Summary
Background
Central nervous system (CNS) metastases is inevitable for epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC). AZD3759 is a novel EGFR-TKI with impressive CNS penetration.
Methods
We initiated a phase 2, multi-center, umbrella trial (CTONG1702, NCT03574402). The eighth arm assessed the efficacy and safety of AZD3759 in untreated EGFR-mutated NSCLC with CNS metastases. The primary objective was the objective response rate (ORR). Simon’s minimax two-stage design was used to calculate the sample size. Dose optimal selection was performed using 200- and 300-mg bid cohorts.
Findings
Between Oct 18, 2018 and Sep 14, 2020, 30 patients received AZD3759 at 200 mg (n = 15) or 300 mg (n = 15) bid. At data cutoff (Dec 31, 2022), median follow-up was 35.4 months. The primary endpoint was reached, with a confirmed ORR of 70% (21/30) (200 mg, 80%; 300 mg, 60%). The median progression-free survival was 12.9 months (200 mg, 15.8 months; 300 mg, 10.7 months). Grade 3 or 4 treatment-related adverse events occurred in 73% (22/30) of the patients (200 mg: 60%; 300 mg: 87%). 59% (10/17) of the patients developed a T790M mutation at disease progression. The median overall survival was 33.7 months, and 34.1 months and 25.3 months in patient treated with or without osimertinib in a later-line setting, respectively.
Interpretation
AZD3759 showed promising efficacy and tolerable safety as a first-line therapy in EGFR-mutated NSCLC with CNS metastases. The 200-mg bid cohort had better clinical outcomes. Sequential use of AZD3759 and third-generation EGFR-TKIs represents a new option.
Funding
Chinese Thoracic Oncology Group (CTONG).
Keywords: EGFR mutation, CNS metastasis, AZD3759, Lung cancer, First-line setting
Research in context.
Evidence before this study
We searched PubMed for reports published between January 1, 2010 and April 20, 2023, with the terms “EGFR”, “epidermal growth factor receptor”, “tyrosine kinase inhibitor”, “TKI”, “lung cancer”, “CNS metastases”, “brain metastasis”, “BM”, “leptomeningeal metastasis”, or “LM”; and the terms “dose selection”, “dose finding”, or “dose optimization”. Independently, we searched the terms “dose” or “dosage” and “AZD3759.” We did not restrict our search by language. The data indicated that epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) had limited effects on patients with central nervous system (CNS) metastases. A prespecified analysis of data from phase III studies indicated that third-generation EGFR-TKIs showed potential clinical activity in a first-line setting in this population. AZD3759 is a novel agent with favourable penetration of the blood–brain barrier. The BLOOM study generated 300 mg as maximum tolerated dose and selected 200 mg as the recommended phase 2 dose. However, the sample size for comparison of the two doses was limited, and the survival data are immature. Dose optimal trials are expected to perform in pre-market settings.
Added value of this study
This treatment arm of an umbrella study showed promising clinical activity and safety of AZD3759 in previously untreated patients with EGFR-mutated non-small cell lung cancer (NSCLC) and CNS metastases. A dose of 200 mg twice daily elicited a higher response, was associated with longer survival, and had lower toxicity compared with 300 mg. EGFR T790M mutation was the major resistance mechanism. The sequential use of AZD3759 and third-generation EGFR-TKIs led to good overall survival (OS) in the target population.
Implications of all the available evidence
This prospective clinical trial assessed overall objective tumour response and survival benefit in patients with NSCLC and CNS metastases treated with AZD3759. Our data suggest that AZD3759 is valuable as a first-line therapy for this group. While dose selection could be optimized via a randomized study, this is time consuming and labour-intensive. We conducted a comprehensive comparation of two treatment doses of AZD3759 for a registered clinical trial and aimed to improve patient care via a dose-finding method. Our encouraging OS data suggest the possibility of sequential use of AZD3759 and third-generation EGFR-TKIs in this patient group.
Introduction
Central nervous system (CNS) metastases of non-small cell lung cancer (NSCLC), such as brain metastasis (BM) and leptomeningeal metastasis (LM), are associated with a poor prognosis and low quality of life.1 The development of molecular targeted therapy has changed the landscape of CNS metastasis management.2 The BRAIN study indicated that the epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) icotinib was superior to whole-brain irradiation plus chemotherapy for treatment of patients with EGFR-mutated NSCLC and brain metastases.3 However, despite treatment with first-generation EGFR-TKIs, up to 40% of patients developed CNS metastases over the course of the disease.4
Third-generation EGFR-TKIs, such as osimertinib, have been approved as standard first-line treatments for patients with EGFR-mutated NSCLC.5, 6, 7 The prespecified analysis of the AURA3 and other phase III studies revealed encouraging results in patients with CNS metastasis.8, 9, 10 The approved EGFR-TKIs have limited ability to cross the blood–brain barrier (BBB), with Kpuu values (the ratio of cerebrospinal fluid [CSF] to plasma concentration) ranging from 0.066 to 0.29.11 Therefore, novel molecular targeted agents with improved Kpuu values may offer improved CNS disease control, and are thus a clinical priority.
AZD3759 is a potent, orally administered EGFR-TKI with high BBB penetrability (Kpuu value = 1.1–1.4), that could inhibit phosphorylation of EGFR in CSF tumour cells.12 Preclinical data have indicated that this is the first drug to be found in equivalent concentrations in the blood, CSF, and brain tissue following administration.13 In phase I of the BLOOM study, 300 mg was defined as the maximum tolerated dose (MTD) in dose-escalation cohorts, and doses of both 200 mg and 300 mg were evaluated in expansion cohorts.14 Although 200 mg was established as the recommended phase 2 dose (RP2D), data were limited with a small sample size in the 300 mg group. Furthermore, the follow-up duration was insufficient for calculation of progression-free survival (PFS) and duration of response (DoR).
Several ongoing studies are investigating the effects of high doses of EGFR-TKIs as a first-line therapy in patients with CNS metastasis in a post-market setting. For some drugs, such as the KRAS-G12C inhibitor sotorasib and the ALK-TKI ceritinib, the recommended doses are needed to be revised post approval because of safety concerns.15,16 To address the challenges associated with dose optimization in targeted therapies, Project Optimus gathered a wide range of experts to reform the dose optimization and dose selection paradigm in oncology, and to draft Food and Drug Administration (FDA) documents to provide guidance for industries and investigators.17,18 Dose-finding clinical trials in pre-market settings are urgently needed, especially those that characterize two or more doses rather than only the highest dose tolerated or tested.19
Herein, we report the results of a phase 2, multi-center, umbrella study (CTONG1702) conducted to assess the efficacy, safety, and dose selection of AZD3759 in untreated patients with EGFR-mutated NSCLC and CNS metastases.
Methods
Study design and participants
CTONG1702 was an open-label, multicentre, phase 2 adaptive umbrella trial. A detailed description of the study design has been previously published.20 Briefly, patients from whom we obtained sufficient formalin-fixed, paraffin-embedded tumour tissue within 6 months before enrolment underwent next-generation sequencing (NGS) screening, with at least 400 genes being screened in the CTONG Joint Laboratory. Based on their genomic variation data, the patients were assigned to different parallel treatment arms and each of them is single arm. In the eighth arm, we evaluated the efficacy, safety, and dose (200- and 300-mg cohorts) of AZD3759 as monotherapy in previously untreated patients with EGFR-sensitive mutations and locally advanced or metastatic NSCLC with BM or LM.
Participants eligible for study enrolment were aged ≥18 years and had cytologically or pathologically confirmed NSCLC; stage IV disease (8th AJCC); EGFR-sensitive mutations; no history of systemic antitumor therapy for metastatic disease; brain parenchymal metastasis confirmed by imaging or LM confirmed by imaging or CSF cytology; an Eastern Cooperative Oncology Group performance score of 0–2; at least one disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; adequate hepatic, kidney, and bone marrow function; and a normal QT interval. Patients with asymptomatic or symptomatic BM or LM could be enrolled at the investigator’s discretion if no local treatment was needed. Both male and female patients were eligible. The key exclusion criteria were EGFR T790M mutation, EGFR exon 20 insertion, or MET amplification (copy number ≥5); active interstitial lung disease; active hepatitis or infectious disease; and prior radiotherapy for ≥30% of bone marrow within the 4 weeks before study treatment. The full eligibility criteria are listed in the protocol (appendix).
The study protocol was approved by the independent ethics committee for each participating center: Guangdong Provincial People’s Hospital (2018-23-59); Tongji Medical College, Huazhong University of Science and Technology (2019-S916-4 for Cancer Center, Union Hospital); the First Affiliated Hospital of Anhui Medical University (PJ2020-07-07 (1)); the First Hospital of Jilin University (19Y033-010); Tongji Medical College, Huazhong University of Science and Technology (2019-S916-3 for Department of Oncology, Tongji Hospital); Liaoning Cancer Hospital (202101115); The Northern Jiangsu People’s Hospital (2019031-4); and Henan Provincial People's Hospital (2020-089-02). And the procedures were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent before the study procedures, sampling, and analyses. The CTONG1702 study was registered on ClinicalTrials.gov (No. NCT03574402).
Procedures
Patients received AZD3759 orally at a treatment dose of 300 mg bid (first stage of enrolment) or 200 mg bid (second stage of enrolment) in a 28-day treatment cycle until disease progression, unacceptable toxicity, withdrawal of consent, or a decision by the investigator to withdraw the treatment. Treatment delays and dose modifications as a result of toxic effects were allowed and specified in the protocol (see Appendix). Patients who were determined to have achieved a complete response (CR) or partial response (PR) underwent a follow-up assessment at least 4 weeks after the initial response. Tumour imaging assessments were conducted at baseline, after 4 and 8 weeks of treatment, and every 8 weeks thereafter according to RECIST version 1.1, using contrast-enhanced computed tomography or magnetic resonance imaging (MRI). Enhanced cranial MRI was mandatory at baseline and subsequent assessment visits. AZD3759 treatment could be continued after disease progression if the patient was experiencing clinical benefit according to the investigator’s assessment. Adverse events (AEs) were monitored until 30 days after the last dose and graded according to the Common Terminology Criteria for Adverse Events version 4.03.
Outcomes
The primary endpoint was the objective response rate (ORR), defined as the proportion of patients whose best overall response was confirmed as a CR or PR. The secondary endpoints were the PFS, overall survival (OS), disease control rate (DCR), DoR, and toxicity. Optimal dose selection was performed following subgroup analysis as an exploratory endpoint. PFS was defined as the time from the date of the first dose until the date of the first sign of disease progression, or all-cause death. OS was defined as the time from the date of the first dose until all-cause death. DCR was defined as the proportion of patients whose best overall response was CR, PR, or stable disease. DoR was defined as the time from the date of the first documented response (subsequently confirmed) until the date of the first documented progression, or all-cause death.
Statistical analysis
To determine whether the sub-study treatment elicited sufficient activity to warrant further study, we used Simon's minimax two-stage design for all treatment arms of CTONG1702. For the AZD3759 arm, a true ORR of ≤15% was considered unacceptable (null hypothesis), whereas a true ORR of ≥35% merited further study (alternative hypothesis). In the first stage, 15 patients were enrolled; if there were fewer than three responses observed at the interim analysis of the 15 patients, the sub-study was closed (see Appendix, Supplementary Fig. S1). Otherwise, 13 additional patients could be accrued for a total of 28 patients. At a type I error rate of 5% and with 80% power, all 28 patients needed to be evaluable in terms of the best overall response to the study treatment. A 10% dropout rate was assumed, so the maximum number of patients was set at 31.
All efficacy and safety endpoints were assessed in the full analysis set, which comprised patients who had received at least one dose of AZD3759. ORR and DCR were calculated, and their 95% confidence intervals (CIs) were estimated using the Clopper–Pearson method. DoR, PFS, and OS were estimated using the Kaplan–Meier method. Subgroup analyses were performed to explore the antitumor activity of AZD3759, classified according to age, sex, smoking status, treatment dose, EGFR mutation subtype, and BM or LM. The data cutoff was December 31, 2022. Statistical software (SAS 9.4 [SAS Institute, Carty, NC, USA] and R 4.2.2 [R Development Core Team, Vienna, Austria]) were used for all analyses.
Both male and female patients were eligible. Sex data were extracted from the identity information provided by the patients.
Role of the funding source
The study was designed by the sponsor and the principal investigator (Y.L. Wu). The sponsor provided funding and organizational support, and had a role in the data collection, analysis, and interpretation, as well as the writing of the report.
Results
Between October 18, 2018 and September 14, 2020, 606 previously untreated patients with advanced NSCLC were consecutively screened using NGS. Of these patients, 30 (5%) identified as having EGFR-sensitive mutations and BM or LM were enrolled in the AZD3759 arm. After an interim analysis of first stage (n = 15), this treatment arm stepped into the second stage (n = 15) (Appendix, Supplementary Fig. S2). All 30 patients had received at least one dose of AZD3759 and were eligible for inclusion in the efficacy and safety analysis. A total of 15 (50%) patients were treated with AZD3759 200 mg bid and 15 (50%) were treated with 300 mg bid. At the data cutoff of December 31, 2022, the median follow-up time was 35.4 months (range: 0.9–49.3 months). All of the patients discontinued treatment: the main reason was disease progression, as assessed by the investigator (25 patients; 83%). Four patients discontinued because of withdrawal of consent, and one patient discontinued because of an AE. At the data cutoff, 16 (53%) patients had died, 8 were alive, and 6 were lost to follow-up.
The baseline characteristics of the enrolled patients are shown in Table 1. The median age of all patients, and of those in the 200- and 300-mg cohorts, was 59, 56, and 64 years, respectively; 53%, 53%, and 53% of the patients were female; 40%, 33%, and 47% of the patients had a smoking history; and all patients had adenocarcinoma and stage IV disease. We found that 28 patients (93%) had BM, 1 had LM, 1 had BM and LM, and none received local radiotherapy for CNS lesions. Furthermore, 18 patients (60%) had an EGFR exon 19 deletion, 11 (37%) had an exon 21 L858R mutation, and 1 had an exon 20 S768I mutation. In the 200-mg and 300-mg groups, most patients had BM and an EGFR exon 19 deletion.
Table 1.
Baseline demographic and clinical characteristics in the full analysis population.
| All patients (n = 30) | 200 mg cohort (n = 15) | 300 mg cohort (n = 15) | |
|---|---|---|---|
| Age, years | 59 (35–72) | 56 (35–65) | 64 (45–72) |
| Sex | |||
| Male | 14 (47%) | 7 (47%) | 7 (47%) |
| Female | 16 (53%) | 8 (53%) | 8 (53%) |
| Smoking history | |||
| Current or former | 12 (40%) | 5 (33%) | 7 (47%) |
| Never | 18 (60%) | 10 (67%) | 8 (53%) |
| Pathologic subtypes | |||
| Adenocarcinoma | 30 (100%) | 15 (100%) | 15 (100%) |
| ECOG performance status | |||
| 0 | 1 (3%) | 0 | 1 (7%) |
| 1 | 29 (97%) | 15 (100%) | 14 (93%) |
| Clinical stage | |||
| IVA | 2 (7%) | 1 (7%) | 1 (7%) |
| IVB | 28 (93%) | 14 (93%) | 14 (93%) |
| Brain or leptomeningeal metastasis | |||
| Brain metastasis | 28 (93%) | 13 (87%) | 15 (100%) |
| Leptomeningeal metastasis | 1 (3%) | 1 (7%) | 0 |
| Brain and leptomeningeal metastasis | 1 (3%) | 1 (7%) | 0 |
| EGFR mutation subtypes | |||
| Exon 19 deletion | 18 (60%) | 11 (73%) | 7 (47%) |
| Exon 21 L858R mutation | 11 (37%) | 4 (27%) | 7 (47%) |
| Exon 20 S768I | 1 (3%) | 0 | 1 (7%) |
Data are median (range) or n (%).
ECOG = Eastern Cooperative Oncology Group performance.
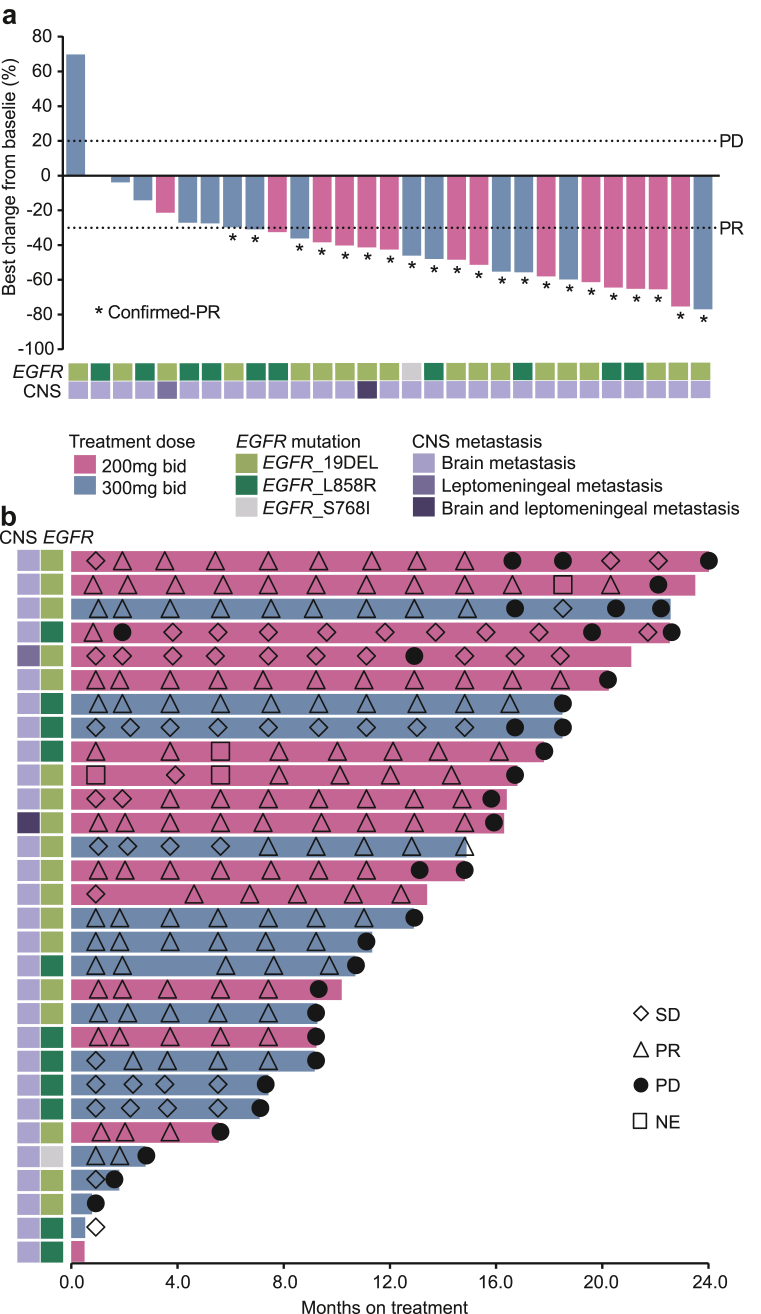
The trial achieved its primary endpoint, with an investigator-assessed confirmed ORR of 70% (95% CI, 51–85). The ORR was 80% and 60% in the 200- and 300-mg cohorts, respectively (Table 2). Most of the patients exhibited disease control and a reduction in tumour size (Fig. 1a), with a DCR of 83% (95% CI, 65–94) in all patients, 87% in the 200-mg cohort, and 80% in the 300-mg cohort. Only one patient experienced a partial response (PR) and progressive disease (PD), at 4 and 8 weeks, respectively. This patient, in the 200-mg cohort, was finally classified as having PD and continued AZD3759 treatment for 21.5 months after the first disease progression (because of the potential benefit, as judged by the investigator). The intracranial ORR of AZD3759 was 73% (95% CI, 54–88) in all patients, and 73% in the 200 mg- and 300 mg-cohorts (Appendix, Supplementary Table S1). Based on subgroup analyses, 200 mg bid dose recipients, females, non-smokers, and EGFR exon 19 deletion patients seemed to attain a favourable response to AZD3759 treatment (Appendix, Supplementary Fig. S3). We summarized the clinical activity of AZD3759 in patients with an EGFR exon 19 deletion or an exon 21 L858R mutation in Supplementary Table S2 (Appendix).
Table 2.
Clinical activity endpoints in the full analysis population.
| All patients (n = 30) | 200 mg cohort (n = 15) | 300 mg cohort (n = 15) | |
|---|---|---|---|
| Confirmed overall response rate | 21 (70%; 51–85) | 12 (80%; 52–96) | 9 (60%; 32–84) |
| Disease control rate | 25 (83%; 65–94) | 13 (87%; 60–98) | 12 (80%; 52–96) |
| Best overall response | |||
| Confirmed partial response | 21 (70%) | 12 (80%) | 9 (60%) |
| Stable disease | 4 (13%) | 1 (7%) | 3 (20%) |
| Progressive disease | 3 (10%) | 1 (7%)a | 2 (13%) |
| Not evaluable | 2 (7%) | 1 (7%) | 1 (7%) |
| Median duration of response, months | 12.0 (8.3–14.9) | 12.2 (8.3–16.9) | 10.2 (1.6–15.7) |
| Median progression free survival, months | 12.9 (9.2–16.6) | 15.8 (9.2–17.0) | 10.7 (2.5–16.6) |
| Median overall survival, months | 33.7 (18.4–46.4) | 28.6 (17.8-NA) | 33.7 (5.2–46.4) |
Data are n (%) or median (95% CI). All assessments were based on investigators’ evaluation.
This patient experienced PR and PD response at 4 and 8 weeks, and continued AZD3759 treatment for 21.5 months because of potential benefit assessed by investigator after first disease progression.
Fig. 1.
Waterfall and swimming plots. Tumour response and maximum change from baseline according to target lesion size (a). Treatment duration of AZD3759 for enrolled patients (b). CNS, central nervous system; NE, not evaluable; PR, partial response; PD, progressive disease; SD, stable disease.
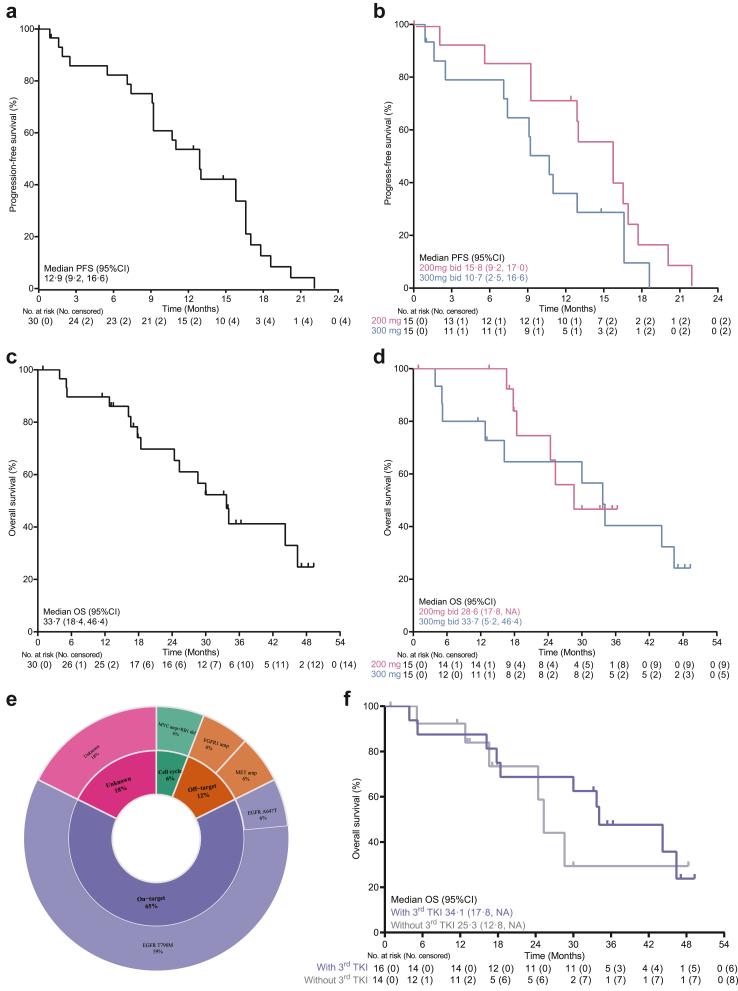
At the data cutoff, the median DoR was 12.0 months (95% CI, 8.3–14.9) in all patients, and 12.2 and 10.2 months in the 200- and 300-mg cohorts, respectively (Appendix, Supplementary Fig. S4). Six (20%, 6/30) patients continued AZD3759 treatment after the first documented sign of disease progression (Fig. 1b). The median PFS was 12.9 months (95% CI, 9.2–16.6) in all patients, and 15.8 and 10.7 months in the 200- and 300-mg cohorts, respectively (Fig. 2a and b). The median OS was 33.7 months (95% CI, 18.4–46.4) in all patients, 28.6 months in the 200 mg cohort, and 33.7 months in the 300 mg cohort (Fig. 2c and d). The median intracranial PFS was 17.1 months in all patients, and 18.5 and 16.9 months in the 200- and 300-mg cohorts, respectively (Appendix, Supplementary Fig. S5).
Fig. 2.
Survival outcomes and molecular mechanisms of acquired resistance. Progression-free survival in all patients (a), and in the 200- and 300-mg cohorts (b). OS in all patients (c), and in the 200- and 300-mg cohorts (d). Acquired molecular mechanisms in on-target, off-target, and cell cycle pathways (e). Median OS in patients who were treated and not treated with third-generation EGFR-TKIs in a later-line setting, respectively (f). CI, confidence interval; NA, not applicable; PFS, progression free survival; OS, overall survival.
We found that 97% (29/30) of the patients who received AZD3759 had at least one treatment-related AE (TRAE), as reported by the investigators, with a frequency of 93% (14/15) and 100% (15/15) in the 200- and 300-mg cohorts, respectively (Table 3). The most common TRAEs of any grade included rash (83%), diarrhea (70%), and increased alanine aminotransferase (ALT, 67%). Grade 3 or 4 TRAEs occurred in 22 (73%) patients, with a frequency of 60% (9/15) and 87% (13/15) in the 200- and 300-mg cohorts, respectively. No patients had grade 5 (i.e. fatal) TRAEs. Two patients in the 300-mg cohort had serious TRAEs, which included grade 4 diarrhea and grade 3 rash. 23 (77%) patients had TRAEs leading to dose adjustment: 22 (73%) had TRAEs leading to dose interruption (200 mg, 67%; 300 mg, 80%); 21 (70%) had TRAEs leading to dose reduction (200 mg, 67%; 300 mg, 73%); and only 1 (3%) patient in the 300-mg cohort had a grade 3 TRAE leading to permanent treatment discontinuation, i.e. increased gamma-glutamyl transferase (GGT).
Table 3.
Treatment-related adverse events that occurred in ≥20% of participants in the safety population.
| All patients (n = 30) |
200 mg cohort (n = 15) |
300 mg cohort (n = 15) |
||||
|---|---|---|---|---|---|---|
| All grades | Grade 3–4 | All grades | Grade 3–4 | All grades | Grade 3–4 | |
| Any events | 29 (97%) | 22 (73%) | 14 (93%) | 9 (60%) | 15 (100%) | 13 (87%) |
| Rash | 25 (83%) | 15 (50%) | 11 (73%) | 6 (40%) | 14 (93%) | 9 (60%) |
| Diarrhea | 21 (70%) | 4 (13%) | 9 (60%) | 1 (7%) | 12 (80%) | 3 (20%) |
| Increased alanine aminotransferas | 20 (67%) | 2 (7%) | 9 (60%) | 1 (7%) | 11 (73%) | 1 (7%) |
| Increased aspartate aminotransferase | 19 (63%) | 1 (3%) | 10 (67%) | 1 (7%) | 9 (60%) | 0 |
| Itch | 18 (60%) | 0 | 8 (53%) | 0 | 10 (67%) | 0 |
| Increased conjugated bilirubin | 16 (53%) | 0 | 7 (47%) | 0 | 9 (60%) | 0 |
| Xerosis cutis | 16 (53%) | 0 | 8 (53%) | 0 | 8 (53%) | 0 |
| Paronychia | 15 (50%) | 1 (3%) | 5 (33%) | 0 | 10 (67%) | 1 (7%) |
| Increased γ-glutamyltransferase | 11 (37%) | 3 (10%) | 6 (40%) | 2 (13%) | 5 (33%) | 1 (7%) |
| Mouth ulcer | 10 (33%) | 2 (7%) | 2 (13%) | 0 | 8 (53%) | 2 (13%) |
| Increased blood bilirubin | 10 (33%) | 0 | 4 (27%) | 0 | 6 (40%) | 0 |
| Cutaneous fissure | 9 (30%) | 1 (3%) | 2 (13%) | 0 | 7 (47%) | 1 (7%) |
| Anorexia | 9 (30%) | 0 | 3 (20%) | 0 | 6 (40%) | 0 |
| Eyelash growth | 8 (27%) | 0 | 4 (27%) | 0 | 4 (27%) | 0 |
| Increased alkaline phosphatase | 8 (27%) | 0 | 6 (40%) | 0 | 2 (13%) | 0 |
| Increased serum creatinine | 7 (23%) | 0 | 5 (33%) | 0 | 2 (13%) | 0 |
| Nausea | 6 (20%) | 1 (3%) | 1 (7%) | 0 | 5 (33%) | 1 (7%) |
| Skin exfoliation | 6 (20%) | 0 | 3 (20%) | 0 | 3 (20%) | 0 |
| Extended QT interval of electrocardiogram | 5 (17%) | 0 | 4 (27%) | 0 | 1 (7%) | 0 |
| Abdominal discomfort | 4 (13%) | 0 | 1 (7%) | 0 | 3 (20%) | 0 |
| Conjunctivitis | 4 (13%) | 0 | 0 | 0 | 4 (27%) | 0 |
| Oral mucositis | 4 (13%) | 0 | 3 (20%) | 0 | 1 (7%) | 0 |
| Alopecia | 4 (13%) | 0 | 1 (7%) | 0 | 3 (20%) | 0 |
Data are n (%). Treatment-related adverse events were assessed by investigators.
QT = time interval between the start of the Q wave and end of the T wave in the electrical cycle of the heart.
At the data cutoff, 26 patients showed signs of disease progression during AZD3759 treatment. Of these patients, 5 (5/26, 19%) had intracranial progression, 15 (15/26, 58%) had extracranial progression, and 6 (6/26, 23%) had both intracranial and extracranial progression. Overall, intracranial progression occurred in approximately 42% of patients. In total, 17 patients had pre- and post-treatment NGS results, with the former involving tumour tissue and the latter involving at least one type of the following specimens: tumour tissue, peripheral blood, CSF, and pleural fluid (Appendix, Supplementary Fig. S6). Multiple treatment-emergent alterations were observed across patients, including alterations in on-target (EGFR mutation), off-target (EGFR downstream or bypass pathway), and cell cycle pathways (Fig. 2e). In total, 59% (10/17) of patients developed an EGFR T790M mutation during disease progression, and all of these patients received osimertinib in the second-line setting (Appendix, Supplementary Table S3). The median OS was 34.1 and 25.3 months in patients who were treated and not treated with third-generation EGFR-TKI in the later-line setting, respectively (Fig. 2f).
Discussion
Traditional phase I clinical trials have used MTD as the default approach for identifying the RP2D. However, targeted therapy with higher doses is not always superior, and may lead to increases in side effects without an added benefit of higher drug activity. Severe toxicity could result in a high rate of dose reductions, permanent discontinuation, and missed opportunities for continued therapeutic benefit from a drug. Following the achievement of increased patient survival, it was required for sponsors to conduct post-marketing trials to evaluate the effects of lower doses.19 However, the best setting for dose optimization is prior to drug approval.
Focusing on dose optimization starting early in clinical development rather than in a post-marketing setting will allow more efficient identification of an optimal dose and prevent exposure of a large number of patients to a dose that may cause excessive toxicity or be less efficacious.16 Although dose-finding studies with randomized designs can yield useful results, they are time consuming, and as a result, not routinely conducted. This may prevent patients from accessing promising new therapies as early as possible.15 Here, we confirmed ideal doses of the EGFR-TKI AZD3759 of 200 and 300 mg in previously untreated patients. Our study complies with FDA requirements for dose-finding studies in a pre-market setting and provides a scientific foundation for dose selection, which could not only optimize treatment for patients, but also support seamless dose updates post approval.19
In our study, the 200-mg bid cohort showed an increased antitumor response compared with the 300-mg bid cohort (80% vs. 60%), an extended survival benefit (median PFS: 15.8 vs. 10.7 months), and a decreased frequency of all grades of AEs (93% vs. 100%) and grade 3–4 AEs (60% vs. 87%). The toxicity profiles were comparable between the two cohorts, but the 200-mg cohort had lower rates of dose interruption, dose reduction, and permanent treatment discontinuation. The ORR rates were consistent with those from phase I of the BLOOM study, at 67% (10/15) and 60% (3/5) in the 200- and 300-mg bid cohorts, respectively.14 Because of the limited follow-up period, data regarding PFS, DoR, and OS were considered too immature for calculation. Additionally, not all patients in the BLOOM study were previously untreated, as 20% (4/20) had a history of chemotherapy. Our study enabled comprehensive comparation of two treatment doses of AZD3759 in terms of efficacy and safety, and indicated that the 200-mg bid cohort had numerically better outcomes. It has provided important information for the phase III study of AZD3759, in which patients were treated with 200 mg bid.
The CNS is protected against the effects of most drugs because of the active BBB, which prevents the entry of foreign materials into the bloodstream in the brain. Third-generation EGFR-TKIs have greater BBB penetration than first- and second-generation EGFR-TKIs, and are considered standard treatment for patients with CNS metastasis.21,22 Phase III studies have reported CNS ORRs of osimertinib and aumolertinib of 57% and 62%, respectively, and CNS PFSs of furmonertinib, and aumolertinib of 29.0 and 20.8 months, respectively.8, 9, 10 However, these findings were generated from prespecified subgroup analyses. Prospective trials with small sample sizes showed preliminary efficacy of third-generation TKIs as first-line therapy in EGFR-mutant patients with brain metastases.23,24 AZD3759, with its impressive CNS penetration, is a promising EGFR-TKI in this population.11,13,14 In our study, the AZD3759 arm reached its primary endpoint, with a confirmed ORR of 70%. Most of the patients achieved disease control, with a DCR of 83%. AZD3759 provided patients with a median PFS of 12.9 months, and greatly extended the median OS to 33.7 months, which was 2.6 fold greater than the PFS. These data were further improved in the 200-mg bid cohort. Specifically, the CNS ORR was 73% and the median CNS PFS was 17.1 months; this was consistent with recent report of a phase III study on AZD3759, with a CNS ORR of 75.0% and a CNS PFS of 15.2 months according to mRECIST 1.1.25 Thus, AZD3759 appears to be an effective EGFR-TKI for untreated patients with EGFR-mutated NSCLC and CNS metastasis. For these patients, the current clinical practice is still the 3rd generation of EGFR-TKI. However, it may change rapidly. Recently, the FLAURA2 study reported that the primary endpoint of a clinically meaningful and statistically significant improvement in PFS was reached when comparing osimertinib plus chemotherapy with osimertinib alone.26 However, detailed information for patients with CNS metastasis is still needed.
Previous studies have produced encouraging results for third-generation EGFR-TKI treatment in EGFR T790M-positive patients who progressed during previous TKI treatment.27,28 In our study, 59% (10/17) of the patients developed the acquired T790M mutation during disease progression, and all of them received osimertinib as a second-line therapy. Multiple factors can influence first-line treatment decisions and subsequent therapy options, including the presence of brain metastases and drug tolerability, which should be considered in the long-term treatment plan. Following afatinib, which is a second-generation EGFR-TKI, with osimertinib appears to be a promising regimen in EGFR-mutated patients, with a median OS of 30.0 months in those with BM.29,30 In the present study, we obtained a median OS of 34.1 months when patients received AZD3759 plus sequential osimertinib in a later-line protocol, indicating that this could be a treatment option for EGFR-mutated patients with BM or LM.
The most common TRAEs for AZD3759 treatment were rash, increased AST, and pruritus, consistent with phase I of the BLOOM study and phase III study.14,25 Grade 3 or 4 TRAEs occurred in 73% of patients, mainly manifesting as rash (50%, 15/30). In total, 73% of our patients experienced TRAEs leading to dose interruption, while 70% of TRAEs led to dose reduction, and 3% of TRAEs led to permanent treatment discontinuation. However, all AEs were generally manageable with standard supportive care. Further research is needed to identify patients at greater risk of dose adjustment and to effectively manage high-grade rash in these patients.
We could not draw firm conclusions regarding the impact of the drug treatment on the CNS in the present study; we mainly focused on optimal dose selection according to safety and efficacy data and were able to confirm the dose for the phase 3 trial.25 There are several limitations to our study. First, our subgroup analysis of treatment doses should be interpreted with caution because of the limited sample size. And the follow-up time for 200 mg bid cohort which enrolled patients at 2nd stage maybe shorter the that of 300 mg bid. However, dose-finding clinical trials can provide meaningful data even with relatively small sample sizes. Second, the data on later-line treatment were incomplete because some of the patients were lost to follow-up. However, our data indicate that the sequential use of AZD3759 and osimertinib extended OS. Third, tumour samples for treatment resistance analysis were limited. Thus, the incidence of EGFR T790M may be underestimated. We have already prospectively collected peripheral blood during treatment and upon disease progression. The resistance mechanism, and the relationship between plasma AZD3759 levels and outcomes, merit further research.28
In summary, we assessed the efficacy, safety, and optimal dose of AZD3759 in untreated patients with EGFR-mutated NSCLC and CNS metastases. We found that 200 mg bid improved antitumor activity and decreased toxicities, which allowed more patients to benefit from AZD3759 for a longer period of time. The sequential use of AZD3759 and osimertinib gave the target population a treatment option that may extend OS. These results should be confirmed in the phase 3 randomized clinical trial of AZD3759 (NCT03653546).
Contributors
The authors conceived and designed the study: Y.L. Wu, Q. Zhou, S.M. Liu; the authors collected and assembled the data: S.M. Liu, J.Y. Deng, C. Lu, X.W. Wei, M.M. Zheng; the authors directed and performed the statistical analysis: Y.L. Wu, Q. Zhou, S.M. Liu, H.H. Yan, J.Y. Deng, M.Y. Yang; the authors recruited patients: X.R. Dong, Z. Wang, Y. Du, J.W. Cui, B.F. Xu, Q. Chu, M.Y. Zheng, Y.S. Li, J. Huang, X.Y. Bai, Y.L. Sun, A. Li, C.R. Xu, B.C. Wang, H.J. Chen, J.J. Yang, W.Z. Zhong, Q. Zhou. All authors were involved in data interpretation, writing, revision, and critical review of the article. Y.L. Wu, Q. Zhou, and S.M. Liu accessed and were responsible for the raw data associated with the study. All authors have approved the submitted version and are accountable for their contributions and the integrity of the work.
Data sharing statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data. Datasets can be requested once the clinical study report has been completed, if there is not a reasonable likelihood of participant re-identification. As that time, access to patient-level data can be requested by sending email to the leading principal investigator Prof. Yi-Long Wu (syylwu@live.cn), who will decide whether data could be provided. No custom code was used for data analysis in this study.
Declaration of interests
Prof. Yi-Long Wu reports grants and personal fees from AstraZeneca, BMS, Pfizer; and personal fees from Boehringer Ingelheim, Eli Lilly, Hengrui, MSD, Sanofi, Roche, outside the submitted work. Prof. Qing Zhou reports honoraria from AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, MSD, Pfizer, Roche, and Sanofi. Prof. Wen-Zhao Zhong reports speech honoraria from AstraZeneca, Roche, Eli Lilly and Pfizer. Dr. Chong-Rui Xu reports grants from Hengrui Pharmaceutical and Pfizer. The other authors have no competing interests to declare.
Acknowledgement
This work was funded by Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (2017B030314120, Yi-Long Wu), Guangdong Provincial People's Hospital Scientific Research Funds for Leading Medical Talents in Guangdong Province (KJ012019426, Yi-Long Wu), National Natural Science Foundation of China (Grant No. 82072562, Qing Zhou), National Natural Science Foundation of China (82202997, Si-Yang Maggie Liu), and the High-level Hospital Construction Project (DFJH201810, Qing Zhou).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102238.
Contributor Information
Qing Zhou, Email: gzzhouqing@126.com.
Yi-Long Wu, Email: syylwu@live.cn.
Appendix ASupplementary data
References
- 1.Brower J.V., Saha S., Rosenberg S.A., Hullett C.R., Ian Robins H. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci. 2016;27:130–137. doi: 10.1016/j.jocn.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Mok T.S., Wu Y.L., Ahn M.J., et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J.J., Yan H.H., Wu Y.L. BRAIN study: it is hard to draw a conclusion - authors' reply. Lancet Respir Med. 2017;5(11):e34. doi: 10.1016/S2213-2600(17)30385-5. [DOI] [PubMed] [Google Scholar]
- 4.Rangachari D., Yamaguchi N., VanderLaan P.A., et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria J.C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y., Chen G., Wang X., et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 2022;10(11):1019–1028. doi: 10.1016/S2213-2600(22)00168-0. [DOI] [PubMed] [Google Scholar]
- 7.Lu S., Dong X., Jian H., et al. AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or MetastaticNon-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;40(27):3162–3171. doi: 10.1200/JCO.21.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reungwetwattana T., Nakagawa K., Cho B.C., et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018 doi: 10.1200/JCO.2018.78.3118. Jco2018783118. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y., Chen G., Wang X., et al. Central nervous system efficacy of furmonertinib (AST2818) versus gefitinib as first-line treatment for EGFR-mutated NSCLC: results from the FURLONG study. J Thorac Oncol. 2022;17(11):1297–1305. doi: 10.1016/j.jtho.2022.07.1143. [DOI] [PubMed] [Google Scholar]
- 10.Lu S., Dong X., Jian H., et al. Aumolertinib activity in patients with CNS metastases and EGFR-mutated NSCLC treated in the randomized double-blind phase III trial (AENEAS) J Clin Oncol. 2022;40(16_suppl):9096. [Google Scholar]
- 11.Colclough N., Chen K., Johnström P., et al. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res. 2021;27(1):189–201. doi: 10.1158/1078-0432.CCR-19-1871. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Q., Wang J., Cheng Z., et al. Discovery and evaluation of clinical candidate AZD3759, a potent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor. J Med Chem. 2015;58(20):8200–8215. doi: 10.1021/acs.jmedchem.5b01073. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z., Guo Q., Wang Y., et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med. 2016;8(368) doi: 10.1126/scitranslmed.aag0976. [DOI] [PubMed] [Google Scholar]
- 14.Ahn M.J., Kim D.W., Cho B.C., et al. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study. Lancet Respir Med. 2017;5(11):891–902. doi: 10.1016/S2213-2600(17)30378-8. [DOI] [PubMed] [Google Scholar]
- 15.Fourie Zirkelbach J., Shah M., Vallejo J., et al. Improving dose-optimization processes used in oncology drug development to minimize toxicity and maximize benefit to patients. J Clin Oncol. 2022;40(30):3489–3500. doi: 10.1200/JCO.22.00371. [DOI] [PubMed] [Google Scholar]
- 16.Shah M., Rahman A., Theoret M.R., Pazdur R. The drug-dosing conundrum in oncology - when less is more. N Engl J Med. 2021;385(16):1445–1447. doi: 10.1056/NEJMp2109826. [DOI] [PubMed] [Google Scholar]
- 17.Project Optimus | FDA. https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus
- 18.Murphy R., Halford S., Symeonides S.N. Project Optimus, an FDA initiative: considerations for cancer drug development internationally, from an academic perspective. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1144056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Optimizing the dosage of human prescription drugs and biological products for the treatment of oncologic diseases. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/optimizing-dosage-human-prescription-drugs-and-biological-products-treatment-oncologic-diseases
- 20.Liu S.M., Yan H.H., Wei X.W., et al. Biomarker-driven studies with multi-targets and multi-drugs by next-generation sequencing for patients with non-small-cell lung cancer: an open-label, multi-center, phase II adaptive umbrella trial and a real-world observational study (CTONG1702&CTONG1705) Clin Lung Cancer. 2022;23(7):e395–e399. doi: 10.1016/j.cllc.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Ozcan G., Singh M., Vredenburgh J.J. Leptomeningeal metastasis from non-small cell lung cancer and current landscape of treatments. Clin Cancer Res. 2023;29(1):11–29. doi: 10.1158/1078-0432.CCR-22-1585. [DOI] [PubMed] [Google Scholar]
- 22.Liu S.M., Zheng M.M., Pan Y., Liu S.Y., Li Y., Wu Y.L. Emerging evidence and treatment paradigm of non-small cell lung cancer. J Hematol Oncol. 2023;16(1):40. doi: 10.1186/s13045-023-01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peled N., Kian W., Inbar E., et al. Osimertinib in advanced EGFR-mutant lung adenocarcinoma with asymptomatic brain metastases: an open-label, 3-arm, phase II pilot study. Neurooncol Adv. 2022;4(1) doi: 10.1093/noajnl/vdab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.367P High-dose aumolertinib in EGFR-mutant NSCLC patients with brain metastases: primary data from ACHIEVE. Ann Oncol. 2022;33 [Google Scholar]
- 25.Zhou Q., Wang J., Yu Y., et al. Randomized phase 3 study of first-line AZD3759 (zorifertinib) versus gefitinib or erlotinib in EGFR-mutant (EGFRm+) non–small-cell lung cancer (NSCLC) with central nervous system (CNS) metastasis. J Clin Oncol. 2023;41(16_suppl):9001. [Google Scholar]
- 26.Tagrisso plus chemotherapy demonstrated strong improvement in progression-free survival for patients with EGFR-mutated advanced lung cancer in FLAURA2 Phase III trial. https://www.astrazeneca.com/media-centre/press-releases/2023/tagrisso-plus-chemo-improved-pfs-in-lung-cancer.html
- 27.Yang J.C.H., Kim S.W., Kim D.W., et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38(6):538–547. doi: 10.1200/JCO.19.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi H., Wakuda K., Fukuda M., et al. A phase II study of osimertinib for radiotherapy-naive central nervous system metastasis from NSCLC: results for the T790M cohort of the OCEAN study (LOGIK1603/WJOG9116L) J Thorac Oncol. 2021;16(12):2121–2132. doi: 10.1016/j.jtho.2021.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Hochmair M.J., Morabito A., Hao D., et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol. 2020;16(34):2799–2808. doi: 10.2217/fon-2020-0740. [DOI] [PubMed] [Google Scholar]
- 30.Popat S., Jung H.A., Lee S.Y., et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: a global non-interventional study (UpSwinG) Lung Cancer. 2021;162:9–15. doi: 10.1016/j.lungcan.2021.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.