Abstract
Herbal plants comprise potent bioactives, and they have a potential for the development of functional foods. Ultrasonication technology can be used to enhance the efficiency and quality of these bioactivities. The present review discussed the ultrasound-assisted novel extraction technologies (supercritical carbon dioxide (CO2) and high pressurized liquid), including mechanistic understanding, influencing factors, extract process efficiency, and the recovery of bioactives with an industrial perspective. The strong observations of this study are the novel ultrasound-induced extraction process variables, such as ultrasound amplitude, sonication time, temperature, solid-solvent ratio, and pressure, are significantly influenced and must be optimized for maximum recovery of bioactives. The novel green technologies (ultrasound and assisted) could remarkably improve the extraction efficiency and enhance the quality of green extract. This review will support technological understanding about the impact on process parameters for the extraction of bioactives for the development of functional foods and nutraceuticals.
Keywords: Bioactives, Green extraction, Herbal plants, Supercritical fluid, Ultrasonication
Introduction
Herbal plants always played an important role in the maintenance of health, well-being, and everyday life of a population worldwide. Throughout the centuries, herbal plant leaves, stems, flowers, seeds, berries, and roots were used for healing and maintenance of different compulsive conditions, as well as in the pharmacological preparation of various functional foods and nutraceuticals. Due to the immense popularity of medicinal or herbal plants in many developed and developing nations, the international market trade on herbal plants and their value-added products are in huge demand. The value was estimated to be 60 billion (USD) in 2010; however, it is predicted to attain 5 trillion (USD) by 2050 (Zahra et al., 2020). Moreover, 80% of the global population quest for herbal drugs to treat their primary health disorders as per the WHO (World Health Organization). The presence of bioactives, which have potential biological benefits are phytochemicals (i.e., polyphenol, flavonoids, alkaloids, polysaccharides, carotenoids, azadirachtin content, aloin, ginsenosides) and has a significant positive impact on human health, such as, antimicrobial, antioxidant, anti-inflammatory, anti-allergenic, antithrombotic, neuroprotective, and vasodilatory characteristics (Santos et al., 2021). Moreover, most of the underdeveloped nations were greatly relying on traditional plant-based therapies because the pharmaceutical-based drugs are expensive (Chatterjee and Ghosh., 2020). However, an incredible number of medicinally active herbal plants are notably higher in bioactives, which will have a synergistic effect on the human body to deliver unique medicinal properties with limited adverse side effects. The common herbal plants containing bioactives are: tulsi, neem (Bhuyan, 2019), ashwagandha (Tsaltaki et al., 2019), rosemary (Munekata et al., 2020), thymus fontanesii (Nabet et al., 2019), eucalyptus (Palma et al., 2021), turmeric root (Raheem et al., 2023); coriander (Senrayan & Venkatachalam, 2019), etc.
As these plants are abundantly available and inexpensive, the extraction and value addition of the bioactives from herbal plants is one of the primary investigative areas for the nutraceutical, pharmaceutical, and cosmetics industries in modern times. However, many bioactive chemicals that may be utilized to produce functional or value-added food products are lost due to the lack of effective extraction techniques. Conventional extraction methods including infusion, reflux, maceration, percolation, and soxhlet extraction typically require a large amount of organic solvent and are associated with low extraction yield and prolonged extraction time (Zhang et al., 2018). Whereas non-conventional extraction methods involve ultrasound-assisted extraction (UAE), supercritical-fluid extraction (SFE), accelerated solvent extraction (ASE), and microwave-assisted extraction (MAE), have been well recognized as environmentally friendly and efficient methods for the extraction of natural bioactives (Zhang et al., 2018). It has been observed that novel-extraction methods have some drawbacks, which include large capital investment in supercritical fluid extraction, requirement of high temperature in accelerated solvent extraction, and poor extraction efficiency for microwave-assisted extraction when the solvent and target compound are non-polar (Kunene and Mahlambi., 2023; Yusoff et al., 2022). However, UAE offers benefits such as lower extraction time temperature, energy consumption, and preservation of extract quality.
To the best of our knowledge, there are some good reviews regarding the UAE of bioactive compounds form spices (Rao et al., 2021a, 2021b), and food and natural products (Chemat et al., 2017a, 2017b). Additionally, Yusoff et al., (2022) reviewed the UAE technology for extraction of bioactives from the plants and mainly focused on the UAE alone with optimization of extraction condition. However, the present review focusing on ultrasound assisted novel technologies (i.e., supercritical fluid extraction and pressurized liquid extraction) based extraction of bioactives from commonly available herbal plants which is not been previously explored. In this regard, the current review intends to discuss the comprehensive and up-to-date overview on the various technological aspects of UAE with mechanistic understanding, critical factors affecting, and various bioactives.
Mechanistic understanding of ultrasonication technology
UAE technology has been acclaimed as a green and innovative technique as it enables the scope of utilizing green solvents by replacing traditionally used organic solvents. The physical and biochemical impacts of UAE are aligned according to its frequency range, for example, physical effects could dominate at lower frequencies (20–100 kHz), while the chemical effects at 200 to 500 kHz; however, the acoustic streaming impacts were found at higher frequencies (> 1 MHz) as reported by Rao et al., (2021a, 2021b). The ultrasonic equipment was designed based on the piezoelectric transducer to treat solid materials in liquid media, such as ultrasound-based probe sonicator (direct application) and ultrasonic bath (indirect applications) as shown in Fig. 1(A) (Kumar et al., 2022). Ultrasonic water baths or cleaners have been used extensively for cleaning, sanitation, and extraction purposes in pharmaceutical, cosmeceutical and ornamental industries. It consists of a transducer, a tank, a heater, and a time and temperature probe as shown in Fig. 1(B). Indirect sonication is most effective for a small amount of sample because foaming and losses of bioactives are eliminated, and the sonication bath has the advantage of non-invasive exposure to keep the sample intact and to ensure a more uniform distribution of US intensity in the sample liquid (Wu, 2019). On other hand, the ultrasonic probe is the most frequently used direct sonication method where an ultrasonic probe tip is directly transferred US intensity to the test sample (Mikheev et al., 2021). It shows higher efficiency and significantly reduces the extraction time as compared to an ultrasonic bath as in a probe system, ultrasonic energy is intensified in a particular zone creating a higher cavitation effect (Low et al., 2022).
Fig. 1.
Ultrasonic devices: (A) Ultrasound sonicator (B) Ultrasonic bath
Ultrasound waves are made up of a series of compression and rarefaction cycles (high and low-pressure regions), which can propagate through a solid, liquid, or gaseous medium, causing the molecules to be dislodged and displaced from their starting positions leading to the generation of immense energy (Pandiselvam et al., 2022). Moreover, the rarefaction cycle overcomes the attraction forces of the liquid molecules when negative pressure is applied at a high intensity, and the cavitation bubbles emerges (Dash et al., 2021; Rao et al., 2021a, 2021b). Many compression and rarefaction cycles of sonication generate stable cavities, which are long-lasting gas bubbles, while ephemeral cavitation or inertial cavitation bubbles exist for a very short time, often lower than a single cycle, and then disintegrate violently (Dong et al., 2020). On a microscopic scale, thousands of bubbles will form in a liquid; some are relatively stable, while others develop to an unsustainable level and increase temperatures and pressures over 5000 K and 1000 atm respectively (Pandiselvam et al., 2022; Rao et al., 2021a, 2021b). Acoustic cavitation can result in various phenomena of modifications in the cellular matrix of plants, such as cellular tissue fragmentation, shear force, cell erosion, enhanced absorption, formation of pores, and slightly diminishes in the swelling index (Vernes et al., 2019). Moreover, cavitation also generates microjets, shock waves, and turbulence which in turn induce the changes in the plant tissues and accelerates the extraction yield (Rao et al., 2021a, 2021b; Pandiselvam et al., 2022). Also, ultrasound induces alterations in temperature and pressure through implosions, resulting in shear disruption, thinning of cell membranes, and subsequent cell destruction, leading to an increase of solvent infiltrate within the cells matrices (Malakar et al., 2023).
Generally, three types of effects are commonly observed in cavitation phenomena such as thermal, chemical, and mechanical. In the last century, the chemical effects of ultrasound were extensively studied due to their significant impact on extraction phenomena. The degradation of target compounds occurs mainly due to the chemical effect of the cavitation process which might be due to the production of free radicals at a higher frequency (Gavahian et al., 2022). Mechanical effects are responsible for the extraction of bioactive compounds from vegetal tissue, following into a two-stage phenomenon. Correspondingly, mechanical effects help to penetrate the solvent into the matrix through the cell wall so that it shows disruption of the acellular matrix and reduction in particle size (Deng et al., 2022). Therefore, the solution diffuses quickly from the plant tissues to the solvent with an increased mass transfer across the plant membranes, and this action continues till the equilibrium attains (Rao et al., 2021a, 2021b). UAE associated with the suitable solvents applied to the target bioactives is a complicated process involving mass transfer and chemical reactions that influence the yield and biological activities. The mass transfer phenomenon happens because of sonication which is directly linked with the ultrasound energy and frequency (Tamidi et al., 2021). In direct ultrasonication systems, the ultrasound probe comes into direct contact with the sample. To prevent further damage to the vessel’s wall and to reduce the deprivation of ultrasonic intensity, it should have a minimal gap between the vessel and the probe (Yusoff et al., 2022; Rao et al., 2021a, 2021b). The system consists of three components: the transducer, the amplifier, and the probe. For the extraction of bioctives from natural products, the piezoelectric transducer is the most commonly utilized. Because of its corrosion resistance and ability to withstand greater temperatures, titanium is the most utilized material in US probes (Chemat et al., 2017a, 2017b; Rao et al., 2021a, 2021b).
The most difficult aspect of any ultrasound-assisted system is the design of the extraction cell, which results in a deprivation of ultrasonic power. In case of a bath type sonication, the thick walls of the extraction cell must bear the pressure, which attenuates the ultrasonic waves from the transducers, limiting the impact that reaches the extraction medium efficiently (Taha et al., 2022). Furthermore, the vibrations in ultrasonic bath must pass through the thick walls of the pressured vessel. The ultrasonic probe, on the other hand, can be placed in the pressurized vessel and come into direct contact with the sample (Yusoff et al., 2022). Furthermore, other factors affecting ultrasonic efficiency include pressure, temperature, sample size, transducer form, generator capacity, and the type of material (Singla and Sit, 2021). The ultrasonic amplitude also has an individual impact on almost all responses such as TPC, TFC, naringin content during extortion of bioactive compounds from grapefruit peels (Islam et. al., 2023b). However, the sonication time had only a significant effect on the TPC and the antioxidant activity performed by the FRAP assay. In many circumstances, the only information offered about the ultrasound equipment's operation is the amount of electrical energy supplied. The characterization of acoustic fields is a complex process due to the increased surface area of the sonicator, design of the reactor, and the uniformity of the acoustic field. (Yusoff et al., 2022; Rao et al., 2021a, 2021b).
Ultrasound-assisted novel technologies
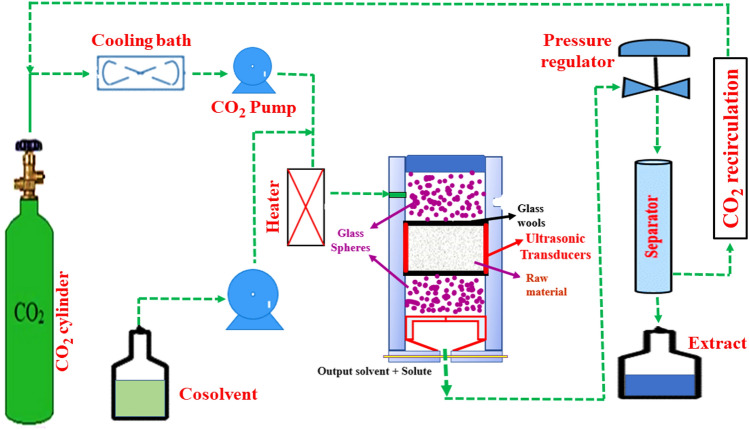
Green and environment-friendly techniques, which can improve extraction yields, and lowers energy usage and solvent with faster heat and mass transfer, have become increasingly popular in recent decades (Rao et al., 2021a, 2021b). To achieve a better balance among the production costs, product quality, and solvent usage, UAE-assisted novel (microwave, pulsed electric field, pressurized liquid, and supercritical fluid)/Novel assisted UAE technologies would be a reasonable approach. Among these, UAE has been broadly utilized to enhance high-pressure extraction procedures, but on a much lesser scale due to practical considerations. The acronym UAE-assisted high-pressure technologies (i.e., pressurized and supercritical fluid extraction) is cited in this article to refer to high-pressure techniques intensified with ultrasonication approaches. The use of UAE with high-pressure technologies (supercritical and pressurized liquid extraction) is new, but it has shown significant potential in improving natural product extraction, with applications in a variety of fields (Zabot et al., 2021). The systems consist similar to those used in UAE with supercritical fluids extraction in concept, using probes and baths to propagate ultrasound waves in the extraction solvent medium is highlighting among the various configurations used, as shown in Fig. 2. The most frequently used ultrasound type equipment for UASEF is water bath (US bath) or ultrasonic transducer coupled with probe (US probe). A stainless-steel extractor cell where the US probe was in direct contact with the sample. A glass wool layer and a filter in extremities to avoid blocking the extraction line, and two layers of glass beads to complete the volume of the cell. A CO2 cylinder from where the CO2 is emitted and mixed with the solvent, finally its mixture is entered in the stain-less extraction chamber. The output solvent and solute were separated through a separation chamber and CO2 is again recirculated for the next extraction cycle (Koubaa et al., 2018).
Fig. 2.
Schematic diagram of ultrasound-assisted supercritical fluid extraction system. (Redrawn from Koubaa et al., 2018)
Ultrasound-assisted supercritical carbon dioxide (CO2) based extraction technology
The ultrasound-assisted supercritical carbon dioxide (CO2) technology is a potent role in the enhancement of the extraction process that ensured effective and safe extraction assists in the substitution of versatile solvents and provides lower energy consumption (Koubaa et al., 2018). In this context, SFE combined with UAE (UASFE) is a unique and environmentally friendly technology that has gotten a lot of attention. The goal of utilizing UAE is to soften and break down seed walls to allow supercritical fluids to penetrate the seed matrix more easily, leading to faster dissolution and oil extraction (Yang et al., 2020). UAE causes cell content leaking by disrupting the cell walls of plant seeds with repeated ultrasonic waves. In an aqueous solution, such waves form bubbles, and the breaking of these bubbles produces energy powerful enough to damage the seed cellular wall tissues (Senrayan & Venkatachalam, 2020). In actuality, the UAE principle is based on acoustic cavitation, which causes the pressure and temperature in the extraction vessel to rise as a result of the generated bubbles. When these bubbles burst, they cause a “shock wave” that causes the cell walls to disintegrate. UAE also improves solvent diffusion into plant cells and oil mass transfer across the cellular tissues. Furthermore, in solution and organic liquids, free radicals such as superoxide anions and hydroxyl radicals (OH−) can be formed. The use of UAE in conjunction with SFE, or seed particle pre-treatment, improves extraction yield while cutting down on time. As a result, the SFE-CO2 extraction and ultrasound assisted approaches are the best-suitable for thermolabile materials (Čižmek et al., 2021).
The UAE alone, on the other hand, is not selective in extracting specified compounds, and the extract also leaves a significant quantity of residues (Kate et al., 2018). Recently, few demonstrations of integrated UAE-assisted SFE-CO2 extraction have been reported. An illustrative investigation is exhibited by Liu et al., (2020b) in case of oil extraction from Iberis amara seed and resulted in UAE-assisted assisted SFE-CO2 (i.e., ultrasonic energy mass of 1.25 W/mL and 10 min) enhanced the oil yield by 28% as compared with UAE treated seeds oils. Furthermore, the integrated impacts of temperature and pressure on the UAE-assisted SFE-CO2 extraction of natural matter dominates the understanding of the behaviour of the mass transfer phenomena included in the operation. These factors are also needed to forecast the impact of vapour pressure and CO2 density on the target compounds. The search for the best temperature and pressure conditions is critical for maximizing extraction yields while lowering operational expenses and extraction duration (Koubaa et al., 2018).
Higher pressure application from supercritical and pressurized liquid extraction and ultrasound power can decline the void percentage and distance between molecules, which limits the ultrasound's convective effects. Furthermore, when using ultrasound, the system's internal temperature may rise. Internal temperature impacts may affect CO2 density and its density at higher temperatures and lower pressures. In general, UASFE trials outperform SFE procedures in terms of recovering the desired chemicals. Temperature and pressure impact the mass transfer between the fluid and the sample matrix, determining fluid density and consequently target compound solubility (Dassoff et al., 2019). As a result of the simultaneous impacts of temperature and pressure on the fluid's characteristics, bubble dynamics, ultrasonic propagation, and the reported extraction yields are obtained.
However, the information from the experiments cited above suggests that higher ultrasonic powers are better suited to lower pressures since the internal temperature can be precisely controlled. At later stages of the extraction, higher pressures are encouraged, especially when diffusional mechanisms are more prevalent. Yang et al., (2019) discovered a similar pattern when they looked at the flavonoids extracted from Scutellaria barbata D. Don. The flavonoid content was raised by 19% when ultrasound was combined with temperature (up to 52 °C) and higher pressure (up to 28 MPa). Cucurbitacin oil was extracted from Iberis amara seeds by Liu et al., (2020a), who found that at 50 °C and 25 MPa, the total yield for UASFE increased by 26.1 percent compared to SFE.
Another important parameter to investigate in UASFE experiments is the CO2 flow rate interval, which might have a direct impact on extraction performance and cost. Lower CO2 flow rates may result in longer residence durations inside the extraction vessel, allowing SC-CO2 to infiltrate into the vegetable matrix and so improve extraction yield. When the CO2 flow rate is increased, the intermolecular contact between the solute and the solvent is increased, allowing the analytes to dissolve in the solvent (Dias et al., 2021). Furthermore, increasing the flow rate of CO2 may diminish the molecules' external mass transfer barrier (Yang et al., 2019). However, increasing the flow rate of CO2 will cause the solvent to flow around the channels at high speeds rather than diffusing through the vegetable matrix. Because the solute–solvent interaction occurs in a shorter contact period, the extraction yield may be reduced. Extraction of bioactive ingredient increase by 24% in yield for the UAE with single bond CO2 extraction technique at a frequency of 37 kHz, optimal pressure of 180 bar, and temperature of 65 °C, in comparison to the super critical single bond CO2 technique (Vaeli et al., 2021).
Ultrasound-assisted pressurized liquid-based extraction technology (UAPLE)
The use of sonication in pressure liquid extraction methods has gotten a lot of attention recently. Although there are some obvious similarities between pressurized liquid and supercritical fluid extraction, many issues about the influence of process factors (i.e., ultrasound power and frequency, pressure, solvent type, experimental apparatus, temperature, particle size, etc.,). However, from a phenomenological perceptive, the extraction process with pressured liquids assisted by ultrasound is complex, as diverse mechanisms of energy and mass co-occur and interact with one another. Furthermore, the ultrasonic application impacts the sample matrix through a variety of mechanisms, including sonoporation, erosion, capillary effect, fragmentation, shear forces, and detexturation, sometimes in a sequential manner, further complicating the extraction process. As a result, scientists and engineers face a significant barrier in understanding the individual and cumulative effects of process factors.
In the UAPLE procedures, the system pressure has a direct impact on the phenomenon of cavitation. Greater acoustic pressure will be needed to generate cavitation as the external pressure rises (Albero et al., 2019). However, if the threshold limits of cavitation are reached under external pressure, the collapsing intensity of the cavitation bubbles is greater than when the cavitation threshold is reached without pressure. There will be an increase in sonochemical effects as a result (Rao et al., 2021a, b). According to Martínez et al., (2020) in the manosonication process, enhancing the medium’s hydrostatic pressure results in higher extraction yields, which is attributed to the effect of static pressure on the dynamics of bubble collapsing and their formations. Moreover, the temperature has also a notable impact on the solvent properties during UAPLE processes. For example, increased temperature causes an improvement in vapour pressure as well as a drop in the solvent’s viscosity and surface tension directly impacting the extraction process' effectiveness (Sun et al., 2019). Martínez et al., (2020) studied the impact of temperature on manosication and found that temperature was the critical factor in carotenoid extraction yield. The influence of temperature on extraction yield was found to be typical of solid–liquid extraction methods of intracellular chemicals from plant matrix, according to the findings. When ultrasonic is applied at ambient pressure, it is known that increasing the temperature declines the intensity of cavitation by increasing the vapour pressure within the bubble (Jiang et al., 2018).
Moreover, the quantity of energy that enters the extraction medium has a direct impact on the cavitation occurrence in the UAPLE. In addition to the collapse pressure of bubbles, it is connected to the number of bubbles and their longevity (Rao et al., 2021a, 2021b). Because the amount of energy is proportional to ultrasonic power, it is critical to assess the impact of this variable on the extraction process. The actual applied acoustic power in the extraction process with pressured liquid assisted by ultrasound was evaluated by certain authors (Santos et al., 2019; Sumere et al., 2018; Vigano et al., 2020). Because it reflects the amount of energy that effectively penetrates the liquid, the actual applied acoustic power is an important measure to assess the effects of power on the extraction process. The most common physical methods involved sound pressure measurement with optical microscopes or hydrophones, the calorimetric approach, and the aluminium foil method. Indirect measurements of OH· radicals generated by chemical dosimeters, or sonoluminescence can be employed for chemical procedures (Chemat et al., 2017a, 2017b). The majority of the investigations found that a higher ultrasonic power causes significant changes in the matrix by causing increased shear forces at constant pressure (Tamidi et al., 2021, Bernaidi et al., 2021). A common strategy is to investigate the effect of power on the target compound’s extraction yield to achieve the best results with the least amount of energy. However, ultrasound can cause free radicals to form when water molecules dissociate, especially in aqueous solutions. These free radicals can cause compounds to oxidize and bonds to break, resulting in changes to macromolecular structures and a drop in molecular weight (Rahman et al 2020).
Factors governing the effectiveness of ultrasound-assisted extraction (UAE)
Ultrasonic amplitude or power
The ultrasonic amplitude is directly proportional to the ultrasonic power and the intensity. High amplitude may not inherently increase the effectiveness and cavitation of systems. The cavitation impact depends on the US systems amplitude, but greater amplitude may not necessarily improve the extraction performance and cavitation because UAE mainly depends on the other operating conditions such as target compounds and type of medicinal or herbal plants (Rao et al., 2021a, 2021b). High amplitude may cause probe erosion and decreases the formation of cavitation, which in turn can lead to decreased cavitation effectiveness (Kumar et al., 2023). This can be proven by the fact that the destruction of the cavity bubble becomes more aggressive with an improvement in amplitude or power because the volume of the bubble is proportional to the ultrasonic wave amplitude (Tao et al., 2022). Although the extraction yields of the bioactive compounds increase with the amplitude or power density of UAE up to a certain time, it decreases over time (Liao et al., 2021). High amplitude could be chosen based on the physicochemical nature of a sample for optimization the amplitude or power for highly viscous solvents to achieve optimal agitation and cavitation improved extraction efficiencies. (Wen et al., 2018).
The larger amplitude of ultrasonic waves produced by a higher power passing through a solvent contributes to the forming of more amount of cavitation bubbles and created collapse. The created shock wave induces cellular uptake into cells, which increases the mass transfer coefficient and the extraction rate Islam et al., 2023a; Senrayan, & Venkatachalam, 2020). The yield and other phenolic compounds extraction from Nepeta (Nepeta binaludensis Jamzad) increased with the increase of ultrasonic amplitude during ultrasonic extraction techniques Azimi Mahalleh et al., 2020). The optimum condition found at 60% amplitude with response of 10.93%, 402.63 mg GA/kg, 0.33 mg/mL and 2838.34 µmol Fe2 + /kg, of yield, TPC, IC50 and FRAP respectively. However, a medium ultrasonic amplitude (40%) shows highest of yield of TPC, TFC and volatile compounds from tea (Bakht et al., 2019). Some extraction factors, like temperature, time, and pH of the extraction fluid, often depend on the influence of power on the yield (Nishad et al., 2019).
Ultrasonic temperature
A wide range of research has been published on the impact of ultrasonic temperature on the extraction yield of bioactive compounds from different medicinal and herbal plants. Khemakhem et al., (2018) studied the effect of different extraction temperatures (10–70°C) on total phenolic content (TPC), and oleuropein content of olive leaf extracts using the UAE process and observed that increase in the temperature, the extraction of total phenolic and oleuropein content increased significantly. As the temperature increased from 10 to 70 °C, the kinematic of TPC showed a significant increment and the TPC value at 70 °C was almost 2.5 times more than the TPC value at 10 °C. This may be because high temperatures seem to have stimulated the mechanisms of permeation and solubilization to flush away the intracellular ingredients from the matrix.
Jiskani et al., (2021) exhibited that at high temperature shows highest extraction yield (11.58%) and maximum TPC (total phenolic content), TFC (total flavonoids content) and metal chelating capacity, as high temperatures increased the number of cavitation nuclei accountable for cell disruption, which amplified the mass transfer and then the accessibility of solvent to cellular constituents. According to Dzah et al., (2020), if the sonication variables have reached their optimal values, further changes in the process variables have been observed to be insignificant and, in some situations, may be negative in terms of output, so that oleuropein content did not show any significant effect when the extraction temperature reached up to 70 °C. Almusallam et al., (2021) found that extraction using sonication at 40.8 °C had a maximum yield of TPC from date palm spikelets. Numerous experiments conducted in the UAE have shown that oil extraction is most successful at temperatures between 15 and 60 °C, despite the lower yield that results from extended heating (Thilakarathna et al., 2023). Ultrasound-assisted oil extraction from Moringa peregrina seeds was studied (Mohammadpou et al., 2019). The yield of Moringa peregrina oil in the UAE was revealed to decline as the temperature increased. At 30 °C, the yield of oil was about 35%, and at 60 °C, it was about 32%. Similarly, Mahua oil recovery reached its peak at 35 °C using UAE, and further decreased (Thilakarathna et al., 2023). This may be explained by the fact that as the vapour pressure of solvent increases with an increase in temperature more cavitation bubbles were created, but they collapsed with less intensity due to a lower pressure difference between the inside and outside of bubbles. Moreover, at lower temperatures, few cavitation bubbles were created even with the high acoustic cavitation due to the low vapour pressure of the solvent. However, ultrasonic time and temperature are insignificant on the allicin content of the garlic slices drying in an infrared drying system due to the rapture the cell’s structure incurred by ultrasound treatment (Malakar et al., 2022).
Sonication frequency
The frequency with the range of 18 to 100 kHz has been used for the extraction of bioactives from herbal plants and other plant-based materials. In the UAE method, low-frequency high-intensity ultrasound induces heavy shear and mechanical energy, while high-frequency low-power density creates a significant number of reactive radicals. Most of the researchers have used constant frequency for the extraction of bioactive compounds from plant-based materials. A particular frequency range of 18 to 20 kHz has been applied for the extraction of different bioactive compounds. A frequency range of 20 to 40 kHz was applied for the extraction of Neem oil (40 kHz) (Bhuyan, 2019) and 35 kHz frequency was applied for the extraction of total phenolic contents from Ashwagandha roots (Dhanani et al., 2017). The ultrasonic frequency is inversely proportional to the rarefaction cycle, as at the higher frequency the cavitation bubbles will not get changes for aquatic cavitation due to get a very short time. As more cavitation bubbles formed due to high frequency create more resistance to mass transfer (Kumar et al., 2021). So, low frequency in the range of 18 to 80 kHz has been used for the extraction of bioactive compounds from different herbal plants. However, the lower frequency at 26 kHz shows more efficient and thermodynamically more acceptable as compared to the ultrasonic bath at 40 kHz (Bakht et al., 2019).
Selection of extracting solvent
The choice of suitable solvents for the ultrasonic extraction of bioactive compounds depends upon their physical properties such as surface tension, viscosity, and vapour pressure as these physical properties affect the cavitation energy in an aqueous solution (Thilakarathna et al., 2023; Kumar et al., 2021). Cavities have been seen for the solvents with high vapor pressure, low viscosity, and low surface tension but on the other hand, high cavitation intensity has been observed for the solvent with low vapor pressure, high viscosity, and high surface tension (Rao et al., 2021a, 2021b). Different types of organic solvents such as ethanol, methanol, acetone, hexane, ethyl acetate, water, etc. have been used for the extraction of different bioactives using UAE. The most used solvents for the extraction of total phenolic compounds, total flavonoid content, carotenoids, and other bioactive compounds from different herbal plants are ethanol, and methanol diluted with water at different ratios because alcohol-based natural deep eutectic solvents exhibited relatively higher extraction efficiencies Different level of ethanol and methanol mixture with water has been used for the extraction of bioactive compounds from plant material like Rosemary leaves (Jin et al., 2019), neem fruits (Bhuyan, 2019), Ashwagandha roots (Dhanani et al., 2017), Olive kernel (Chanioti & Tzia, 2018), etc. Ethanol has been found to have the highest affinity for phenolics, making it a preferred solvent for extracting phenolic compounds from various herbal plants.
Similarly, Dhanani et al., (2017) have seen that ashwagandha root extract prepared with ethanol had the highest TPC followed by ethanol–water, and water. The recovery of TPC and DPPH radical scavenging behaviour from date plum spikelets were influenced by ethanol concentration and maximum TPC and DPPH have been shown at 50% ethanol concentration (Almusallam et al., 2021). The ethanol content exceeds its maximum limit, it has been shown to have a detrimental influence on extraction yield. Acetone, hexane, and ethyl acetate have been used as a solvent for the extraction of oil from different types of plants such as neem fruits (Bhuyan, 2019) and olive kernels (Chanioti & Tzia, 2018).
Moreover, the green deep eutectic solvents (DES) can also be used as a sustainable option to hazardous organic solvents because of their non-toxicity and biocompatibility. Jamshaid et al., (2022) explored that UAE with a combination of green DES (glycerol-ammonium acetate) resulted the yields of 17.11 mg gallic acid equivalent (GAE)/g of dry weight DW) (TPC), 11.33 mg rennet/g of DW (TFC), 52.66% (iron chelating activity-ICA), and 72.84% (DPPH activity) for Melia azedarach fruit under the optimized conditions of temperature-50 °C, DES concentration-50%, and amplitude-100%. Similiarly, tul Kubra et al., (2023) also reported that using the glycerine based deep eutectic solvents (glycerol-ammonium acetate and glycerol-choline chloride) resulted the greater extraction yield from Glycyrrhizin from licorice (Glycyrrhiza glabra) than the conventional organic solvents (methanol). Jamshaid and Ahmed (2022) also reported that ultrasound with the combination of low-cost glycerol-choline chloride resulted the 9.154 mg of GAE/g DW, TFC 21.880 mg rennet/g DW, and 33.254% of ICA from the Melia azedarach fruit under the conditions of 46.4 °C temperature, 100% of US amplitude and 50% of DES concentration.
UAE of bioactive compounds from various herbal plant sources
The herbal bioactives such as polyphenols (phenolic compounds, flavonoids), volatile oils, alkaloids, and other bioactives such as anthraquinone glycoside, azadirachtin, ginsenosides, curcuminoids, rhizomes, lutein and β-Carotene carotenoids, total chlorophyll have been extracted through ultrasound-assisted extraction technology and their optimum conditions are discussed below.
Polyphenolic compounds
The UAE technology is recognized as one of the highly efficient and emerging extraction technologies for the extraction of polyphenols from plant matrixes. The TPC, TFC, and the antioxidant activity compounds have been extracted from Rosemary leaves (Munekata et al., 2020;), Ashwagandha roots (Tsaltaki et al., 2019), Olive pomace (Susy et al., 2019), Lemon-scented tea tree leaves (Saifullah et al., 2020), Eucalyptus robusta leaves (Bhuyan et al., 2017), Rumex hastatus (Jiskani et al., 2021), Date palm leaves (Almusallam et al., 2021).
The different UAE variables and their optimized ultrasonication parameters for extraction of polyphenols for the herbal plants were summarised in Table 1. Among all the UAE parameters, solvent to solid ratio, temperature, time, and power were the most effective variables for accomplishing maximum recovery of TPC, TFC, and antioxidant activity from the plant materials. For example, Bhuyan et al., (2017) demonstrated that time, temperature, power, and solvent to solid ratio significantly influenced the total phenol extraction from Eucalyptus leaves (Eucalyptus robusta), but among these, the temperature has a more significant impact on the total yield of TPC as compared to ultrasound power and time. Similarly, Saifullah et al., (2020) also reported that ultrasound-treated Lemon scented tea tree (Leptospermum petersonii) leaves resulted in a greater TPC value (15.27%) than compared the shaking water bath extraction method. The higher extraction of TPC and TFC from rosemary and thyme leaves has observed in conventional extraction (CE) than UAE (Munekata et al., 2020). The finding might be the degradation or modification of the bioactive compounds due to the high energy input and temperature generated during the extraction process. This can lead to a decrease in the concentration of TPC and TFC in the extract. Additionally, Kumar et al., (2023) utilized UAE for the extract recovery from giloy stem powder and observed that the maximum phenolic content yield was obtained at low to moderate power amplitude and high extraction time.
Table 1.
Optimized extraction parameters for ultrasonic-assisted extraction of phenolic, flavonoids, and antioxidant activity for different herbal and medicinal plants
| Plant materials | Extraction variables and solvent | Ultrasonic device | Optimum operating conditions/results | Reference |
|---|---|---|---|---|
| Date palm leaves (Phoenix dactylifera L.) | S: ethanol: water (25–50% v/v); T: 25–60; t: 20–40; F:40; P: 110, | Ultrasonic bath |
• S: ethanol: water (50%); T: 40.8; t: 21.6; show maximum recovery of TPC and DPPH radical scavenging • Temperature and ethanol concentration were the major significant factor for the recovery of TPC and DPPH radical scavenging activity |
(Almusallam et al., 2021) |
| Rumex hastatus | S: hydro-ethanol: water (20–80% v/v); A: 50–100 m; T: 50–70 | Ultrasonic probe |
• S: hydro-ethanol: water (20%); T: 50; A: 50 m show maximum yield (10.58%). • Hydro-ethanol: water (80%); T: 60; A: 70 m show maximum TPC • Hydro-ethanol: water (80%); T: 50; A: 50 m show maximum TFC • Hydro-ethanol: water (20%); T: 50; A: 100 m show maximum metal chelating activity |
(Jiskani et al., 2021) |
|
Rosemary leaves (Rosmarinus officinalis L.) |
S: water (100% v/v); ethanol: water (50:50% v/v); T: 40; t:10; P: 400; SLR: 1:50; | Ultrasonic probe |
• S: ethanol: water (50:50%v/v); shows the maximum recovery of TPC, TFC, and antioxidant activity • UAE shows more extraction of carotenoids than CE • But TPC, TFC value was more for CE than UAE |
(Munekata et al., 2020) |
| Lemon-scented tea tree leaves (Leptospermum petersonii) | S: water, acetone, ethanol, acetone: water (50% v/v) ethanol: water (50% v/v); t: 30–60; T:30–50; P: 150–250 | ultrasonic probe |
• S: acetone: water (50% v/v); T: 50; t: 60; P:200 show maximum recovery of TPC, TFC and Pro-anthocyanidins • UAE was found to be more efficient than conventional shaking water bath extraction for the TPC and antioxidant activity |
(Saifullah et al., 2020) |
| Ashwagandha roots (Withania somnifera) | S: ethanol, ethanol: water (10% v/v), water; t: 5, 10, 20, T: 25, P: 480, F:35, SLR: 1:10 | Ultrasonic bath |
• S: ethanol; t: 20; shows the maximum recovery of TPC • S: water; t: 5 shows the maximum antioxidant activity • S: ethanol; t: 25 show maximum recovery of total withanolide content |
(Tsaltaki et al., 2019) |
|
Olive pomace (Olea europaea L) |
S: n-Hexane, methanol; T:40–60; SLR: 1:4–1:12; p:0.5 -2; P: 280 | Ultrasonic bath |
• S: n-Hexane, T: 50, LSR: 1:8, p:0.9, show the maximum recovery of TPC of olive oil extract • S: n-Hexane, T: 55, LSR: 1:8, p:0.9, show the maximum antioxidant activity of olive oil extract |
(Chanioti & Tzia, 2018) |
|
Eucalyptus leaves, (Eucalyptus robusta) |
S: water: ethanol (70–100%); acetone, acetonitrile, ethyl acetate; t: 30–90; T:30–60; P: 150–250; LSR: 1:50; F:50; | Ultrasonic bath |
• S: water; t:90; T:60; P:250; show maximum recovery of TPC • Significant of different extraction parameters in the following order: temperature > time > power |
(Bhuyan et al., 2017) |
|
Thyme leaves (Thymus serpyllum L.) |
S: ethanol: water (30–70% v/v); t: 5–30; p:0.3–1.5; SLR:1:10–1:30, F:20, P:600, | Ultrasonic probe |
• S: ethanol: water (50%); T:25, t: 15, p:0.3, SLR:1:30 show the maximum recovery of TPC and TFC • The significant level of different extraction methods in the following order: UAE > HAE > maceration |
(Jovanović et al., 2017) |
T temperature (°C), t sonication time (min), P power (W), F frequency (kHz), SLR solid liquid ratio (g/mL), EC ethanol concentration (%), A amplitude (%), UED ultrasonic energy density, (w/cm3), S solvent, TPC total phenolic content, UAE ultrasonic assisted extraction, CE conventional extraction, TFC total flavonoid content, p partical size (mm)
Additionally, Dhanani et al., (2017) have observed that water as an extracting solvent showed more antioxidant activity but on the other hand, ethanol shows more TPC and total withanolides contents from ashwagandha (Withania somnifera) roots. Almusallam et al., (2021) studied the different levels of ultrasonic process variables (temperature, time, and ethanol concentration) to obtain the maximum recovery of TPC and antioxidant activity from data palm spikelets. The results revealed that ultrasonic temperature and ethanol concentration are the two most important factors affecting TPC yield and antioxidant activity from date palm spikelets. Interestingly, Amin et al., (2022) reported that ethanol with the combination of DES (glycerol-ammonium acetate) as extraction solvent resulted the better TPC, TFC, ICA, and anti-radical activity than the alone DES and ethanol based bioactives extract from bottle gourd (Lagenaria siceraria) fruit.
Recovery of oil
Recovery and yield of the oils can be improved by using UAE and ultrasound-assisted supercritical fluid extraction with varying the, temperature, extraction time, particle size, pressure, CO2 flow rate, and solid-solvent ratio. The use of supercritical fluids (G CO2) as solvents is an intriguing option for acquiring high-quality natural products without leaving harmful residues. The optimized ultrasonic process parameters and oil extraction yield from different herbal and medicinal plants are described in Table 2. Moreover, Chanioti & Tzia, (2018) extracted olive oil from olive pomace and found that the optimal ultrasonic-assisted extraction (UAE) conditions resulted in an oil yield of 88.93% exhibited superior quality compared to the solvent extraction (SE) technique. It has been seen that the optimized UAE method significantly reduces the time (1 h), temperature (60 °C), and solid to solvent ratio (1:12 g/mL) compared to SE obtained time 8 h, 76 °C, and solid to solvent ratio 1:8 g/mL.
Table 2.
Optimized extraction parameters for ultrasonic-assisted extraction of oil from different herbal/medicinal plants
| Plant Materials | Extraction variable and Solvents | Ultrasonic device | Optimum operating condition/results | Yield | Reference |
|---|---|---|---|---|---|
| Iberis l amara flower seeds |
S: hexane; t:5–10; SLR: 1:6; UED: 0.625–2.5 F:25; P: 500; CO2 FR: 3 (mL/min);T:55; t: 80; Pc: 25 Mpa |
Ultrasonic unit SFE units |
• Ultrasonic pre-treatment at S: hexane; t:10; SLR: 1:6; UED: 1.25 F:25; P: 500 USE combined with SFE shows a 28% higher yield Ultrasonic extraction combined with SFE shows a better quality of the oil |
22.28% | (Liu et al., 2020a) |
|
Neem fruits (Azadirachta indica) |
S: n-Hexane: ethyl acetate (1:1); ethyl acetate: acetone (0.92:1); hexane: acetone (1.06:1); t:20; SLR: 1:6; F:40 | Ultrasonic bath | • S: ethyl acetate: acetone (0.92:1); show the maximum yield | 20–30% | (Bhuyan, 2019) |
|
Umbu seeds (Spondias tuberosa) |
S: ethanol: water; t:4; SLR: 1:6; F:20; P: 500; CO2 FR: 11.6 (g/min);T: 40; t: 180; Pc: 15–30 Mpa |
Ultrasonic units SFE unit |
• S: ethanol: water; t:4; SLR: 1:6; F:20; P: 500; CO2 FR: 11.6 (g/min); T: 40; t: 180; Pc: 15–30 MPa show the optimum condition • Post-treatment of ultrasonication with SFE increased the extraction yield compared to the UAE and SFE alone |
10.9% | (Dias et al., 2019) |
|
Coriander seeds (Coriandrum sativum) |
S: Hexane; T:40–50; t:5–15; A:70–90%; SLR: 1:5–1:15; P:400; F:25; | Ultrasonic processor |
• S: Hexane; T:44.5; t:9; A: 82%; SLR:1:12.48; show the maximum yield and antioxidant activity • UASE gives better oil yield with 84% MUFA and good thermal stability within less extraction time |
30.94% | (Senrayan & Venkatachalam, 2019) |
|
Olive kernel (Olea europaea) |
S: n-Hexane, methanol; T:40–60; LSR: 1:4 -1:12; p:0.5 -2 | Ultrasonic cleaning bath |
• S: n-Hexane; T: 60; SLR: 1:12; p:0.5 show maximum yield • Soxhlet extraction shows more extraction yield, but less TPC and DPPH activity compared to the UAE |
11.03% | (Chanioti & Tzia, 2018) |
T Temperature (°C); t sonication time (min); P power (W); F frequency (kHz); SFE supercritical fluid extraction; UASE ultrasonic-assisted supercritical fluid extraction; HPU high pressure ultrasonic unit; UED ultrasonic energy density (W/ml); S solvent; p particle size (mm); SLR solid liquid ratio (g/mL); A amplitude (%); FR flow rate (L/hr); Pc pressure (bar); TPC total phenolic content (mg GAE/ g); TFC total flavonoid content (mg QE/g)
Moreover, Senrayan & Venkatachalam, (2019) used UAE for the extraction of coriander seeds oil and optimize the extraction process parameter, including sample solvent ratio, amplitude level, temperature and time, and at optimized conditions oil yield and antioxidant activity 30.74% and 71.05% respectively. Dias et al., (2019) have sonicate the umbu seeds powder after the SFE treatment and found a high yield capacity compared to the SEF and ultrasound extraction alone. It might be due the fact that non-polar materials like rasins and waxes modification will enhance the extraction yields.
Alkaloids
Plant alkaloids are secondary metabolites and often include nitrogen in a ring and it consists of 20% of plant species. Alkaloids possess significant therapeutic value, with their traditional and contemporary applications ranging from 25 to 75% in medicines (Zahra et al., 2020). The optimum conditions for the extraction of alkaloids from different herbal plants have been summarized in Table 3. They are abundant in several medicinal and herbal plants, and UAE has discovered how to extract them more efficiently from plant matrices. In comparison to traditional solvent extraction, UAE of alkaloids from Pocoa pod husk (Theobroma cacao) (Nguyen et al., 2022) and Soursop fruit (Annona muricata L.) pulp, peel, seed, and columella (Aguilar-Hernández et al., 2020) yielded more within less time and at a lower temperature. On the other hand, Wang et al., (2018) have optimized the process variables of the ultrasound-assisted enzymatic hydrolysis extraction method (UAEH) for the extraction of Quinolizidine alkaloids from Sophora alopecuroides L seeds and elaborated that UAEH has shown significantly higher extraction yield within a short period as compared to the UAE and enzymatic extraction (EE). Moreover, Aguilar-Hernández et al., (2020) have used ethanol as an extracting solvent for extraction of total alkaloid content (TAC), from Soursop fruit pulp, peel, seed, and columella.
Table 3.
Optimized extraction parameters for ultrasonic-assisted extraction of alkaloids from different herbal/medicinal plants
| Bioactive compounds | Plants | Extraction variable and Solvents | Ultrasonic device | Optimum operating condition/results | Reference |
|---|---|---|---|---|---|
| Total alkaloids (TA) | Stephania tetrandra roots | S: deep eutectic solvents (DESs): water (23%v/v); t: 82; T:52; SLR: 1:23; | Ultrasonic bath |
• S: deep eutectic solvents (DESs): water (23%v/v); t: 82; T:52; SLR: 1:23; show maximum extraction yield of total alkaloids (20.59 mg/g) • Optimized DESs extraction efficiency is 2.2, 3.3, and 4.1 times higher than methanol, 95% ethanol, and water respectively |
(He et al., 2023) |
| Total alkaloids content (TAC) |
Cocoa pod husk (Theobroma cacao) |
t: 50; T:62; SLR: 1:50; | Ultrasonic bath | • t: 50; T:62; SLR: 1:50; show maximum total alkaloid content (TAC; 45.62 mg AE/g dried sample (DS), alkaloid extraction efficiency (AEE; 63.28%), DPPH radical scavenging capacity (DRSC;16.57,), and ferric reducing antioxidant power (FRAP; 2.15 mg TE/g DS) | (Nguyen et al., 2022) |
| Total alkaloids contents | Soursop fruit pulp, peel, seed, and columella (Annona muricata L.) | S: methanol; t: 5–15; PC: 0.4,0.7,1; T:25; SLR:1:7.5; F: 24; P:400 | Ultrasonic probe |
• S: methanol; t: 5; PC: 0.4,0.7,1; T:25; SLR:1:7.5; F: 24; P:400 shows maximum of total alkaloid content (TAC) in peel, sheed and columella respectively • UAE shows 56.31, 5.45, 3.06, and 2.96 times higher the extraction of alkaloids from the peel, pulp, seed, and columella, respectively, then the extraction by maceration |
(Aguilar-Hernández et al., 2020) |
| Quinolizidine alkaloids |
Kudouzi (Sophora alopecuroides L) |
Cellulase enzyme, pH: 4–6; T:40–60; t:20–70; SLR: 1:60–1:140 | Ultrasonic bath |
• pH: 5; T:54; t:60; SLR: 112:1 show maximum extraction yield of quinolizidine alkaloids • Ultrasound-assisted enzymatic hydrolysis (UAEH) increases the extraction yield and shorter extraction time compared to UAE, Enzymatic extraction (EE), |
(Wang et al., 2018) |
T temperature (°C); t sonication time (min); P power (W); F frequency (kHz); S solvent; p particle size (mm); SLR solid liquid ratio (g/mL); PC pulse cycle
Polysaccharides
Polysaccharides are high-molecular-weight (carbohydrate) macromolecules found in different plant tissues that have been identified with various bioactivity including anti-oxidation, immunomodulation, anti-tumour, anti-cancer, and hypoglycaemic action. Due to its large bioactivity, the popularity of polysaccharides has been increasing for the development of functional foods and medicinal products. The remarkable extraction efficiency of ultrasonic extraction made it popular for extracting polysaccharides from various unutilized plant materials. Optimized conditions for the extraction of polysaccharides from different herbal or medicinal plants are shown in Table 4.
Table 4.
Optimized extraction condition for the extraction of polysaccharides from different herbal/medicinal plants
| Plant materials | Extraction variables and solvent | Ultrasonic device | Optimum operating conditions/results | Reference |
|---|---|---|---|---|
|
Ginkgo biloba leaves (Ginkgo biloba L.) |
S: distilled water; t: 40–60; SLR: 1:15–1:35; P: 288–360 | Ultrasonic bath | • S: distilled water; t: 50; SLR: 1:30; P: 340 shows the maximum Ginkgo biloba leaves polysaccharide yield of 5.37% | (Li et al., 2023) |
| Scutellaria baicalensis root | cellulase concentration: 100–200; T: 40–60; SLR: 1: 44.8; t: 50, P: 225 W; | Ultrasonic bath |
• cellulase concentration: 165.6 U/mL, T: 57.3, SLR: 1: 44.8; t: 50, P: 225 W; show the maximum of Scutellaria baicalensis root polysaccharide yield of 12.27 • Scutellaria baicalensis root polysaccharide has a potent immunomodulatory activity on dendritic cell maturation |
(Yun et al., 2023) |
| Choerospondias axillaris peels | S: distilled water; t: 40; T: 50; SLR: 1:40; F; 40; P; 240 | Ultrasonic bath |
• S: distilled water; t: 40; T: 50; SLR: 1:40; F; 40; P; 240 show the maximum polysaccharide yield • At optimum condition shows the lower molecular weight (127.7 kDa) and lower neutral sugar content (35.1%) but higher contents of protein (4.8%) and phenolic compounds (5.1%) than those of polysaccharides extracted by hot water extraction methods |
(Wang et al., 2023) |
|
Vietnamese Red (Delonix regia), lingzhi mushroom (Ganoderma lucidum) |
S: distilled water with vicozyme and chitinase enzyme (1:1); PH: 6–10; T:30–70; t: 40–200; P:120–600; | Ultrasonic bath |
• S: distilled water with vicozyme and chitinase enzyme (1: 1); PH: 5.5; T:45, t: 30, P:480; show maximum recovery of polysaccharide content (32.08 mg/g) • UAE combined with enzyme show an effective extraction method for crude polysaccharide from G. lucidum |
(Do et al., 2021) |
| Glycyrrhiza (Glycyrrhiza uralensis Fisch) | S: distilled water, T:50–80; t: 10–60; SLR: 1:10–1:20; P:420–600; | Ultrasonic bath |
• S: distilled water; T:70.15; t:60; SLR: 1:15; P:600 show the maximum recovery of polysaccharide yield (3.53%) • Polysaccharide shows good thermal stability and DPPH scavenging activities |
(Y. Wang et al., 2019) |
|
Eyrea vernalis whole (Turpinia arguta (lindl.) |
S: ethanol: water (1–4% v/v); t: 15–50; SLR: 1:15–1:35; | Ultrasonic bath | • S: ethanol: water (2.78% v/v); t: 40.78; SLR:1:27.07 shows maximum recovery of polysaccharide yields (3.11%) | (Zhao et al., 2018) |
|
Sijiaoling stem (Trapa quadrispinosa Roxb) |
S: distilled water; T:50–70; t:30–50; SLR: 1:25–1:35; | Ultrasonic bath |
• S: distilled water; T:58; t:41; SLR: 1:31.5; show maximum recovery of polysaccharide yields (2.78 ± 0.16%) • But,S: distilled water; T: 56; t:38, SLR: 1: 32 shows maximum antioxidant capacity (19.02 μmol Fe2 + /g), |
(Raza et al., 2017) |
| Rhododendron arboreum (Rhododendron L. Ericaceae) | S: distilled water; T:50–60; t:1–3; P:150–250; SRL: 1:25; F:45 | Ultrasonic bath |
• S: distilled water; T:55; t:2.2; P: 200 shows maximum polysaccharide yield (9.418%) • UAE show 1.12-time higher extraction than hot water extraction |
(Guo et al., 2017) |
T temperature (°C), t sonication time (min), P power (W), F frequency (kHz), S solvent, p particle size (mm), SLR solid liquid ratio (g/mL, UAEE Ultrasonic-assisted enzymatic extraction)
Other bioactives
Different bioactives including curcuminoids from Curcuma longa L. root and rhizomes (Raheem et al., 2023), saponins forms Panax notoginseng (Wang et al., 2020), volatile contents from tea (Bakht et al., 2019), Gingerols from Ginger (Hsieh et al., 2020) has been extracted by different researchers using ultrasound-assisted extraction technology. The optimized ultrasonic conditions for the different bioactives are given in Table 5. Aydar et al., (2022) reported that ultrasound treatment followed by microwave drying enhanced the dry matter Inula viscosa (L.) leaves with improved TPC, TFC, total chlorophylls and antioxidant properties. Inula viscosa (L.) leaves were treated to 30 min of sonication, the greatest DPPH and TPC levels were reported. The improved phenolics attributes to the greater release of bioactive from cell walls caused the US induced cavitation effects on the cell walls (Pandiselvam et al., 2022). Raheem et al., (2023) reported that UAE with combination of isopropyl as extracting solvent resulted the greater curcumin recovery than maceration-based extraction. The optimum conditions of UAE were 50 mL/g solvent-to-solid ratio, 10 min sonication time, and 40 °C temperature with maximum yield of 0.43–5.59 mg/g as compared to other extraction techniques i.e., maceration and microwave assisted extraction. On the other hand, ultrasonic extraction of volatile compounds from tea exhibits more extraction efficiency compared to the conventional extraction and also found that 26 kHz ultrasonic probe better extraction techniques compare to the 40 kHz ultrasonic bath technique. Wang et al., (2020) reported that vesicle-based UAE was found to be a suitable extraction method of saponins from Panax notoginseng. Another study by Hadidi et al., (Hadidi et al., 2020) also found that the rate contant of ultrsonic extraction of saponis from alfalfa is two time more than heat-reflux method. UAE of ginderols from ginger by using alcohol-based deep eutectic solvents and found that at higher temperature leads to degradation of phenolic compounds (Hsieh et al., 2020).
Table 5.
Optimized extraction parameters for ultrasonic-assisted extraction of other bioactive compounds from different herbal/medicinal plants
| Bioactive | Plant materials | Extraction variable and solvent use | Ultrasonic device | Optimum operating condition/results | Reference |
|---|---|---|---|---|---|
| Curcuminoids |
Turmeric (Curcuma longa) |
S: isopropyl alcohol; t: 10 T: 40: LSR: 50 |
Ultrasonic probe |
• S: isopropyl alcohol; t:10; T: 40; LSR: 50 shows maximum recovery (1.43–5.59) of curcumin recovery • UAE shows better extraction methods than maceration, but MAE shows the best recovery |
Raheem et al., 2023) |
| Saponins |
Chinese ginseng (Panax notoginseng) |
S: methanol: t:20; | Ultrasonic bath |
• As Compared to the traditional extraction method, ultrasound has enhanced the four saponins yields • With potential advantages involving shorter extraction time, it decreased the consumed solvent |
(Wang et al., 2020) |
| Volatile compounds |
Tea (Camellia sinensis) |
S: methanol: water (80% w/w); T:30–40; t:30; F:26,40; A 30–50%; P:200; | Ultrasonic probe, ultrasonic bath |
• S: methanol: water (80% w/w); T: 40; t:30; F:26,40; A 40%; P:200; shows maximum recovery of volatile compounds (mg/100 g DW), total phenolic content (mg gallic acid/g DW), flavonoids (mg/g DW) and DPPH radical scavenging activity (%) in all branded tea • Ultrasonic probe (26 kHz) is more appropriate for the extraction purpose and thermodynamically more acceptable as compared to ultrasonic bath (40 kHz) |
(Bakht et al., 2019) |
| Saponins |
Alfalfa (Medicago sativa) |
S: ethanol: water (60–90% w/w); LSR: 5–15; T:50–80; t: 60–180; A:30–50%; P:50–150; | Ultrasonic cleaning bath |
• S: ethanol: water (78.2% w/w); LSR: 1: 11.4; T:76.8; t: 170.4; P:112 shows the maximum yield of total saponins from alfalfa • The extraction rate constant of ultrasonic extraction is two time more than heat-reflux method |
(Hadidi et al., 2019) |
| Gingerols |
Ginger (Zingiber officinale) |
S: (deep eutectic solvents) DES (75% v/v); LSR: 30; F: 40; P:300; | Ultrasonic cleaning bath |
• S: DES (75% v/v); LSR: 30; F: 40; P:300; shows the maximum gingerols from the ginger • Higher temperatures more than 50℃ induced degradation and loss of phenolic compounds during extraction |
(Hsieh et al., 2020) |
T temperature (°C), t sonication time (min), S solvent, P power (W), F frequency (kHz), SLR solid liquid ratio (g/mL), DC duty cycle (%), PD/IT plused duration/interval time(s/s), A amplitude (%), UED ultrasonic energy density(w/cm3), UAE ultrasonic assisted extraction, CE conventional extraction; DGL dry gel loading; p partical size, CUAE continuous ultrasonic-assisted extraction; PUAE pulsed ultrasonic-assisted extraction; MAE microwave assisted extraction; IPA isopropyl alcohol
Industrial relevance of herbal plant extracts in the food and pharma sectors
The pharmaceutical and food sectors have rekindled interest in herbal health care formulations, herbal-based cosmetics, and herbal nutritional supplements in response to the rising demand for herbal plant products. The global and national markets for medicinal and herbal plants have been expanding. India is the second highest exporter of herbal, medicinal and aromatic plants (MAPs), accounting for 8.75% of the Asian trade in MAPs (Riaz et al., 2021). Developing nations like India have tremendous potential for cultivating medicinal and herbal plants, however appropriate use of such plants is still a challenge owing to a lack of adequate innovative extraction techniques. In the pharmaceutical and food sector, herbal oils are widely utilized in a variety of applications, including medicine, sanitary, flavoring, fragrance, preservatives, and food additives (e.g., antioxidant and growth promoter) as alternative medicine. As example of herbal oils such as Adlay oil can help avoid the loss of white blood cells during chemical treatment by inhibiting cancer cell growth with an effectiveness of 87% (He et al., 2020).
Herbal plant extracts are becoming more significant food additives due to their high content of bioactives such as polyphenols, flavonoids, and carotenoids, which have antibacterial and antioxidant properties, particularly against low-density lipoprotein (LDL) and DNA oxidative alteration (Proestos, 2020). This natural extract may be used in a variety of food matrix, including meat, oils, and salad dressings. Nieto et al., (2018) have used rosemary extract as a natural preservative because it delays lipid oxidation, and microbiological spoilage, and extends the shelf life of food but their uses have been limited due to negative organoleptic properties, such as odour and taste. The use of thyme extract in different meat products such as (minced beef, minced pork, and chicken sausages) has been proven to increase their self-life by inhabiting the microbial (E. coli, lactic acid bacteria, Pseudomonads) growth (Lorenzo et al., 2019). These medicinal and herbal plants have evolved as important components of civilization, and they are now generally regarded as symbols of the country's rich cultural and scientific legacy.
Future perspective and conclusion
This review ascertains the potentialities of ultrasound assisted novel technologies (i.e., supercritical fluid extraction and pressurized liquid extraction) for the bioactives extraction from medicinal and herbal plants. In comparison to the conventional extraction methods, UAE appeared to have numerous advantages, including a shorter extraction time, higher extraction yield, and lower solvent consumption. Moreover, ultrasound assisted with combined green technologies has more potential for better extraction approaches i.e., obtaining higher extraction efficiency of bioactivities from herbal plants without altering their functional characteristics. The combined application of ultrasound with novel techniques for the extraction of bioactives from herbal plants gives substantial intensification benefits in terms of time, temperature, and solvent consumption in the extraction procedure.
However, scaling up ultrasonic extraction technology can be challenging due to certain limitations that affect its effectiveness. Maintaining consistent and efficient extraction at larger scales may require significant modifications to equipment design and processing parameters, optimized extraction conditions. Even though a wide range of pieces of evidence and prospective applications have been published but the majority of the researcher are working on basic ultrasonic devices designs, such as ultrasonic probes and ultrasonic baths, that might or might not be successful at the commercial level, so further research is needed to enhance the method’s industrial usability. Generally small scale ultrasonication probe is used for extraction, which can make it difficult to achieve consistent and efficient extraction across large volumes of material. This can result in uneven extraction and lower yields. Therefore, more research might be employed to design novel reactor based on the utilization of many transducers. Technological adjustments, such as the use of UAE based pre-treatment approach and sequential extraction must be investigated. Ultrasonic operation energy measurement is presently uncommon, but it will be required in the future to compare ultrasonic technology to conventional and non-conventional technology. Combination/ Hybridization with other novel techniques (i.e., ohmic assisted extraction, and pulsed electric field) would make a great balance among the product quality, production costs, and solvent consumption. However, the process variables of these combinational technologies have a significant impact on extraction performance and needs to be carefully optimized. The technological difference in the utilization of these combined technologies to herbal plants as a coupled/simultaneous extraction and sequential extraction needs to be further investigated. Investigations on the stability of targeted bioactives extracted from herbal plants during the ultrasound assisted novel technologies /ultrasound assisted extraction offer a novel strategy for future study.
Acknowledgements
All the authors are thankful to the National Institute of Food Technology Entrepreneurship and Management, Haryana, India, for providing support during the preparation of this review paper.
Author contributions
MI: Conceptualization, Methodology, Writing—original draft. SM: Formal analysis, writing review & editing, MVR: Writing review & editing. NK: Conceptualization, supervision, writing review. JKS: Review and editing.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Makdud Islam, Email: makubkv96@gmail.com.
Santanu Malakar, Email: santanuinfo10@gmail.com.
Madaraboina Venkateswara Rao, Email: venkatmadanaboina123@gmail.com.
Nitin Kumar, Email: nitinkumar.iit@gmail.com.
Jatindra K. Sahu, Email: jksahu@iitd.ac.in
References
- Aguilar-Hernández G, Zepeda-Vallejo LG, García-Magaña MDL, Vivar-Vera MDLÁ, Pérez-Larios A, Girón-Pérez MI, Montalvo-González E. Extraction of alkaloids using ultrasound from pulp and by-products of soursop fruit (Annona muricata L.) Applied Sciences. 2020;10(14):4869. doi: 10.3390/app10144869. [DOI] [Google Scholar]
- Albero B, Tadeo JL, Pérez RA. Ultrasound-assisted extraction of organic contaminants. Trends in Analytical Chemistry. 2019;118:739–750. doi: 10.1016/j.trac.2019.07.007. [DOI] [Google Scholar]
- Almusallam IA, Mohamed Ahmed IA, Babiker EE, Al Juhaimi FY, Fadimu GJ, Osman MA, Al Maiman SA, Ghafoor K, Alqah HAS. Optimization of ultrasound-assisted extraction of bioactive properties from date palm (Phoenix dactylifera L.) spikelets using response surface methodology. LWT-Food Science and Technology. 2021;140:110816. doi: 10.1016/j.lwt.2020.110816. [DOI] [Google Scholar]
- Altemimi A, Lightfoot DA, Kinsel M, Watson DG. Employing response surface methodology for the optimization of ultrasound assisted extraction of lutein and β-carotene from spinach. Molecules. 2015;20:6611–6625. doi: 10.3390/molecules20046611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin R, Ahmed D, Aydar AY, Qamar MT. Modelling of polyphenol and flavonoid extraction from bottle gourd fruit using green and cost effective LTTM glycerol-ammonium acetate in neat and diluted forms. Journal of Food Measurement and Characterization. 2022;16:3372–3384. doi: 10.1007/s11694-022-01445-8. [DOI] [Google Scholar]
- Aydar AY, Aydın T, Yılmaz T, Kothakota A, Terezia SC, Leontin CF, Pandiselvam R. Investigation on the influence of ultrasonic pretreatment on color, quality and antioxidant attributes of microwave dried Inula viscosa (L.) Ultrasonics Sonochemistry. 2022;90:106184. doi: 10.1016/j.ultsonch.2022.106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi Mahalleh A, Sharayei P, Azarpazhooh E. Optimization of ultrasonic-assisted extraction of bioactive compounds from Nepeta (Nepeta binaludensis Jamzad) Journal of Food Measurement and Characterization. 2020;14:668–678. doi: 10.1007/s11694-019-00314-1. [DOI] [Google Scholar]
- Bakht MA, Geesi MH, Riadi Y, Imran M, Ali MI, Ahsan MJ, Ajmal N. Ultrasound-assisted extraction of some branded tea: optimization based on polyphenol content, antioxidant potential and thermodynamic study. Saudi Journal of Biological Sciences. 2019;26:1043–1052. doi: 10.1016/j.sjbs.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi S, Lupatini-Menegotto AL, Kalschne DL, Moraes Flores ÉL, Bittencourt PRS, Colla E, Canan C. Ultrasound: a suitable technology to improve the extraction and techno-functional properties of vegetable food proteins. Plant Foods for Human Nutrition. 2021;76:1–11. doi: 10.1007/s11130-021-00884-w. [DOI] [PubMed] [Google Scholar]
- Bhuyan P. Extraction of Neem (Azadirachta indica) oil using blends of hexane, ethyl acetate and acetone by sonication. International Journal of Advance Research, Idras and Innovations in Technology. 2019;5:78–84. [Google Scholar]
- Bhuyan DJ, Vuong QV, Chalmers AC, Van Altena IA, Bowyer MC, Scarlett CJ. Phytochemical, antibacterial and antifungal properties of an aqueous extract of Eucalyptus microcorys leaves. South African Journal of Botany. 2017;112:180–185. doi: 10.1016/j.sajb.2017.05.030. [DOI] [Google Scholar]
- Chanioti S, Tzia C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT - Food Science and Technology. 2018;79:178–189. doi: 10.1016/j.lwt.2017.01.029. [DOI] [Google Scholar]
- Chatterjee T, Ghosh B. Micropropagation of medicinal plants: a review. International Journal of Economic Plants. 2020;7:066–072. doi: 10.23910/2/2020.0368. [DOI] [Google Scholar]
- Chemat F, Vian MA, Cravotto G. Green extraction of natural products: concept and principles. International Journal of Molecular Sciences. 2012;13:8615–8627. doi: 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemat F, Rombaut N, Meullemiestre A, Turk M, Perino S, Abert-vian M. Review of green food processing techniques. Preservation, transformation, and extraction. Innovative Food Science and Emerging Technologies. 2017;41:357–377. doi: 10.1016/j.ifset.2017.04.016. [DOI] [Google Scholar]
- Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols, and applications: a review. Ultrasonics Sonochemistry. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Čižmek L, Bavcon Kralj M, Čož-Rakovac R, Mazur D, Ul’yanovskii N, Likon M, Trebše P. Supercritical carbon dioxide extraction of four medicinal mediterranean plants: Investigation of chemical composition and antioxidant activity. Molecules. 2021;26(18):5697. doi: 10.3390/molecules26185697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dary C, Baghdikian B, Kim S, Mabrouki F, Hul S, Jabbour F, Bun SS. Optimization of ultrasound-assisted extraction of bioactive alkaloids from Stephania cambodica using response surface methodology. Comptes Rendus Chimie. 2017;20(11–12):996–1005. doi: 10.1016/j.crci.2017.09.004. [DOI] [Google Scholar]
- Dash DR, Pathak SS, Pradhan RC. Improvement in novel ultrasound-assisted extraction technology of high value-added components from fruit and vegetable peels. Journal of Food Process Engineering. 2021;44:e13658. doi: 10.1111/jfpe.13658. [DOI] [Google Scholar]
- Dassoff ES, Li YO. Mechanisms and effects of ultrasound-assisted supercritical CO2 extraction. Trends in Food Science & Technology. 2019;86:492–501. doi: 10.1016/j.tifs.2019.03.001. [DOI] [Google Scholar]
- Deng Y, Wang W, Zhao S, Yang X, Xu W, Guo M, Liu D. Ultrasound-assisted extraction of lipids as food components: mechanism, solvent, feedstock, quality evaluation and coupled technologies–a review. Trends in Food Science & Technology. 2022 doi: 10.1016/j.tifs.2022.01.034. [DOI] [Google Scholar]
- Dhanani T, Shah S, Gajbhiye NA, Kumar S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arabian Journal of Chemistry. 2017;10:S1193–S1199. doi: 10.1016/j.arabjc.2013.02.015. [DOI] [Google Scholar]
- Dias JL, Mazzutti S, de Souza JA, Ferreira SR, Soares LA, Stragevitch L, Danielski L. Extraction of umbu (Spondias tuberosa) seed oil using CO2, ultrasound and conventional methods: evaluations of composition profiles and antioxidant activities. The Journal of Supercritical Fluids. 2019;145:10–18. doi: 10.1016/j.supflu.2018.11.011. [DOI] [Google Scholar]
- Dias ALB, de Aguiar AC, Rostagno MA. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: current status and trends. Ultrasonics Sonochemistry. 2021;74:105584. doi: 10.1016/j.ultsonch.2021.105584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do DT, Lam DH, Nguyen T, Phuong Mai TT, Phan LTM, Vuong HT, Nguyen DV, Linh NTT, Hoang MN, Mai TP, Nguyen HH. Utilization of response surface methodology in optimization of polysaccharides extraction from Vietnamese red Ganoderma lucidum by ultrasound-assisted enzymatic method and examination of bioactivities of the extract. Scientific World Journal. 2021;2021:1–14. doi: 10.1155/2021/7594092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Delacour C, Carogher KM, Udepurkar AP, Kuhn S. Continuous ultrasonic reactors: Design, mechanism and application. Materials. 2020;13:344–358. doi: 10.3390/ma13020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzah CS, Duan Y, Zhang H, Wen C, Zhang J, Chen G, Ma H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: a review. Food Bioscience. 2020;35:100547. doi: 10.1016/j.fbio.2020.100547. [DOI] [Google Scholar]
- Fernando CD, Soysa P. Extraction Kinetics of phytochemicals and antioxidant activity during black tea (Camellia sinensis L.) brewing. Nutrition Journal. 2015;14(1):1–7. doi: 10.1186/s12937-015-0060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavahian M, Manyatsi TS, Morata A, Tiwari BK. Ultrasound-assisted production of alcoholic beverages: from fermentation and sterilization to extraction and aging. Comprehensive Reviews in Food Science and Food Safety. 2022;21:5243–5271. doi: 10.1111/1541-4337.13043. [DOI] [PubMed] [Google Scholar]
- Guo X, Shang X, Zhou X, Zhao B, Zhang J. Ultrasound-assisted extraction of polysaccharides from Rhododendron aganniphum: antioxidant activity and rheological properties. Ultrasonics Sonochemistry. 2017;38:246–255. doi: 10.1016/j.ultsonch.2017.03.021. [DOI] [PubMed] [Google Scholar]
- Hadidi M, Ibarz A, Pagan J. Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chemistry. 2020;309:125786. doi: 10.1016/j.foodchem.2019.125394. [DOI] [PubMed] [Google Scholar]
- He W, Yin M, Yang R, Zhao W. Optimization of adlay (Coix lacryma-jobi) bran oil extraction: Variability in fatty acids profile and fatty acid synthase inhibitory activities. Biocatalysis and Agricultural Biotechnology. 28: 101740 (2020) 10.1016/j.bcab.2020.101740
- He Q, Lei Q, Huang S, Zhou Y, Liu Y, Zhou S, Qiu H. Effective extraction of bioactive alkaloids from the roots of Stephania tetrandra by deep eutectic solvents-based ultrasound-assisted extraction. Journal of Chromatography A. 1689: 463746 (2023) [DOI] [PubMed]
- Hojnik M, Škerget M, Knez Ž. Extraction of lutein from marigold flower petals–experimental kinetics and modelling. LWT-Food Science and Technology. 2008 doi: 10.1016/j.lwt.2007.11.017. [DOI] [Google Scholar]
- Hsieh YH, Li Y, Pan Z, Chen Z, Lu J, Yuan J, Zhang J. Ultrasonication-assisted synthesis of alcohol-based deep eutectic solvents for extraction of active compounds from ginger. Ultrasonics Sonochemistry. 2020;63:104915. doi: 10.1016/j.ultsonch.2019.104915. [DOI] [PubMed] [Google Scholar]
- Islam M, Saini P, Das R, Shekhar S, Sinha AS, Kumar M, Kamlesh P. Rice straw as a source of nanocellulose for sustainable food packaging materials: a review. Bipresources. 2023 doi: 10.15376/biores.18.1.Islam. [DOI] [Google Scholar]
- Islam M, Malakar S, Dwivedi U, Kumar N, Prabakar PK, Kishore A, Kumar A. Impact of different drying techniques on grapefruit peels and subsequent optimization of ultrasonic extraction conditions for bioactive compounds. Journal of Food Process Engineering. 2023 doi: 10.1111/jfpe.14331. [DOI] [Google Scholar]
- Jacotet-navarro M, Rombaut N, Fabiano-tixier A, Danguien M, Bily A, Chemat F. Ultrasound versus microwave as green processes for extraction of rosemarinic, carnosic and ursolic acids from rosemary. Ultrasonics Sonochemistry. 2015;27:102–109. doi: 10.1016/j.ultsonch.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Jamshaid S, Ahmed D. Optimization of ultrasound-assisted extraction of valuable compounds from fruit of Melia azedarach with glycerol-choline chloride deep eutectic solvent. Sustainable Chemistry and Pharmacy. 2022;29:100827. doi: 10.1016/j.scp.2022.100827. [DOI] [Google Scholar]
- Jamshaid S, Ahmed D, Aydar AY. Ultrasound-assisted extraction optimization of polyphenols, flavonoids, and antioxidant compounds from fruit of Melia azedarach using a glycerol-based green deep eutectic solvent. Journal of Food Processing and Preservation. 2022;46:e16657. doi: 10.1111/jfpp.16657. [DOI] [Google Scholar]
- Jiang Y, Ning Z, Li S. Extraction and purification of isochlorogenic acid C from Chrysanthemum morifolium using ionic liquid-based ultrasound-assisted extraction and aqueous two-phase system. Food Science & Nutrition. 2018;6:2113–2122. doi: 10.1002/fsn3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Liu Z, Liu D, Shi G, Liu D, Yang Y, Gu H. Industrial Crops & Products Natural antioxidant of rosemary extract used as an additive in the ultrasound-assisted extraction of anthocyanins from lingonberry (Vaccinium vitis-idaea L.) pomace. Industrial Crops & Products. 2019;138:111425. doi: 10.1016/j.indcrop.2019.05.074. [DOI] [Google Scholar]
- Jiskani AH, Aydar AY, Ahmed D. Optimization of ultrasound-assisted extraction of antioxidant compounds from Rumex hastatus with response surface methodology. Journal of Food Processing and Preservation. 2021;45(11):e15983. doi: 10.1111/jfpp.15983. [DOI] [Google Scholar]
- Jovanović AA, Đorđević VB, Zdunić GM, Pljevljakušić DS, Šavikin KP, Gođevac DM, Bugarski BM. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Separation and Purification Technology. 2017;179:369–380. doi: 10.1016/j.seppur.2017.01.055. [DOI] [Google Scholar]
- Kate AE, Singh A, Shahi NC, Pandey JP, Singh TP, Prakash O. Impact of polar bio-solvent, particle size and soaking time on microwave-assisted extraction of edible oil from black soybean. Journal of Food Measurement and Characterization. 2018;11(1):272–280. doi: 10.1007/s11694-016-9394-0. [DOI] [Google Scholar]
- Khemakhem I, Ahmad-Qasem MH, Catalán EB, Micol V, García-Pérez JV, Ayadi MA, Bouaziz M. Kinetic improvement of olive leaves’ bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrasonics Sonochemistry. 2018;34:466–473. doi: 10.1016/j.ultsonch.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Koubaa M, Mhemdi H, Fages J. Recovery of valuable components and inactivating microorganisms in the agro-food industry with ultrasound-assisted supercritical fluid technology. The Journal of Supercritical Fluids. 2018;134:71–79. doi: 10.1016/j.supflu.2017.12.012. [DOI] [Google Scholar]
- Kumar K, Srivastav S, Sharanagat VS. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrasonics Sonochemistry. 2021;70:105325. doi: 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Upadhyay S, Yadav DK, Malakar S, Dhurve P, Suri S. Application of ultrasound technology for extraction of color pigments from plant sources and their potential bio-functional properties: a review. Journal of Food Process Engineering. 2022 doi: 10.1111/jfpe.14238. [DOI] [Google Scholar]
- Kumar N, Kumar G, Prabhakar PK, Sahu JK, Naik S. Ultrasound-assisted extraction of bioactive compounds from giloy (Tinospora cordifolia) stem: quantitative process optimization and bioactives analysis. Journal of Food Process Engineering. 2023 doi: 10.1111/jfpe.14259. [DOI] [Google Scholar]
- Kunene P, Mahlambi P. Assessment of antiretroviral drugs in vegetables: evaluation of microwave-assisted extraction performance with and without solid-phase extraction cleanup. Separation Science plus. 2023;6:2200059. doi: 10.1002/sscp.202200059. [DOI] [Google Scholar]
- Li J, Chen Z, Shi H, Yu J, Huang G, Huang H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrasonics Sonochemistry. 2023 doi: 10.1016/j.ultsonch.2023.106295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Guo Z, Yu G. Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrasonics Sonochemistry. 2021;76:105661. doi: 10.1016/j.ultsonch.2021.105661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ou H, Xiang Z, Gregersen H. Ultrasound pretreatment combined with supercritical CO2 extraction of Iberis amara seed oil. Journal of Applied Research on Medicinal and Aromatic Plants. 2020;18:100265. doi: 10.1016/j.jarmap.2020.100265. [DOI] [Google Scholar]
- Liu X, Ou H, Gregersen H. Ultrasound-assisted supercritical CO2 extraction of cucurbitacin E from Iberis amara seeds. Industrial Crops and Products. 2020;145:112093. doi: 10.1016/j.indcrop.2020.112093. [DOI] [Google Scholar]
- Lorenzo JM, Khaneghah AM, Gavahian M, Eş I, Munekata PES, Ferreira ICFR, Barba FJ, Lorenzo JM, Khaneghah AM, Gavahian M, Eş I, Munekata PES, Ferreira ICFR, Barba FJ. Understanding the potential benefits of thyme and its derived products for food industry and consumer health : from extraction of value-added compounds to the evaluation of and antimicrobial activities. Critical Reviews in Food Science and Nutrition. 2019;59(18):2879–2895. doi: 10.1080/10408398.2018.1477730. [DOI] [PubMed] [Google Scholar]
- Low ZL, Low DYS, Tang SY, Manickam S, Tan KW, Ban ZH. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrasonics Sonochemistry. 2022;90:106176. doi: 10.1016/j.ultsonch.2022.106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakar S, Arora VK, Munshi M, Yadav DK, Pou KR, Deb S, Chandra R. Application of novel pretreatment technologies for intensification of drying performance and quality attributes of food commodities: a review. Food Science and Biotechnology. (2023) 10.1007/s10068-023-01322-0 [DOI] [PMC free article] [PubMed]
- Malakar S, Dhurve P, Arora VK. Modeling and optimization of osmo-sonicated dehydration of garlic slices in a novel infrared dryer using artificial neural network and response surface methodology. Journal of Food Process Engineering. (2022) 10.1111/jfpe.14261
- Martínez JM, Delso C, Aguilar DE, Álvarez I, Raso J. Organic-solvent-free extraction of carotenoids from yeast Rhodotorula glutinis by application of ultrasound under pressure. Ultrasonics Sonochemistry. 2020;61:104833. doi: 10.1016/j.ultsonch.2019.104833. [DOI] [PubMed] [Google Scholar]
- Mikheev IV, Pirogova MO, Usoltseva LO, Uzhel AS, Bolotnik TA, Kareev IE, Proskurnin MA. Green and rapid preparation of long-term stable aqueous dispersions of fullerenes and endohedral fullerenes: the pros and cons of an ultrasonic probe. Ultrasonics Sonochemistry. 2021;73:105533. doi: 10.1016/j.ultsonch.2021.105533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour H, Sadrameli SM, Eslami F, Asoodeh A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Industrial Crops and Products. 2019;131:106–116. doi: 10.1016/j.indcrop.2019.01.030. [DOI] [Google Scholar]
- Munekata PES, Alcántara C, Žugčić T, Abdelkebir R, Carmen M, García-pérez JV, Režek A, Gavahian M, Barba FJ, Lorenzo JM. Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Food Research International. 2020;134:109242. doi: 10.1016/j.foodres.2020.109242. [DOI] [PubMed] [Google Scholar]
- Nabet N, Gilbert-López B, Madani K, Herrero M, Ibáñez E, Mendiola JA. Optimization of microwave-assisted extraction recovery of bioactive compounds from Origanum glandulosum and Thymus fontanesii. Industrial Crops and Products. 2019;129:395–404. doi: 10.1016/j.indcrop.2018.12.032. [DOI] [Google Scholar]
- Nguyen VT, Tran NTH, Tran TG. Central composite experimental design for ultrasound-assisted extraction optimization of alkaloid compounds and antioxidant properties from cocoa pod husk (Theobroma cacao L.) Journal of Food Processing and Preservation. 2022;46(11):e17084. doi: 10.1111/jfpp.17084. [DOI] [Google Scholar]
- Nieto G, Ros G, Castillo J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis L.): a review Gema. Medicines. 2018 doi: 10.3390/medicines5030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishad J, Saha S, Kaur C. Enzyme- and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: a comparative study. Journal of Food Processing and Preservation. 2019;43:1–13. doi: 10.1111/jfpp.14046. [DOI] [Google Scholar]
- Palma A, Díaz MJ, Ruiz-Montoya M, Morales E, Giráldez I. Ultrasound extraction optimization for bioactive molecules from Eucalyptus globulus leaves through antioxidant activity. Ultrasonics Sonochemistry. 2021;76:105654. doi: 10.1016/j.ultsonch.2021.105654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiselvam R, Aydar AY, Kutlu N, Aslam R, Sahni P, Mitharwal S, Kothakota A. Individual and interactive effect of ultrasound pre-treatment on drying kinetics and biochemical qualities of food: a critical review. Ultrasonics Sonochemistry. 2022;92:106261. doi: 10.1016/j.ultsonch.2022.106261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proestos C. The benefits of plant extracts for human health. Foods. 2020;9(11):1653. doi: 10.3390/foods9111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheem M, Ahmed D, Aydar AY. The optimization of curcumin extraction from turmeric with modern techniques using response surface methodology. Latin American Applied Research. 2023;53(2):95–102. doi: 10.52292/j.laar.2023.961. [DOI] [Google Scholar]
- Rahman MM, Byanju B, Grewell D, Lamsal BP. High-power sonication of soy proteins: hydroxyl radicals and their effects on protein structure. Ultrasonics Sonochemistry. 2020;64:105019. doi: 10.1016/j.ultsonch.2020.105019. [DOI] [PubMed] [Google Scholar]
- Rao MV, Sengar AS, Sunil CK, Rawson A. Ultrasonication-a green technology extraction technique for spices: a review. Trends in Food Science & Technology. 2021;116:975–991. doi: 10.1016/j.tifs.2021.09.006. [DOI] [Google Scholar]
- Rao MV, Akhil KG, Sunil CK, Venkatachalapathy N, Jaganmohan R. Effect of microwave treatment on physical and functional properties of foxtail millet flour. Int. J. Chem. Stud. 2021;9(1):2762–2767. doi: 10.22271/chemi.2021.v9.i1am.11641. [DOI] [Google Scholar]
- Raza A, Li F, Xu X, Tang J. Optimization of Ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. International Journal of Biological Macromolecules. 2017;94:335–344. doi: 10.1016/j.ijbiomac.2016.10.033. [DOI] [PubMed] [Google Scholar]
- Riaz U, Iqbal S, Sohail MI, Samreen T, Ashraf M, Akmal F, Siddiqui A, Ahmad I, Naveed M, Khan NI, Akhter RM. A comprehensive review on emerging importance and economical potential of medicinal and aromatic plants (MAPs) in current scenario. Pakistan Journal of Agricultural Research. 2021;34:68–73. doi: 10.17582/journal.pjar/2021/34.2.381.392. [DOI] [Google Scholar]
- Riera E, Golás Y, Blanco A, Gallego JA, Blasco M, Mulet A. Mass transfer enhancement in supercritical fluids extraction by means of power ultrasound. Ultrasonics Sonochemistry. 2004;11:241–244. doi: 10.1016/j.ultsonch.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Riera Enrique, Blanco A, García J, Benedito J, Mulet A, Gallego-Juárez JA, Blasco M. High-power ultrasonic system for the enhancement of mass transfer in supercritical CO2 extraction processes. Physics Procedia. 2010;3(1):141–146. doi: 10.1016/j.phpro.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Fernandes FAN, Sousa E, Brito D, Dutra A, Narain N. Ultrasound extraction of phenolics and anthocyanins from jabuticaba peel. Industrial Crops & Products. 2015;69:400–407. doi: 10.1016/j.indcrop.2015.02.059. [DOI] [Google Scholar]
- Saifullah M, McCullum R, McCluskey A, Vuong Q. Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon. 2020;6:e03666. doi: 10.1016/j.heliyon.2020.e03666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MP, Souza MC, Sumere BR, da Silva LC, Cunha DT, Bezerra RMN, Rostagno MA. Extraction of bioactive compounds from pomegranate peel (Punica granatum L.) with pressurized liquids assisted by ultrasound combined with an expansion gas. Ultrasonics Sonochemistry. 2019;54:11–17. doi: 10.1016/j.ultsonch.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Santos L, Janet Z, Uribe AG, Benedito J. Effect of solvent composition on ultrasound - generated intensity and its influence on the ultrasonically assisted extraction of bioactives from agave bagasse (Agave salmiana) Food Engineering Reviews. 2021;13:713–725. doi: 10.1007/s12393-020-09260-x. [DOI] [Google Scholar]
- Senrayan J, Venkatachalam S. Optimization of ultrasound-assisted solvent extraction (UASE) based on oil yield, antioxidant activity and evaluation of fatty acid composition and thermal stability of Coriandrum sativum L. seed oil. Food Science and Biotechnology. 2019;28:377–386. doi: 10.1007/s10068-018-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senrayan J, Venkatachalam S. Ultrasonic acoustic-cavitation as a novel and emerging energy efficient technique for oil extraction from kapok seeds. Innovative Food Science & Emerging Technologies. 2020;62:102347. doi: 10.1016/j.ifset.2020.102347. [DOI] [Google Scholar]
- Singla M, Sit N. Application of ultrasound in combination with other technologies in food processing: a review. Ultrasonics Sonochemistry. 2021;73:105506. doi: 10.1016/j.ultsonch.2021.105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumere BR, de Souza MC, Dos Santos MP, Bezerra RMN, da Cunha DT, Martinez J, Rostagno MA. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.) Ultrasonics Sonochemistry. 2018;48:151–162. doi: 10.1016/j.ultsonch.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Sun Q, Du B, Wang C, Xu W, Fu Z, Yan Y, Zhang H. Ultrasound-assisted ionic liquid solid-liquid extraction coupled with aqueous two-phase extraction of naphthoquinone pigments in Arnebia euchroma (Royle) Johnst. Chromatographia. 2019;82:1777–1789. doi: 10.1007/s10337-019-03804-y. [DOI] [Google Scholar]
- Susy J, Araújo F. De, Leite E, Souza D, Ribeiro J, Cristina A, Gomes A, Ryan L, Kotzebue V, Lincon D, Lomonaco D, Oliveira V. De, Elaine S, Leandro A, Tejo M. Microencapsulation of sweet orange essential oil (Citrus aurantium var. dulcis ) by liophylization using maltodextrin and maltodextrin/gelatin mixtures : preparation, characterization, antimicrobial. International Journal of Biological Macromolecules. 2019;143:991–999. doi: 10.1016/j.ijbiomac.2019.09.160. [DOI] [PubMed] [Google Scholar]
- Taha A, Mehany T, Pandiselvam R, Anusha Siddiqui S, Mir NA, Malik MA, Hu H. Sonoprocessing: mechanisms and recent applications of power ultrasound in food. Critical Reviews in Food Science and Nutrition. 2022 doi: 10.1080/10408398.2022.2161464. [DOI] [PubMed] [Google Scholar]
- Tamidi AM, Lau KK, Khalit SH. A review of recent development in numerical simulation of ultrasonic-assisted gas-liquid mass transfer process. Computers & Chemical Engineering. 2021;155:107498. doi: 10.1016/j.compchemeng.2021.107498. [DOI] [Google Scholar]
- Tang W, Li D, Lv Y, Jiang J. Extraction and removal of caffeine from green tea by ultrasonic-enhanced supercritical fluid. Journal of Food Science. 2010;75:C363–C368. doi: 10.1111/j.1750-3841.2010.01604.x. [DOI] [PubMed] [Google Scholar]
- Tao Y, Wu P, Dai Y, Luo X, Manickam S, Li D, Show PL. Bridge between mass transfer behavior and properties of bubbles under two-stage ultrasound-assisted physisorption of polyphenols using macroporous resin. Chemical Engineering Journal. 2022;436:135158. doi: 10.1016/j.cej.2022.135158. [DOI] [Google Scholar]
- Thilakarathna RCN, Siow LF, Tang TK, Chan ES, Lee YY. Physicochemical and antioxidative properties of ultrasound-assisted extraction of mahua (Madhuca longifolia) seed oil in comparison with conventional soxhlet and mechanical extractions. Ultrasonics Sonochemistry. 2023;92:106280. doi: 10.1016/j.ultsonch.2022.106280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-valenzuela LS, Ballesteros-gómez A. Green solvents for the extraction of high added-value compounds from agri-food Waste. Food Engineering Reviews. 2020;12(1):83–100. doi: 10.1007/s12393-019-09206-y. [DOI] [Google Scholar]
- Tsaltaki C, Katsouli M, Kekes T, Chanioti S, Tzia C. Comparison study for the recovery of bioactive compounds from Tribulus conventional extraction methods. Industrial Crops & Products. 2019;142:111875. doi: 10.1016/j.indcrop.2019.111875. [DOI] [Google Scholar]
- Tul Kubra K, Ahmed D, Aydar AY, Qamar MT. Ultrasound-and heat-assisted extraction of glycyrrhizin from licorice by two glycerol-based DESs-modeling and optimization as per response surface methodology. Sustainable Chemistry and Pharmacy. 2023;31:100910. doi: 10.1016/j.scp.2022.100910. [DOI] [Google Scholar]
- Vaeli N, Honarvar B, Esfandiari N, Aboosadi ZA. A laboratory study on extracting active ingredients from scrophularia striata boiss using ultrasound-assisted supercritical fluid extraction technique. South African Journal of Chemical Engineering. 2021;35:111–117. doi: 10.1016/j.sajce.2020.09.006. [DOI] [Google Scholar]
- Venkateswara Rao M, CK S, Rawson A, C DV. Modifying the plant proteins techno-functionalities by novel physical processing technologies: a review. Critical Reviews in Food Science and Nutrition. 2021 doi: 10.1080/10408398.2021.1997907. [DOI] [PubMed] [Google Scholar]
- Vernes L, Abert-Vian M, El Maâtaoui M, Tao Y, Bornard I, Chemat F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrasonics Sonochemistry. 2019;54:48–60. doi: 10.1016/j.ultsonch.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Vigano J, de Paula Assis BF, Nathia-Neves G, Dos Santos P, Meireles MAA, Veggi PC, Martinez J. Extraction of bioactive compounds from defatted passion fruit bagasse (Passiflora edulis sp.) applying pressurized liquids assisted by ultrasound. Ultrasonics Sonochemistry. 2020;64:104999. doi: 10.1016/j.ultsonch.2020.104999. [DOI] [PubMed] [Google Scholar]
- Wang H, Tong Y, Li W, Zhang X, Gao X, Yong J, Zhao J, Koike K. Enhanced ultrasound-assisted enzymatic hydrolysis extraction of quinolizidine alkaloids from Sophora alopecuroides L. seeds. Journal of Natural Medicines. 2018;72:424–432. doi: 10.1007/s11418-017-1165-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang X, Ma X, Zhang K, Li S, Wang X, Liu X, Liu J, Fan W, Li Y, Li Q, Zhu X. Study on the kinetic model, thermodynamic and physicochemical properties of Glycyrrhiza polysaccharide by ultrasonic assisted extraction. Ultrasonics Sonochemistry. 2019;51:249–257. doi: 10.1016/j.ultsonch.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Wang QY, Dong X, Yang J, Hu YH, Peng LQ, Zheng H, Cao J. Vesicle based ultrasonic-assisted extraction of saponins in Panax notoginseng. Food Chemistry. 2020;303:125394. doi: 10.1016/j.foodchem.2019.125394. [DOI] [PubMed] [Google Scholar]
- Wang C, Li J, Cao Y, Huang J, Lin H, Zhao T, Li Q. Extraction and characterization of pectic polysaccharides from Choerospondias axillaris peels: comparison of hot water and ultrasound-assisted extraction methods. Food Chemistry. 2023;401:134156. doi: 10.1016/j.foodchem.2022.134156. [DOI] [PubMed] [Google Scholar]
- Wen C, Zhang J, Zhang H, Dzah CS, Zandile M, Duan Y, Luo X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops–a review. Ultrasonics Sonochemistry. 2018;48:538–549. doi: 10.1016/j.ultsonch.2018.07.018. [DOI] [PubMed] [Google Scholar]
- Wu J. Power ultrasound for extraction and modification of polysaccharides from medicinal fungi. In: Shalu V, editor. Advances in food processing technology. Singapore: Springer; 2019. [Google Scholar]
- Yang Y-C, Wei M-C. Ethanol solution-modified supercritical carbon dioxide extraction of triterpenic acids from Hedyotis corymbosa with ultrasound assistance and determination of their solubilities. Separation and Purification Technology. 2015;150:204–214. doi: 10.1016/j.seppur.2015.01.008. [DOI] [Google Scholar]
- Yang YC, Wang CS, Wei MC. Development and validation of an ultrasound-assisted supercritical carbon-dioxide procedure for the production of essential oils from Perilla frutescens. LWT. 2020;128:109503. doi: 10.1016/j.lwt.2020.109503. [DOI] [Google Scholar]
- Yang Y, Wang C, Wei M. Kinetics and mass transfer considerations for an ultrasound-assisted supercritical CO2 procedure to produce extracts enriched in fl avonoids from Scutellaria barbata. Journal of CO2 Utilization. 2019;32:219–231. doi: 10.1016/j.jcou.2019.04.008. [DOI] [Google Scholar]
- Yun C, Ji X, Chen Y, Zhao Z, Gao Y, Gu L, Wang H. Ultrasound-assisted enzymatic extraction of Scutellaria baicalensis root polysaccharide and its hypoglycemic and immunomodulatory activities. International Journal of Biological Macromolecules. 2023;227:134–145. doi: 10.1016/j.ijbiomac.2022.12.115. [DOI] [PubMed] [Google Scholar]
- Yusoff IM, Taher ZM, Rahmat Z, Chua LS. A review of ultrasound-assisted extraction for plant bioactive compounds: phenolics, flavonoids, thymols, saponins and proteins. Food Research International. 2022 doi: 10.1016/j.foodres.2022.111268. [DOI] [PubMed] [Google Scholar]
- Zabot GL, Viganó J, Silva EK. Low-frequency ultrasound coupled with high-pressure technologies: impact of hybridized techniques on the recovery of phytochemical compounds. Molecules. 2021;26(17):5117. doi: 10.3390/molecules26175117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahra W, Rai SN, Birla H, Singh SS, Rathore AS, Dilnashin H, Singh SP. Economic importance of medicinal plants in Asian countries. Bioeconomy for Sustainable Development. 2020 doi: 10.1007/978-981-13-9431-7_19. [DOI] [Google Scholar]
- Zhang Z, Wang L, Li D, Jiao S, Dong X, Mao Z. Ultrasound-assisted extraction of oil from flaxseed. Separation and Purification Technology. 2008;62:192–198. doi: 10.1016/j.seppur.2008.01.014. [DOI] [Google Scholar]
- Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medicine (united Kingdom) 2018;13(1):1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L-J, Xia B-H, Lin L-M, Xiong S-H, Tang J, Liao D-F. Optimization of ultrasound-assisted enzymatic polysaccharide extraction from turpiniae folium based on response surface methodology. Digital Chinese Medicine. 2018;1:239–246. doi: 10.1016/s2589-3777(19)30031-x. [DOI] [Google Scholar]