Summary
Background
Despite being the second least populated state, Mizoram exhibits the highest incidence rate of cancer in India. Its inhabitants, constituting an endogamous and isolated population, have embraced their own distinct culture, way of life and dietary preferences, setting them apart from the rest of mainland India. In 2003, the Mizoram Population Based Cancer Registry (PBCR) was established under the auspices of the National Centre for Disease Informatics and Research (NCDIR), a division of the Indian Council of Medical Research (ICMR), in collaboration with the Department of Health & Family Welfare of the Government of Mizoram, India.
Methods
Cancer incidence and mortality data were extracted from the Mizoram PBCR spanning the years 2003–2020. The Age Standardized Incidence Rate (ASIR) and Age Standardized Mortality Rate (ASMR) were computed per 100,000 individuals, utilizing Segi's World Standard Population as the benchmark. The trajectory of these changes was analysed employing the Joinpoint Regression Analysis Program, Version 4.9.1.0.13, to unveil the Annual Percent Change (APC) with a 95% Confidence Interval and a Significance test (p < 0.05) using Monte Carlo Permutation. The resulting graphical visualizations were generated using Flourish Studio.15.
Findings
The overall ASIR for all cancer sites among men was 197.2 per 100,000, while for women, it was 164.9 per 100,000. Among men, the most prevalent cancer site was the Stomach (ASIR = 41.4), followed by Head & Neck, Lung, Oesophagus, Colorectal, Liver, Urinary, Non-Hodgkin's Lymphoma and Prostate cancers. Conversely, among women, Lung cancer exhibited the highest incidence (ASIR = 26.7), succeeded by Cervical, Breast, Stomach, Head & Neck, Colorectal, Oesophagus, Liver and Ovarian cancers. Stomach cancer emerged as the leading cause of death among men (ASMR = 22.6) and among women, Lung cancer held the highest ASMR (15.9). Joinpoint regression analysis revealed a rising trend in incidence and mortality over time for overall cancer sites. Among the primary cancer sites contributing to incidence and mortality, an increase in APC was observable for all, except Stomach cancer, in both men and women. The diagnostic approach, except for cases of cancer with unknown primary sites, involved a microscopic method.
Interpretation
This cross-sectional study examines PBCR reports spanning from 2003 to 2020, shedding light on a consistent uptick in cancer incidence and mortality trends in Mizoram. Stomach cancer emerges as the most prevalent and primary cause of cancer-related deaths among men, while Lung cancer takes a parallel role in women. The elevated cancer incidence and the growing trend among younger generations might stem from the static lifestyle and dietary patterns prevalent within the endogamous tribal population, potentially contributing to a genetic predisposition. The escalation in mortality rates could be attributed to a dearth of specialized diagnostic facilities and skilled human resources, treatment strategies guided by genomic research and transportation challenges. This underscores the urgent requirement for comprehensive scientific exploration across diverse facets. The implementation of easily accessible diagnostic facilities in proximity and genetic testing for pharmacogenomics to enhance prognoses would also aid in mitigating the burden and advancing the healthcare system's effectiveness.
Funding
Population Based Cancer Registry (PBCR) was supported by National Centre for Disease Informatics and Research (NCDIR) of the Indian Council of Medical Research (ICMR), India.
Keywords: Mizoram, Endogamous, Health care systems, Northeast India, Cancer incidence & mortality
Research in context.
Evidence before this study
The primary evidence and the data source are from the Population Based Cancer Registry, Mizoram of Indian Council for Medical Research, New Delhi. The literature search included Mizoram, Age Standardized Incidence Rates (ASIRs), Age Standardized Mortality Rates, Risk Factors, Lifestyle Habits, Cancer Trend from various hospitals and centres, however, there have been inconsistencies in depicting Mizoram's cancer rates, often combining data from different years in reports. Cancer incidence and mortality data were collected from the Mizoram Population Based Cancer Registry spanning 18 years (from 2003 to 2020). The Mizoram state is within the top 10 in ASIR of cancers among several sites, in both men and women. The patients who were diagnosed within the Mizoram state alone in both men and women of all ages, all cancer sites were included in the study. The cancer sites which were not diagnosed among the population were excluded.
Added value of this study
This paper provides a comprehensive yearly overview of 18 years of cancer incidences and mortality, including the top 10 leading sites and their trends among rural and urban areas and distribution among different age groups in both men and women for all sites. Moreover, this paper highlights the etiology of Cancers and will create an awareness among the general public worldwide and subsequently it highlights the need for attention and intervention regarding the potential risk factors in a tribal community in a developing nation.
Implications of all the available evidence
Upgrading Primary Health Care Centres in rural areas of developing countries with skilled technicians and proper infrastructure for diagnostic and prognostic purposes is crucial. There is a lack of more precise cancer-specific diagnostic methods and genetic counselling practices for understanding the genetic predisposition at the population level. Conducting comprehensive cancer research encompassing epidemiology, etiology, lifestyle factors, food habits and population-wide genomic screening is essential for improving the healthcare system and addressing the urgent needs of rural populations. This paper raises the need for awareness among the international scientific community, funding agencies and government bodies about the critical cancer situation in Mizoram and advocate for collaborative efforts to mitigate the problem, considering the socioeconomic, genetic and traditional backgrounds of the population.
Introduction
Mizoram, situated in India's north-eastern region, holds the distinction of being the second least populous state in the country, with Aizawl serving as its capital.1 As of the 2011 Census, the population stands at 1,097,206.2 Encompassing an area of 21,087 square kilometres, the state boasts a forest cover that extends around 91% of its land area.2,3 The people of Mizoram are characterized by their unique culture and lifestyle that distinguishes them from other regions within India. Notably, the practice of endogamy has been deeply rooted in this population for an extended period.4
In India, the National Cancer Registry Programme (NCRP), under the National Centre for Disease Informatics and Research (NCDIR) of the Indian Council of Medical Research (ICMR), has been overseeing the collection of cancer data through Population Based Cancer Registries (PBCRs) and Hospital Based Cancer Registries (HBCRs) since 1982. These efforts have resulted in the publication of reports encompassing various types of cancer from diverse registries across the country.5 In 2003, the Mizoram Population Based Cancer Registry (PBCR) was jointly established by the NCRP of ICMR and the Department of Health &Family Welfare of the Government of Mizoram, India. This registry diligently records all instances of cancer diagnosis within the geographical boundaries of Mizoram.6
Between 2003 and 2010, Mizoram consistently held the distinction of reporting the highest Age Standardized Incidence Rate (ASIR) for all cancer sites in both men and women among all the Indian cancer registries.5 However, in the subsequent three-year period covered by PBCR reports (2012–2014), Mizoram's ASIR shifted to 211.5 for men and 165.8 for women, ranking it fourth overall among all cancer sites. It's noteworthy that the Aizawl District demonstrated the highest ASIR for men (270.7) and the second highest for women (207.7).5 According to the most recent five-year NCRP report (spanning 2012 to 2016), Aizawl district sustained its lead with an ASIR of 269.4 for men, while Mizoram state secured fourth place with 207.0. For women, Aizawl district's ASIR stood at 214.1, maintaining the second highest position and Mizoram state climbed to third with a rate of 172.3.6 Comparatively, on the global scale in 2014, the Aizawl District was ranked 11th for both men and women cancers (273.4 and 227.8, respectively). Mizoram State landed at the 14th spot for men cancers (189.5) and the 15th spot for women cancers (153.7).7 The Age Standardized Mortality Rate (ASMR) depicted a similar pattern, with Aizawl district reporting the highest rates in the country: 154.1 for men and 110.1 for women. Mizoram State followed closely with ASMR figures of 110.3 for men and 76.5 for women with cancers.7 The aim of our study is to spotlight the yearly trends in cancer incidence and mortality over the past 18 years, aiming to provide a comprehensive perspective on the cancer burden in Mizoram.
Disparities in ancestry, socioeconomic factors, cultural attributes, dietary preferences and lifestyle choices are mirrored in the diversity of cancer prevalence, both nationally and within the global context.7, 8, 9 To address the mounting incidence of malignancies in Mizoram, coupled with a lack of corresponding reduction in mortality rates, an in-depth scientific inquiry is imperative to unearth the underlying trends. This study aims to unravel crucial aspects, including the prevalent cancers among men and women, the intricate patterns of incidence and mortality, commonly employed diagnostic methodologies, and areas within the healthcare system that offer potential for enhancement, effectively countering the burden of cancer. Conducted as a cross-sectional study, our analysis delves into an 18-year span of PBCR reports, spanning from its inception in 2003 until 2020. Through this comprehensive investigation, our objective is to establish a nuanced comprehension of the elevated incidence and mortality rates, pinpoint gaps in diagnosis and treatment, and chart a path towards more effective progress in alleviating the burden of cancer in the state of Mizoram.
Methods
Collection of data
To ensure the precise recording and reporting of cancer cases, every medical staff member employed at District hospitals in Mizoram has undergone thorough training. This training initiative aims to guarantee the accuracy of data collection and reporting. These staff members are entrusted with the responsibility of submitting monthly reports detailing cancer incidence and mortality figures within their respective districts. In addition to their reporting duties, the staff members in charge of the registry undertake visits to approximately 40 distinct sources of registration. This encompassing list includes both public and private hospitals, nursing homes, public health centres, diagnostic laboratories, as well as the official registry of births and deaths. This meticulous process is essential to amass comprehensive and accurate data.6 Furthermore, when it comes to patients who have received a cancer diagnosis in other states but fall within the jurisdiction of the PBCR area, the corresponding registries collaborate with us by providing the necessary data.6
Mizoram operates with a comprehensive network of 832 Registrars of Births and Deaths (RBDs), who play a crucial role in documenting mortality rates across the region. To facilitate this process, the government has appointed local school teachers within their respective village council (panchayat) areas. These individuals are entrusted with the task of recording deaths in their communities. The dataset compiled by the RBDs is rich in details, encompassing crucial information such as the name of the deceased, the date and time of death, the cause of death, age, gender, parental names, place of residence, hospital of admission, religious affiliation, occupation and various lifestyle habits like smoking, smokeless tobacco use and alcohol consumption. Once gathered, these vital records are submitted to the Additional Chief Registrar of Births and Deaths, who holds the position of Director within the Economics and Statistics Department. This hierarchical process ensures that accurate and comprehensive data is systematically compiled and maintained.6
The task of collecting mortality data presents a challenge due to the absence of a mandatory requirement for a medical doctor to certify the cause of death. Nevertheless, the dedicated PBCR staff undertakes the collection of all deaths caused by cancer from the registrar of Births and Deaths. Subsequently, this data is meticulously cross-referenced with existing records or hospital documentation to ensure accuracy. A comprehensive outline detailing the data collection methodology is available in our prior publications.6 For this specific study, a total of 25,803 cancer patients (comprising 13,917 men and 11,886 women) registered by the PBCR from 2003 to 2020 were included as subjects for further analysis. This dataset serves as the foundational cornerstone for our investigation into the trends of cancer incidence and mortality within Mizoram over the stipulated 18-year timeframe. The methodologies and outcomes of this study were reported in accordance with the guidelines set forth by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) initiative, ensuring adherence to best practices for observational studies.10
Data processing
Cancer incidence and mortality data spanning an 18-year period (2003–2020) were collected from the Mizoram Population Based Cancer Registry (PBCR). The registry encompasses all malignant neoplasms classified under morphology behaviour codes '3' and '6' according to the International Classification of Disease—Oncology, 3rd Edition, by the World Health Organization (ICD-O 3), along with primary site classification by ICD-10.11 Crude Rates for both Incidence and Mortality were computed by employing the Estimated Population Census data for each year between 2003 and 2020. The estimated population census for the respective 5-year age groups was derived from the population growth rate recorded between 2001 and 2011, utilizing the Difference Distribution Method developed by Takiar and Shobana in 2009.5 To assess the cancer burden, the ASIR and ASMR per 100,000 population were calibrated, employing Segi's World Standard Population as the reference.12
A comprehensive outline of the data collection strategies and statistical methodologies can be found in our prior publications.5,6 To discern the long-term trends spanning from 2003 to 2020, in both men and women, for the prominent cancer sites, we employed Joinpoint Regression Analysis through Joinpoint Regression Program Version 4.9.1.0.13 The Annual Percent Change (APC) was calculated, with a 95% Confidence Interval and a Significance Test (with a p-value threshold of &<0.05), utilizing the Monte Carlo permutation method.14 The findings were visually depicted through graphical representations generated using Flourish Studio.15
Results
Cancer sites were classified into 36 distinct categories based on the ICD-10 classification (see Supplementary Table S1). Inclusion in our study was limited to those cancer sites that had diagnosed cases within the population, with any non-diagnosed sites being excluded. Specifically, 30 different cancer sites were identified among men, while the corresponding count for women was 32. Visual representation of the trends in overall cancer incidence and mortality across all sites, as well as within both men and women, can be observed in Supplementary Figure S1. Additionally, a detailed enumeration of patients for each cancer site is provided in Supplementary Table S2. The scope of overall incidence rates spanned from 156 cases (recorded in 2006) to a peak of 197.2 cases (observed in 2011), while the spectrum of mortality rates extended from 67.5 (in 2003) to 114.2 (in 2020). Among men, the lowest ASIR was noted in 2006 at 167.8, while the highest occurred in 2013 at 221.8. A similar pattern was observed among women, with the lowest ASIR in 2006 at 144.1 and the highest in 2016 at 182.2. The most noteworthy finding was the prominence of cancer incidences and mortality among individuals aged 65–69 years, as depicted in Supplementary Figure S1. Interestingly, a higher rate of cancer was also observed among women in the younger age groups, specifically those aged between 25 and 44. A discernible decline in cancer incidence within the age bracket of 70–74 was followed by a significant spike in the 75+ years age group. This pattern results from the inclusion of cases above 75 years, in line with WHO guidelines (Supplementary Figure S1). Detailed ASIR of each cancer site for men and women throughout the studied years (2003–2020) are presented in Supplementary Table S3 a and b, respectively. Correspondingly, the ASMR for all cancers among men and women are outlined in Supplementary Table S4 a and b. The distribution of cancer patients in leading sites, categorized by age groups of 45 years and below and above (as per the American Cancer Society Guidelines for Early Detection of Cancer, https://www.cancer.org/), is outlined in Supplementary Table S5. A visual depiction of the overall ASIR and ASMR for each cancer site spanning 2003 to 2020, both for men and women, can be observed in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5. Finally, the trends in leading sites among men and women are illustrated in Figs. 6 and 7 respectively.
Fig. 1.
Trend in Age Standardized Incidence and Mortality Rates (2003–2020). Trend in incidence and mortality in men, women and both genders (overall) for a span of 2003–2020 for all cancer sites. Age Standardized Rates for incidence and mortality were calculated per 100,000 population using Segi's World Standard Population.
Fig. 2.
Overall ASIRs (2003–2020) for all sites in men. Overall Age Standardized Incidence Rates (ASIRs) were calculated for each cancer site from 2003 to 2020 to illustrate the lowest to highest rate in men.
Fig. 3.
Overall ASIRs (2003–2020) for all sites in women. Overall Age Standardized Incidence Rates (ASIRs) were calculated for each cancer site from 2003 to 2020 to illustrate the lowest to highest rate in women.
Fig. 4.
Overall ASMRs (2003–2020) for all sites in men. Overall Age Standardized Mortality Rates (ASMRs) were calculated for each cancer site from 2003 to 2020 to illustrate the lowest to highest rate in men.
Fig. 5.
Overall ASMRs (2003–2020) for all sites in women. Overall Age Standardized Mortality Rates (ASMRs) were calculated for each cancer site from 2003 to 2020 to illustrate the lowest to highest rate in women.
Fig. 6.
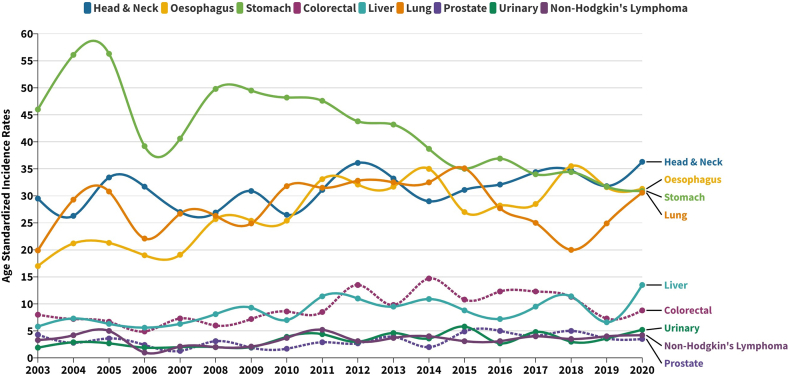
Trend in ASIR for leading sites in men (2003–2020). Trends in Age Standardized Incidence Rates (ASIRs) for leading sites in men from 2003 to 2020.
Fig. 7.
Trend in ASIR for leading sites in women (2003–2020). Trends in Age Standardized Incidence Rates (ASIRs) for leading sites in women from 2003 to 2020.
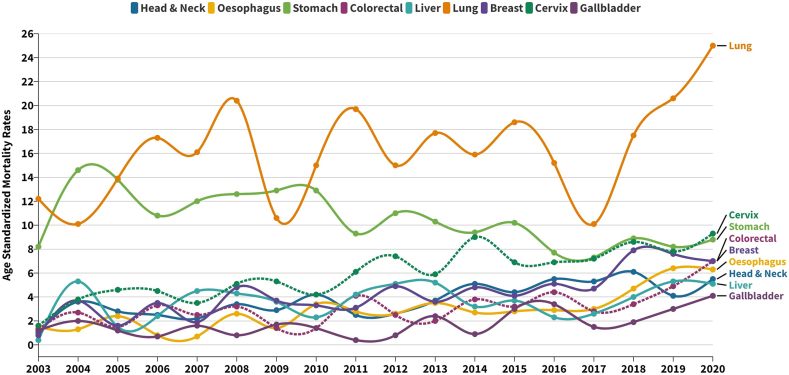
Figs. 8 and 9 depict the primary sites of mortality for male and female cancers, respectively. Stomach cancer emerges as the leading causative factor of cancer-related mortality among men. However, a noteworthy paradigm shift becomes evident post-2013, with escalating mortality rates attributed to Oesophageal, Head & Neck and Lung cancers, subsequently surpassing those of Stomach cancer. Conversely, Lung cancer consistently maintains its paramount position as the principal source of cancer-related deaths in the female cohort over the comprehensive 18-year temporal expanse. The trajectory of ASMR underscores a gradual yet consistent upward trajectory across varied cancer sites, encompassing the Cervix, Breast, Oesophagus, Head & Neck, Liver and Gallbladder. Remarkably, the ASMR for Stomach cancer exhibits a progressive decline in both male and female cohorts across the studied time span.
Fig. 8.
Trend in ASMR for leading sites in men (2003–2020). Trends in Age Standardized Mortality Rates (ASMRs) for leading sites in men from 2003 to 2020.
Fig. 9.
Trend in ASMR for leading sites in women (2003–2020). Trends in Age Standardized Mortality Rates (ASMRs) for leading sites in women from 2003 to 2020.
Supplementary Table S6 presents the outcomes of the Joinpoint regression analysis applied to cancer incidence across all sites and the primary sites. Supplementary Figures S2 and S3 illustrate the graphical trends resulting from the Joinpoint analyses for incidences. According to the findings of the Joinpoint regression analysis, the trajectory of overall cancer incidences across all sites, as well as within the male and female populations, demonstrated marginal or negligible variations throughout the course of the study. The Joinpoint regression analysis performed on the leading cancer sites among men, spanning from 2003 to 2020, revealed distinct APC patterns. Notably, an upward trend in incidence was observed for Head &Neck (1.1%), Liver (3.2%), Urinary (4.6%), Non-Hodgkin's Lymphoma (NHL) (1.2%) and Prostate (2.6%) cancers, whereas Stomach exhibited a decrease of −3.0%. Oesophageal cancer displayed an increase of 6.7% annually from 2003 to 2012, followed by a subsequent decline. Colorectal cancer demonstrated an ascent of 6.3% from 2003 to 2016, succeeded by an 11.5% reduction from 2016 onward. The trajectory of Lung cancer saw a steady rise of 2.9% per annum from 2003 until 2015, followed by a sharp decline of 16.2% up to 2018, and ultimately a resurgence of 23% from 2018 to 2020. Similarly, among women, the Joinpoint regression analysis indicated an upward trajectory from 2003 to 2020 for Head &Neck (0.3%), Oesophagus (5.2%), Liver (0.9%), Cervix (1%), Breast (3.6%) and Ovary (4.2%) cancers. Conversely, the trend displayed a decline for Stomach (−3.7%) and Lung (−0.3%) cancers during the same studied period.
Supplementary Table S7 provides an overview of the Joinpoint trend analysis applied to cancer mortality across all sites and the primary sites. Supplementary Figures S2 and S4 offer graphical depictions of the Joinpoint analyses for mortality patterns. The analysis underscores a consistent escalation in mortality rates across all sites, recorded at a rate of 1.5% per year for men, 1.7% for women and an overall 1.5%. From 2003 to 2020, a discernible surge in mortality rates was observed among both men and women for Head &Neck (5.8% & 5.4%), Oesophagus (5.8% & 9.2%), Colorectal (3.4% & 6.1%), Liver (4.8% & 2.0%) and Lung (2.1% & 2.4%) cancers. Conversely, a decrease was evident for Stomach (1.5% & 2.9%) cancer mortality rates during the same period. Noteworthy is the striking trajectory of Pancreatic cancer in men: a precipitous drop of 52.7% from 2003 to 2005, succeeded by a significant upswing of 16.8% from 2005 to 2015, followed by a decline of 32.6% until 2018, and a subsequent resurgence of 63.8% from 2018 to 2020. Furthermore, the analysis identified a mortality trend increase of 7.4% annually for Urinary Cancer and Prostate Cancer in men, alongside an elevation in mortality for Gallbladder (7.4%), Breast (7.1%) and Cervix (5.6%) cancers among women.
The microscopic method stands out as the most frequently utilized diagnostic technique across the primary sites, except for cases of Cancer of Unknown Primary sites (CUPs), where detection generally relies on the Death Certificate Only (DCO) during post-mortem examinations (Supplementary Figure S5). X-ray imaging techniques find application in the diagnosis of Lung, Liver, Ovary, Prostate and Urinary cancers. The distribution of cancer incidences across rural and urban areas of Mizoram, along with the gender-based breakdown of incidences among men and women, as well as the distribution of various sub-sites within the primary cancer sites, provides additional insights into the spatial and gender-related disparities in cancer incidence within the region (Supplementary Figures S6 and S7).
Discussion
This cross-sectional study provides an extensive analysis of the upward trends in cancer incidence and mortality spanning a period of 18 years in Mizoram. Among men, the most prevalent cancer sites include Stomach, Head & Neck, Lung, Oesophagus, Cancer of Unknown Primary, Colorectal, Liver, Urinary and Non-Hodgkin's Lymphoma. Among women, the leading sites are Lung, Cervix, Breast, Stomach, Cancer of Unknown Primary, Head & Neck, Colorectal, Oesophagus, Liver and Ovary. Notably, Stomach cancer stands out as the primary cause of cancer-related deaths in men, whereas Lung cancer holds this position among women. Several publications have underscored that Mizoram state boasts the highest cancer incidence rates within India.5, 6, 7,16,17 In a comparative assessment of cancer incidence in Northeast India and five continents, based on the IARC report, Aizawl district ranked eleventh, with an ASIR of 273.4 in men and 227.8 in women.7,18 Over this 18-year period, the lowest ASIR recorded for men was 167.8 and for women was 144.1, while the highest ASIR stood at 221.8 for men and 182.2 for women. These figures starkly illustrate the escalating burden of cancer cases over almost two decades, becoming an enduring concern within the population. Each neighbouring northeastern state in India exhibits a unique cancer profile. For instance, Nagaland leads in Nasopharyngeal cancer for both men and women, while Meghalaya (East Khasi Hills) leads in Oesophagus cancer (for both men and women) and Hypopharynx cancer (men) and Arunachal Pradesh (Papumpare District) takes the lead in Stomach cancer (women), as well as Cervix and Liver cancer (men).6 Within Mizoram, Aizawl District, Mizoram State and Mizoram excluding Aizawl consistently top several cancer sites, including Colon and Lung cancers in both men and women. This state also consistently ranks first among all cancer sites in (men).6 The gravity of the cancer burden is further quantified, with Mizoram having the highest Disability Adjusted Life Years (DALYs) at 3424 per 100,000 and also the highest Years Lived with Disability (YLDs) at 153 per 100,000.19 These statistics portray the harsh reality of cancer incidence when compared to mainland India and neighbouring states. Despite being the second-least populous state in the nation, Mizoram confronts a disconcerting surge in cancer incidence and mortality rates, leading to its unfortunate moniker as the “cancer capital of India”.
Unravelling the underlying cause of the escalating cancer incidence during the study period poses a challenging endeavour. Current scientific exploration lacks the requisite depth and comprehensive coverage across diverse facets, encompassing epidemiology, cancer genomics, population genetics (predisposition), pharmacogenomics and beyond. Our prior publications have unveiled a spectrum of mutations in cancer-associated genes, spanning both familiar and novel variants. Notable examples encompass APC, AKT1, and CDH1 in Gastric Cancer, KRT18, CYP4A11, SLC4A3, SLC26A5, KCNS1, ABCD1, YTHDC2, PINX1, TNRC6A, TACO1, LAMA1, ACP7 and ACP7 in Type 2 Diabetes, as well as MT-ND2, MT-ND6, NOTCH1 and FLT3 in Pediatric Leukaemia patients from Mizoram.20, 21, 22, 23, 24, 25 These genetic deviations may owe their existence to the distinctive attributes of the population, potentially serving as pivotal research entry points for exploring potential predisposition mechanisms. As an illustration, the DPYD gene encodes DPD, a determinant governing the bioavailability of 5-Fluorouracil, thus determining treatment efficacy and toxicity for solid tumour patients.26 Intriguingly, our unpublished data reveals an absence of genotypic shifts in the highly polymorphic DPYD gene. This insight prompts the notion that other genes could potentially serve as pharmacogenomic biomarkers within the population. This underscores the exigency of genomic screening to uncover unique biomarkers tailored to this population. In the epoch of genomic medicine, embracing population-wide genome screening for identifying driver mutations or pharmacogenomic markers holds promise in aiding diagnosis and treatment decisions. Identification and utilization of targeted, distinctive SNP panels, harmonized with the clinical requisites of the population, stand as pivotal tasks. These panels could effectively serve as diagnostic instruments and guide the optimal selection of treatment strategies. Earlier investigations have spotlighted a notable pattern: individuals with a first-degree family member afflicted by cancer face an elevated risk of developing Breast cancer.27 This observation underscores the imperative of rigorously probing this hypothesis across all cancer sites. Should congruent patterns emerge, it may lead us to infer a potential inherent predisposition to cancer within the broader population.
According to the latest Globocan 2018 &; 2020 data, the prevalent cancers among men included Lung, Prostate, Colorectum, Stomach, Liver, Bladder, Oesophagus, NHL and Leukaemia. In women, the most common cancers were Breast, Colorectum, Lung, Cervix, Thyroid, Corpus uteri, Stomach, Ovary, Liver and NHL.28 The incidence of Stomach cancer was highest in Eastern Asia from 1980 to 2018, although the global trend for Stomach cancer incidence has been declining in developed nations.29 Given that Stomach cancer has consistently ranked as the most common cancer among men in Mizoram, and considering the rise in Oesophageal cancer cases, it's plausible that significant variables, such as dietary and lifestyle choices, as well as genetic factors specific to South and East Asians, play a role.7 Various risk factors for Stomach cancer have been identified in Mizoram, including the regular consumption of certain foods like processed meat, “sa-um” (a traditional food fermented pork fat), fish and soda as a food additive. These dietary habits have also been linked to an elevated risk of developing diabetes.30,31 Moreover, a study in the Mizoram population revealed that a high intake of animal fats, primarily from red meat during premenopausal years, is associated with an increased risk of Breast cancer.32 Additionally, the consumption of imported fish preserved with formaldehyde (a Group 1 carcinogen) has been found to elevate the risk of Breast cancer.33
In the traditional Mizo diet, foods are typically prepared through boiling, stewing, smoking, or fermentation, encompassing both vegetables and non-vegetable items.34 Wild edible vegetables constitute a valuable food source alongside garden-grown or cultivated vegetables, with diverse parts of the vegetables utilized in various preparations.34 While a substantial portion of rural inhabitants are conscious of the adverse effects linked to carcinogenic substances like smoking, consumption of smoked foods and alcohol consumption, challenges persist in certain rural areas where limited resources preclude access to food storage solutions such as refrigerators. Consequently, reliance on smoking and fermentation as preservation methods is imperative, with these processed foods often used as seasonings, additives, or consumed directly.35 Fermentation and smoking are well-established practices in food preparation and their utilization is prevalent. However, the frequency of consuming such fermented and smoked items can significantly impact health. Particularly, in the northeastern states of India, including Mizoram, a high rate of consumption has been observed. Notably, while these food items hold a place of culinary significance in the northeastern states, certain items such as “Sa-um”, fermented pork fat, are exclusive to Mizoram and not commonly consumed in neighbouring states. The heightened consumption of red meat, whether processed or not, remains a known risk factor for various malignancies, including Colorectal cancer.36 This could also hold relevance in our study, possibly due to extended digestion and assimilation processes within the colon. A scenario analysis conducted within a sub-population in France underscores that restricting consumption to under 65 g of meat daily could mitigate the risk of Colorectal cancer.37 Although precise statistical data regarding daily meat intake eluded us, cultural factors indicate a propensity toward high meat consumption.33,38,39
Alongside dietary habits, the act of smoking in any form has been correlated with escalated risks of Stomach cancer and Head & Neck Cancer.4,30 Tobacco consumption in Mizoram encompasses “Tuibur”, tobacco-infused smoked water, and the use of non-filtered, locally crafted cigarettes known as “Zozial”. Prior investigations conducted within Mizoram have revealed a heightened risk (OR > 1) of Gastric Cancer among tuibur users, with a noteworthy correlation (OR > 1) between Helicobacter pylori infection (a bacterium linked to gastritis and Stomach cancer) and the consumption of tuibur and smoked food, including “sa-um”.38,40 Moreover, cigarette smoking, alcohol consumption and insufficient intake of fruits and vegetables are well-recognized risk factors for distinct subtypes of Gastric Cancer.41 The prevailing rates of Stomach cancer and H. pylori infection, coupled with their connection to specific lifestyle practices within the Mizo population, imply the presence of distinct population-specific lifestyle and demographic factors that warrant thorough investigation to effectively tackle the disease. Pertaining to Head & Neck Cancer, established classical risk factors include smoking and alcohol usage.42 The risk of developing Head & Neck Cancer escalates in a dose-dependent manner with smoking duration, reaching up to a 35-fold increase with alcohol consumption. Mizoram exhibits parallel trends, with the risk of Head & Neck Cancer rising in tandem with greater quantities and extended periods of smoking and alcohol use.43 A similar pattern emerges in Mizoram, where the risk elevates with greater quantities and longer durations of smoking and alcohol use.4 Additionally, the consumption of tobacco-related products (both smoking and smokeless forms) stands as the principal risk factor for the emergence of Head & Neck cancer. This cancer subtype ranks as the second most prevalent among men in the state.6
As per the latest data from the National Family Health Survey, Mizoram holds the highest rate of tobacco usage among men and women across the country.44 The prevalence of tobacco use in men aged 15 and above is notably elevated in Mizoram (73%), followed by Andaman & Nicobar Islands (59%) and Manipur (58%).43 Among women, Mizoram also records the highest tobacco use (62%), trailed by Tripura (51%) and Manipur (43%).43 It is noteworthy that 69.5% of men aged 15 and above belong to urban areas, contrasting with 77.4% in rural regions. For women, the corresponding percentages are 56.8% in urban areas and 68.5% in rural areas. Amid the research period, Lung cancer emerged as the foremost malignancy in women and a leading cause of cancer-related mortality. This trend is potentially linked to the prevalent addiction to Tuibur and Zozial. Additionally, it becomes evident that Zozial smoking significantly elevates cancer risk within the population.4,45 Notably, approximately 77.64% of Head & Neck cancer patients were found to smoke Zozial rather than commercially manufactured cigarettes, showcasing a significant association with cancer risk.4 It is pertinent to acknowledge that smoking, consuming smoked foods and alcohol consumption are deeply ingrained in Mizo customs and traditions, often masking their adverse effects. Furthermore, in rural areas, certain individuals might hold the belief that smoking, or tobacco usage doesn't lead to cancer, possibly due to personal experiences of not having the disease or encountering cancer patients who have not used tobacco products. This underscores the pressing need for comprehensive awareness and education regarding the risks associated with tobacco use. Across the country, the most prevalent cancer sites in each state frequently correlate with dietary habits and tobacco usage. A survey indicates that approximately 70% of Indian cancers are attributable to modifiable and preventable risk factors.46 This underscores the urgency of crafting initiatives for heightened awareness, constituting a pivotal initial stride toward addressing the mounting trend in cancer incidence.
The remote regions of Mizoram exhibit elevated rates of Stomach and Lung cancers (Supplementary Figure S6 l and m), with constrained transportation access and limited availability or exorbitant costs of Liquified Petroleum Gas (LPG). An alternative recourse to the resource deficit involves cooking with firewood, which emits Polycyclic Aromatic Hydrocarbon (PAH).47 A substantial majority (76.4%) of rural inhabitants depend on wood for cooking.48 Notably, certain cooking practices in various Asian countries, including the recycling of cooking oil, have been correlated with an escalated risk of Lung cancer.49, 50, 51 Insufficient kitchen ventilation further compounds health hazards. Regrettably, rural dwellings in Mizoram grapple with inadequate exhaust systems, thereby exacerbating the risks linked to poor ventilation.50 Mizoram has long practiced “Jhum cultivation”, also recognized as “Shifting Cultivation”, for over a century. This technique entails land clearance through controlled burning, followed by abandonment to facilitate regeneration before moving to a different location. A study encompassing over two million Canadians highlights that residing within 50 km of a wildfire over a decade elevates the risk of Lung and Brain cancer.52 Generational exposure to the emissions resulting from these practices across a century may also contribute as a potential factor.
Cervical cancer's correlation with Human Papilloma Virus (HPV) infection is evident, and the community transmission of HPV in Mizoram is supported by a prior pilot study. This study revealed the presence of high-risk HPV genotypes 16, 31, 18, 33, 35, 52 and 58 in both cervicitis and cervical cancer patients.1 According to the NCDIR report of 2021, a mere 6.9% of women in Mizoram have undergone cervical cancer screening.53 This underscores a lack of awareness regarding the significance of screening in the realm of cervical cancer prevention and intervention within the state.
Similar to the trend in incidence, the rise in mortality rates among cancer patients is a distressing phenomenon. Our study recorded the highest mortality rates in the year 2020: 130.1 for men and 98.3 for women. A comparison of these rates with the latest Globocan data reveals that Mizoram's mortality rates are comparable to other regions. For instance, France's La Reunion reports a rate of 130.2 (ranked 45th) for men, while China's rate for women is 98.1 (ranked 28th).28 Within India, the National Cancer Registry Programme (NCRP) report indicates the highest ASMR rate for Aizawl district (Mizoram state capital) at 152.7 for men and 89.5 for women, followed by the entire Mizoram State with ASMR of 121.4 for men and 76.4 for women.6 Multiple factors might contribute to the gradual increase in mortality rates in Mizoram. A notable concern is the absence of early diagnosis, personalized treatment, pharmacogenomics and palliative care. The scarcity of specialized facilities and qualified personnel is a prominent driver of high mortality in Northeast India.54,55 Indeed, the significant discrepancy in ASMR between Mizoram and other Indian regions underscores Mizoram's elevated cancer-related mortality. Mizoram's ASMR ranks highest at 130.1 for men and 98.3 for women, followed by an ASMR of 95 for men in the East Khasi Hills District of Meghalaya State and 61.4 for women in Mumbai (the capital of Maharashtra state), depicting a pronounced mortality rate in Mizoram. This stark variation underscores the pressing need for further research, awareness campaigns and the advancement of enhanced early diagnostic and prognostic methodologies in Mizoram.6
Pinpointing the factors contributing to the heightened mortality rates such as late-stage diagnoses, limited access to timely and suitable treatment and inadequate awareness of preventive measures is vital for devising targeted interventions. These observations have given rise to a plausible hypothesis that the endogamous population and the unaltered cultural and traditional lifestyles may have cumulated risk factors and mutations that potentially contribute to carcinogenesis. This underscores the urgency for additional research, utilizing scientific methodologies across various domains and platforms, to address this pressing public health issue.
The elevated ASMR observed in rural areas can likely be attributed to various factors, including transportation difficulties, poverty and limited access to early diagnostic assessments. In rural regions, the incidence of Cancers of Unknown Primary (CUPs) is notably high, and these cases typically only receive confirmation through DCO (Supplementary Figures S5 and S6g). This pattern suggests a potential deficiency in timely and precise diagnoses, considering that most cancers are identified using microscopic methods. The presence of CUPs in both men and women might indicate either patient neglect or the utilization of suboptimal diagnostic techniques.56 The integration of tumour-specific diagnostic and prognostic biomarkers, along with advanced imaging technologies, is still a challenge to achieve within the state. Consequently, the heightened mortality rate is primarily attributed to the scarcity of accessible infrastructure for diagnostic and treatment facilities, the absence of genetic testing for pharmacogenomic significance, and the lower socioeconomic status prevalent among rural inhabitants in Mizoram. Furthermore, the hurdles in transportation from these remote areas to hospitals located in Aizawl compound the challenges faced by rural communities in accessing quality healthcare.
The discussion underscores the pressing requirement to upgrade Primary Health Care Centres in rural areas like Mizoram. These centres must be furnished with skilled technicians and sufficient infrastructure to perform essential diagnostic tests. Currently, the predominant diagnostic approach relies on microscopy, with limited access to imaging technologies such as X-ray and CT scans for specific cancers (Supplementary Figure S5). The absence of more advanced and precise cancer-specific diagnostic techniques like Fluorescence In-situ Hybridization (FISH) or DNA sequencing methods like Sanger and Next Generation Sequencing (NGS) is evident. Furthermore, genetic counselling practices at the population level are deficient, impeding the comprehension and application of pharmacogenomic insights for cancer treatment. There is an urgent necessity for comprehensive cancer research that spans integrated aspects such as epidemiology, aetiology, lifestyle factors and dietary habits, along with population-wide genomic screening that could be developed as diagnostic and prognostic panels for rural populations. This imperative underscore the need for international agencies to prioritize such research endeavours, given the state's limited resources for enhancing its healthcare system. The cross-sectional design of this study does have limitations in fully exploring all cancer types and the factors driving the rise in incidences and mortalities. Nevertheless, this paper's primary intent is to bring the grave and alarming condition of cancer prevalence in Mizoram to the attention of the international scientific community, funding organizations and governmental regulatory bodies. The aim is to encourage greater involvement in addressing the cancer crisis in the state. This study underscores the necessity for Governmental and Non-Governmental organizations to intervene through robust awareness campaigns, promoting better education on dietary choices, and the hazards of tobacco consumption, as well as implementing cessation programs for chewing and smoking. Initiatives like HPV vaccination and enhanced screening for Oral cavity, Lung, Cervical and Breast cancers are also essential. The socioeconomic, genetic and traditional contexts of the Mizo population all hold significance within this study. Elucidating potential risk factors and outlining a path toward an improved cancer-related healthcare system also holds relevance for regions confronting similar challenges globally.
Contributors
EZ, EVH, NSK, JZ conceptualized and supervised the study. EZ and EVH provided the resources and curated the data. LT, LC, LP and JP clinically validated the data. ZZ, AV and EZ did the formal analysis, visualisation and wrote the original draft of the manuscript. EZ and EVH administered the project. All the authors reviewed and revised the manuscript.
Data sharing statement
The data used for this study were retrieved from Population Based Cancer Registry, National Centre for Disease Information and Research (NCDIR) of Indian Council of Medical Research (ICMR). The corresponding author is responsible for acquiring the data. The NCDIR policy on data processing and disclosure protects the data and permission to access the data is given based on Scientific merit and for medical/public health research or the administration of cancer related public health programs. Researchers may request ‘Chief Data controller’ of ICMR-NCDIR to access any data.
Declaration of interests
The authors declare that they have no conflict of interests.
Acknowledgement
The authors would like to thank the Medical Superintendent, Civil Hospital Aizawl for the support. The authors are deeply grateful to Dr. Prashant Mathur, Director of NCDIR as this undertaking would not have been possible without their contribution. The authors would like to express their sincere gratitude to the registry staff members of Population Based Cancer Registry (PBCR) and all the sources of registrations - Hospital Based Cancer Registry (HBCR) in government and private hospitals, nursing homes and diagnostic laboratories within the state for their diligent work in collection of data. The authors thank the Directorate of Economics and Statistics Department, Government of Mizoram, India and the Health and Family Welfare Department, Government of Mizoram, India for their unhindered cooperation. The authors would like to thank Department of Science & Technology, New Delhi for providing DST-INSPIRE fellowship to the author ZZ (DST/INSPIRE Fellowship/IF180827). The authors are extremely grateful to DBT, New Delhi for providing Advanced State Level Biotech Hub, MZU (BT/NER/143/SP44475/2021) for computational analysis.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2023.100281.
Appendix A. Supplementary data
References
- 1.Sailo C.V., Tonsing M.V., Sanga Z., et al. Risk factors of tuberculosis in Mizoram: first report of the possible role of water source. Indian J Tuberc. 2022;69(4):675–681. doi: 10.1016/j.ijtb.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Census of India 2011, Government of India. https://www.censusindia.co.in/states/mizoram
- 3.Forest Survey of India Ministry of environment, forest & climate change. https://fsi.nic.in/cover_2011/mizoram.pdf
- 4.Pachuau L., Zami Z., Nunga T., et al. First-degree family history of cancer can be a potential risk factor among head and neck cancer patients in an isolated Mizo tribal population, northeast India. Clin Epidemiol Glob Health. 2022;13 [Google Scholar]
- 5.National Centre for Disease Informatics and Research Consolidated report of population based cancer registries, 2006-2008, 2009-2011, 2012-2014, Bengaluru, India, National Cancer Registry Programme (NCRP-ICMR) https://ncdirindia.org/Reports.aspx
- 6.Report of National cancer registry Programme (2012-2016) Bangaluru; India: 2020. [Google Scholar]
- 7.Sharma J.D., Kalit M., Nirmolia T., Saikia S.P., Sharma A., Barman D. Cancer: scenario and relationship of different geographical areas of the globe with special reference to North East-India. Asian Pac J Cancer Prev. 2014;15(8):3721–3729. doi: 10.7314/apjcp.2014.15.8.3721. [DOI] [PubMed] [Google Scholar]
- 8.Omran A.R. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49:509–538. [PubMed] [Google Scholar]
- 9.Gersten O., Wilmoth J.R. The cancer transition in Japan since 1951. Demogr Res. 2002;7:271–306. [Google Scholar]
- 10.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 11.WHO: international statistical classification of diseases and related health problems. World Health Organization; Geneva, Switzerland: 1994. [Google Scholar]
- 12.Segi M. The Department of Public Health, Tohoku University School of Medicine; 1960. Cancer mortality for selected sites in 24 countries 1950-1957. Sendai, Japan. [Google Scholar]
- 13.Joinpoint Regression Program, version 4.9.1.0; Statistical Methodology and Applications Branch, Surveillance Research Program. National Cancer Institute; 2023. [Google Scholar]
- 14.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for Joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. (correction: 2001; 20: 655) [DOI] [PubMed] [Google Scholar]
- 15.Flourish Studio Kiln enterprises Ltd. 2020. https://flourish.studio/
- 16.Shanker N., Mathur P., Das P., Sathishkumar K., Martina Shalini A.J., Chaturvedi M. Cancer scenario in North-East India & need for an appropriate research agenda. Indian J Med Res. 2021;154(1):27–35. doi: 10.4103/ijmr.IJMR_347_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.India State-Level Disease Burden Initiative Cancer Collaborators The burden of cancers and their variations across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Oncol. 2018;19(10):1289–1306. doi: 10.1016/S1470-2045(18)30447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferlay J., Curado M.P., Edwards B., et al. IARC Scientific publications No. 160; Lyon, IARC: 2007. Cancer incidence in five continents, VOL. IX. [Google Scholar]
- 19.Kulothungan V., Sathishkumar K., Leburu S., et al. Burden of cancers in India–estimates of cancer crude incidence, YLLs, YLDs and DALYs for 2021 and 2025 based on National Cancer Registry Program. BMC Cancer. 2022;22:527. doi: 10.1186/s12885-022-09578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghatak S., Chakraborty P., Sarkar S.R., et al. Novel APC gene mutations associated with protein alteration in diffuse type gastric cancer. BMC Med Genet. 2017;18:61. doi: 10.1186/s12881-017-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghatak S., Lalnunhlimi S., Lalrohlui F., et al. Novel AKT1 mutations associated with cell-cycle abnormalities in gastric carcinoma. Per Med. 2018;15(2):79–86. doi: 10.2217/pme-2017-0053. [DOI] [PubMed] [Google Scholar]
- 22.Lalrohlui F., Zohmingthanga J., Hruaii V., Vanlallawma A., Kumar N.S. Whole exome sequencing identifies the novel putative gene variants related with type 2 diabetes in Mizo population, northeast India. Gene. 2021;769 doi: 10.1016/j.gene.2020.145229. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty P., Ghatak S., Yadav R., et al. Novel somatic mutations of the CDH1 gene associated with gastric cancer: prediction of pathogenicity using comprehensive in silico methods. Curr Pharmacogenomics Person Med. 2020;17(1):182–196. [Google Scholar]
- 24.Vanlallawma A., Zami Z., Pautu J.L., et al. Pediatric leukemia could be driven predominantly by non-synonymous variants in mitochondrial complex V in Mizo population from Northeast India. Mitochondrial DNA A DNA Mapp Seq Anal. 2020;31(6):245–249. doi: 10.1080/24701394.2020.1786545. [DOI] [PubMed] [Google Scholar]
- 25.Vanlallawma A., Lallawmzuali D., Pautu J.L., Scaria V., Sivasubbu S., Kumar N.S. Whole exome sequencing of pediatric leukemia reveals a novel InDel within FLT-3 gene in AML patient from Mizo tribal population, Northeast India. BMC Genom Data. 2022;23(1):23. doi: 10.1186/s12863-022-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hariprakash J.M., Vellarikkal S.K., Keechilat P., et al. Pharmacogenetic landscape of DPYD variants in south Asian populations by integration of genome-scale data. Pharmacogenomics. 2018;19(3):227–241. doi: 10.2217/pgs-2017-0101. [DOI] [PubMed] [Google Scholar]
- 27.Zodinpuii D., Pautu J.L., Zothankima B., Pachuau L., Kumar N.S. Clinical features and first-degree relative breast cancer, their correlation with histological tumor grade: a 5-year retrospective case study of breast cancer in Mizoram, India. Environ Sci Pollut Res Int. 2020;27(2):1991–2000. doi: 10.1007/s11356-019-06944-8. [DOI] [PubMed] [Google Scholar]
- 28.Ferlay J., Ervik M., Lam F., et al. International Agency for Research on Cancer; Lyon, France: 2020. Global cancer observatory: cancer today.https://gco.iarc.fr/today Available from: [Google Scholar]
- 29.Wong M.C.S., Huang J., Chan P.S.F., et al. Global incidence and mortality of gastric cancer, 1980-2018. JAMA Netw Open. 2021;4(7):1–14. doi: 10.1001/jamanetworkopen.2021.18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phukan R.K., Narain K., Zomawia E., Hazarika N.C., Mahanta J. Dietary habits and stomach cancer in Mizoram, India. J Gastroenterol. 2006;41(5):418–424. doi: 10.1007/s00535-006-1761-x. [DOI] [PubMed] [Google Scholar]
- 31.Lalrohlui F., Ghatak S., Zohmingthanga J., Hruaii V., Kumar N.S. Fermented pork fat (Sa-um) and lifestyle risk factors as potential indicators for type 2 diabetes among the Mizo population, Northeast India. J Health Popul Nutr. 2021;40(1):32. doi: 10.1186/s41043-021-00257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thapa S., Lalrohlui F., Ghatak S., et al. Mitochondrial complex I and V gene polymorphisms associated with breast cancer in mizo-mongloid population. Breast Cancer. 2016;23(4):607–616. doi: 10.1007/s12282-015-0611-1. [DOI] [PubMed] [Google Scholar]
- 33.Zodinpuii D., Pautu J.L., Zothankima B., et al. Breast cancer is significantly associated with cancers in the first- and second-degree relatives in ethnic mizo-mongoloid population, Northeast India. Natl J Community Med. 2022;13(9):606–611. [Google Scholar]
- 34.Kar A., Bora D., Borthakur S.K., Goswami N.K., Saharia D. Wild edible plant resources used by the Mizos of Mizoram, India. Kathmandu University. J Sci Eng Technol. 2013;9(1):106–126. [Google Scholar]
- 35.Lalthanpuii P.B., Lalruatfela B., Zoramdinthara L.H. Traditional food processing techniques of the Mizo people of Northeast India. Sci Vis. 2015;15(1):39–45. [Google Scholar]
- 36.Farvid M.S., Sidahmed E., Spence N.D., et al. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2021;36:937–951. doi: 10.1007/s10654-021-00741-9. [DOI] [PubMed] [Google Scholar]
- 37.De Oliveira Mota J., Boué G., Guillou S., Pierre F., Membré J.M. Estimation of the burden of disease attributable to red meat consumption in France: influence on colorectal cancer and cardiovascular diseases. Food Chem Toxicol. 2019;130:174–186. doi: 10.1016/j.fct.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty P., Ghatak S., Chenkual S., et al. Panel of significant risk factors predicts early stage gastric cancer and indication of poor prognostic association with pathogens and microsatellite stability. Genes Environ. 2021;43(1):1–15. doi: 10.1186/s41021-021-00174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadav R.P., Ghatak S., Chakraborty P., et al. Lifestyle chemical carcinogens associated with mutations in cell cycle regulatory genes increases the susceptibility to gastric cancer risk. Environ Sci Pollut Res Int. 2018;25(31):31691–31704. doi: 10.1007/s11356-018-3080-1. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee S., Madathil S.A., Ghatak S., et al. Association of tobacco smoke–infused water (tuibur) use by Mizo people and risk of Helicobacter pylori infection. Environ Sci Pollut Res. 2020;27(8):8580–8585. doi: 10.1007/s11356-019-07543-3. [DOI] [PubMed] [Google Scholar]
- 41.Rawla P., Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leemans C.R., Snijders P.J.F., Brakenhoff R.H. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18(5):269–282. doi: 10.1038/nrc.2018.11. [DOI] [PubMed] [Google Scholar]
- 43.Wyss A., Hashibe M., Chuang S.C., et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the international head and neck cancer epidemiology consortium. Am J Epidemiol. 2013;178(5):679–690. doi: 10.1093/aje/kwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.International Institute for Population Sciences (IIPS) and ICF . IIPS; Mumbai: 2021. National family health survey (NFHS-5), India, 2019-21: Mizoram. [Google Scholar]
- 45.Tonsing M.V., Ghatak S., Lalrohlui F., Kumar N.S., Zohmingthanga J. Five year record on cancer incidence from a diagnostic centre in Mizoram, Northeast India. Cancer Health Disparities. 2019;3:e1–e15. [Google Scholar]
- 46.Gandhi A.K., Kumar P., Bhandari M., Devnani B., Rath G.K. Burden of preventable cancers in India: time to strike the cancer epidemic. J Egypt Natl Canc Inst. 2017;29(1):11–18. doi: 10.1016/j.jnci.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Moorthy B., Chu C., Carlin D.J. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci. 2015;145(1):5–15. doi: 10.1093/toxsci/kfv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Report on monitoring survey of cancer risk factors and health system response in North East Region (NER) https://ncdirindia.org/All_Reports/ner2022/Mizoram.aspx
- 49.Chiang T.A., Wu P.F., Ko Y.C. Prevention of exposure to mutagenic fumes produced by hot cooking oil in Taiwanese kitchens. Environ Mol Mutagen. 1998;31(1):92–96. [PubMed] [Google Scholar]
- 50.Kim C., Gao Y.T., Xiang Y.B., et al. Home kitchen ventilation, cooking fuels, and lung cancer risk in a prospective cohort of never smoking women in Shanghai, China. Int J Cancer. 2015;136(3):632–638. doi: 10.1002/ijc.29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen T.Y., Fang Y.H., Chen H.L., et al. Impact of cooking oil fume exposure and fume extractor use on lung cancer risk in non-smoking Han Chinese women. Sci Rep. 2020;10:6774. doi: 10.1038/s41598-020-63656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korsiak J., Pinault L., Christidis T., Burnett R.T., Abrahamowicz M., Weichenthal S. Long-term exposure to wildfires and cancer incidence in Canada: a population-based observational cohort study. Lancet Planet Health. 2022;6(5) doi: 10.1016/S2542-5196(22)00067-5. [DOI] [PubMed] [Google Scholar]
- 53.ICMR-NCDIR . Bengaluru; India: 2021. Profile of cancer and related health indicators in the North East region of India. [Google Scholar]
- 54.Ngaihte P., Zomawia E., Kaushik I. Cancer in the NorthEast India: where we are and what needs to be done? Indian J Public Health. 2019;63:251–253. doi: 10.4103/ijph.IJPH_323_18. [DOI] [PubMed] [Google Scholar]
- 55.Vaiphei S.D., Sisodia D.S. Terminal cancer in Northeast India: an analytical study on its rapid growth, causes and solutions. Eur J Res. 2020;6(3):248–256. [Google Scholar]
- 56.Rassy E., Assi T., Pavlidis N. Exploring the biological hallmarks of cancer of unknown primary: where do we stand today? Br J Cancer. 2020;122(8):1124–1132. doi: 10.1038/s41416-019-0723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.