Abstract
Changes in kynurenine metabolites are reported in users of estrogen containing contraception. We have assessed kynurenines, vitamin B6, vitamin B2 and the inflammation markers, C-reactive protein (CRP) and neopterin, in healthy, never-pregnant women between 18 and 40 years (n = 123) and related this to their use of hormonal contraception. The population included 58 women, who did not use hormonal contraceptives (non-users), 51 users of estrogen-containing contraceptives (EC-users), and 14 users of progestin only contraceptives (PC-users). EC-users had significantly lower plasma kynurenic acid (KA) and higher xanthurenic acid (XA) levels compared to non-users. Serum CRP was significantly higher and negatively associated with both vitamin B6 and B2 status in EC-user compared to non-users. No significant differences in any parameters were seen between PC-users and non-users (p > 0.1). The low KA and high XA concentration in users of estrogen containing contraception resemble the biochemical profile observed in vitamin B6 deficiency. The hormonal effect may result from interference with the coenzyme function of vitamin B6 and B2 for particular enzymes in the kynurenine metabolism. KA has been suggested to be neuroprotective and the significantly reduced concentration in EC-users may be of importance in the observed increased risk of mood disorders among users of oral contraceptives.
Subject terms: Biochemistry, Biomarkers, Medical research
Introduction
Hormone contraceptive use is linked to changes in basal neuroendocrine and inflammatory profiles, potentially increasing the sensitivity to mood disturbances 1. Oral contraceptives contain either a combination of estrogen and progesterone or progesterone alone. Both hormones have been related to mood alterations2 and epidemiologic data have suggested an increased risk for incident depression among users of oral contraceptives, particularly in adolescents 3,4.
Estrogens are also reported to affect tryptophan (Trp) degradation through the kynurenine (Kyn) pathway5,6, and changes in Kyn metabolites have been implicated in the pathogenesis of neuropsychiatric disorders 7–9.
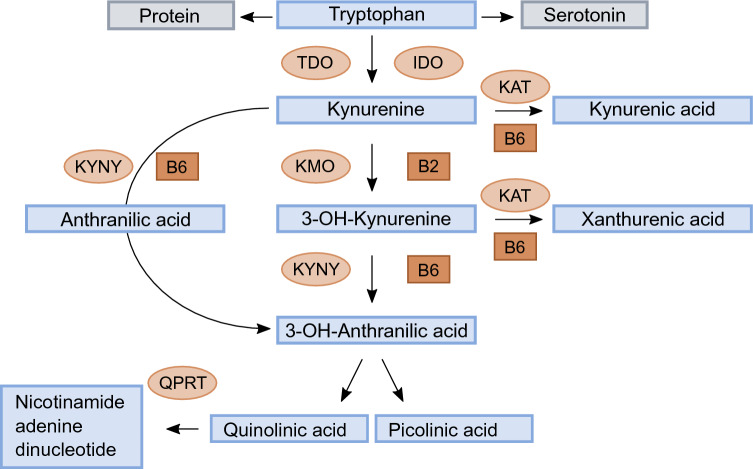
The essential amino acid Trp is mainly (90%) metabolised to Kyn, while a small portion serves as precursor of serotonin (Fig. 1). Conversion of Trp to Kyn is catabolized by tryptophan 2,3-dioxygenase (TDO) or indole 2,3-dioxygenase (IDO). The activity of TDO is activated mainly by cortisol, while IDO is stimulated by proinflammatory cytokines, like interferon gamma, tumor necrosis factor alpha (TNF-a) and interleukin-6. Kyn is further metabolised by the enzyme kynurenine aminotransferase (KAT) to kynurenic acid (KA) or by kynurenine 3-monooxygenase (KMO) to 3-hydroxykynurenine (HK)10. HK can be converted to xanthurenic acid (XA) by KAT, or metabolised through 3- hydroxyanthranilic acid (HAA) by kynureninase (KYNU) to either picolinic acid (Pic) or quinolinic acid (QA). Kyn can also be converted to anthranilic acid (AA) by the enzyme KYNU.
Figure 1.
Schematic drawing of the tryptophan metabolism through the kynurenine pathway. Enzymes: tryptophan 2,3-dioxygenase (TDO), indole 2,3-dioxygenase (IDO), kynurenine aminotransferase (KAT), kynurenine 3-monooxygenase (KMO) kynureninase (KYNU), quinolinate phosphoribosyl transferase (QPRT). Cofactors: vitamin B6 (pyridoxal 5´-phosphate, PLP) and vitamin B2 (flavin adenine dinucleotide, FAD).
The enzymes KAT and KYNU require vitamin B6 in the form of pyridoxal 5′-phosphate (PLP) as a cofactor, while KMO requires vitamin B2 in the form of flavin adenine dinucleotide (FAD) as a cofactor. Deficiency of either vitamins will cause disturbances in the kynurenine metabolites 11. In patients with low levels of PLP, the concentrations of KA will be low, while HK, XA and QA will be high compared to healthy controls. Deficiency of PLP will therefore increase the ratio between HK and KA, termed HK/KA ratio, as well as the ratio between HK and the sum of kynurenic acid (KA) + anthranilic acid (AA) + xanthurenic acid (XA) + 3-hydroxyanthranilic acid (HAA), termed HK ratio (HKr), a proposed marker of vitamin B6 deficiency 12.
Treatment with estrogen-containing oral contraceptives are reported to cause elevated urinary excretions of XA, Kyn, HK, and HAA, changes which are similar to the changes observed in dietary vitamin-B6 deficiency 13. The use of combined oral contraceptives has also been reported to increase circulating C-reactive protein (CRP) concentrations14. Plasma PLP is the most commonly used marker of vitamin B6 status and has consistently been shown to be low in inflammatory conditions15.
Although oral contraceptives have been used for more than 50 years by millions of women, there are still questions regarding the biological consequences of these medications16. In order to study estrogen-related changes in Kyn metabolism, we have investigated Trp, Kyn metabolites, inflammation markers and B vitamins in healthy, never-pregnant women aged 18 to 40 years in relation to their use of hormonal contraception.
Results
Demographics
The total study population included 158 healthy, never-pregnant women between 18 and 40 years. In order to diminish variation in Trp intake, women with a special diet were excluded (n = 35) and the 123 women, who were included in the study, were all omnivores.
Demographic data according to reported current use of contraceptives (non-users, n = 58, PC-users, n = 14 and EC-users, n = 51) are given in Table 1. A minority were regular users of micronutrient supplements and users of tobacco. Apart from a higher consumption of alcohol in women who used hormonal contraceptives compared to non-users, there were no significant differences in demographic data between the three groups (Table 1, Supplemental Table 1).
Table 1.
Baseline characteristics of healthy women according to use of hormonal contraceptives (n = 123).
| (25th-75th percentile) | Non-users N = 58 | Users of hormonal contraceptives | P value | |
|---|---|---|---|---|
| Progestin-only N = 14 | Estrogen and progestin N = 51 | |||
| Age, years, median (IQR) | 24 (22–29) | 24 (22–25) | 24 (22–26) | 0.711 |
| Body mass index, kg/m2, median (25th-75th percentile) | 22.2 (20.8–24.7) | 20.8 (20.0–21.6) | 22.3 (20.5–23.5) | 0.061 |
| Regular users of micronutrient supplements, n (%) | 12 (21%) | 1 (7%) | 14 (28%) | 0.262 |
| Smokers3, n (%) | 1 (2%) | 1 (7%) | 7 (14%) | 0.062 |
| Alcohol, number of units/week, median (25th-75th percentile) | 1.0 (0.4–3.0) | 4.0 (1.0–4.3) | 2.0 (1.0–4.0) | 0.011 |
1Comparison by Kruskal–Wallis test.
2Comparison by Pearson Chi-square test.
3Smokers defined as plasma cotinine ≥ 85 nmol/L.
Inflammation markers and vitamins according to use of hormonal contraceptives
There were significant differences in the concentrations of serum CRP and plasma neopterin according to use of hormonal contraception (Table 2; Supplemental Table 2). EC-users had the highest median serum CRP concentration and the lowest median plasma neopterin concentrations compared to PC-users and non-users. Serum CRP in EC-users was significantly higher (p = 0.007) compared to non-users and PC-users (p = 0.002), while plasma neopterin was lower, compared to non-users (p = 0.04) and PC-users (p = 0.009) (Supplemental Table 2). No significant differences were seen in CRP or neopterin concentrations between non-users and PC-users (p ≥ 0.07) (Supplemental Table 2).
Table 2.
Concentrations of inflammation parameters, vitamins and tryptophan/kynurenine metabolites according to use of hormonal contraceptives (n = 123).
| Parameters Median (25th-75th percentile) | Non-users N = 58 | Users of hormonal contraceptives | P value1 | |
|---|---|---|---|---|
| Progestin-only N = 14 | Estrogen and progestin N = 51 | |||
| Markers of inflammation, serum/plasma | ||||
| C-reactive protein, mg/L | 1.0 (0.6–2.0) | 1.0 (0.7–1.0) | 2.0 (1.0–5.0) | < 0.001 |
| Neopterin, nmol/L | 11.9 (9.6–14.5) | 14.7 (10.9–19.0) | 10.1 (8.4–13.8) | 0.01 |
| Vitamins, plasma | ||||
| Pyridoxal 5-phosphate, nmol/L | 63.8 (51.2–94.3) | 91.9 (58.0–108.0) | 58.1 (42.7–103.0) | 0.07 |
| Pyridoxal, nmol/L | 11.6 (9.0–15.0) | 14.5 (10.6–19.5) | 11.4 (8.4–15.6) | 0.13 |
| Pyridoxic acid, nmol/L | 20.5 (19.8–26.6) | 25.1 (15.6–39.9) | 22.3 (15.3–28.1) | 0.69 |
| PAr2 | 27 (22–34) | 25 (18–31) | 32 (24–40) | 0.04 |
| Riboflavin, nmol/L | 8.9 (5.9–14.1) | 9.2 (4.6–12.1) | 7.4 (5.7–13.0) | 0.65 |
| Flavin mononucleotide, nmol/L | 11.4 (8.8–15.5) | 15.6 (12.0–18.7) | 12.0 (9.1–17.6) | 0.04 |
| Nicotinamide, nmol/L | 191 (146–264) | 188 (138–235) | 202 (147–271) | 0.72 |
| Tryptophan and kynurenine metabolites, plasma | ||||
| Tryptophan, µmol/L | 70.7 (59.6–81.1) | 72.7 (65.3–84.3) | 70.4 (62.9–82.7) | 0.62 |
| Kynurenine, µmol/L | 1.48 (1.33–1.64) | 1.56 (1.37–1.77) | 1.43 (1.30–1.62) | 0.30 |
| 3-Hydroxykynurenine, nmol/L | 41.7 (35.5–50.8) | 42.7 (35.2–49.1) | 43.2 (35.4–54.7) | 0.92 |
| Kynurenic acid, nmol/L | 52.8 (41.5–65.8) | 60.9 (47.0–73.3) | 38.1 (28.7–50.4) | < 0.001 |
| Anthranilic acid, nmol/L | 13.1 (11.0–16.2) | 15.3 (10.1–18.0) | 12.3 (10.0–15.3) | 0.15 |
| 3-Hydroxyanthranilic acid, nmol/L | 45.4 (39.0–57.1) | 52.3 (39.7–63.9) | 50.3 (36.6–64.3) | 0.64 |
| Xanthurenic acid, nmol/L | 18.0 (13.3–22.5) | 18.1 (12.8–25.0) | 23.7 (15.5–29.5) | 0.07 |
| Picolinic acid, nmol/L | 55.3 (49.0–71.7) | 63.5 (53.5–85.7) | 64.7 (41.3–73.7) | 0.50 |
| Quinolinic acid, nmol/L | 336 (268–383) | 321 (287–383) | 342 (299–425) | 0.35 |
| Kynurenine metabolite ratios | ||||
| Kynurenine/Tryptophan ratio3 | 21 (19–25) | 21 (18–24) | 20 (18–22) | 0.12 |
| Kynurenic acid/Quinolinic acid3 | 17 (13–22) | 17 (14–21) | 11 (9–13) | < 0.001 |
| HKr4 | 33 (28–38) | 29 (27–33) | 36 (29–43) | 0.01 |
1Comparison by Kruskal–Wallis test.
2PAr: Pyridoxic acid/( Pyridoxal 5-phosphate + Pyridoxal). The ratio was multiplied by 100.
3The ratio was multiplied by 100.
4 HKr: 3-Hydroxykynurenine/(Kynurenic acid + Anthranilic acid + 3-Hydroxyanthranilic acid + Xanthurenic acid). The ratio was multiplied by 100.
There were no significant differences in B6 and B2 vitamers between EC-user and non-users (p > 0.2), but the PAr index was higher in EC-users (p = 0.03) (Table 2, Supplemental Table 2). The highest median plasma PLP concentration was seen in PC-users and the lowest in EC-users, while the opposite was seen for the PAr index. PC-users had higher median FMN concentration compared to non-users (p = 0.01), but not to EC-users (p = 0.07) (Table 2, Supplemental Table 2).
Correlations between inflammatory marker and B-vitamers
Serum CRP was significantly negative correlated to plasma PLP, PL PA, riboflavin and FMN in EC-users (rho: − 0.3, − 0.5, p < 0.01) and non-users (rho = − 0.3–− 0.4, p < 0.03). No significant correlations were seen between CRP and B6 and B2 species in PC-users.
Plasma neopterin was significantly positive correlated to the PAr index in EC-users (rho: 0.4, p = 0.005), but no other significant correlations were seen between neopterin and B6 and B2 species in any group.
Kynurenine metabolites according to use of hormonal contraceptives
Plasma concentrations of Trp and Kyn metabolites according to use of hormonal contraception are given in Table 2. The lowest median plasma KA concentrations and highest median plasma XA concentrations were seen in EC-users. Median plasma KA concentration was 28% lower, while median plasma XA concentration was 32% higher in EC-users compared to non-users and the median ratio between KA and QA was reduced by 35% (Table 2, Supplemental Table 2). There were no significant differences in median plasma Trp, Kyn and any other Kyn metabolite between EC-users and non-users (p > 0.08) (Table 2).
Compared to PC-users, EC-users had lower median plasma KA concentrations and KA/QA ratio (Table 2, Supplemental Table 2).
There were no significant differences in any Kyn-related parameters between PC-users and non-users (p > 0.1) (Table 2).
Vitamin B6 status by PLP and HKr
Plasma PLP was significantly negative correlated to HKr in EC-users (rho: − 0.6, − 0.5, p < 0.001), PC-users (rho: − 0.7, − 0.5, p < 0.001), and non-users (rho = − 0.3, p < 0.009). Median HKr was increased by 9% (p = 0.04) in EC-users compared to non-users, indicative of impaired vitamin B6 status related to use of estrogen containing contraception.
Correlations involving KA and XA
Plasma KA and XA were not significantly related to serum CRP or plasma neopterin in any groups. Plasma KA was positive correlated to PLP (rho: 0.3, p = 0.07), riboflavin and FMN (rho = 0.3, p = 0.02) in EC-users, to PLP (rho = 0.3, p = 0.3), riboflavin (rho: 0.7, p = 0.008), FMN (rho = 0.5, p = 0.09) in PC-users, and weakly only to PLP (rho = 0.2, p = 0.08) in non-users.
No significant correlations were observed of plasma XA with B6 and B2 vitamers in EC-users (rho < 0.2, p = > 0.2), weaker correlations were seen for PLP( rho: 0.3, p = 0.3) and riboflavin (rho: 0.5, p = 0.06) in PC-users, and more significant correlations to PLP (rho = 0.4, p = 0.004), riboflavin (rho: 0.2, p = 0.1) and FMN (rho = 0.3, p = 0.04) were seen in non-users.
Predictors of KA and XA
Plasma Kyn was the strongest predictor for plasma KA (beta = 0.37, p < 0.001), followed by use of hormonal contraceptives (beta = − 0.29, p = 0.001) and serum CRP (beta = -0.19, p = 0.04), in a multiple linear regression model which additionally included age, BMI, use of alcohol and smoking (plasma cotinine), plasma Trp, PLP and FMN. Including plasma neopterin in the model did not change the results. By applying stepwise regression, only use of hormonal contraceptives (beta = -0.39 p < 0.001), plasma Kyn (beta = 0.35, p < 0.001) and PLP (beta = 0.21, p = 0.008) remained as predictors for plasma KA.
The strongest predictor for XA in the full multiple linear regression model was plasma cotinine (beta = − 0.26, p = 0.009), plasma Trp (beta = 0.25, p = 0.02), followed by use of hormonal contraceptives (beta = 0.23, p = 0.02). Including plasma neopterin did not change the models. By applying stepwise regression, plasma Trp (beta = 0.34, p < 0.001), plasma cotinine (beta = − 0.23, p = 0.01) and use of hormonal contraceptives (beta = 0.20, p = 0.03) remained as significant predictors for plasma XA.
Discussion
In healthy, never-pregnant women aged 18 to 40 years, use of estrogen containing contraception was associated with significantly lower plasma concentrations of KA and higher concentrations of XA compared to non-users of hormonal contraception.
Compared to non-users, in EC-users serum CRP was higher and all vitamin B6 and B2 species were significantly negatively associated with CRP and positive related to plasma KA.
Estrogen has a number of effects on the modulation of the inflammatory response and immune cell function 17, and many of these are mediated by estrogen receptors present on monocytes and macrophages18,19. CRP is mainly produced in hepatocytes in response to increased levels of inflammatory cytokines, especially interleukin-620, while neopterin is synthesized by monocytes and macrophages in response to interferon-γ produced by activated T cells 21. Median concentrations of CRP and neoterin differed according to use of contraception. EC-users had higher serum CRP, and lower plasma neopterin compared to non-users, which is in accordance with published data 14,22,23. The use of combined oral contraceptives and postmenopausal hormone therapy have repeatedly been reported to elevate circulating CRP concentrations14,17,24. A reduced concentration of neopterin by estrogen therapy in menopause has been reported by several authors18,22,23, but data on neopterin concentrations in premenopausal women using hormonal therapy is not available.
In inflammatory conditions, plasma PLP and liver PLP are low, while erythrocyte and muscle PLP are unaffected and functional vitamin B6 biomarkers are not changed 15. Transfer of vitamin B6 to the sites of inflammation, where it may serve as a co-factor in pathways producing metabolites with immunomodulating effects, has therefore been suggested as a cause of low plasma PLP observed in inflammation 15. Also increased catabolism of the vitamin in inflammatory conditions have been proposed as a possible explanation. An increase in PA, the vitamin B6 catabolite that is excreted into the urine, relative to PL plus PLP measured as the PAr index has been interpreted as evidence of increased vitamin B-6 catabolism11. More than 90% of the variations in the PAr index are reported to be explained by inflammatory markers such as CRP, neopterin, white blood cell count and the Kyn to Trp ratio, indicating that there might be an increased catabolism of vitamin B-6 during inflammation25. We observed a significant negative correlation between CRP and PLP, while there was a positive correlation between CRP and the PAr index in EC-users, data which might support the hypothesis of increased B6 catabolism in inflammatory conditions.
Significant negative correlations were also observed between CRP and riboflavin and FMN in both EC-users and non-users. Riboflavin plays multiple roles in human health, and has recently been proposed as a possible new antimicrobial agent, as the vitamin may suppress or inactivate the growth of different microbes including bacteria, viruses, fungi and parasites 26. Data on change in riboflavin and FMN concentrations in inflammatory conditions are, however, to our knowledge unavailable.
The EC-users in our study had a 9% lower median plasma PLP and 9% higher HKr, indicating a poorer vitamin B6 status in EC-users. A recent study found a 10% lower median plasma PLP level in oral contraceptive users compared to non-users27, an observation in agreement with our results. Oral contraceptives with estrogens have been reported to reduce the level of several vitamins, including B6 and B2 28–31, however most of these studies were published more than 40 years ago when the level of estrogen in oral contraceptives were much higher32. The lower levels of B6 vitamin in oral contraceptive users have also been suggested to be due to tissue redistribution of PLP, rather than actual vitamin B-6 deficiency27,28,30. However, our observation of an increase in the functional B6 marker HKr in EC users suggests an impaired B6 status in these women.
We observed significantly lower plasma KA and higher plasma XA concentrations in women taking estrogen containing hormonal contraceptives compared to non-users. Meier et al. found significantly lower levels of KA in women taking oral contraceptives compared to non-users and the same has been reported by others 27,33. Increased urinal excretion of XA has also been reported in women taking estrogen containing contraceptives34.
The observed profile in EC-users is similar to the biochemical profile observed in vitamin B6 deficiency, with low KA and high XA and HKr35. One prevailing hypothesis for the metabolic changes resembling B6 deficiency in EC-users is that estrogen conjugates, which are typically elevated during pregnancy, inhibit the binding of PLP to both KAT and KYNU36,37. A marked fall in the excretion of XA after pyridoxine treatment has been observed in women taking progestogen-estrogen, suggesting that the hormonal effect may result from interference with the coenzyme function of PLP for these particular enzymes38.
There are neuroactive metabolites in the kynurenic pathway, including the N-methyl-d-aspartate (NMDA) receptor antagonist KA and agonist QA15. KA is the only naturally occurring antagonist of the glutamatergic NMDA receptor in the human brain39 and has under experimental conditions been shown to be neuroprotective40. A main finding in our study is lower KA concentrations and KA/QA ratio in EC-users compared to non-users. A recent meta-analysis reported that kynurenic acid and the KA/QA ratio were decreased in mood disorders, such as major depressive disorder, bipolar disorder and schizoaffective disorder41,42.
By only including women with an omnivore diet, we reduced the possible impact of low B6 vitamin and Trp intake, which might have affected kynurenine metabolism. As we did not have information about the menstrual phase or plasma sex hormone concentrations we were unable to relate our data to plasma estrogen concentrations, which is a limitations of this study. Additionally, the low number of PC-users made it difficult to test the progestin effect on kynurenine metabolism.
In healthy, never-pregnant women aged 18 to 40 years, use of estrogen containing contraception was associated with significantly lower plasma concentrations of KA and higher concentrations of XA, compared to non-users of hormonal contraception. The reason for this is unknown, however, the hormonal effect may result from interference with the coenzyme function of vitamin B6 and B2 for particular enzymes in the kynurenine metabolism.
KA has been suggested to be a neuroprotective amino acid 40 and the significantly reduced KA concentration in EC-users may be related to the reported increased risk for mood disorders among users of oral contraceptives.
Material and methods
Study population and design
Between June 2012 and March 2015 never-pregnant women aged 18 to 40 years were asked to participate in a study on factors related to B-vitamin status in healthy women. Recruitment was done among employees and students at Haukeland University Hospital and the University of Bergen, Norway and no specific randomization method was applied.
Ethical approval of the protocol was granted by the Regional Committee for Medical Research Ethics Western Norway (2011/2447). All procedures were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all women.
Clinical data
The women completed a questionnaire concerning age, body weight, height, health status, diet, as well as use of multiple micronutrient supplements, alcohol, tobacco and use of hormone containing contraceptives,. Women who had a chronic disease and used daily medications were excluded.
Regular use of micronutrient supplements was defined as use more than three days per week and the definition of a regular tobacco user was based on a plasma cotinine concentration > 85 nmol/L 43.
Use of hormone containing contraceptives
Use of hormonal contraception, including oral contraceptives, hormone implants and injections, was recorded. Progestins-only contraceptives contain different forms and doses of progestins, while combination contraceptives additionally contain ethinylestradiol in the range 20–35 µg 44.
Women who did not use hormonal contraception were defined as non-users, women who used estrogen containing contraceptives were defined as the EC-users, whereas women who used progestins-only contraceptives were defined as the PC-users.
Blood sampling and analysis
Non-fasting blood samples were obtained by antecubital venipuncture and collected into Vacutainer Tubes with (for plasma) and without (for serum) EDTA (Becton Dickinson). EDTA tubes were placed in ice water, and plasma was separated within 4 h. The samples were stored at − 80 °C until analysis.
Analysis Trp, Kyn metabolites, vitamin B2 (riboflavin and flavin mononucleotide (FMN)) and B6 vitamers (PLP, pyridoxal and pyridoxic acid), cotinine and neopterin was performed by liquid chromatography on an Agilent series 1100 HPLC system coupled with electrospray ionization tandem mass spectrometry (ESI–MS/MS) on an API 4000 triple-quadrupole tandem mass spectrometer from Applied Biosystems/MSD SCIEX45 at Bevital, Bergen, Norway (www.bevital.no).
Pic was added to an existing method based on LC–MS/MS 45, using multiple reaction monitoring (MRM) in the positive mode, recoding the ion pairs 124 m/z–78 m/z for picolinic acid and 128 m/z–88 m/z for picolinic-d4 acid (internal standard). The within-day and between CV were 6–7% and 5–8%, respectively (Supplemental file 1).
QA was added to an existing method based on LC–MS/MS 45, using multiple reaction monitoring (MRM) in the positive mode, recoding the ion pairs 168 m/z 78 m/z for quinolinic acid and 171 m/z–81 m/z for quinolinic-d3 acid (internal standard). The within-day and between CV were 7.0–7.5% and 7.2–10.3%, respectively (Supplemental file 2).
Serum CRP concentrations were determined using an immunoturbidimetric method using a Cobas 8000 analyzer (Roche diagnostics, Milan, Italy).
Statistical analysis
Results are presented as median and interquartile range (IQR), and compared by Kruskal Wallis test with Dunn’s test for multiple pairwise comparisons. For variables and metabolites that reported a p value of < 0.05, post hoc Dunn’s tests were performed to investigate differences between the individual study groups. Chi-square test was used for categorical data. Spearman correlations and multiple linear regression models were used to explore relationships between parameters. For KA and XA, we interrogated a panel of predictors known to modify serum concentrations of kynurenines, including age, BMI, use of hormonal contraception, alcohol and tobacco (plasma cotinine), serum CRP, plasma Trp, Kyn, PLP and FMN 11,33,46–48 in a multiple linear regression model for the whole study population. Further inclusion of neopterin did not change the estimates. Stepwise regression was applied as an exploratory analysis.
The SPSS statistical program (version 29) was used for statistical analyses. Two-sided p values < 0.05 were considered statistically significant.
Supplementary Information
Acknowledgements
The authors would like to express their gratitude to all the women who participated in the study. We thank Gry Kvalheim at Bevital AS, Bergen, Norway for analysing the blood samples.
Author contributions
A.-L.B.-M. and K.V. conceived, designed and performed the study; analysed the data and wrote the paper, S.T.S. was responsible for the figure and has critically revised the paper, A.U. and C.E. critically revised the paper, P.M.U. was responsible for the laboratory analyses and has critically revised the paper.
All authors have read and approved the final manuscript.
Funding
Open access funding provided by University of Bergen. The study was supported by grants from the Foundation to promote research into functional vitamin B12-deficiency and from Department of Medical Biochemistry and Pharmacology, Haukeland University Hospital. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report or in the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-43196-6.
References
- 1.Masama C, Jarkas DA, Thaw E, Daneshmend AZB, Franklyn SI, Beaurepaire C, et al. Hormone contraceptive use in young women: Altered mood states, neuroendocrine and inflammatory biomarkers. Horm Behav. 2022;144:105229. doi: 10.1016/j.yhbeh.2022.105229. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J. Appl. Physiol. 2001;91(6):2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 3.de Wit AE, Booij SH, Giltay EJ, Joffe H, Schoevers RA, Oldehinkel AJ. Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiat. 2020;77(1):52–59. doi: 10.1001/jamapsychiatry.2019.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skovlund CW, Morch LS, Kessing LV, Lidegaard O. Association of hormonal contraception with depression. JAMA Psychiat. 2016;73(11):1154–1162. doi: 10.1001/jamapsychiatry.2016.2387. [DOI] [PubMed] [Google Scholar]
- 5.Badawy AA, Dougherty DM. Assessment of the human kynurenine pathway: Comparisons and clinical implications of ethnic and gender differences in plasma tryptophan, kynurenine metabolites, and enzyme expressions at baseline and after acute tryptophan loading and depletion. Int. J. Tryptophan. Res. 2016;9:31–49. doi: 10.4137/IJTR.S38189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015;9:37. doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnone D, Saraykar S, Salem H, Teixeira AL, Dantzer R, Selvaraj S. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci. Biobehav. Rev. 2018;92:477–485. doi: 10.1016/j.neubiorev.2018.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J. Affect. Disord. 2007;98(1–2):143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Reus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015;68:316–328. doi: 10.1016/j.jpsychires.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujigaki H, Yamamoto Y, Saito K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology. 2017;112(Pt B):264–274. doi: 10.1016/j.neuropharm.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Ueland PM, Ulvik A, Rios-Avila L, Midttun O, Gregory JF. Direct and functional biomarkers of vitamin B6 status. Annu. Rev. Nutr. 2015;35:33–70. doi: 10.1146/annurev-nutr-071714-034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulvik A, Midttun O, McCann A, Meyer K, Tell G, Nygard O, et al. Tryptophan catabolites as metabolic markers of vitamin B-6 status evaluated in cohorts of healthy adults and cardiovascular patients. Am. J. Clin. Nutr. 2020;111(1):178–186. doi: 10.1093/ajcn/nqz228. [DOI] [PubMed] [Google Scholar]
- 13.Rose DP. The interactions between vitamin B6 and hormones. Vitam. Horm. 1978;36:53–99. doi: 10.1016/s0083-6729(08)60982-6. [DOI] [PubMed] [Google Scholar]
- 14.Divani AA, Luo X, Datta YH, Flaherty JD, Panoskaltsis-Mortari A. Effect of oral and vaginal hormonal contraceptives on inflammatory blood biomarkers. Mediators Inflamm. 2015;2015:379501. doi: 10.1155/2015/379501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueland PM, McCann A, Midttun O, Ulvik A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 2017;53:10–27. doi: 10.1016/j.mam.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Pletzer BA, Kerschbaum HH. 50 years of hormonal contraception-time to find out, what it does to our brain. Front. Neurosci. 2014;8:256. doi: 10.3389/fnins.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakoski SG, Herrington DM. Effects of hormone therapy on C-reactive protein and IL-6 in postmenopausal women: A review article. Climacteric. 2005;8(4):317–326. doi: 10.1080/13697130500345109. [DOI] [PubMed] [Google Scholar]
- 18.Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm. 2014;2014:615917. doi: 10.1155/2014/615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy AJ, Guyre PM, Wira CR, Pioli PA. Estradiol regulates expression of estrogen receptor ERalpha46 in human macrophages. PLoS One. 2009;4(5):e5539. doi: 10.1371/journal.pone.0005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J. Exp. Med. 1984;160(1):310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toniolo A, Fadini GP, Tedesco S, Cappellari R, Vegeto E, Maggi A, et al. Alternative activation of human macrophages is rescued by estrogen treatment in vitro and impaired by menopausal status. J. Clin. Endocrinol. Metab. 2015;100(1):E50–E58. doi: 10.1210/jc.2014-2751. [DOI] [PubMed] [Google Scholar]
- 23.Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 2015;5:15224. doi: 10.1038/srep15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Clos TW. Function of C-reactive protein. Ann. Med. 2000;32(4):274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 25.Ulvik A, Midttun O, Pedersen ER, Eussen SJ, Nygard O, Ueland PM. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am. J. Clin. Nutr. 2014;100(1):250–255. doi: 10.3945/ajcn.114.083196. [DOI] [PubMed] [Google Scholar]
- 26.Farah N, Chin VK, Chong PP, Lim WF, Lim CW, Basir R, et al. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. 2022;3:100111. doi: 10.1016/j.crmicr.2022.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deac OM, Mills JL, Shane B, Midttun O, Ueland PM, Brosnan JT, et al. Tryptophan catabolism and vitamin B-6 status are affected by gender and lifestyle factors in healthy young adults. J. Nutr. 2015;145(4):701–707. doi: 10.3945/jn.114.203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aly HE, Donald EA, Simpson MH. Oral contraceptives and vitamin B6 metabolism. Am. J. Clin. Nutr. 1971;24(3):297–303. doi: 10.1093/ajcn/24.3.297. [DOI] [PubMed] [Google Scholar]
- 29.Palmery M, Saraceno A, Vaiarelli A, Carlomagno G. Oral contraceptives and changes in nutritional requirements. Eur. Rev. Med. Pharmacol. Sci. 2013;17(13):1804–1813. [PubMed] [Google Scholar]
- 30.Price SA, Rose DP, Toseland PA. Effects of dietary vitamin B 6 deficiency and oral contraceptives on the spontaneous urinary excretion of 3-hydroxyanthranilic acid. Am J Clin Nutr. 1972;25(5):494–498. doi: 10.1093/ajcn/25.5.494. [DOI] [PubMed] [Google Scholar]
- 31.Webb JL. Nutritional effects of oral contraceptive use: A review. J. Reprod. Med. 1980;25(4):150–156. [PubMed] [Google Scholar]
- 32.De Leo V, Musacchio MC, Cappelli V, Piomboni P, Morgante G. Hormonal contraceptives: Pharmacology tailored to women's health. Hum. Reprod. Update. 2016;22(5):634–646. doi: 10.1093/humupd/dmw016. [DOI] [PubMed] [Google Scholar]
- 33.Meier TB, Drevets WC, Teague TK, Wurfel BE, Mueller SC, Bodurka J, et al. Kynurenic acid is reduced in females and oral contraceptive users: Implications for depression. Brain Behav. Immun. 2018;67:59–64. doi: 10.1016/j.bbi.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose DP, Adams PW. Oral contraceptives and tryptophan metabolism: effects of oestrogen in low dose combined with a progestagen and of a low-dose progestagen (megestrol acetate) given alone. J. Clin. Pathol. 1972;25(3):252–258. doi: 10.1136/jcp.25.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oxenkrug G, Tucker KL, Requintina P, Summergrad P. Neopterin, a marker of interferon-gamma-inducible inflammation, correlates with pyridoxal-5'-phosphate, waist circumference, HDL-cholesterol, insulin resistance and mortality risk in adult Boston community dwellers of Puerto Rican origin. Am. J. Neuroprot. Neuroregen. 2011;3(1):48–52. doi: 10.1166/ajnn.2011.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason M, Gullekson EH. Estrogen-enzyme interactions: Inhibition and protection of kynurenine transaminase by the sulfate esters of diethylstilbestrol, estradiol, and estrone. J. Biol. Chem. 1960;235:1312–1316. [PubMed] [Google Scholar]
- 37.Mason M, Manning B. Effects of steroid conjugates on availability of pyridoxal phosphate for kynureninase and kynurenine aminotransferase activity. Am. J. Clin. Nutr. 1971;24(7):786–791. doi: 10.1093/ajcn/24.7.786. [DOI] [PubMed] [Google Scholar]
- 38.Rose DP. Excretion of xanthurenic acid in the urine of women taking progestogen-oestrogen preparations. Nature. 1966;210(5032):196–197. doi: 10.1038/210196a0. [DOI] [PubMed] [Google Scholar]
- 39.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993;45(3):309–379. [PubMed] [Google Scholar]
- 40.Bratek-Gerej E, Ziembowicz A, Godlewski J, Salinska E. The mechanism of the neuroprotective effect of kynurenic acid in the experimental model of neonatal hypoxia-ischemia: the link to oxidative stress. Antioxidants (Basel) 2021;10(11):1775. doi: 10.3390/antiox10111775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marx W, McGuinness AJ, Rocks T, Ruusunen A, Cleminson J, Walker AJ, et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol. Psychiatry. 2021;26(8):4158–4178. doi: 10.1038/s41380-020-00951-9. [DOI] [PubMed] [Google Scholar]
- 42.Wurfel BE, Drevets WC, Bliss SA, McMillin JR, Suzuki H, Ford BN, et al. Serum kynurenic acid is reduced in affective psychosis. Transl Psychiatry. 2017;7(5):e1115. doi: 10.1038/tp.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim, S. overview of cotinine cutoff values for smoking status classification. International Journal of Environmental Research and Public Health. 13(12) (2016). [DOI] [PMC free article] [PubMed]
- 44.Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther. Clin. Risk. Manag. 2008;4(5):905–911. doi: 10.2147/tcrm.s2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Midttun O, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23(9):1371–1379. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 46.Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity. 2015;23(10):2066–2074. doi: 10.1002/oby.21199. [DOI] [PubMed] [Google Scholar]
- 47.Sorgdrager FJH, Vermeiren Y, Van Faassen M, van der Ley C, Nollen EAA, Kema IP, et al. Age- and disease-specific changes of the kynurenine pathway in Parkinson's and Alzheimer's disease. J. Neurochem. 2019;151(5):656–668. doi: 10.1111/jnc.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng H, Teague TK, Yeh FC, Burrows K, Figueroa-Hall LK, Aupperle RL, et al. C-Reactive protein and the kynurenic acid to quinolinic acid ratio are independently associated with white matter integrity in major depressive disorder. Brain Behav. Immun. 2022;105:180–189. doi: 10.1016/j.bbi.2022.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.