Summary
Background
Effective methods of preventing and identifying childhood wasting are required to achieve global child health goals. Family mid-upper arm circumference (MUAC) programs train caregivers to screen their child for wasting with MUAC tapes. We assessed the effectiveness of a two-way short message service (SMS) platform (referred to as the Maternally Administered Malnutrition Monitoring System [MAMMS]) in western Kenya.
Methods
In this individual-level randomised controlled trial in two rural countries in western Kenya, children (aged 5–12 months) were randomly allocated (1:1) to receive either standard care (SOC) or MAMMS. Randomisation method was permuted-block randomisation with a block size of 10. Eligible participants were children attending maternal child health clinics in the two counties whom had a MUAC between 12.5 and 14.0 cm. The MAMMS group received two MUAC tapes and weekly SMS reminders to screen their child's MUAC. The SOC group received routine community health volunteer services and additional quarterly visits from the study team. The primary analysis used a cox proportional hazards model to compare SOC and MAMMS time-to-diagnosis of wasting (MUAC <12.5 cm) confirmed by a health professional during 6-months follow-up. Secondary outcomes were days from enrolment to treatment initiation among children with wasting, proportion of all children with wasting who were identified by the two approaches (treatment coverage), mean MUAC at treatment initiation, and duration of wasting treatment. This trial was registered on ClinicalTrials.gov, NCT03967015.
Findings
Between August 1, 2019 and January 31, 2022, 1200 children were enrolled, among whom the incidence of confirmed wasting was 37% lower in the MAMMS group (hazard ratio: 0.63, 95% CI: 0.42–0.94, p = 0.022). Among children with wasting, the median number of days-to-diagnosis was similar between study groups (MAMMS: 63 days [interquartile range (IQR): 23–92], SOC: 58 days [IQR: 22–94]). Treatment coverage in the MAMMS group was 83.3% (95% CI: 39.9–100.0) while coverage in the SOC group was 55.6% (95% CI: 22.3–88.9%, p = 0.300). Treatment duration and mean MUAC at treatment initiation were similar between groups.
Interpretation
Family MUAC supported by SMS was associated with a 37% reduction in wasting among young children. Empowering caregivers to monitor their child's nutritional status at home may prevent a substantial proportion of moderate wasting.
Funding
Thrasher Research Foundation and Pamela and Evan Fowler.
Keywords: Family MUAC, Childhood wasting, Acute malnutrition
Research in context.
Evidence before this study
Programs that teach caregivers to monitor their children's Mid-Upper Arm Circumference (MUAC) at home, known as Family MUAC, are an increasingly popular method of identifying children with wasting (low weight-for-height) among Community Management of Acute Malnutrition programs. To understand the effectiveness of Family MUAC programs we searched PubMed on January 3, 2023 using the terms “Family MUAC” OR “Mother MUAC” for published studies in English, French or Spanish. Of 169 records identified, four observational studies and one cluster randomised trial reported implementing a Family MUAC program. Observational studies found that caregivers could be trained to use MUAC tapes effectively, that standard insertion MUAC tapes outperformed other MUAC tapes when used by caregivers, and that Family MUAC approaches had been deployed in many settings to fill gaps in screening coverage during the COVID-19 pandemic. A cluster randomised trial showed comparable treatment coverage in areas randomised to Family MUAC and community health worker led screening, but earlier detection of wasting and a lower cost-per-annum in areas randomised to Family MUAC. No individual-level randomised trials of Family MUAC were identified, and multiple studies highlighted concerns about sustaining caregiver MUAC screening over long time periods.
Added value of this study
This large individual level trial found that children randomised to a Family MUAC program supported by a two-way short message service (SMS) mHealth platform had a one-third lower incidence of wasting than those in the standard of care group. Among children who progressed to wasting in the intervention group, there was a similar median number of days between enrolment and diagnosis, severity of wasting at treatment initiation, and mean treatment duration compared with a strong community healthcare worker implemented screening program (standard of care). However, there was also some evidence that after 6-months of follow-up, treatment coverage was higher in the Family MUAC group compared with standard of care.
Implications of all the available evidence
Family MUAC, with the support of two-way SMS, may serve as a useful adjuvant or alternative to community health worker led screening for wasting, as well as a method for preventing wasting in some settings. Over one-third of child mortality is attributable to wasting, suggesting that improved methods for the prevention and identification of wasting could make an important contribution to achieving global child health goals.
Introduction
Childhood wasting affects 45 million children globally and is the underlying cause of approximately 30% of under-five mortality each year.1,2 Community Management of Acute Malnutrition (CMAM) programs identify and treat children with wasting, defined by a mid-upper arm circumference (MUAC) below 12.5 cm if between 6 and 59 months of age, a weight-for-height z-score (WHZ) below −2, or nutritional oedema. Wasted children enrolled in community management programs typically have a case fatality rate below 2%.3, 4, 5 However, children with severe wasting and medical complications (those who are acutely unwell with diarrhoea, pneumonia, sepsis or other diseases) have mortality rates as high as 16%.6,7 UNICEF estimates that only 17% of children with wasting receive CMAM management prior to developing complications.4 New strategies to expand the early identification of children with wasting are urgently needed to reduce global child mortality and achieve Sustainable Development Goal 3.2.
Training caregivers to monitor their child's MUAC and seek care if they identify wasting is increasingly being used to expand malnutrition screening.8, 9, 10, 11, 12 Research in West Africa has suggested that these “Family MUAC” programs may lead to the earlier identification of wasted children compared with community health worker facilitated screening.8,9 However, there is minimal evidence regarding methods to support caregivers to sustain Family MUAC measurements and to access care if their measurement indicates childhood wasting. Two-way short message service (SMS) platforms, in which families and healthcare providers communicate via text messages, provide a low cost approach to improve health literacy and reduce barriers to accessing health care services.13, 14, 15, 16, 17 A Family MUAC program supported by a two-way SMS platform may be an effective and low-cost strategy to increase the early identification of childhood wasting. Earlier identification may also reduce the severity of wasting at treatment initiation and therefore the time, cost, and morbidity associated with treatment of childhood wasting.
In this large randomised controlled trial, we examined whether the Maternally Administered Malnutrition Monitoring System (MAMMS), a Family MUAC intervention supported by a two-way SMS platform, affected the time-to-diagnosis of moderate or severe wasting in children compared with standard of care (SOC) community health worker active screening. We also compared the effect of the MAMMS intervention on the severity of wasting, and the duration of wasting treatment.
Methods
Trial design and participants
We conducted an individual-level randomised controlled trial of MAMMS compared with SOC among children aged 5–12 months in Homa Bay and Migori Counties, Kenya. These are rural, agriculturally productive counties on the shores of Lake Victoria in western Kenya with an estimated 2% prevalence of wasting among children under five years of age.18 Measles vaccine is typically given at 9 months of age, the middle of our eligible age band, and has a coverage of 85% in these counties.18 The trial was registered on clinicaltrials.gov (NCT03967015) and the protocol published in an open journal.19
We recruited eligible participants from among caregivers with children attending maternal child health clinics at the Migori County Referral and Teaching Hospital, St. Joseph's Mission Hospital, Macalder Hospital, and Homa Bay Teaching and Referral Hospital between August 2019 and January 2022. Children aged 5–12 months with a MUAC between 12.5 and 14.0 cm were eligible. Children with MUAC less than 12.5 cm, or with nutritional oedema, were referred to nutritional services for management and those with MUAC higher than 14.0 cm were excluded as they were unlikely to be at risk of wasting in the 6-month follow-up period (<13.5 cm is often considered “at risk” for wasting).20,21 Additional exclusion criteria included a mobile phone number that was not part of the program associated SMS network (Safaricom), the caregiver unable to read or write and did not have someone to help them read or write, the caregiver did not plan to reside in the catchment area for at least 6-months, the caregiver was unable to complete MUAC measurement training, the child was acutely unwell and required hospitalisation, or the child or child's sibling was currently or previously enrolled in the study.
Ethical approval was obtained from the Kenya Medical Research Institute Scientific and Ethics Review Unit (0121/3821) and the University of Washington Institutional Review Board (00006221). All primary caregivers provided written informed consent in their preferred language (Kiswahili, Luo, Kuria, or English).
Procedures
At enrolment, study staff collected sociodemographic, medical, nutritional, and household characteristics from the primary caregiver. Anthropometric measurements (weight, length/height, and MUAC) were obtained in duplicate by study staff from all children and their caregiver. The research team worked with the local HIV voluntary counselling and testing program to offer HIV testing to all caregivers and children without a documented result per Kenyan National Guidelines.
After completion of the enrolment procedures and prior to randomisation, study staff delivered a 10-min standardised training to all enrolled caregivers demonstrating how to measure their child's MUAC using a colour-coded insertion MUAC tape with numbered 1 mm gradations. Study staff and caregivers then measured the child's MUAC independently of each other as a validation check. Caregivers who did not pass this validation check, defined as a difference of ≥0.05 cm between the study staff and caregiver's MUAC measurement, were provided further instruction. If a caregiver was not able to satisfactorily complete the MUAC training after receiving a second training, they were excluded from the study, prior to randomisation. However, no caregivers were excluded due to failed training.
Randomisation and masking
After completion of MUAC training, eligible caregiver-child dyads were randomised in a 1:1 allocation to MAMMS or SOC groups using a permuted-block randomisation with a block size of 10. Allocations were generated by the study biostatistician using a computer-generated random number and concealed in sequentially numbered and sealed envelopes by participant ID number. Investigators and participants were not blinded to the interventions as it was considered impractical to do so.
The screening programs in both study groups were delivered by salaried professional staff which we referred to as community health workers. This cadre is distinct from the community health volunteers who sometimes deliver CMAM screening as they receive a salary rather than a stipend and they have a longer period of training.
MAMMS intervention
Caregivers randomised to the MAMMS group were provided with two colour coded insertion MUAC tapes, numbered to 1 mm gradations, and enrolled in the two-way Mobile WACh SMS mHealth platform.15,22,23 The mHealth platform sent a weekly automated text that included both a health education message and requested the caregiver to respond with either the colour or the colour and numeric value of their child's MUAC measurement. Study staff, including community health workers and two nutritionists, reviewed all text messages from caregivers and responded with a text message assuring the caregiver that the measurement appeared to be normal if the child's MUAC was reported to be greater than or equal to 12.5 cm or in the green coloured section of the MUAC tape. Study staff responded with a text message asking caregivers to bring their child to clinic if their child's MUAC was reported to be less than 12.5 cm or in the yellow-coloured section of the MUAC tape (moderate wasting). All messages were sent in the caregiver's preferred language (Kiswahili, Luo, Kuria, or English). Both sending and receiving study messages were free of charge to participants.
Health education messages were included in weekly text messages to increase caregiver interest and engagement. The health education messages in the weekly text included adapted content based on validated messages used in our team's prior studies and new messages relevant to this age group.15, 16, 17,22 The topics and content of these messages were based on UNICEF's Guidance on Childhood Development and the Integrated Management of Childhood Illness recommendations,24,25 and tailored to the cultural setting through engagement with clinical and community health workers. The messages were piloted and further tailored following five focus group discussions with caregivers of children between 6 and 12 months of age attending maternal child health clinics at the Migori County Referral Hospital in May 2019. The health education topics included: developmental milestones, sanitation and hygiene practices, timing of vaccinations, the utility of a kitchen garden, prevention of malaria, recognition of fever, home management of diarrhoea, recognition of ear infections, and severe infection danger signs. To avoid modifying the underlying incidence of wasting in the MAMMS group, the health education messages avoided content related to breastfeeding and child or caregiver nutrition with the exception of starting a kitchen garden.
SOC group
The SOC group included active and passive case identification to replicate a “gold standard” approach used in CMAM programs. In CMAM programs, active case identification may include door-to-door MUAC screening conducted by community health workers. As part of the trial, study staff conducted home visits for participants in the SOC group once per calendar quarter. These SOC home visits were in addition to regular active case finding conducted by Ministry of Health organised community health services and aimed to ensure the MAMMS group was compared with a strong community screening program. For passive case identification, where caregivers present to health care services seeking care for their child who is subsequently diagnosed with wasting, study staff worked with the hospital at each clinic site to monitor attendances and hospital admissions and identify any children in the SOC or MAMMS group that were admitted to the paediatric ward. In such cases, nutritional status was ascertained at the time of hospital admission or outpatient visit. Passive identification was strengthened by asking caregivers to present to study staff if they believed their child required medical attention, after which study staff would accompany the caregiver to consult the hospital paediatric team to ensure the child received prompt clinical attention. To capture information on hospital admission or receipt of malnutrition diagnosis at non-study facilities, we asked each caregiver whether their child had been admitted to a paediatric ward or received a diagnosis of malnutrition at the 6-month visit. If a hospitalisation or malnutrition diagnosis occurred, study staff visited the facility and reviewed the patient's notes to confirm the diagnosis of wasting.
Follow-up
One research follow-up visit occurred 6 months after enrolment in each study group. At the 6-month visit, study staff obtained anthropometric measurements (weight, length, and MUAC) on the child and administered a standardised questionnaire to ascertain the child's current health status, any wasting diagnosis since enrolment, and other illnesses and hospitalisations. Caregivers in the MAMMS group were asked to measure their child's MUAC at the visit and the measure was compared with that taken by study staff.
Primary outcome ascertainment
The primary analysis was a time-to-diagnosis of confirmed wasting (termed acute malnutrition in the protocol registration). Wasting was defined as MUAC <12.5 cm as measured by a trained health professional, identified by either the SOC or MAMMS screening program during the 6-month follow-up period. In the MAMMS group, study staff confirmed the child's MUAC measurement if the caregiver reported via text message a MUAC <12.5 cm or a MUAC in the yellow-coloured section of the tape. In the SOC group, children with MUAC <12.5 cm were identified during active case finding either during a home visit conducted by study staff, by clinical staff at hospital admission or outpatient visit, or during routine active or passive screening conducted by the county nutritional services. In both groups, children identified as wasted at the 6-month visit who had not been detected by either the SOC or MAMMS screening program were considered “missed cases” and were not classified as wasted in the primary analysis because the 6-month visit was a research activity that would not be part of a routine screening program. However, these missed cases were included in a secondary analysis to estimate the overall incidence of wasting, as described below.
Children in both study groups identified as wasted were offered treatment by study nutritionists according to Kenya National Guidelines in collaboration with Ministry of Health nutritional services.26 Study staff reviewed the clinical progress of these children at two, four, eight, twelve, and sixteen weeks after identification of wasting to ascertain the time to treatment recovery, defined as MUAC ≥12.5 cm. Children with wasting in both study groups were sent text messages to remind them to pick up supplementary foods and return to the clinic for treatment follow-up visits. Any child who recovered from wasting and was discharged from treatment within the study's 6-month follow-up period continued with their regularly scheduled follow-up visit at 6 months. Children whose treatment course went beyond the 6-month follow-up visit, were followed until they recovered or had received at least sixteen weeks of treatment.
Statistical analysis
The study was powered to detect a two-fold difference in wasting identified in the MAMMS group compared with the SOC group based on a cumulative incidence of 4% identified wasting among children in the SOC group.18 A sample size of 1200 caregiver-child dyads (600 per group), accounting for 10% attrition, was estimated to provide at least 80% power to detect a two-fold difference in wasting identification between the two groups at an alpha = 0.05. An interim analysis was conducted after 50% of participants had been recruited to enable the Data and Safety Monitoring Board to examine interim results for evidence of benefit, harm or futility.
The primary analysis was intention-to-treat based on randomised allocation. To ensure confounders were balanced between the two groups, baseline characteristics were compared using a Chi-square test for proportions and Mann Whitney U test for medians, as appropriate. The effect of any chance imbalance in baseline characteristics were evaluated as potential confounders in a sensitivity analysis. To compare the time-to-confirmed wasting diagnosis between the MAMMS and SOC groups, we used Cox proportional hazard regression models estimating hazard ratios (HRs) with 95% confidence intervals (95% CI) derived from the model. The event was defined as any wasting diagnosis prior to the 6-month follow-up visit. All hypothesis tests were two-sided and performed at alpha = 0.046 to account for the interim analysis. Because fewer than 50% of the children progressed to wasting, we did not calculate a median survival time, but rather compared the median days between enrolment and diagnosis among wasted children in both groups.
While the primary analysis included only cases identified by the SOC and MAMMS screening programs prior to the 6-month visit, an a priori planned secondary analysis added children missed by these two approaches but diagnosed at the 6-month follow-up visit in an otherwise identical time-to-event analysis. This secondary analysis estimated the total incidence of wasting in the two groups. Post-hoc secondary analyses were also added to the pre-specified primary analysis to compare the two screening approaches between CMAM program outcomes. We estimated the treatment coverage in each group at the 6-month visit by dividing the number of children who were currently in wasting treatment by the total number of children who were currently wasted at the 6-month follow-up visit, i.e., the numerator plus any missed cases discovered at the 6-month timepoint. To understand the effect of the MAMMS versus SOC screening programs on severity of wasting at treatment initiation, a post-hoc secondary analysis compared the mean MUAC measurement and number of severe wasting cases (defined as MUAC <11.5 cm) at treatment initiation between the two groups. Finally, a post-hoc cox proportional hazard model was used to compare the duration of malnutrition treatment once a child was identified as wasted, in addition to a chi-squared comparison of cumulative treatment success rate defined as recovery (MUAC ≥12.5 cm) from wasting, and comparison of mortality and hospitalisations in both groups.
To understand the fidelity and acceptability of the MAMMS and SOC programs, we described the proportion of MAMMS participants who responded to at least one SMS, and the median number of messages received from these participants. In an exit survey, we also asked MAMMS participants how confident they felt using the MUAC tapes, and used Pearson correlation coefficient to measure the association between caregiver and CHW measured MUAC at the six month visit. In a post-hoc analysis we calculated the positive predictive value of a caregiver reporting their child to be wasted based on their MUAC measurements during follow-up and calculated the Kappa statistic based on caregiver and CHW measurements at the 6-month follow-up visit. For the SOC children, we described the number of home visits in which the CHWs were able to locate and measure the participant child. Analyses were conducted in R4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Role of the funding source
The funders had no role in the design of the study; the collection, analysis or interpretation of the data; nor the writing of the manuscript.
Results
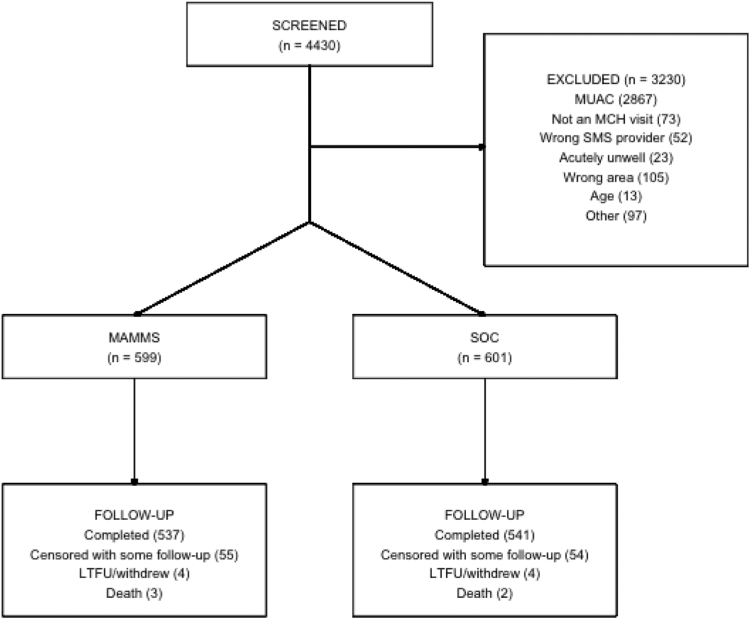
Among 4430 caregiver-child dyads screened for study eligibility, 1200 were enrolled (Fig. 1). The most common exclusion criterion was child's MUAC greater than 14.0 cm (n = 2867). Few caregivers were excluded due to an incompatible SMS service provider (n = 52). No caregiver was excluded because they were unable to read or write and did not have someone to help them read or write. Among caregiver-child dyads who were eligible, 599 were randomised to MAMMS and 601 to SOC.
Fig. 1.
Trial CONSORT diagram. Reasons for exclusion: MUAC above or below eligible range; not a routine MCH visit—caregiver/child came to clinic but not for a routine visit; wrong SMS provider—caregiver did not have access to Safaricom; acutely unwell—child required hospitalization; wrong area—caregiver planned to leave the study area in the next 6-months; child age—below 5 months or older than 12 months. Abbreviations: LTFU, lost to follow-up; MAMMS, Maternally Administered Malnutrition Monitoring System; MCH, maternal child health clinic; MUAC, mid-upper arm circumference; SOC, Standard of care.
Median age of children enrolled was 7 months (interquartile range [IQR], 6–9 months). Median age of primary caregivers was 26 years (IQR, 22–31 years) and 302 (25.2%) caregivers shared their phone with another person. Caregiver and child characteristics were balanced between study groups (Table 1), with the exception of small differences in prior clinic visit frequency, breastfeeding status and maternal education. In the MAMMS group, 353 (58.9%) children had previously visited the clinic when they had been unwell while 390 (64.9%) SOC randomised families reported previous clinic visits. MAMMS children also had a slightly higher prevalence of breastfeeding, with 575 (96%) reporting at least some ongoing breastfeeding compared with 558 (93%) SOC caregivers. Similarly, 536 (89.5%) MAMMS caregivers had attended primary school or a higher level of education, while 506 (84.2%) SOC caregiver had this level of education.
Table 1.
Baseline characteristics by randomization group.
| MAMMS group |
SOC group |
|||
|---|---|---|---|---|
| N = 599 | (%) | N = 601 | (%) | |
| Child characteristics | ||||
| Female | 322 | (53.8%) | 334 | (55.6%) |
| Male | 277 | (46.2%) | 267 | (44.4%) |
| Age (months) | ||||
| <6 | 31 | (5.2%) | 33 | (5.5%) |
| 6–9 | 380 | (63.4%) | 361 | (60.1%) |
| >9 | 188 | (31.4%) | 207 | (34.4%) |
| Previous clinic visit | 353 | (58.9%) | 390 | (64.9%) |
| Currently breastfeeding | ||||
| No | 24 | (4.0%) | 43 | (7.2%) |
| Partial | 502 | (83.8%) | 493 | (82.0%) |
| Exclusive | 69 | (11.5%) | 60 | (10.0%) |
| HIV status | ||||
| Infected | 5 | (0.8%) | 0 | (0%) |
| Exposed uninfected | 185 | (30.9%) | 191 | (31.8%) |
| Unexposed | 405 | (67.6%) | 407 | (67.7%) |
| Unknown | 4 | (0.7%) | 3 | (0.5%) |
| MUAC (median, IQR) | 13.6 | (13.2, 13.9) | 13.6 | (13.1, 13.9) |
| Underweight (WAZ < −2 SD) | 55 | (9.2%) | 59 | (9.8%) |
| Stunting (LAZ < −2 SD) | 79 | (13.2%) | 100 | (16.6%) |
| Caregiver characteristics | ||||
| Body mass index | ||||
| Underweight (<18.5 kg/m2) | 28 | (4.7%) | 32 | (5.3%) |
| Obese (>30 kg/m2) | 69 | (11.5%) | 81 | (13.5%) |
| Age (years) | ||||
| ≤24 | 208 | (34.7%) | 206 | (34.3%) |
| 25–35 | 318 | (53.1%) | 334 | (55.6%) |
| >35 | 73 | (12.2%) | 61 | (10.1%) |
| Education | ||||
| No formal educationa | 63 | (10.5%) | 95 | (15.8%) |
| Any primary educationb | 132 | (22.0%) | 123 | (20.5%) |
| Any secondary and abovec | 404 | (67.4%) | 383 | (63.7%) |
| Shared phone | 142 | (23.7%) | 160 | (26.6%) |
| Help sending SMS | 20 | (3.3%) | 40 | (6.7%) |
| Married | 485 | (81.0%) | 505 | (84.0%) |
| Employed | 304 | (50.8%) | 306 | (50.9%) |
| Household conditions | ||||
| Crowding (≥3 people/room) | 177 | (29.5%) | 180 | (30.0%) |
| Improved sanitation | 182 | (30.4%) | 167 | (27.8%) |
| Owns animals | 389 | (64.9%) | 361 | (60.1%) |
| Food insecurity | ||||
| Secure/Mild | 250 | (41.7%) | 257 | (42.8%) |
| Moderate | 216 | (36.1%) | 192 | (31.9%) |
| Severe | 126 | (21.0%) | 150 | (25.0%) |
| Minutes to clinic (median, IQR) | 30 | (15, 30) | 25 | (15, 30) |
Abbreviations: IQR, interquartile range; kg/m2, kilograms per meter squared; LAZ, length-for-age z-score; MAMMS, Maternally Administered Malnutrition Monitoring System, MUAC, mid-upper arm circumference; SMS, short message service; SOC, standard of care; WAZ, weight-for-age z-score.
Includes caregivers who had no formal education or less than primary education.
Includes caregivers who started or completed primary education.
Includes caregivers who started or completed secondary education, and those who had above secondary education.
Retention and child deaths did not differ between groups with eight (0.7%) children being lost to follow-up and five (0.4%) deaths occurring during the study. Further, 109 caregiver-child dyads were administratively censored prior to their 6-month visit as COVID-19 social distancing regulations prevented them from attending clinic.
Primary outcome
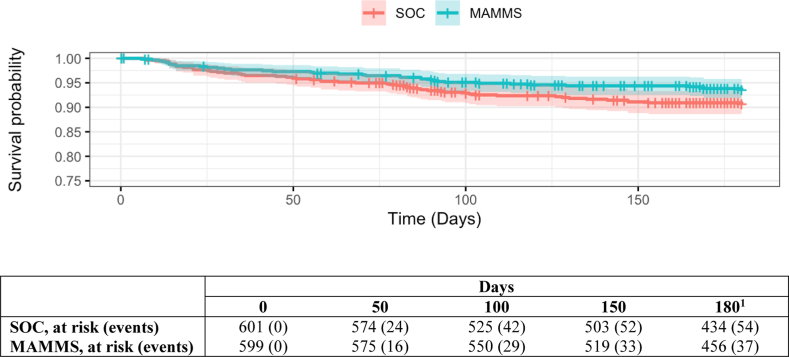
101 (8.4%) children progressed to wasting during the 6-month follow-up period, with 91 (7.6%) of these events identified before the 6-month follow-up visit (Table 2). In the MAMMS group, 37 children progressed to wasting and were identified by the MAMMS group, yielding an incidence rate of 13.4 diagnoses of wasting per 100 child-years (95% confidence interval [CI], 9.3–17.4). In the SOC group, 54 children progressed to wasting and were identified by active or passive identification, yielding an incidence rate of 19.8 diagnoses of wasting per 100 child-years (95% CI, 15.0–24.6). The incidence of wasting was 33% lower in the MAMMS group over 6 months than the SOC group (Fig. 2), although this association was not statistically significant (HR: 0.67 [95% CI, 0.44–1.02]; p = 0.063). Ten (1%) of 1078 children attending the 6-month visit were identified as wasting cases that had been missed by either the MAMMS (n = 2) or SOC (n = 8) groups (Table 2). Including these 10 missed cases of wasting incidence, MAMMS was significantly associated with a 37% reduction in the incidence of wasting compared with SOC (HR, 0.63 [95% CI, 0.42–0.94]; p = 0.022). Among the children who progressed to wasting, the median days-to-diagnosis was comparable between the two groups, 63 days (95% CI: 23–92) in the MAMMS group and 58 (95% CI: 22–94) in the SOC group.
Table 2.
Effect of the MAMMS screening program on incidence of wasting and days to diagnosis and malnutrition treatment coverage.
| Primary outcomea |
Secondary outcome |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Child-years | Events | Incidence rate per 100 child-years (95% CI) | Median days to wasting diagnosis (IQR) | Missed cases | Treatment coverage at 6-months | Mean duration of treatment in weeks (95% CI)b | Treatment successc | |
| SOC | 601 | 265.4 | 54 | 19.8 (15.0, 24.6)d | 58 (22, 94) | 8 (13.1%) | 55.6% | 9.14 (8.8–9.5)e | 50 (86.2%) |
| MAMMS | 599 | 271.4 | 37 | 13.4 (9.3, 17.4)d | 63 (23, 92) | 2 (5.1%) | 83.3% | 8.8 (8.4–9.2)e | 35 (92.1%) |
Abbreviations: CI, confidence interval; IQR, interquartile range; MAMMS, Maternally Administered Malnutrition Monitoring System; SOC, standard of care.
Includes only cases identified by the SOC or MAMMS screening programs prior to the 6-month follow-up visit.
Includes all children identified with wasting, including those identified at the 6-month follow-up visit, and those who initiated malnutrition treatment.
Treatment success was defined as recovery from wasting (MUAC ≥12.5 cm) among those who started malnutrition treatment, i.e., excluding the caregivers of three children in the SOC group and one in the MAMMS group that chose not to start treatment for wasting.
p-value = 0.063 for a difference in the incidence of wasting detected by the MAMMS versus SOC programs.
p-value = 0.712 for a difference in the mean duration of treatment between MAMMS and SOC groups.
Fig. 2.
Kaplan–Meier curve of wasting free survival by randomization group. 1Day 180 visit were conducted between day 170 and 180, so some participants successfully exited the trial before day 180. Abbreviations: MAMMS, Maternally Administered Malnutrition Monitoring System group; SOC, Standard of care group.
Secondary outcomes
Using this 6-month visit as a cross-section of the study population, the estimated treatment coverage (the proportion of all children with wasting who were identified as wasted by their screening program) in the MAMMS group was 83.3% (95% CI: 39.9–100.0%), while the SOC intervention had a treatment coverage of 55.6% (95% CI: 22.3–88.9%, p = 0.300) (Table 2).
There was no indication that severity of wasting at treatment initiation differed between MAMMS and SOC groups. Mean MUAC at diagnosis was 12.1 cm (95% CI: 12.0–12.2 cm) in the MAMMS group and 12.2 cm (95% CI: 12.1–12.3 cm) in the SOC group. Three children in the MAMMS group and three in the SOC group were severely wasted (MUAC <11.5 cm) at treatment initiation (Table 3). There was no difference in the mean treatment duration or proportion of children that successfully completed treatment (defined as MUAC ≥12.5 cm) between the two groups. Mean treatment duration was 8.8 weeks (95% CI: 8.4–9.2) in the MAMMS group and 9.1 weeks (95% CI: 8.8–9.5, p = 0.712) in the SOC group. Overall, 35 (92.1%) of the MAMMS children and 50 (86.2%) of the SOC children recovered (MUAC ≥12.5 cm) after treatment. Among the children who did not meet the criteria for successfully completing treatment, eight children completed four months of treatment but did not achieve a MUAC ≥ 12.5 cm, one child passed away, and one child was lost to follow-up.
Table 3.
Adverse health outcomes by randomization group.
| Hospitalizationa | Death | Severe malnutritionb | |
|---|---|---|---|
| SOC | 14 (2.3%) | 2 (0.3%) | 3 (5.2%) |
| MAMMS | 19 (3.2%) | 3 (0.5%) | 3 (7.9%) |
Abbreviations: MAMMS, Maternally Administered Malnutrition Monitoring System; SOC, standard of care.
Two children (both in the SOC group) were reported to have been hospitalized twice during follow-up.
Mid-upper arm circumference less than 11.5 cm at the initiation of treatment for malnutrition.
There was no statistical evidence of a difference in the number of children who were hospitalised or died during 6-month follow-up (Table 3). In the MAMMS group there were 19 (3%) hospitalisations and three (<1%) deaths, while in the SOC group 10 (2%) children were hospitalised once, two children were hospitalised twice (<1%), and two (<1%) children died.
Sensitivity analyses designed to account for the imbalance in baseline characteristics by dropping children living with HIV and adjusting for breastfeeding status, previous clinic visits and caregivers’ educational attainment did not substantively alter the results (Appendix 1a). Sensitivity analysis excluding children younger than 6-months of age at enrolment showed similar results to original analysis (Appendix 1b).
Fidelity and acceptability to MAMMS and SOC
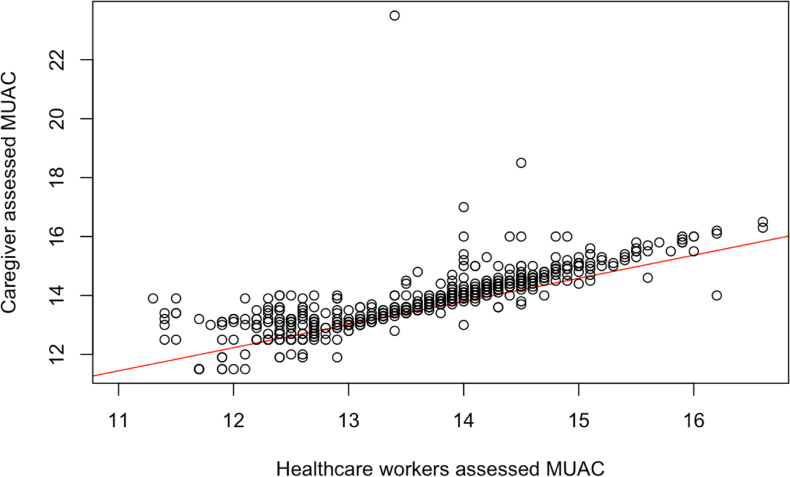
There was high uptake of the intervention among families randomised to MAMMS. Among the 599 in the MAMMS group, 563 (94%) responded to at least one text over the 6-month follow-up period (Appendix 1c), with caregivers sending a median of 20 text messages (IQR: 15–24) to the system. At the 6-month visit, 532 caregivers in the MAMMS group completed the exit survey (55 were censored due to COVID-19, four lost to follow-up, three children died, five were non-responders) and 502 (94%) reported being very comfortable using the MUAC tapes, 26 (5%) were somewhat comfortable, and four (1%) were not comfortable using the MUAC tape. During study follow-up, study staff confirmed the MUAC measurements of any caregiver in the MAMMS group that reported their child to be wasted. Of 41 such reports, four caregivers were identified to have incorrectly classified their child (positive predictive value of 0.90). The measurements of caregivers attending the 6-month visit were strongly correlated with the measurements of trained study staff (correlation coefficient = 0.78, Fig. 3). At this time point, all caregivers in the MAMMS group correctly classified their child as wasted (n = 3) or non-wasted (n = 529, Kappa = 1.00). Similarly, the SOC group was successfully implemented. In 1129 (95%) of 1192 SOC home visits (excluding visits that would have occurred after censoring or the child's death), study staff were able to successfully locate and measure the MUAC of the enrolled SOC child.
Fig. 3.
Correlation between caregiver and healthcare workerMUAC measurements at 6-month visit (Pearson correlation coefficient = 0.78). Abbreviations: MUAC, mid-upper arm circumference.
Discussion
In this randomised controlled trial, family MUAC supported by a two-way SMS mHealth platform was associated with a one-third reduction in incidence of wasting over 6-month follow-up as compared with a strong community health worker delivered SOC screening program. Both groups had a similar mean time to wasting diagnosis, severity of disease at treatment initiation, and duration of treatment among the children who progressed to wasting. These findings suggest that Family MUAC supported by two-way SMS may have a dual role in CMAM programs, offering both an adjuvant or alternative to door-to-door active screening approaches but also a method to reduce the risk of moderate childhood wasting among participating families.
Family MUAC studies in West Africa have demonstrated comparable outcomes to community health worker or health volunteer led malnutrition screening.8,9 In our trial, greater than 95% of SOC home visits successfully located and screened the enrolled child, and the estimated treatment coverage in the SOC group was 55.6%. The Sphere Project, which sets humanitarian response standards, suggests a target treatment coverage of 50% in rural non-emergency settings,3 while an analysis of 34 CMAM programs in rural settings found the mean treatment coverage to be 35%.27 Similarly, Sphere cites a target treatment success rate of 75%, and the SOC group achieved 86% treatment recovery. The Sphere targets may not be directly comparable to our results as they provide a reference for entire communities, rather than families that attended a health facility and self-selected to participate in a clinical trial, but they do illustrate that our SOC group performed above international targets and current practice in many settings. Our Family MUAC with two-way SMS platform appeared to be comparable to this strong SOC program across key outcomes including severity of wasting at enrolment into treatment, duration of treatment, and treatment success rate. Additionally, the Family MUAC group had a higher treatment coverage 6-months after enrolment than the SOC group, although this difference was not statistically significant.
Homa Bay and Migori counties are agriculturally productive, the prevalence of wasting is far below the cutoff for an emergency setting, and the population has a high literacy (>80%) rate. Despite these favourable conditions, our prior studies show that up to 50% of paediatric hospital admissions are moderately or severely wasted,6,28 and nearly 20% of families in this community-based trial reported severe food insecurity. Our results suggest that Family MUAC combined with two-way SMS messaging in this setting was highly acceptable and may have led to a reduced incidence of wasting. It seems reasonable to suggest that families who regularly assess their child's nutritional status in non-emergency settings may be able to alter their child's diet in response to the MUAC measurement and avert a certain proportion of wasting cases. A recent review of growth monitoring in high resource settings noted that in all identified studies parents reported changing their child's diet based on growth monitoring.29 Similarly, a cluster randomised trial in Zambia found that regular growth monitoring among young children led to altered feeding practices and may have promoted growth among stunted children,30 and in a qualitative study of clinic based growth monitoring in Ethiopia, mothers reported making “corrections” to the child's diet based on MUAC and weight measurements.31 However, the clinical significance of averted cases of moderate wasting remains unclear, and a larger study must determine if reductions in moderate wasting translates to fewer cases of severe wasting, fewer hospital admissions or deaths, improved linear growth and better neurodevelopmental outcomes.
The high literacy, mobile phone ownership, and access to agriculturally productive land may limit the generalisability of our findings. Emergency settings are rightly the focus of most international funding that supports nutritional programs. However, many wasted children live outside these settings,4 in areas that do not receive additional programmatic funding to support CMAM. Consequently, effective and feasible approaches to identify wasted children in settings like Migori and Homa Bay counties could greatly increase the global coverage of CMAM. In this study, we purposely intervened at the time the children became moderately wasted, as we felt it was unethical to receive weekly messages plotting the child's descent into severe wasting. It is unclear how these findings would be generalised to severe wasting programs and those in emergency settings, although as noted above, several studies have suggested Family MUAC may have a role in identifying severely wasted children.8,9
This randomised controlled trial has several strengths, including high fidelity to the intervention in both study groups and high retention over 6-month follow-up. We also observed more wasting events than anticipated in our sample size calculation, suggesting we were well powered to evaluate the wasting incidence and duration of treatment outcomes. However, there are several limitations. Given the nature of the MAMMS SMS platform, investigators and participants were not able to be blinded. We did not adjust our analyses for multiple testing, but we did reduce the significance level (alpha) of the final analysis to account for the interim analysis. The trial was also affected by the COVID-19 pandemic, resulting in interrupted follow-up visits for a small number of children. These children were evenly distributed between the groups, and we do not believe they have introduced any bias into our sample. Additionally, caregivers presenting to a health facility for routine care and willing to provide consent to participate in this clinical trial may not reflect caregivers in the broader community and may have found the Family MUAC and SMS interventions to be more acceptable than families who do not come to clinic for routine care or those that elected to not participate in the trial. MUAC is the predominant anthropometric measure used to identify wasted children in community settings, however, MUAC screening misses some children with low weight-for-height. All MUAC screening programs should be accompanied by rigorous weight and height measures at routine health or sick visits. Finally, it is possible that some degree of contamination occurred between study groups that might have reduced the ability to detect a difference between study groups. However, we are unaware of any contact between participants in the SOC and intervention groups, and by virtue of the rural nature of the study area we feel this bias is likely to be minimal.
This randomised controlled trial showed that Family MUAC supported by a two-way SMS mHealth platform was significantly associated with a one-third reduction in the overall incidence of wasting and associated with a non-significant reduction in the wasting incidence detected by the two screening programs. Additionally, the Family MUAC with two-way SMS was comparable to a successful community healthcare worker screening program in the time between enrollment and diagnosis among wasted children, the duration of treatment, and the severity of wasting at treatment initiation. In agriculturally productive areas with relatively high literacy, tools which empower caregivers to monitor their children's nutritional status may lead to reductions in the incidence of moderate wasting.
Contributors
The study was designed by KDT, BOS, CJM, BAR, KR, JAU, ARM, CL, EMC, and CA. Study implementation was overseen by BOS, EY, CA, MM, MA, NA. MMD and KDT accessed and verified the underlying data. Data were analysed by MMD and KDT. KDT and CJM wrote the first draft of the manuscript. All authors edited and approved the content of the final manuscript.
Data sharing statement
The underlying data and code have been made publicly available on Dryad (https://doi.org/10.5061/dryad.nvx0k6dz8).
Declaration of interests
KR reports payments to their institution for grants or contracts from the National Institutes of Health. KR also reports payments or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from University of California, San Francisco. All other authors declare no competing interests.
Acknowledgements
This work was supported by the Thrasher Research Foundation (ID#14656) and an unrestricted gift from Pamela H. Fowler.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102218.
Appendix A. Supplementary data
References
- 1.Black R.E., Victora C.G., Walker S.P., et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 2.UNICEF/WHO/World Bank Joint child malnutrition estimates, 2023 edition. https://data.unicef.org/resources/jme-report-2023/
- 3.Project SPHERE . 2004. SPHERE handbook: humanitarian charter and minimum standards in disease response. [DOI] [PubMed] [Google Scholar]
- 4.UNICEF . 2015. Management of severe acute malnutrition in children: working towards results at scale. [Google Scholar]
- 5.World Health O . World Health Organisation; Geneva: 2013. Guideline: updates on the managment of severe acute malnutrition in infants and children. [PubMed] [Google Scholar]
- 6.Diallo A.H., Sayeem Bin Shahid A.S.M., Khan A.F., et al. Childhood mortality during and after acute illness in Africa and south Asia: a prospective cohort study. Lancet Global Health. 2022;10:e673–e684. doi: 10.1016/S2214-109X(22)00118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hossain M., Chisti M.J., Hossain M.I., Mahfuz M., Islam M.M., Ahmed T. Efficacy of World Health Organization guideline in facility-based reduction of mortality in severely malnourished children from low and middle income countries: a systematic review and meta-analysis. J Paediatr Child Health. 2017;53(5):474–479. doi: 10.1111/jpc.13443. [DOI] [PubMed] [Google Scholar]
- 8.Ale F.G., Phelan K.P., Issa H., et al. Mothers screening for malnutrition by mid-upper arm circumference is non-inferior to community health workers: results from a large-scale pragmatic trial in rural Niger. Arch Public Health. 2016;74:38. doi: 10.1186/s13690-016-0149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackwell N., Myatt M., Allafort-Duverger T., Balogoun A., Ibrahim A., Briend A. Mothers Understand And Can do it (MUAC): a comparison of mothers and community health workers determining mid-upper arm circumference in 103 children aged from 6 months to 5 years. Arch Public Health. 2015;73:26. doi: 10.1186/s13690-015-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ALIMA . 2016. Mother-MUAC: teaching mothers to screen for malnutrition. [Google Scholar]
- 11.Wrabel M., Stokes-Walters R., King S., Funnell G., Stobaugh H. Programmatic adaptations to acute malnutrition screening and treatment during the COVID-19 pandemic. Matern Child Nutr. 2022;18 doi: 10.1111/mcn.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daures M., Phelan K., Issoufou M., et al. New approach to simplifying and optimising acute malnutrition treatment in children aged 6-59 months: the OptiMA single-arm proof-of-concept trial in Burkina Faso. Br J Nutr. 2020;123:756–767. doi: 10.1017/S0007114519003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S.H., Nurmatov U.B., Nwaru B.I., Mukherjee M., Grant L., Pagliari C. Effectiveness of mHealth interventions for maternal, newborn and child health in low- and middle-income countries: systematic review and meta-analysis. J Glob Health. 2016;6 doi: 10.7189/jogh.06.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronen K., Choo E.M., Wandika B., et al. Evaluation of a two-way SMS messaging strategy to reduce neonatal mortality: rationale, design and methods of the Mobile WACh NEO randomised controlled trial in Kenya. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-056062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unger J.A., Ronen K., Perrier T., et al. Short message service communication improves exclusive breastfeeding and early postpartum contraception in a low- to middle-income country setting: a randomised trial. BJOG. 2018;125:1620–1629. doi: 10.1111/1471-0528.15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake A.L., Unger J.A., Ronen K., et al. Evaluation of mHealth strategies to optimize adherence and efficacy of Option B+ prevention of mother-to-child HIV transmission: rationale, design and methods of a 3-armed randomized controlled trial. Contemp Clin Trials. 2017;57:44–50. doi: 10.1016/j.cct.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronen K., Unger J.A., Drake A.L., et al. SMS messaging to improve ART adherence: perspectives of pregnant HIV-infected women in Kenya on HIV-related message content. AIDS Care. 2018;30:500–505. doi: 10.1080/09540121.2017.1417971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenyan National Bureau of Statistics, Kenyan Ministry of Helath, National AIDS Control Council . National Council for Population and Development, The DHS Program II. Kenya Demographic and Health Survey; 2015. Kenya medical research Institute.https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf [Google Scholar]
- 19.Tickell K.D., Diakhate M.M., Goodman J.L., et al. Impact of a two-way short message service (SMS) to support maternally administered childhood mid-upper arm circumference monitoring and expand malnutrition screening in Kenya: the Mama Aweza trial protocol. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-036660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dairo M.D., Fatokun M.E., Kuti M. Reliability of the mid upper arm circumference for the assessment of wasting among children aged 12-59 months in Urban Ibadan, Nigeria. Int J Biomed Sci. 2012;8:140–143. [PMC free article] [PubMed] [Google Scholar]
- 21.Eaton-Evans J. In: Encyclopedia of human nutrition. 3rd ed. Caballero B., editor. Academic Press; Waltham: 2013. Nutritional assessment: anthropometry; pp. 227–232. [Google Scholar]
- 22.Unger J.A., Kinuthia J., John-Stewart G. Texting condolences: adapting mHealth programs after unexpected pregnancy and infant outcomes. JMIR Mhealth Uhealth. 2017;5:e176. doi: 10.2196/mhealth.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington E. Evaluation of an mHealth SMS dialogue strategy to meet women's and couples' postpartum contraceptive needs in Kenya (mobile WACh XY) https://clinicaltrials.gov/ct2/show/NCT02781714?term=Mobile+WACh&rank=2
- 24.UNICEF . 2017. UNICEF’S programme guidance for early childhood development UNICEF. [Google Scholar]
- 25.WHO . 2014. Integrated management of childhood illness chart booklet. Module 2: the sick young infant. [Google Scholar]
- 26.Kenyan Ministry of Health . 2009. National guideline for integrated management of acute malnutrition. [Google Scholar]
- 27.Rogers E., Myatt M., Woodhead S., Guerrero S., Alvarez J.L. Coverage of community-based management of severe acute malnutrition programmes in twenty-one countries, 2012-2013. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tickell K.D., Pavlinac P.B., John-Stewart G.C., et al. Impact of childhood nutritional status on pathogen prevalence and severity of acute diarrhea. Am J Trop Med Hyg. 2017;97:1337–1344. doi: 10.4269/ajtmh.17-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansoor Y., Hale I. Parent perceptions of routine growth monitoring: a scoping review. Paediatr Child Health. 2021;26:154–158. doi: 10.1093/pch/pxaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fink G., Levenson R., Tembo S., Rockers P.C. Home- and community-based growth monitoring to reduce early life growth faltering: an open-label, cluster-randomized controlled trial. Am J Clin Nutr. 2017;106:1070–1077. doi: 10.3945/ajcn.117.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilal S.M., Moser A., Blanco R., Spigt M., Dinant G.J. Practices and challenges of growth monitoring and promotion in Ethiopia: a qualitative study. J Health Popul Nutr. 2014;32:441–451. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.