scott HA, Berthon BS, Jensen M, Wood LG. Diet and asthma: is it time to adapt our message? Nutrients. 2017;9(11):1227. doi: 10.3390/nu9111227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Cao L, Wang Z, Wang Z. Dietary meat intake and risk of asthma in children: evidence from a meta-analysis. Medicine. 2020;99(1):e18235. doi: 10.1097/MD.0000000000018235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey OJ, Cookson JB, Britton J, Tattersfield AE. The effect of lifestyle on wheeze, atopy, and bronchial hyperreactivity in Asian and white children. Am J Respir Crit Care Med. 1996;154(2 Pt 1):537–540. doi: 10.1164/ajrccm.154.2.8756835 [DOI] [PubMed] [Google Scholar]
- 28.Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ. Consumption of oily fish and childhood asthma risk. Med J Aust. 1996;164(3):137–140. doi: 10.5694/j.1326-5377.1996.tb122010.x [DOI] [PubMed] [Google Scholar]
- 29.Forastiere F, Pistelli R, Sestini P, et al. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment). Thorax. 2000;55(4):283–288. doi: 10.1136/thorax.55.4.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hijazi N, Abalkhail B, Seaton A. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax. 2000;55(9):775–779. doi: 10.1136/thorax.55.9.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang SL, Lin KC, Pan WH. Dietary factors associated with physician-diagnosed asthma and allergic rhinitis in teenagers: analyses of the first Nutrition and Health Survey in Taiwan. Clin Exp Allergy. 2001;31(2):259–264. doi: 10.1046/j.1365-2222.2001.00938.x [DOI] [PubMed] [Google Scholar]
- 32.Takemura Y, Sakurai Y, Honjo S, et al. The relationship between fish intake and the prevalence of asthma: the Tokorozawa childhood asthma and pollinosis study. Prev Med. 2002;34(2):221–225. doi: 10.1006/pmed.2001.0978 [DOI] [PubMed] [Google Scholar]
- 33.Nafstad P, Nystad W, Magnus P, Jaakkola JJ. Asthma and allergic rhinitis at 4 years of age in relation to fish consumption in infancy. J Asthma. 2003;40(4):343–348. doi: 10.1081/JAS-120018633 [DOI] [PubMed] [Google Scholar]
- 34.Wijga AH, Smit HA, Kerkhof M, et al. Association of consumption of products containing milk fat with reduced asthma risk in pre-school children: the PIAMA birth cohort study. Thorax. 2003;58(7):567–572. doi: 10.1136/thorax.58.7.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demir AU, Karakaya G, Bozkurt B, Sekerel BE, Kalyoncu AF. Asthma and allergic diseases in schoolchildren: third cross-sectional survey in the same primary school in Ankara, Turkey. Pediatr Allergy Immunol. 2004;15(6):531–538. doi: 10.1111/j.1399-3038.2004.00202.x [DOI] [PubMed] [Google Scholar]
- 36.Wong GW, Ko FW, Hui DS, et al. Factors associated with difference in prevalence of asthma in children from three cities in China: multicentre epidemiological survey. BMJ. 2004;329(7464):486. doi: 10.1136/bmj.329.7464.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JL, Elfman L, Mi Y, Johansson M, Smedje G, Norbäck D. Current asthma and respiratory symptoms among pupils in relation to dietary factors and allergens in the school environment. Indoor Air. 2005;15(3):170–182. doi: 10.1111/j.1600-0668.2005.00334.x [DOI] [PubMed] [Google Scholar]
- 38.Lewis SA, Antoniak M, Venn AJ, et al. Secondhand smoke, dietary fruit intake, road traffic exposures, and the prevalence of asthma: a cross-sectional study in young children. Am J Epidemiol. 2005;161(5):406–411. doi: 10.1093/aje/kwi059 [DOI] [PubMed] [Google Scholar]
- 39.Njå F, Nystad W, Lødrup Carlsen KC, Hetlevik O, Carlsen KH. Effects of early intake of fruit or vegetables in relation to later asthma and allergic sensitization in school-age children. Acta Paediatrica. 2005;94(2):147–154. doi: 10.1080/08035250410023638 [DOI] [PubMed] [Google Scholar]
- 40.Wickens K, Barry D, Friezema A, et al. Fast foods - are they a risk factor for asthma? Allergy. 2005;60(12):1537–1541. doi: 10.1111/j.1398-9995.2005.00945.x [DOI] [PubMed] [Google Scholar]
- 41.Hong SJ, Lee MS, Lee SY, et al. High body mass index and dietary pattern are associated with childhood asthma. Pediatr Pulmonol. 2006;41(12):1118–1124. doi: 10.1002/ppul.20372 [DOI] [PubMed] [Google Scholar]
- 42.Pastorino AC, Rimazza RD, Leone C, Castro AP, Solé D, Jacob CM. Risk factors for asthma in adolescents in a large urban region of Brazil. J Asthma. 2006;43(9):695–700. doi: 10.1080/02770900600925544 [DOI] [PubMed] [Google Scholar]
- 43.Tabak C, Wijga AH, de Meer G, Janssen NA, Brunekreef B, Smit HA. Diet and asthma in Dutch school children (ISAAC-2). Thorax. 2006;61(12):1048–1053. doi: 10.1136/thx.2005.043034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatzi L, Torrent M, Romieu I, et al. Diet, wheeze, and atopy in school children in Menorca, Spain. Pediatr Allergy Immunol. 2007;18(6):480–485. doi: 10.1111/j.1399-3038.2007.00596.x [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Marcos L, Canflanca IM, Garrido JB, et al. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax. 2007;62(6):503–508. doi: 10.1136/thx.2006.060020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okoko BJ, Burney PG, Newson RB, Potts JF, Shaheen SO. Childhood asthma and fruit consumption. Eur Respir J. 2007;29(6):1161–1168. doi: 10.1183/09031936.00097806 [DOI] [PubMed] [Google Scholar]
- 47.Tsai HJ, Tsai AC. The association of diet with respiratory symptoms and asthma in schoolchildren in Taipei, Taiwan. J Asthma. 2007;44(8):599–603. doi: 10.1080/02770900701539509 [DOI] [PubMed] [Google Scholar]
- 48.Waser M, Michels KB, Bieli C, et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy. 2007;37(5):661–670. doi: 10.1111/j.1365-2222.2006.02640.x [DOI] [PubMed] [Google Scholar]
- 49.Corbo GM, Forastiere F, De Sario M, et al. Wheeze and asthma in children: associations with body mass index, sports, television viewing, and diet. Epidemiology. 2008;19(5):747–755. doi: 10.1097/EDE.0b013e3181776213 [DOI] [PubMed] [Google Scholar]
- 50.de Batlle J, Garcia-Aymerich J, Barraza-Villarreal A, Antó JM, Romieu I. Mediterranean diet is associated with reduced asthma and rhinitis in Mexican children. Allergy. 2008;63(10):1310–1316. doi: 10.1111/j.1398-9995.2008.01722.x [DOI] [PubMed] [Google Scholar]
- 51.Foliaki S, Annesi-Maesano I, Tuuau-Potoi N, et al. Risk factors for symptoms of childhood asthma, allergic rhinoconjunctivitis and eczema in the Pacific: an ISAAC Phase III study. Int J Tuberc Lung Dis. 2008;12(7):799–806. [PubMed] [Google Scholar]
- 52.Garcia E, Aristizabal G, Vasquez C, Rodriguez-Martinez CE, Sarmiento OL, Satizabal CL. Prevalence of and factors associated with current asthma symptoms in school children aged 6–7 and 13–14 yr old in Bogotá, Colombia. Pediatr Allergy Immunol. 2008;19(4):307–314. doi: 10.1111/j.1399-3038.2007.00650.x [DOI] [PubMed] [Google Scholar]
- 53.Gutiérrez-Delgado RI, Barraza-Villarreal A, Escamilla-Núñez MC, Solano-González M, Moreno-Macías H, Romieu I. Consumo de alimentos y asma en niños escolares de Cuernavaca [Food consumption and asthma in school children in Cuernavaca, Morelos, Mexico]. Salud Publica Mex. 2009;51(3):202–211. Spanish. doi: 10.1590/S0036-36342009000300010 [DOI] [PubMed] [Google Scholar]
- 54.Mai XM, Becker AB, Liem JJ, Kozyrskyj AL. Fast food consumption counters the protective effect of breastfeeding on asthma in children? Clin Exp Allergy. 2009;39(4):556–561. doi: 10.1111/j.1365-2222.2008.03169.x [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez Barcala FJ, Pertega S, Bamonde L, et al. Mediterranean diet and asthma in Spanish schoolchildren. Pediatr Allergy Immunol. 2010;21(7):1021–1027. doi: 10.1111/j.1399-3038.2010.01080.x [DOI] [PubMed] [Google Scholar]
- 56.Rodríguez-Rodríguez E, Perea JM, Jiménez AI, Rodríguez-Rodríguez P, López-Sobaler AM, Ortega RM. Fat intake and asthma in Spanish schoolchildren. Eur J Clin Nutr. 2010;64(10):1065–1071. doi: 10.1038/ejcn.2010.127 [DOI] [PubMed] [Google Scholar]
- 57.Arvaniti F, Priftis KN, Papadimitriou A, et al. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10–12 years old children: the PANACEA study. Pediatr Allergy Immunol. 2011;22(3):283–289. doi: 10.1111/j.1399-3038.2010.01113.x [DOI] [PubMed] [Google Scholar]
- 58.Arvaniti F, Priftis KN, Papadimitriou A, et al. Salty-snack eating, television or video-game viewing, and asthma symptoms among 10- to 12-year-old children: the PANACEA study. J Am Diet Assoc. 2011;111(2):251–257. doi: 10.1016/j.jada.2010.10.051 [DOI] [PubMed] [Google Scholar]
- 59.Goksör E, Alm B, Thengilsdottir H, Pettersson R, Åberg N, Wennergren G. Preschool wheeze - impact of early fish introduction and neonatal antibiotics. Acta Paediatrica. 2011;100(12):1561–1566. doi: 10.1111/j.1651-2227.2011.02411.x [DOI] [PubMed] [Google Scholar]
- 60.Willers SM, Wijga AH, Brunekreef B, et al. Childhood diet and asthma and atopy at 8 years of age: the PIAMA birth cohort study. Eur Respir J. 2011;37(5):1060–1067. doi: 10.1183/09031936.00106109 [DOI] [PubMed] [Google Scholar]
- 61.Kiefte-de Jong JC, de Vries JH, Franco OH, et al. Fish consumption in infancy and asthma-like symptoms at preschool age. Pediatrics. 2012;130(6):1060–1068. doi: 10.1542/peds.2012-0875 [DOI] [PubMed] [Google Scholar]
- 62.Goksör E, Alm B, Pettersson R, et al. Early fish introduction and neonatal antibiotics affect the risk of asthma into school age. Pediatr Allergy Immunol. 2013;24(4):339–344. doi: 10.1111/pai.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawson JA, Rennie DC, Dosman JA, Cammer AL, Senthilselvan A. Obesity, diet, and activity in relation to asthma and wheeze among rural dwelling children and adolescents. J Obes. 2013;2013:315096. doi: 10.1155/2013/315096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akcay A, Tamay Z, Hocaoglu AB, Ergin A, Guler N. Risk factors affecting asthma prevalence in adolescents living in Istanbul, Turkey. Allergologia Et Immunopathologia. 2014;42(5):449–458. doi: 10.1016/j.aller.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 65.D’Innocenzo S, Matos SM, Prado MS, et al. Padrão alimentar, asma e sibilo atópico e não atópico em crianças e adolescentes: estudo SCAALA, Salvador, Bahia, Brasil [Dietary pattern, asthma, and atopic and non-atopic wheezing in children and adolescents: SCAALA study, Salvador, Bahia State, Brazil]. Cad Saude Publica. 2014;30(9):1849–1860. Spanish. doi: 10.1590/0102-311x00165513 [DOI] [PubMed] [Google Scholar]
- 66.Cepeda AM, Del Giacco SR, Villalba S, et al. A traditional diet is associated with a reduced risk of eczema and wheeze in colombian children. Nutrients. 2015;7(7):5098–5110. doi: 10.3390/nu7075098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomes de Luna Mde F, Gomes de Luna JR, Fisher GB, de Almeida PC, Chiesa D, Carlos da Silva MG. Factors associated with asthma in adolescents in the city of Fortaleza, Brazil. J Asthma. 2015;52(5):485–491. doi: 10.3109/02770903.2014.984841 [DOI] [PubMed] [Google Scholar]
- 68.Han YY, Forno E, Brehm JM, et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann Allergy Asthma Immunol. 2015;115(4):288–293.e281. doi: 10.1016/j.anai.2015.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lumia M, Takkinen HM, Luukkainen P, et al. Food consumption and risk of childhood asthma. Pediatr Allergy Immunol. 2015;26(8):789–796. doi: 10.1111/pai.12352 [DOI] [PubMed] [Google Scholar]
- 70.Papadopoulou A, Panagiotakos DB, Hatziagorou E, et al. Antioxidant foods consumption and childhood asthma and other allergic diseases: the Greek cohorts of the ISAAC II survey. Allergologia Et Immunopathologia. 2015;43(4):353–360. doi: 10.1016/j.aller.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 71.Rice JL, Romero KM, Galvez Davila RM, et al. Association between adherence to the mediterranean diet and asthma in peruvian children. Lung. 2015;193(6):893–899. doi: 10.1007/s00408-015-9792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saadeh D, Salameh P, Caillaud D, et al. Prevalence and association of asthma and allergic sensitization with dietary factors in schoolchildren: data from the French six cities study. BMC Public Health. 2015;15(1):993. doi: 10.1186/s12889-015-2320-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alqahtani JM, Asaad AM, Awadalla NJ, Mahfouz AA. Environmental determinants of bronchial asthma among Saudi school children in Southwestern Saudi Arabia. Int J Environ Res Public Health. 2016;14(1):22. doi: 10.3390/ijerph14010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feng M, Yang Z, Pan L, et al. Associations of early life exposures and environmental factors with asthma among children in rural and urban areas of Guangdong, China. Chest. 2016;149(4):1030–1041. doi: 10.1016/j.chest.2015.12.028 [DOI] [PubMed] [Google Scholar]
- 75.Mascarenhas JM, Silva Rde C, Assis AM, Pinto Ede J, Conceição JS, Barreto ML. Symptoms of asthma and associated factors in adolescents from Salvador, Bahia. Revista brasileira de epidemiologia. 2016;19(1):181–193. doi: 10.1590/1980-5497201600010016 [DOI] [PubMed] [Google Scholar]
- 76.Hallit S, Raherison C, Abou Abdallah R, Hallit R, Salameh P. Correlation of types of food and asthma diagnosis in childhood: a case-control study. J Asthma. 2018;55(9):966–974. doi: 10.1080/02770903.2017.1379535 [DOI] [PubMed] [Google Scholar]
- 77.Kang SY, Song WJ, Kim MH, et al. Dietary assessment and the development of asthma in Korean adolescents and adults. Allergy. 2018;73(11):2254–2256. doi: 10.1111/all.13554 [DOI] [PubMed] [Google Scholar]
- 78.Melo B, Rezende L, Machado P, Gouveia N, Levy R. Associations of ultra-processed food and drink products with asthma and wheezing among Brazilian adolescents. Pediatr Allergy Immunol. 2018;29(5):504–511. doi: 10.1111/pai.12911 [DOI] [PubMed] [Google Scholar]
- 79.Elias BC, Silva JB, Mais LA, Warkentin S, Konstantyner T, Solé D. Factors associated with asthma in Brazilian adolescents: National Adolescent School-Based Health Survey (Pense-2012). Revista Paulista de Pediatria. 2019;37(4):406–413. doi: 10.1590/1984-0462/;2019;37;4;00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malaeb D, Hallit S, Sacre H, Malaeb B, Hallit R, Salameh P. Diet and asthma in Lebanese schoolchildren: a cross-sectional study. Pediatr Pulmonol. 2019;54(6):688–697. doi: 10.1002/ppul.24280 [DOI] [PubMed] [Google Scholar]
- 81.Øien T, Schjelvaag A, Storrø O, Johnsen R, Simpson MR. Fish consumption at one year of age reduces the risk of eczema, asthma and wheeze at six years of age. Nutrients. 2019;11(9):1969. doi: 10.3390/nu11091969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lawrence WR, Lin S, Lin Z, et al. Interactions between dietary habits and home environmental exposures on respiratory symptoms in Romanian school children: an analysis of data from the SINPHONIE project. Environ Sci Pollut Res Int. 2020;27(3):2647–2657. doi: 10.1007/s11356-019-07129-z [DOI] [PubMed] [Google Scholar]
- 83.Nkosi V, Rathogwa-Takalani F, Voyi K. The frequency of fast food consumption in relation to wheeze and asthma among adolescents in Gauteng and North West Provinces, South Africa. Int J Environ Res Public Health. 2020;17(6):1994. doi: 10.3390/ijerph17061994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reis WP, Chai E, Gaio J, Becerra MB, Banta JE, Dos Santos H. Dietary Factors Associated with Asthma Prevalence Among Children in California. Pediatr Allergy Immunol Pulmonol. 2020;33(2):85–91. doi: 10.1089/ped.2020.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tarazona-Meza CE, Hanson C, Pollard SL, et al. Dietary patterns and asthma among Peruvian children and adolescents. BMC Pulm Med. 2020;20(1):63. doi: 10.1186/s12890-020-1087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Antonogeorgos G, Priftis KN, Panagiotakos DB, et al. Exploring the relation between atopic diseases and lifestyle patterns among adolescents living in greece: evidence from the Greek Global Asthma Network (GAN) Cross-Sectional Study. Children. 2021;8(10):932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antonogeorgos G, Priftis KN, Panagiotakos DB, et al. Parental education and the association between fruit and vegetable consumption and asthma in adolescents: the Greek Global Asthma Network (GAN) Study. Children. 2021;8(4):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mendes FC, Paciência I, Cavaleiro Rufo J, et al. Higher diversity of vegetable consumption is associated with less airway inflammation and prevalence of asthma in school-aged children. Pediatr Allergy Immunol. 2021;32(5):925–936. doi: 10.1111/pai.13446 [DOI] [PubMed] [Google Scholar]
- 89.Wang JG, Liu B, Kroll F, et al. Increased advanced glycation end product and meat consumption is associated with childhood wheeze: analysis of the National Health and Nutrition Examination Survey. Thorax. 2021;76(3):292–294. doi: 10.1136/thoraxjnl-2020-216109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molnar D, Galffy G, Horvath A, et al. Prevalence of asthma and its associating environmental factors among 6–12-year-old schoolchildren in a metropolitan environment-a cross-sectional, questionnaire-based study. Int J Environ Res Public Health. 2021;18(24):13403. doi: 10.3390/ijerph182413403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. doi: 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 92.Lv N, Xiao L, Ma J. Dietary pattern and asthma: a systematic review and meta-analysis. J Asthma Allergy. 2014;7:105–121. doi: 10.2147/JAA.S49960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phillips JA. Dietary guidelines for Americans, 2020–2025. Workplace Health Saf. 2021;69(8):395. doi: 10.1177/21650799211026980 [DOI] [PubMed] [Google Scholar]
- 94.Penard-Morand C, Raherison C, Charpin D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36(1):33–40. doi: 10.1183/09031936.00116109 [DOI] [PubMed] [Google Scholar]
- 95.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S [DOI] [PubMed] [Google Scholar]
- 96.Slavin JL, Jacobs D, Marquart L, Wiemer K. The role of whole grains in disease prevention. J Am Diet Assoc. 2001;101(7):780–785. doi: 10.1016/S0002-8223(01)00194-8 [DOI] [PubMed] [Google Scholar]
- 97.Castro-Rodriguez JA, Garcia-Marcos L, Alfonseda Rojas JD, Valverde-Molina J, Sanchez-Solis M. Mediterranean diet as a protective factor for wheezing in preschool children. J Pediatr. 2008;152(6):823–828, 828 e821–822. doi: 10.1016/j.jpeds.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 98.Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP; Group IPTS. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax. 2010;65(6):516–522. doi: 10.1136/thx.2009.128256 [DOI] [PubMed] [Google Scholar]
- 99.Ellwood P, Asher MI, Garcia-Marcos L, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax. 2013;68(4):351–360. doi: 10.1136/thoraxjnl-2012-202285 [DOI] [PubMed] [Google Scholar]
- 100.Patrick H, Nicklas TA. A review of family and social determinants of children’s eating patterns and diet quality. J Am Coll Nutr. 2005;24(2):83–92. doi: 10.1080/07315724.2005.10719448 [DOI] [PubMed] [Google Scholar]
- 101.Feunekes GI, de Graaf C, Meyboom S, van Staveren WA. Food choice and fat intake of adolescents and adults: associations of intakes within social networks. Prev Med. 1998;27(5 Pt 1):645–656. doi: 10.1006/pmed.1998.0341 [DOI] [PubMed] [Google Scholar]
- 102.Ellwood P, Asher MI, Bjorksten B, Burr M, Pearce N, Robertson CF. Diet and asthma, allergic rhinoconjunctivitis and atopic eczema symptom prevalence: an ecological analysis of the International Study of Asthma and Allergies in Childhood (ISAAC) data. ISAAC Phase One Study Group. Eur Respir J. 2001;17(3):436–443. doi: 10.1183/09031936.01.17304360 [DOI] [PubMed] [Google Scholar]
- 103.Nagel G, Linseisen J. Dietary intake of fatty acids, antioxidants and selected food groups and asthma in adults. Eur J Clin Nutr. 2005;59(1):8–15. doi: 10.1038/sj.ejcn.1602025 [DOI] [PubMed] [Google Scholar]
- 104.Sausenthaler S, Kompauer I, Borte M, et al. Margarine and butter consumption, eczema and allergic sensitization in children. The LISA birth cohort study. Pediatr Allergy Immunol. 2006;17(2):85–93. doi: 10.1111/j.1399-3038.2005.00366.x [DOI] [PubMed] [Google Scholar]
- 105.Bolte G, Frye C, Hoelscher B, Meyer I, Wjst M, Heinrich J. Margarine consumption and allergy in children. Am J Respir Crit Care Med. 2001;163(1):277–279. doi: 10.1164/ajrccm.163.1.2006004 [DOI] [PubMed] [Google Scholar]
- 106.Te Morenga L, Montez JM. Health effects of saturated and trans-fatty acid intake in children and adolescents: systematic review and meta-analysis. PLoS One. 2017;12(11):e0186672. doi: 10.1371/journal.pone.0186672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sievert K, Lawrence M, Naika A, Baker P. Processed foods and nutrition transition in the pacific: regional trends, patterns and food system drivers. Nutrients. 2019;11(6):1328. doi: 10.3390/nu11061328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.World Health Organization. Essential Nutrition Actions: Mainstreaming Nutrition Throughout the Life-Course. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]