Abstract
The O/W emulsions were prepared using perilla seed oil (PSO) dispersed in soy sauce (PSE) and in distilled water (PWE), respectively. Octenyl succinic anhydride-modified starch (OSA starch, 3 wt%) showed the most efficient emulsifying ability and its stabilities of emulsion and oxidation in PSE and PWE were studied at different storage periods (0, 4, and 8 weeks) and temperatures (4, 25, and 40 °C). Negligible change in droplet diameter of PSE was observed without coalescence or flocculation during storing for 8 weeks at 4 °C. The stabilizing ability of OSA-starch despite the high ionic strength of soy sauce is attributed to the starch backbone, which promotes steric repulsions between droplets. A lower oxidation degree was observed for PSE prepared than PWE and PSO under all storage conditions. Thus, the O/W emulsion prepared from PSO and soy sauce can be applied to the production of ω-3 fatty acid-enriched Asian-style emulsified products.
Keywords: Perilla seed oil, Octenyl succinic anhydride-modified starch, Oil-in-water emulsion, Oxidation stability, Soy sauce
Introduction
Perilla is an Asiatic crop widely cultivated in India, China, Korea, Japan, and Southeast Asia (Nitta et al., 2003). Var. crispa and var. frutescens are two distinct varieties of perilla that are cross-fertile (Nitta et al., 2003; Honda et al., 1990). The var. crispa variety is recognized as a Chinese medicinal herb and is mainly cultivated in southern China and Japan, whereas the var. frutescens variety, an oilseed crop, is widely cultivated in other East Asian regions (Duke and Ayensu, 1985). In Korea, var. frutescens is the major variety of Perilla. In addition, its seeds are used for producing dietary oil that adds flavors to traditional foods in Korean cuisine. Perilla seed oil (PSO) contains a significant amount of α-linolenic acid (ALA), a well-known healthy omega-3 unsaturated fatty acid (Shin et al., 2016; Wang et al., 2014). In the metabolic pathway of omega-3 fatty acids, ALA might be converted into eicosapentaenoic acid and docosahexaenoic acid (Anderson and Ma, 2009; Visentainer et al., 2005), which reduce the risk of coronary heart diseases (Visentainer, 2005), mental disorders (Lucas et al., 2011), and cancer (Bougnoux, 1999). However, the ALA is susceptible to oxidation and produces low molecular weight off-flavor compounds that impair the nutritional quality and nutritional aspects (Wang et al., 2014; Shim and Lee, 2011; McClements and Decker, 2000). Thus, the short shelf-life of PSO limits its use in the food processing industry.
Soy sauce originated in China and became popular across East and Southeast Asia. Soy sauce has a rich umami flavor and is widely used as a liquid condiment for cooking and dipping in Asian cuisine. It is traditionally prepared from soybean paste, wheat, and brine and fermented by Aspergillus oryzae or Aspergillus sojae molds (Gao et al., 2011; Chou and Ling, 1998; Lee et al., 2015).
The core sensory effects of all soy sauce varieties are due to free amino acids, water soluble peptides, sodium, sugar, and Maillard reaction products. However, nowadays, soy sauce has a large variety of tastes, consistencies, flavors, and saltiness depending on the region and culture. In Korea, soy sauce has a salt content of 16–35% (Lee et al., 2015). The saltiness of sodium chloride (NaCl) dominates the taste of soy sauce, followed by the umami taste due to free amino acids and water soluble peptides and the sweetness of hydrolyzed sugar from starch. Previous studies have reported that the colored fraction of soy sauce contains pigments such as melanoidins, which are Maillard reaction products and show high antioxidant activity (Lee et al., 2015; Wang et al., 2007). However, the influence of the antioxidant activity of soy sauce in the oil-in-water (O/W) emulsions has not been studied previously.
An emulsion is a dispersion of two or more immiscible liquids stabilized by suitable emulsifiers (i.e., surfactants). Emulsification is commonly employed in the food industry to prepare emulsified products such as sauces and beverages, which involve blending two immiscible substances. Recently, the food industry has shown an interest in the different variations of ALA-enriched emulsions (Liu et al., 2018a; Sharif et al., 2017; Manshadi et al., 2019). PSO and soy sauce have great potential in producing traditional emulsified sauces in East Asia because of their sensorial and ALA-enriched nutritional properties. However, the susceptibility of fatty acid composition to oxidation, particularly ALA in PSO (Shin et al., 2016), and the high ionic strength due to the high concentration of NaCl in soy sauce destabilizes the emulsion (Liu et al., 2018a). Reducing the emulsion droplet size increases the stability of the emulsion; however, this also increases the droplet surface area and rate of oxidation, due to increased interaction between the oil phase and the continuous phase (Gallego et al., 2013). Thus, the nutritional properties of PSO are significantly impaired.
In this study, the stabilities of emulsions prepared from soy sauce and PSO were investigated based on emulsifier type and concentration ratio. The emulsifying abilities of glyceryl monolaurate (MAG), sucrose fatty acid ester (F160), polyoxyethylene sorbitan monolaurate (Tween-20), and octenyl succinic anhydride-modified starch (OSA-starch) were compared with respect to the high salinity of the continuous phase. Subsequently, stability towards oxidation was compared using peroxide value (POV), headspace-gas chromatography/mass spectrometry analysis (HS-GC/MS), and proton nuclear magnetic resonance (1H-NMR) spectroscopy. Oxidation stability was monitored for 8 weeks of storage at different temperatures (4 °C, 25 °C, and 40 °C).
Materials and methods
Materials
Soy sauce (Sampyo Food Co., Seoul, Korea) and PSO (Sajo Haepyo Corp., Seoul, Korea) were purchased from a local market. The emulsifiers were obtained as follows: octenyl succinic anhydride-modified starch (OSA-starch) from Daesang Corp. (Seoul, Korea), polyoxyethylene sorbitan monolaurate (Tween-20) from Sigma-Aldrich Korea Co. (Seoul, Korea), sucrose fatty acid ester (F160), and glyceryl monolaurate (MAG) from Ilshinwells Co. (Seoul, Korea). All other chemicals used in this study were of analytical grade. Secondary distilled water was used in this study.
Preparation of emulsions
In this study, PSO was dispersed in soy sauce and distilled water to form 10 wt% O/W emulsions; PSE (PSO + soy sauce) and PWE (PSO + distilled water), respectively. Tween-20 (0.4 wt%), OSA-starch (1, 2, and 3 wt%), and a mixture of F160: MAG (0.1:0.3 wt% and 0.2:0.6 wt%) were used to prepare the emulsions (Fig. 1). The OSA-starch was entirely dispersed in the continuous phase at 60 °C for 15 min. Tween-20 and F160 were dispersed at 35 °C, while MAG was mixed with PSO at 35 °C and held overnight. The continuous phase containing the emulsifier was mixed in a 90:10 (w/w) ratio with PSO before being homogenized in an ultrasonic processor (VC750, Sonic & Material Inc., US) for 3 min to obtain the final emulsion.
Fig. 1.
Effect of emulsifier type and ratio (wt%) on stability after 48 h of storage at room temperature. A Tween-20 0.4 wt%; B MAG:F160 = 0.3:0.1 wt%; C MAG:F160 = 0.6:0.2 wt%; D OSA-starch 1 wt%; E OSA-starch 2 wt%; (F) OSA-starch 3 wt%. A oil-off; B–E creaming; F stable
Droplet size distribution analysis
Microscopic images of droplets for each emulsion were obtained using an Olympus CX21 microscope (Olympus Optical Co. Ltd., Japan). One sample drop was placed on a coverslip (0.17 mm thickness). The absence of air gap or bubbles between the sample and coverslip was ensured before analysis using a 100 × lens. The mean droplet diameter (MDD) of the emulsion was measured using a laser diffraction particle size analyzer (Master Sizer S, Malvern Instrument, Worcestershire, UK) and MDD was expressed as the volume-weighted mean diameter (d43 = Σnidi4/Σnidi3), where ni is the number of particles with diameter di.
Determination of oxidation stability
Each 45 mL of PSE and PWE prepared using OSA-starch (3 wt%) were capped and stored in a 50 mL vial at 4 °C, 25 °C, and 40 °C in the dark for 8 weeks. Data were collected at the beginning of oxidation, and at 4 and 8 weeks to study the oxidation stability of the emulsion. PSO (4.5 g) was also prepared as described above.
Determination of peroxide value
The primary products of lipid oxidation, hydroperoxides, were measured according to the International Fragrance Association guideline with some modifications (IFRA, 2019). PSO (0.2 g) or 2 g of emulsion (about 0.2 g oil) were transferred into a 250 mL Erlenmeyer flask. A 25 mL mixture of acetic acid: chloroform in a 3:2 ratio (v/v) was added to break the emulsion, followed by 1 mL of saturated KI solution. The solution was mixed in a vortex mixer for 1 min and allowed to stabilize in the dark for 10 min before adding 30 mL of distilled water and 1 mL of 1% (w/v) starch indicator. The solution was titrated with 0.01 N sodium thiosulfate (Na2S2O3) until the blue color disappeared. The POV of the sample was calculated as follows:
where a is the volume of Na2S2O3 used to titrate the samples in mL, b is the volume of Na2S2O3 used for blank titration, 0.01 is the concentration of Na2S2O3 solution, f is the factor of 0.01 N Na2S2O3 solution, and S is the weight in g of the emulsion.
HS-GC/MS analysis
Samples were analyzed using 6890N HS-GC/MS with a DB-WAX column (Agilent Co., Ltd., USA). Five milliliters of each PSE and PWE were transferred into a 20 mL headspace vial sealed with an aluminum cap, and placed into the autosampler chamber. The volatile organic compound profiles from samples were extracted and injected into the GC–MS instrument using an automated static HS sampler (Agilent 7697A, Santa Clara, CA) at the Chungnam National University Chemistry Core Facility. The equilibrated sample was heated at 80 °C for 20 min before being injected by a filling flow of 1 mL/min, and the injection time was 0.5 min. PSO (0.5 g) was also prepared as described above. The oven temperature was programmed as follows: initial temperature of 35 °C for 5 min, a temperature rises of 3 °C/min until 80 °C, temperature rise by 10 °C/min, and a temperature held at 195 °C for 5 min. Analysis was performed using argon as the carrier gas with an electron energy of 70 eV and a frequency of 5.1 scans/s. Propanal and hexanal peaks were identified using NIST MS 2.3 library.
1H-NMR analysis
1H-NMR analysis was performed using a Bruker Avance III 600 spectrometer (Bruker Corporation, Billerica, MA, USA). After extraction of lipids using the Folch solution from PSE, 100 µL of each extracted lipid and PSO was mixed with 500 µL of CDCl3 containing 0.1% tetramethylsilane (TMS) as an internal standard. The mixtures were placed into a 5 mm diameter NMR tube (NORELL, Landisville, PA, USA). The acquisition parameters used were: spectral width of 12,018.23 Hz, 16 scans, and acquisition time of 2.726 s. Chemical shifts (δ) were measured relative to tetramethylsilane (TMS) at δ = 0 ppm.
Statistical analysis
Significant differences in the analytical results were evaluated using the Statistical Analysis System (SAS, version 9.2, SAS Institute Inc., Cary, USA). Duncan’s multiple range tests were conducted to determine the statistical significance with differences at a 95% confidence interval (p < 0.05). Statistical analysis was performed on the samples after storage.
Results and discussion
Effect of different emulsifiers on emulsion stability
The chemical properties of the continuous phase significantly affected the stability of the emulsions. In this study, soy sauce having 18% salinity and pH 4.5 was used as the continuous phase. The isoelectric point of protein-based emulsifiers is near pH 3–5 (whey protein, casein, perilla protein, etc.), at which the protein molecules become electrically neutral. In addition, because of the high concentration of Na+ and Cl− ions (i.e., high ionic strength), which significantly reduces the stability of electrostatically stabilizable emulsions, other ionic e mulsifiers are not applicable to soy-sauce-based emulsions (Liu et al., 2018a). Therefore, nonionic emulsifiers (Tween-20, F160, MAG) and modified starch (OSA-starch) were used to investigate the stability of PSE emulsions.
As shown in Fig. 1, after 1 h of preparation, a cream layer was observed in the PSE prepared using a mixture of MAG and F160 regardless of the concentration ratio. In addition, PSE prepared using Tween-20 showed an oil-off phenomenon after one day of storage. However, PSE with OSA-starch seemed more stable than those with Tween-20 and the mixture of MAG and F160, but only PSE prepared with 3 wt% OSA-starch was stable after 48 h of storage at room temperature.
In previous studies, OSA-starch was reported to show high emulsion stability and even anti-oxidation activity in O/W emulsions, which are rich in omega-3 fatty acids (Sharif et al., 2017). However, its ability to stabilize high-ionic-strength emulsions has not been previously investigated. Further, the main interaction between droplets in OSA-starch based emulsion was reported as steric stabilization rather than electrostatic repulsion (Yan et al., 2019; Liu et al., 2018b). Therefore, the emulsifying capacity of OSA-starch was not much affected by the high ionic strength of the soy sauce compared to that of other emulsifiers. In this present study, it is suggested that OSA-starch may have the ability to develop a thicker layer which prevents aggregation by covering the surface of oil droplets in PSE, promoting steric hindrance between oil droplets while Tween-20, MAG and F160 would not be able to show such similar mechanism. Based on the results of the emulsion stability, PSE and PWE prepared with 3 wt% OSA-starch were selected for further investigation. The emulsions were stored for 8 weeks at 4 °C, 25 °C, and 40 °C, and the emulsion stability and oxidation degree were monitored.
Droplet size distribution
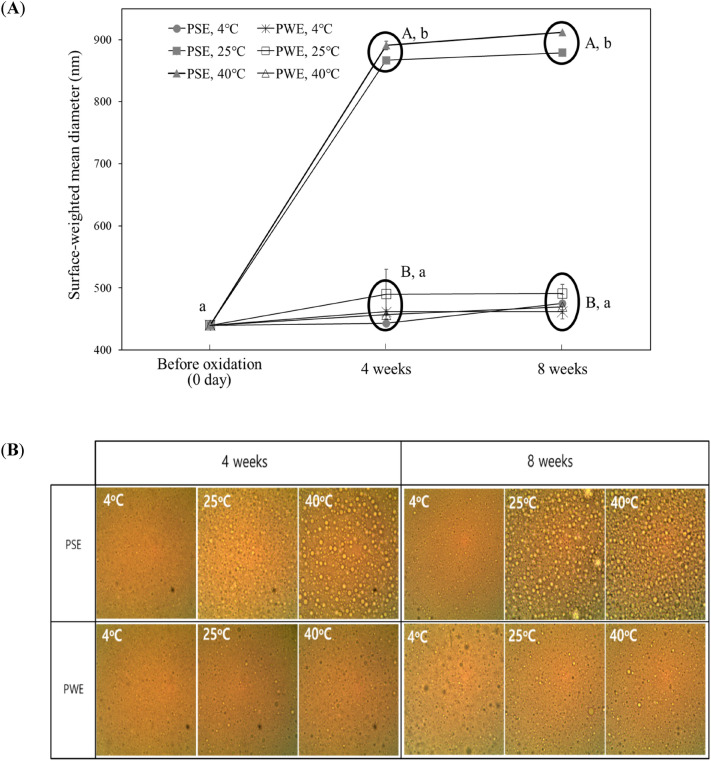
Droplet diameter is a critical parameter for evaluating the stability of an emulsion system. The large size of the droplets readily results in flocculation and coalescence, leading to phase separation. The mean droplet diameter (MDD) in the present study is expressed as the volume-weighted mean diameter (d43), and Fig. 2A shows the effect of temperature and dispersion media on the O/W emulsion during 8 weeks of storage.
Fig. 2.
Mean droplet diameter (A) and microscopic images (B) of PSE and PWE prepared with 3 wt% OSA-starch emulsions stored under different conditions (storage time and temperature). Values with A and B are significantly different (p < 0.05) at the same storage time. Values with a and b are significantly different (p < 0.05) during the 8 weeks of storage time
Initially, the MDD of PWE and PSE were 440 ± 3 and 440 ± 4 nm, respectively, which is a commonly acceptable droplet size for O/W emulsions. No significant change (p > 0.05) in the MDD was observed for all emulsions after storing for 8 weeks at 4 °C, regardless of the continuous phase. However, after 4 weeks, MDD in PSE increased to 867 ± 40.3 nm and 891 ± 5.1 nm at 25 °C and 40 °C, respectively, showing droplet coalescence. Thereafter, the increase in droplet diameters up to 8 weeks at 25 °C and 40 °C were statistically insignificant (p > 0.05). At present, it is not clear why PSE shows a larger droplet size than PWE when the temperature is increased to 25℃ and 40℃. The difference between these two emulsions is the continuous phase in which PSE contains the salts such as NaCl and some organic acids. If then, the free carboxyl group of OSA-starch is probably one of the reasons. Although the main interaction between emulsion droplets in OSA-starch was steric repulsion, free carboxyl groups in the octenyl succinate may result in a negative charge according to the pH of the continuous phase (Lars & Björn, 2007). Hence, in addition to steric hindrance, OSA-modified starches, to some degree, also stabilize droplets by electrostatic repulsive forces. Therefore, the change in electrostatic force and low pH due to the organic acids may lower the interaction between droplets, increasing the droplet size as the temperature rises. From the result, PSE was stable for 8 weeks only at 4 °C. This means that the emulsifying ability of OSA-starch, which is considered to be not much affected by the ionic strength of the aqueous phase, varies depending on the storage temperature. In particular, when the storage temperature is above room temperature, eventually the high ionic strength of soy sauce negatively affects the stability of the O/W emulsion. The result was supported by observing the microscopy image, in which the droplet size of the PSE for 4 and 8 weeks was similar to that initially observed only when PSE was stored at 4 °C (Fig. 2B).
Influence of temperature on oxidation stability of emulsion
Determination of peroxide value
The peroxide value (POV) is widely used for assessing oxidation in fats and oils, providing a degree of rancidity. Figure 3 shows the POV of PSO, PWE, and PSE at different storage temperatures (4 °C, 25 °C, and 40 °C) for 8 weeks. Initially, the POV of PSO, PWE, and PSE were 3.7 ± 0.3, 4.3 ± 0.3, and 5.0 ± 0.0 mEq/kg, respectively. During the storage, it is assumed that oxidation of oil present in the O/W emulsion will occur more rapidly than the oil itself because the large surface area of oil droplets in the emulsion leads to an increase in interaction with oxidative factors (Sharif et al., 2017) such as oxygen.
Fig. 3.
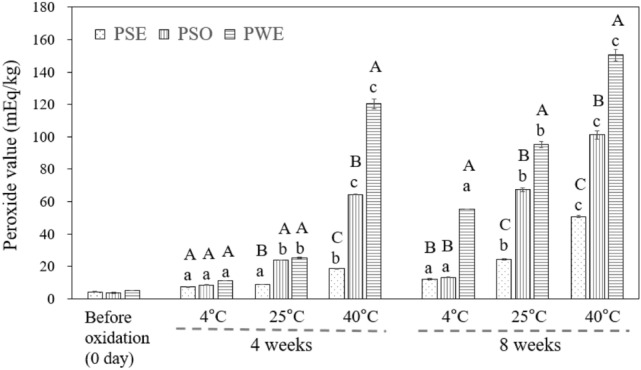
Effect of storage time and temperature on peroxide value of 3 wt% OSA-starch PSE, 3 wt% OSA-starch PWE, and PSO (n = 3). Values with A, B, C are significantly different (p < 0.05) within the same temperature and storage time. Values with a, b, and c are significantly different (p < 0.05) at only the same storage time within the same samples (PSO, PSE, and PWE), regardless of storage temperature
After 4 weeks of storage, the oxidation stability of PSO and PWE at 40ºC was 64.3 ± 0.3 mEq/kg and 120.2 ± 3.3 mEq/kg, respectively (p < 0.05), while that of PSE was the lowest among the samples with the statistical difference (Fig. 3). Furthermore, POV of PWE at 8 weeks was 55.3 ± 0.2, 95.0 ± 2.0, and 150.6 ± 3.5 mEq/kg at 4 °C, 25 °C, and 40 °C (p < 0.05), respectively while those of PSO were 67.1 ± 0.9 and 101.2 ± 2.5 mEq/kg at 25 °C and 40 °C, respectively (p < 0.05). However, POV of PSE was lower than that of PSO and PWE at any storage temperature during the 8 week-storage period, suggesting that PSE showed the highest oxidation stability among the samples (Fig. 3). This phenomenon was particularly evident at 25ºC and 40ºC rather than 4ºC, and at 8 weeks rather than 4 weeks of storage. For 8 weeks of storage, POV of PSE were 12.1 ± 0.3 and 50.7 ± 0.6 mEq/kg at 4 °C and 40 °C, respectively. Soy sauce was reported to show noticeable anti-oxidation activity because of the presence of melanoidins (Lee et al., 2015; Wang et al., 2007; Kobayashi, 2005). Thus, PSE exhibited better oxidation stability, regardless of the surface area of the droplets (Fig. 2A). Meanwhile, the lower oxidation stability of PWE compared to PSO was due to the large surface area of the droplets.
Determination of propanal and hexanal content using HS-GC/MS
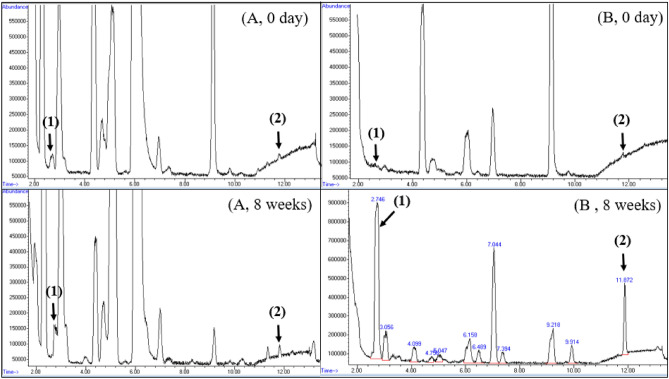
PSO is reported to have a fatty acid composition rich in ALA (ω-3) and linoleic acid (ω-6) (Shin et al., 2016). In GC–MS analysis, the headspace concentration of propanal and hexanal is accepted as an oxidation stability indicator for fatty acids (Sharif et al., 2017). Figure 4 shows the total ion chromatogram (TIC) of PSE and PWE before and after oxidation (at 8 weeks, 40 °C). Propanal and hexanal peaks were identified at 2.7 min and 11.8 min, respectively, and a distinctive increase in their peak areas was observed in PWE rather than in PSE.
Fig. 4.
TIC of HS-GC/MS from (A) PSE and (B) PWE stabilized with 3 wt% OSA-starch emulsion before oxidation (row 1) and after 8 weeks of storage at 40 °C (row 2). (1) Propanal peak; (2) Hexanal peak
Figure 5 shows the propanal (Fig. 5A) and hexanal (Fig. 5B) content during the 8-week storage at different temperatures. Between the 4 and 8 weeks, a noticeable increase in propanal and hexanal was observed in PWE and PSO rather than in PSE, and such increase was distinctive when stored at 25 °C and 40 °C rather than at 4 °C. For example, the propanol peak area of PSE (8.8 × 106) was much lower than that of PWE (78.7 × 106), showing a significant difference after 8-week storage at 40 °C (p < 0.05). At 4 °C, when PSE with high ion concentration showed a stable emulsion state, there was little difference in propanol content between PWE and PSE. When the storage temperature was increased to 40 °C to accelerate the oxidation rate, the propanol content of PSE was significantly lower than that of PWE as well as PSO after 4 and 8 weeks (p < 0.05, Fig. 5A). It can be seen that oxidation was suppressed, which can be complementarily explained with the POV result in Fig. 3.
Fig. 5.
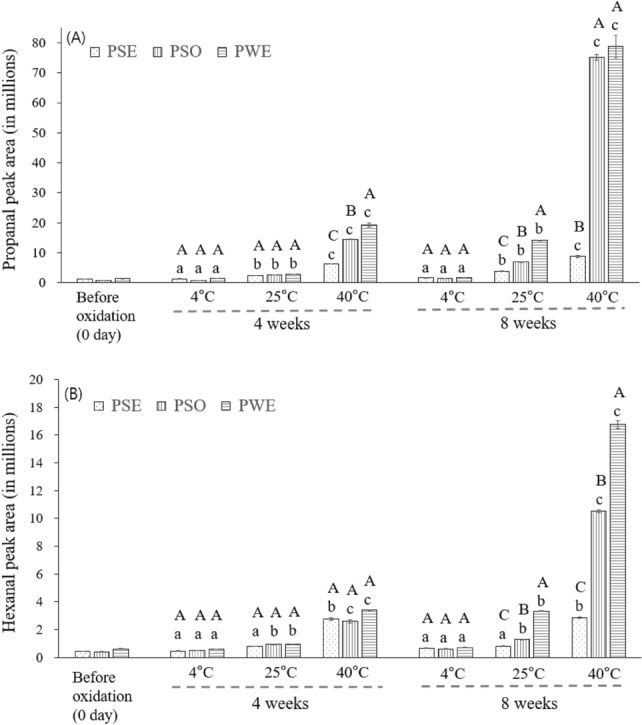
Effect of storage time and temperature on headspace (A) propanal and (B) hexanal content of 3 wt% OSA-starch PSE, 3 wt% OSA-starch PWE, and PSO (n = 3). Values with A, B, C are significantly different (p < 0.05) within the same temperature and storage time. Values with a, b, and c are significantly different (p < 0.05) at only the same storage time within the same samples (PSO, PSE, and PWE), regardless of storage temperature
The hexanal concentration is shown in Fig. 5B, in which the peak area of all samples ranged from 2.6 to 3.4 × 106 after 4 weeks of storage at 40 °C. However, an increase in hexanal content was more pronounced in PWE, showing 16.8 × 106 after 8 weeks while only a minor change occurred in PSE. A high oxidation degree for PWE was due to the increased surface area, which increased the interaction rate between the oil and oxidizing agent in the continuous phase. However, owing to the antioxidant activity of the colored fraction of soy sauce, the oxidation stability observed in PSE was minimized. Overall, the headspace concentration of both propanal and hexanal shows a clear difference in the oxidation degree between PSE and PWE as well as PSO at 25 °C and 40 °C after 8 weeks of storage (p < 0.05), implying that PSE among the samples produced the lowest amount of major aldehydes, which can be used as an indicator of the degree of oxidation, and this was particularly noticeable at high temperature (40 °C) and long storage period (8 weeks).
According to the previous study, different aldehyde species would be expected depending on the fatty acid structures during autoxidation of oil (Shahidi, 2001). Because of the different positions of double bonds in the ALA (ω-3) and linoleic acid (ω-6), ALA would generate 3-hexanal and propanal as well as others (i.e., 2,4-heptadienal and 2,4,7-decatrienal, etc.) from 9-, 12-. 13-, and 16-hydroperoxide while 9- and 13-hydroperoxide would be expected from linoleic acid as major primary oxidation products, mainly generating hexanal and 2,4-decadienal. Therefore, in the process of oxidizing PSO with the highest composition ratio of ALA, which has a higher oxidation rate than linoleic acid, propanal is produced faster than hexanal in both of PWE and PSE (Fig. 5).
Analysis of primary and secondary oxidation product of PSE using 1H-NMR
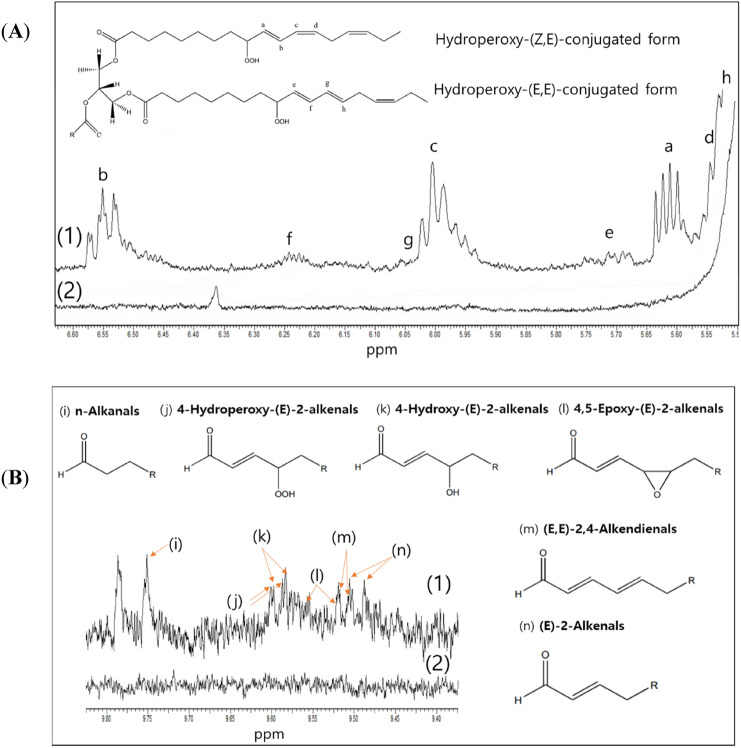
1H-NMR spectrometry represents an alternative tool to conventional methods for evaluating the stability of edible oils (Wang et al., 2014; Shahidi, 2001). The 1H-NMR spectrum of PSE after 8 weeks (stored at 40 °C) was compared with that of PSO under similar storage conditions. As shown in Fig. 6A 1H-NMR signals for primary oxidation products, such as conjugated forms and hydroperoxides were identified according to the previous study (Wang et al., 2014) and observed at 5.45–6.60 ppm. These compounds were quantified as mmol/mol oil (mM oil) by normalizing the peak area of the β-hydrogen atoms in the glycerol backbone to 1. Further, aldehydes were identified as the secondary oxidation products (Almoselhy et al., 2014; Jia et al., 2015) (Fig. 6B). The concentrations of the total conjugated form, total peroxide, and total aldehyde of PSO observed after 8 weeks were 61.8 ± 12.5, 14.5 ± 0.6, and 5.3 ± 0.1 mM oil, respectively. However, none of the above compounds were detected in the PSE sample. The ALA degradation product in PSE was detected in the headspace-GC/MS chromatogram but not in the 1H-NMR spectrum because of the limited sensitivity of the 1H-NMR spectrometer. Nevertheless, the above 1H-NMR results demonstrate the overall oxidation stability of PSE, which is due to the antioxidant activity of soy sauce.
Fig. 6.
1H-NMR spectrum of primary oxidation products (conjugated forms, A) and secondary oxidation products (aldehydes, B) in (1) PSO and (2) PSE after 8 weeks at 40 °C
To overcome the effect of high salt concentration (i.e. high ionic strength) in soy sauce, OSA-starch was used as an emulsifier. OSA-starch promotes steric repulsion between oil droplets and can be used with dispersion media having a high ionic concentration. The change in droplet diameter of PSE was negligible over 8 weeks when stored at 4 °C, exhibiting no coalescence or flocculation. Furthermore, the antioxidant activity of soy sauce and the role of OSA-starch as an oxygen barrier may be beneficial in preventing the oxidation of fatty acid composition in PSO. Headspace-GC/MS and 1H-NMR analysis results show that PSE prepared with 3 wt% OSA-starch can stabilize the emulsion and improve oxidation stability, particularly under refrigeration temperature. Thus, O/W emulsion prepared from PSO and soy sauce in this study has great potential in producing ω-3 fatty acid-enriched Asian-style emulsified products.
Acknowledgements
This work was supported by the research fund of Chungnam National University (2021).
Declarations
Conflict of interest
The authors report no financial or any other conflicts of interest in this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manh-Thang Nguyen, Email: nguyenmanhthang7596@gmail.com.
Jung-Ah Shin, Email: jashin@gwnu.ac.kr.
Ki-Teak Lee, Email: ktlee@cnu.ac.kr.
References
- Almoselhy RIM, Allam MH, El-Kalyoubi MH, El-Sharkawy AA. 1H NMR spectral analysis as a new aspect to evaluate the stability of some edible oils. Annals of Agricultural Sciences. 2014;59:201–206. doi: 10.1016/j.aoas.2014.11.006. [DOI] [Google Scholar]
- Anderson BM, Ma DW. Are all n-3 polyunsaturated fatty acids created equal? Lipids in Health and Disease. 2009;8:1–20. doi: 10.1186/1476-511X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnoux P. n-3 Polyunsaturated fatty acids and cancer. Current Opinion in Clinical Nutrition and Metabolic Care. 1999;2:121–126. doi: 10.1097/00075197-199903000-00005. [DOI] [PubMed] [Google Scholar]
- Chou CC, Ling MY. Biochemical changes in soy sauce prepared with extruded and traditional raw materials. Food Research International. 1998;31:487–492. doi: 10.1016/S0963-9969(99)00017-4. [DOI] [Google Scholar]
- Duke JA, Ayensu ES. Medicinal plants of China. Algonac, MI: Reference Publications Inc.; 1985. [Google Scholar]
- Gallego MG, Gordon MH, Segovia FJ, Skowyra M, Almajano MP. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. Journal of the American Oil Chemists' Society. 2013;90:1559–1568. doi: 10.1007/s11746-013-2303-3. [DOI] [Google Scholar]
- Gao X, Cui C, Ren J, Zhao H, Zhao Q, Zhao M. Changes in the chemical composition of traditional Chinese-type soy sauce at different stages of manufacture and its relation to taste. International Journal of Food Science & Technology. 2011;46:243–249. doi: 10.1111/j.1365-2621.2010.02487.x. [DOI] [Google Scholar]
- Honda G, Koezuka Y, Tabata M. Genetic studies of fruit color and hardness in perilla frutescens. Japanese Journal of Breeding. 1990;40:469–474. [Google Scholar]
- Jia CH, Shin JA, Lee KT. Effects of caffeic acid phenethyl ester and 4-vinylcatechol on the stabilities of oil-in-water emulsions of stripped soybean oil. Journal of Agricultural and Food Chemistry. 2015;63:10280–10286. doi: 10.1021/acs.jafc.5b02423. [DOI] [PubMed] [Google Scholar]
- Kobayashi M. Immunological functions of soy sauce: Hypoallergenicity and antiallergic activity of soy sauce. Journal of Bioscience and Bioengineering. 2005;100:144–151. doi: 10.1263/jbb.100.144. [DOI] [PubMed] [Google Scholar]
- Lars N, Björn B. Adsorption of hydrophobically modified anionic starch at oppositely charged oil/water interfaces. Journal of Colloid and Interface Science. 2007;308(2):508–513. doi: 10.1016/j.jcis.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong Y, Yim SB, Yu S. Antioxidant activity of Korean traditional soy sauce. Journal of the Korean Society of Food Science and Nutrition. 2015;44:1399–1406. doi: 10.3746/jkfn.2015.44.9.1399. [DOI] [Google Scholar]
- Liu N, Chen Q, Li G, Zhu Z, Yi J, Li C, Chen X, Wang Y. Properties and stability of perilla seed protein-stabilized oil-in-water emulsions: Influence of protein concentration, pH. NaCl Concentration and Thermal Treatment. Molecules. 2018;23:1533. doi: 10.3390/molecules23071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Li Y, Chen M, Xu F, Zhong F. Stabilizing oil-in-water emulsion with amorphous and granular octenyl succinic anhydride modified starches. Journal of Agricultural and Food Chemistry. 2018;66:9301–9308. doi: 10.1021/acs.jafc.8b02733. [DOI] [PubMed] [Google Scholar]
- Lucas M, Mirzaei F, O’Reily EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. The American Journal of Clinical Nutrition. 2011;93:1337–1343. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manshadi AD, Peighambardoust SH, Azadmard-Damirchi S, Niakosari M. Oxidative and physical stability, rheological properties and sensory characteristics of ‘salad dressing’ samples formulated with flaxseed oil and n-OSA starch. Journal of Food Measurement and Characterization. 2019;13:26–33. doi: 10.1007/s11694-018-9915-0. [DOI] [Google Scholar]
- McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. Journal of Food Science. 2000;65:1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Nitta M, Lee JK, Ohnishi O. Asian perilla crops and their weedy forms: Their cultivation, utilization and genetic relationships. Economic Botany. 2003;57:245–253. doi: 10.1663/0013-0001(2003)057[0245:APCATW]2.0.CO;2. [DOI] [Google Scholar]
- Shahidi F. Headspace volatile aldehydes as indicators of lipid oxidation in foods. In: Rouseff RL, Cadwallader KR, editors. Headspace analysis of foods and flavors. Boston, MA: Springer; 2001. pp. 113–123. [DOI] [PubMed] [Google Scholar]
- Sharif HR, Williams PA, Sharif MK, Khan MA, Majeed H, Safdar W, Shamoon M, Shoaib M, Haider J, Zhong F. Influence of OSA-starch on the physico chemical characteristics of flax seed oil-eugenol nanoemulsions. Food Hydrocolloids. 2017;66:365–377. doi: 10.1016/j.foodhyd.2016.12.002. [DOI] [Google Scholar]
- Shim SD, Lee SJ. Shelf-life prediction of perilla oil by considering the induction period of lipid oxidation. European Journal of Lipid Science and Technology. 2011;113:904–909. doi: 10.1002/ejlt.201000325. [DOI] [Google Scholar]
- Shin JA, Lee MY, Lee KT. Oxidation stability of O/W emulsion prepared with linolenic acid enriched diacylglycerol. Journal of Food Science. 2016;81:C2373–C2380. doi: 10.1111/1750-3841.13421. [DOI] [PubMed] [Google Scholar]
- The International Fragrance Association (IFRA), Analytical Method, Determination of the peroxide value (POV) (2019). Available from: https://ifrafragrance.org/safe-use/scientific-guidance (accessed on 14 November 2022)
- Visentainer D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Alternative Therapies in Health and Medicine. 2005;11:24–30. [PubMed] [Google Scholar]
- Visentainer JV, de Souza NE, Makoto M, Hayashi C, Franco MRB. Influence of diets enriched with flaxseed oil on the α-linolenic, eicosapentaenoic and docosahexaenoic fatty acid in Nile tilapia (Oreochromis niloticus) Food Chemistry. 2005;90:557–560. doi: 10.1016/j.foodchem.2004.05.016. [DOI] [Google Scholar]
- Wang H, Jenner AM, Lee CYJ, Shui G, Tang SY, Whiteman M, Wenk MR, Halliwell B. The identification of antioxidants in dark soy sauce. Free Radical Research. 2007;41:479–488. doi: 10.1080/10715760601110871. [DOI] [PubMed] [Google Scholar]
- Wang XY, Yang D, Gan LJ, Zhang H, Shin JA, Park SH, Lee KT. Degree of oxidation depending on the positional distribution of linolenic acid in perilla oil and interesterified products. Food Science and Biotechnology. 2014;23:1733–1740. doi: 10.1007/s10068-014-0237-7. [DOI] [Google Scholar]
- Yan C, McClements DJ, Zou L, Liu W. A stable high internal phase emulsion fabricated with OSA-modified starch: An improvement in β-carotene stability and bioaccessibility. Food & Function. 2019;10:5446–5460. doi: 10.1039/C9FO00508K. [DOI] [PubMed] [Google Scholar]