Abstract
Pulmonary hypertension (PH) is common, with an estimated prevalence of approximately 1% that increases with age. Prompt and accurate diagnosis is key to institute timely and appropriate therapy to improve symptoms and prognosis. The international guidelines for the diagnosis and management of PH have recently been updated, with a lowering of the haemodynamic threshold for diagnosis to a mean pulmonary artery pressure >20 mmHg. New diagnostic algorithms and revised indications for screening in at-risk groups have been developed to facilitate early referral to specialist PH centres. This includes fast-track referral pathways for patients who are either clinically high-risk or are at-risk for pulmonary arterial hypertension (PAH) or chronic thromboembolic pulmonary hypertension (CTEPH). This review summarises key changes in the PH guidelines for general physicians who are, most often, the first healthcare professionals to encounter these patients and consequently have a key role as referrers into specialist PH services.
KEYWORDS: Pulmonary hypertension, chronic thromboembolic pulmonary hypertension, pulmonary arterial hypertension, screening, systemic sclerosis
Key points
Pulmonary hypertension is common, with multiple subtypes and aetiologies that have individualised management recommendations.
The haemodynamic definition of PH by right heart catheterisation (RHC) has changed to a lower mean pulmonary arterial pressure (mPAP) at rest of >20 mmHg based upon the upper normal limit in healthy individuals along with supportive prognostic data. However, there is currently no evidence for the efficacy of targeted PH medications in patients with mPAP <25 mmHg and pulmonary vascular resistance (PVR) <3 WU, which remains an evidence gap.
Early detection and referral to a specialist PH centre is crucial to institute targeted management that can improve symptoms and optimise prognosis. Physicians should be familiar with the streamlined pathway for suspicion, detection and confirmation of PH, the latter step being performed at PH centres. Fast-track referral pathways should be taken for clinically high-risk patients and for patient cohorts ‘at-risk’ of developing pulmonary arterial hypertension (PAH) or chronic thromboembolic pulmonary hypertension (CTEPH).
Annual systematic screening using the DETECT algorithm is a class 1 recommendation in asymptomatic adults with systemic sclerosis of more than 3 years' duration, forced vital capacity (FVC) ≥40%, and a transfer factor (DLCO) <60%.
Patients with ongoing dyspnoea after 3 months of anticoagulation in the setting of a history of previous thromboembolic disease require further evaluation for chronic thromboembolic pulmonary disease (CTEPD) or CTEPH.
Introduction
Pulmonary hypertension (PH) is commonly encountered in daily clinical practice. It has an estimated prevalence of 1% of the global population, which considerably increases in people aged over 65 years owing to the higher burden of cardiopulmonary comorbidities encountered with age.1 Among the many aetiologies of PH (Table 1), left heart disease (Group 2) is the most common, followed by lung disease (Group 3), with treatment in both aimed at the underlying cause. Consequently, an awareness of how to identify and manage PH will improve care for the general medical patient and also help identify the rarer, directly treatable forms of PH such as pulmonary arterial hypertension (PAH, Group 1) and chronic thromboembolic PH (CTEPH, Group 4).2 Identifying and differentiating these patients who may benefit from targeted PH therapies is complicated by the non-specific symptoms and signs of PH, which overlap across all PH subtypes and with other common cardiopulmonary conditions. Therefore, a key focus for physicians is ensuring early referral to specialist PH centres when directly treatable PH is suspected, thereby avoiding worse outcomes associated with delays in initiating PH therapies.
Table 1.
Clinical classification of PH3
| Group 1: Pulmonary arterial hypertension (PAH) |
1.1 Idiopathic
|
| 1.2 Heritable |
| 1.3 Associated with drugs and toxins |
1.4 Associated with:
|
| 1.5 PAH with features of venous/capillary (PVOD/PCH) involvement |
| 1.6 Persistent PH of the newborn |
| Group 2: PH associated with left heart disease |
2.1 Heart failure:
|
| 2.2 Valvular heart disease |
| 2.3 Congenital/acquired cardiovascular conditions leading to post-capillary PH |
| Group 3: PH associated with lung diseases and/or hypoxia |
| 3.1 Obstructive lung disease or emphysema |
| 3.2 Restrictive lung disease |
| 3.3 Lung disease with mixed restrictive/obstructive pattern |
| 3.4 Hypoventilation syndromes |
| 3.5 Hypoxia without lung disease (eg high altitude) |
| 3.6 Developmental lung disorders |
| Group 4: PH associated with pulmonary artery obstructions |
| 4.1 Chronic thrombo-embolic PH |
| 4.2 Other pulmonary artery obstructions (including sarcomas, other malignant or non-malignant tumours, arteritis without connective tissue disease, congenital pulmonary arterial stenoses, and hydatidosis) |
| Group 5: PH with unclear and/or multifactorial mechanisms (discussion with +/− referral to PH centres should be considered) |
| 5.1 Haematological disorders (including inherited and acquired chronic haemolytic anaemia and chronic myeloproliferative disorders) |
| 5.2 Systemic disorders (including sarcoidosis, pulmonary Langerhans's cell histiocytosis, and neurofibromatosis type 1) |
| 5.3 Metabolic disorders (including glycogen storage diseases and Gaucher's disease) |
| 5.4 Chronic renal failure with or without haemodialysis |
| 5.5 Pulmonary tumour thrombotic microangiopathy |
| 5.6 Fibrosing mediastinitis |
HIV = human immunodeficiency virus; PAH = pulmonary arterial hypertension; PCH = pulmonary capillary haemangiomatosis; PH = pulmonary hypertension; PVOD = pulmonary veno-occlusive disease.
The recently updated European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines for the diagnosis and treatment of PH include many changes relevant to physicians across a range of specialties.3 Early recognition and detection of who to refer, fast-track referral pathways to PH centres, and a focus on screening at-risk populations are key features. Importantly, the diagnostic criteria and thresholds for PH have been lowered along with the recommendation for haemodynamic evaluation to be undertaken at expert centres. This review aims to provide physicians with an overview of the key advances in the diagnosis and management guidelines of known or suspected PH and the evidence that underpins these changes.
Changes to diagnostic thresholds
The haemodynamic definition of PH has changed to a lower mean pulmonary arterial pressure (mPAP) at rest of >20 mmHg by right heart catheterisation (RHC). This change is based upon the upper normal limit in healthy individuals along with prognostic data in patients beyond this threshold. However, there is currently no evidence for the efficacy of targeted PH medications in patients with mPAP <25 mmHg and pulmonary vascular resistance (PVR) <3 WU (Wood units), which remains an evidence gap. Nevertheless, patients at risk for developing PAH (such as those with systemic sclerosis (SSc) or portal hypertension) but with haemodynamics in the new milder PH range should be closely followed up in a specialist PH centre.
Measuring pulmonary arterial wedge pressure (PAWP) and PVR is pivotal for the accurate haemodynamic classification of PH (Table 2). Hence, RHC is recommended to be performed only in experienced centres following standardised protocols. Thus, the key focus for referring physicians should be early referral if PH is suspected and screening at-risk patient populations.
Table 2.
Haemodynamic definitions of PH3,21
| Definitions | Characteristics | Clinical groups |
|---|---|---|
| Pre-capillary PH | mPAP >20 mmHg PAWP ≤15 mmHg PVR >2 WU |
1, 3, 4 and 5 |
| Isolated post-capillary PH (IpcPH) | mPAP >20 mmHg PAWP >15 mmHg PVR ≤2 WU |
2 and 5 |
| Combined pre- and post-capillary PH (CpcPH) | mPAP >20 mmHg PAWP >15 mmHg PVR >2 WU |
2 and 5 |
mPAP = mean pulmonary arterial pressure; PAWP = pulmonary arterial wedge pressure; PVR = pulmonary vascular resistance; WU = Wood units.
Diagnostic pathway, early referral and the role of echocardiography
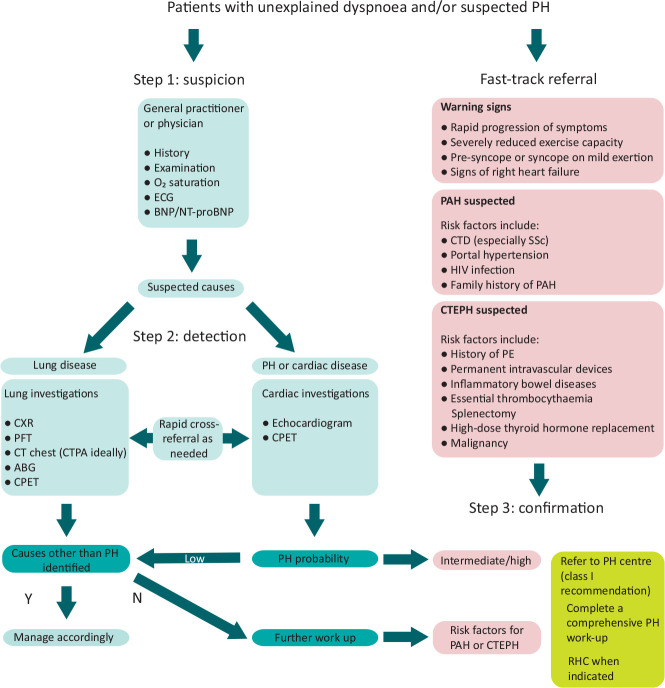
A new diagnostic algorithm, including a fast-track referral pathway, has been proposed to encourage earlier detection of PH in patients (Fig 1). Step 1 is the initial evaluation for suspecting PH, including clinical review, ECG and blood test for brain natriuretic peptide (BNP or NT-proBNP). Step 2 involves first-line investigations, with the investigation pathway diverging according to the suspicion of lung disease versus cardiac disease/PH as the most likely cause. The final step suggests which patients to refer on to a PH centre. These include patients with intermediate or high probability of PH and patients at risk of PH, such as those with a prior history of pulmonary embolism (PE), connective tissue disease, portal hypertension, HIV, or a family history of PAH. Any warning signs such as syncope, tachycardia, hypotension, or signs of right heart failure, all of which suggest advanced PH, should prompt an expedited referral directly to a specialist PH centre.
Fig 1.
Diagnostic algorithm for patients with unexplained dyspnoea and/or suspected pulmonary hypertension. Adapted from the 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension.3 ABG = arterial blood gas; BNP = brain natriuretic peptide; CPET = cardiopulmonary exercise testing; CT = computed tomography; CTEPH = chronic thromboembolic pulmonary hypertension; ECG = electrocardiogram; HIV = human immunodeficiency virus; N = no; NT-proBNP = N-terminal pro-brain natriuretic peptide; PAH = pulmonary arterial hypertension; PE = pulmonary embolism; PFT = pulmonary function tests; PH = pulmonary hypertension; Y = yes.
Echocardiography remains the first-line non-invasive investigation for suspected PH. An echocardiographic probability of PH is given according to the peak tricuspid regurgitation velocity (TRVmax) measured by continuous wave Doppler along with the presence of any accompanying features suggestive of PH: abnormalities of right heart size and function, deleterious interventricular septal dynamics suggesting pressure overload of the right ventricle, pulmonary arterial dilatation, and the ratio of right ventricular longitudinal function to estimated systolic pulmonary arterial pressure (sPAP). These measurements are all within the remit of routine echocardiography studies and are not limited to expert centres. Of note, the TRVmax thresholds to estimate the probability of PH are unchanged from previous guidelines as there are no data to recalibrate them against the new RHC-derived diagnostic criteria. Importantly, echocardiography only provides a probability estimate for PH and cannot be used alone to diagnose PH - this can only be made by RHC. Echocardiography can also offer clues to PH aetiology, especially due to left heart disease in cases of left ventricular systolic dysfunction, severe left-sided valvular heart disease, or isolated left atrial dilatation implying diastolic dysfunction.
Screening in ‘at risk’ patients
Approximately three-quarters of patients with connective tissue disease (CTD)-associated PAH have SSc as the underlying CTD.4 The prevalence of PAH in patients with SSc ranges from 7% to 19% and carries a poor prognosis, which can be improved with early detection and initiation of targeted PH therapy.5 The benefits of systematic screening for PAH in SSc are well known but the screening methods used vary between institutions.6 The latest PH guidelines recommend annual systematic screening for PH in patients with SSc, with the DETECT algorithm recommended for screening asymptomatic adults with SSc of more than 3 years' duration, forced vital capacity (FVC) ≥40%, and a transfer factor (DLCO) <60%.7 This is an evidence-based, non-invasive, multiparametric clinical screening tool that has a high sensitivity and high negative predictive value for PAH detection in SSc.7 Using this tool minimises missed diagnoses, identifies milder PH, and addresses resource utilisation, particularly for echocardiography.
Overall, physicians should have a low threshold to investigate any patient complaining of dyspnoea with an associated condition deeming them at-risk for PH, including SSc, portal hypertension, or HIV. In breathless patients with SSc in whom no other identifiable cause is found, referral to a PH centre for evaluation by RHC is recommended, irrespective of the echocardiographic probability of PH. Unlike patients with SSc, screening asymptomatic patients with portal hypertension or HIV is not recommended given the lower prevalence of PH in these conditions. However, patients under consideration for liver transplantation or a transjugular portosystemic shunt (TIPS) should routinely undergo echocardiography screening for portopulmonary hypertension. Patients with dyspnoea and a history of previous thromboembolic disease require further evaluation for chronic thromboembolic pulmonary disease (CTEPD) or chronic thromboembolic pulmonary hypertension (CTEPH). For example, patients with ongoing dyspnoea after 3 months of anticoagulation for an acute pulmonary embolism (PE) in conjunction with mismatched perfusion defects should be referred to a PH centre after considering the results of an echocardiogram, which is the preferred first-line investigation for suspected CTEPH.
Left heart PH (Group 2) – when to refer?
PH due to left heart disease (PH-LHD), also known as group 2 or post-capillary PH, is the most common type of PH and is defined by mPAP >20 mmHg in the presence of an elevated PAWP (>15 mmHg) and normal PVR (≤2 WU). In general, it reflects the severity of LHD, and treatment should be directed at the underlying cause before further assessing the PH itself. Targeted PH therapies are not recommended in isolated PH-LHD, with studies showing potentially harmful outcomes.8
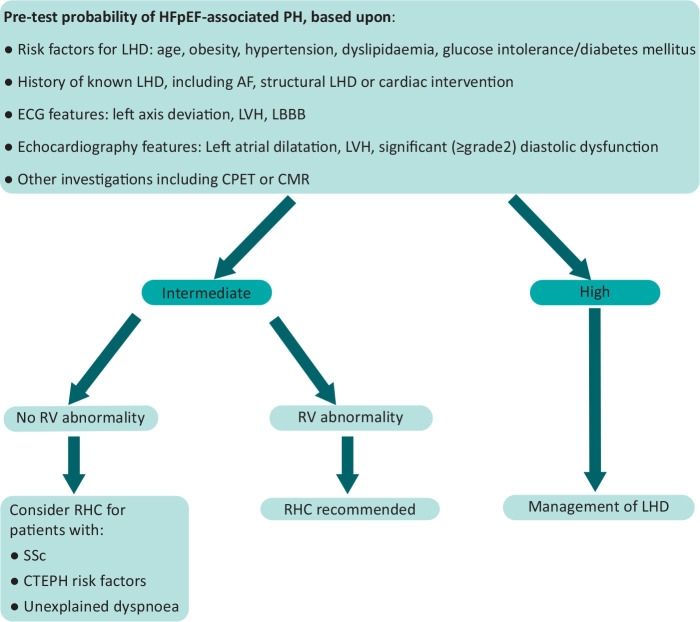
However, referral to a PH centre and RHC should still be considered in specific cases. For example, features of predominant right heart failure or disproportionately severe PH in patients with LHD should be referred to PH services for diagnostic evaluation. These patients may have PAH (Group 1) or CTEPH (Group 4) with LHD comorbidities or combined pre- and post-capillary PH, for which individualised management in a PH service is recommended. Even after assigning a clinical pre-test probability of LHD, differentiating heart failure with preserved ejection fraction (HFpEF) from PAH with comorbidity can be challenging and onward referral should be considered when disproportionate right heart abnormalities are found on echocardiography (Fig 2). Moreover, RHC still has a role in patients with LHD if it will assist in the patient's management but, as discussed previously, should be performed at PH centres.3 In suspected PH-LHD, this enables relevant provocative testing when required, including fluid challenge or exercise testing.
Fig 2.
When to refer patients with possible HFpEF-associated pulmonary hypertension (PH) to specialist centres for further evaluation by right-heart catheterisation (RHC). RHC is recommended in cases with an intermediate probability of HFpEF-associated PH when risk factors of pulmonary arterial hypertension / chronic thromboembolic pulmonary hypertension (PAH/CTEPH) are present and/or if there is evidence of right ventricular (RV) abnormality. If the probability of HFpEF-associated PH is high, management should focus on the underlying left heart disease (LHD). Adapted from Vachiery et al 2019.20 AF = atrial fibrillation; CMR = cardiovascular magnetic resonance; CPET = cardiopulmonary exercise testing; ECG = electrocardiogram; HFpEF = heart failure with preserved ejection fraction; LBBB = left bundle branch block; LVH: left ventricular hypertrophy; SSc: systemic sclerosis.
Lung PH (Group 3) – evidence for targeted treatment?
PH due to lung disease (Group 3 PH) is the second commonest PH subtype. Like PH-LHD, it reflects the severity of the lung disease with treatment of the underlying cause being the focus of management. This includes, where indicated, management of hypoxaemia, alveolar hypoventilation or sleep-disordered breathing. Referral to PH centres should be considered in patients with potential dual diagnosis, in particular, patients with CTD who may have interstitial lung disease (ILD) as well as an independent vasculopathy. Referral for RHC might also assist in management decisions such as referral for transplantation or when the suspected severity of PH gives rise to uncertainty in treatment.
Despite an individualised approach being recommended for the management of group 3 PH, there is a lack of robust evidence for the use of targeted PH therapies currently commissioned in the UK for group 1 PH. Moreover, medications in PAH may have detrimental effects in these patients and should be avoided in most instances. A recent phase 3 randomised controlled trial of inhaled treprostinil in PH due to ILD demonstrated improvements in six-minute walk distance, NT-proBNP, and reduced clinical worsening.9 However, further evidence is required and this is currently not clinically commissioned in the UK.
PAH with cardiopulmonary comorbidities – ‘real world’ phenotypes
Rather than being the primary cause of PH, cardiovascular and respiratory comorbidities are common and further complicate diagnosis and treatment options in PAH. The classic phenotype of young, predominantly female patients without comorbidity only accounted for the minority (12.6%) of patients diagnosed with idiopathic PAH (IPAH) in the COMPERA registry.10 The majority of patients with IPAH (51.6%) were in the cardiopulmonary cluster, mostly consisting of elderly men with LHD risk factors and a significant smoking history who were often hypoxaemic with a low transfer factor. The remaining 35.8% of patients, termed the left heart phenotype, were predominantly elderly women with risk factors for HFpEF, just under one-third of whom had a history of atrial fibrillation.
The presence of cardiopulmonary comorbidities in patients with PAH confers a poorer response to targeted PH therapies with higher discontinuation rates, a lower likelihood of improving their risk profile, and a poorer prognosis. Evidence for targeted PH treatments in these patients is limited as patients with such comorbidities are often excluded from clinical trials. Initial PH monotherapy is recommended on an individual basis, with registry data suggesting a preference for phosphodiesterase-5 inhibitors (PDE5i).
Group 1 PH – current and future treatment options and strategies
For patients with PAH without significant comorbidity, initial combination therapy with PDE5i (eg tadalafil or sildenafil) and endothelin receptor antagonist (eg ambrisentan or macitentan) is the standard of care.11 If the patient is high risk at presentation, intravenous prostanoid (epoprostenol) is also considered.12 The aim of PH therapy is to optimise the patient's risk profile and, consequently, prognosis.13 Addition of the oral prostacyclin receptor agonist selexipag is recommended in patients with an intermediate-low risk profile after initial PH therapy.14 Patients in the intermediate-high or high risk categories should be considered for intravenous prostanoid and/or lung transplantation. Sotatercept, an activin receptor type IIA-Fc fusion protein, is a potential therapy of promise in PAH. A recent double-blind, randomised controlled trial showed significantly improved 6-minute walk distance with sotatercept in patients with PAH already receiving stable background PH therapy, including patients on parenteral prostanoid therapy.15
Treatments options for CTEPH (Group 4)
Medical, percutaneous, and surgical treatment options are now available for CTEPH. In patients with anatomically suitable disease (in terms of distribution and burden), pulmonary endarterectomy (PEA) is the treatment of choice following evaluation by expert centres.16 Percutaneous balloon pulmonary angioplasty (BPA) is a more recent treatment modality recommended in suitable patients with CTEPH who have inoperable disease or with residual CTEPH following PEA surgery.17,18 Furthermore, BPA in combination with medical PH therapy has been upgraded in the CTEPH treatment algorithm. Riociguat, a soluble guanylate cyclase inhibitor, is the recommended medical therapy in patients with inoperable CTEPH or with residual CTEPH following PEA.19 Lifelong anticoagulation is recommended in all patients with CTEPH. Patients with CTEPH should be tested for antiphospholipid syndrome and, when found, treated with vitamin K antagonists.
Conclusions
The early recognition of suspected PH along with systematic screening of at-risk patient groups enables early diagnosis and, consequently, early treatment. Obtaining a prompt and accurate diagnosis determines treatment strategies and impacts on prognosis, with referral to specialist PH centres for RHC being the final step in the diagnostic algorithm. This requires a collaborative multidisciplinary approach between referrers across a wide range of medical specialties, national PH centres, and their associated regional shared care centres.
Funding and disclosures
DSK is supported by a British Heart Foundation (BHF) Clinical Research Leave Fellowship (FS/CRLF/20/23004). TK has received speaker fees from Janssen UK. BES has received consulting fees, speaker fees, and research funding from Johnson & Johnson, and speaker fees from GSK. JGC has received consulting fees from Acceleron, consulting and speaker fees from Bayer, GSK and Johnson & Johnson, and research funding from Johnson & Johnson. DSK has received research support, speaker fees, and advisory board fees from Janssen UK.
References
- 1.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016;4:306–22. [DOI] [PubMed] [Google Scholar]
- 2.Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ 2021;11:;2045894020977300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618–731. [DOI] [PubMed] [Google Scholar]
- 4.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009;179:151–7. [DOI] [PubMed] [Google Scholar]
- 5.Weatherald J, Montani D, Jevnikar M, et al. Screening for pulmonary arterial hypertension in systemic sclerosis. Eur Respir Rev 2019;28:;190023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humbert M, Yaici A, de Groote P, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum 2011;63:3522–30. [DOI] [PubMed] [Google Scholar]
- 7.Coghlan JG, Denton CP, Grunig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014;73:1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermejo J, Yotti R, García-Orta R, et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J 2018;39:1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 2021;384:325–34. [DOI] [PubMed] [Google Scholar]
- 10.Hoeper MM, Pausch C, Grünig E, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020;39:1435–44. [DOI] [PubMed] [Google Scholar]
- 11.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015;373:834–44. [DOI] [PubMed] [Google Scholar]
- 12.Boucly A, Savale L, Jaïs X, et al. Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2021;204:842–54. [DOI] [PubMed] [Google Scholar]
- 13.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017;50:1700740. [DOI] [PubMed] [Google Scholar]
- 14.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015;373:2522–33. [DOI] [PubMed] [Google Scholar]
- 15.Hoeper MM, Badesch DB, Ghofrani HA, et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med 2023;388:1478–90. [DOI] [PubMed] [Google Scholar]
- 16.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:756–62. [DOI] [PubMed] [Google Scholar]
- 18.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:748–55. [DOI] [PubMed] [Google Scholar]
- 19.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369:319–29. [DOI] [PubMed] [Google Scholar]
- 20.Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:1801897.30545974 [Google Scholar]
- 21.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]