Abstract
Dogs were experimentally inoculated with Ehrlichia canis Florida to assess the efficacy of doxycycline hyclate for the treatment of acute ehrlichiosis. Treatment with doxycycline eliminated infection in eight of eight dogs. Untreated infected control dogs appeared to eliminate the infection or, alternatively, suppress the degree of ehrlichiemia to a level not detectable by tissue culture isolation or PCR or by transfusion of blood into recipient dogs. Prior infection did not infer protection against homologous (strain Florida) or heterologous (strain NCSU Jake) strains of E. canis. We conclude that doxycycline hyclate is an effective treatment for acute E. canis infection; however, these results may not be applicable to chronic infections in nature. Spontaneous resolution of infection, induced by the dog’s innate immune response, provides evidence that an E. canis vaccine, once developed, might potentially confer protective immunity against the organism.
Ehrlichia canis is a cause of monocytic ehrlichiosis, a disease of worldwide importance in dogs. Despite several studies that have addressed the therapeutic efficacy of tetracycline hydrochloride (1, 2, 6, 17) or doxycycline hyclate (2, 11, 19, 20) for the treatment of E. canis infection in dogs, the effectiveness of tetracycline derivatives for the elimination of E. canis infection remains controversial. In an experimental infection study, treatment with doxycycline hyclate for 7 days resulted in a carrier status, i.e., E. canis organisms were still present, in three of five dogs (11). As a result of this and because of persistent posttreatment clinical or hematologic abnormalities or the persistence of E. canis-specific serum antibodies, some veterinarians have adopted the practice of treating E. canis infections with doxycycline for months or years (2). Providing medically or scientifically valid documentation that E. canis infection has been therapeutically eliminated has important clinical implications. This documentation is particularly relevant for dogs with persistent unexplained clinical or hematologic abnormalities following treatment for ehrlichiosis (nonresponders).
Recently, important strain differences have been identified among other Ehrlichia species, particularly Ehrlichia chaffeensis and Ehrlichia risticii (4, 7, 8, 21). Differences in in vitro biologic behaviors and antigenicities were found for the two currently characterized E. chaffeensis strains (8). Of particular importance, E. risticii antigenic strain differences appear to be responsible for the failure of commercially available vaccines to consistently prevent Potomac horse fever (4, 21). Despite substantial variation in the type, duration, or severity of disease manifestations attributed to natural E. canis infection, there is minimal clinical, experimental, or microbiologic data to compare the immunopathogenic variability associated with different E. canis strains (16). The extent to which differences in disease manifestations reflect pathogenic diversity among strains, variability in the immunologic response of individual dogs to the rickettsia, repeated exposure to E. canis, coinfection with other tick-transmitted pathogens, or other, unknown factors remains unclear. Although experimental studies conducted nearly three decades ago documented reinfection following homologous E. canis challenge (3), studies using more sensitive techniques for the detection of E. canis have not been conducted to determine whether reinfection occurs following homologous or heterologous E. canis challenge.
The purposes of this study were (i) to assess the efficacy of doxycycline hyclate for the treatment of acute ehrlichiosis in experimentally infected dogs; (ii) to compare the utility of tissue culture isolation, Western immunoblotting, PCR detection of E. canis DNA, and the transfusion of blood into recipient dogs to document therapeutic elimination of the organism; and (iii) to compare the severity of disease manifestations following homologous or heterologous E. canis challenge.
MATERIALS AND METHODS
E. canis strains.
E. canis Florida, the historical reference strain, was obtained in primary canine monocytes from the University of Illinois before 1988. The North Carolina isolate (NCSU Jake) was cultured in 1989 from a sick 2-year-old male pug dog admitted to the North Carolina State University Veterinary Teaching Hospital for evaluation of normocytic normochromic nonregenerative anemia (hematocrit, 20%), neutropenia (2,600 neutrophils/μl), thrombocytopenia (platelets 29,000/μl), and seroreactivity to E. canis antigen (reciprocal immunofluorescent antibody [IFA] titer, 20,840). E. canis NCSU Jake has been maintained in tissue culture since the original isolation and was used for heterologous challenge in this study.
Inoculum preparation.
E. canis organisms (Florida and NCSU Jake strains) were grown in 030 cells (a continuous canine-origin histiocytic cell line) (15). Aliquots of frozen cellular stock were assayed for viable organisms with 10-fold serial dilutions in tubes containing 030 cells. The cellular harvests were diluted to 100 organisms/ml in sterile brain heart infusion broth and were inoculated intravenously into two dogs (donor 1 received 10 ml, but donor 2 received only 2 ml due to the development of anaphylaxis) that served as infected-blood donors for the experimental infection study. Infection of donor dogs with the tissue culture-derived E. canis strains was timed so that experimental recipients were transfused during a period of culture-confirmed ehrlichiemia. Infected blood was collected from donor 1 on postinfection day (PID) 33 for initial infection of dogs in groups II, III, and IV and on PID 103 for homologous challenge of dogs in group III. Blood from donor 2 was collected on PID 25 for heterologous challenge of dogs in group IV. Ehrlichiemia was documented by tissue culture isolation on PIDs 33 and 103 for donor 1 and on PID 25 for donor 2.
Experimental animals and infection transmission.
Sixteen mixed-breed dogs (Hazelton Laboratories) ranging in age from 9 to 12 months were randomized to one of the following four study groups: group I, uninfected control; group II, infected control; group III, homologous challenge; group IV, heterologous challenge (n = 4 dogs/group). Prior to infection, clinical and hematologic test results were within the reference ranges, and all dogs were seronegative for E. canis antigens by IFA testing. Initially, groups II, III, and IV were inoculated with 20 ml of E. canis reference strain (strain Florida)-infected donor blood anticoagulated with citrate dextrose solution (ACD) at a ratio of 1 ml of ACD/20 ml of blood. The uninfected control group (group I) was inoculated by the same procedure with 20 ml of blood from a healthy donor.
Doxycycline treatment.
Beginning on PID 31, group III and IV dogs were treated with oral doxycycline hyclate (mean group dosages, 6 and 5.6 mg/kg of body weight twice daily, respectively) for 14 consecutive days. Group II dogs, although infected, were not treated with doxycycline.
Challenge inoculation.
On PID 90, group III dogs received a homologous E. canis challenge (strain Florida) and group IV dogs received a heterologous E. canis challenge (strain NCSU Jake). Group I and II dogs received blood from a healthy donor not seroreactive for E. canis.
Monitoring.
Beginning 1 week prior to initial inoculation, physical examinations, including rectal temperature measurements and quantitation of behavioral characteristics, were performed daily in an examiner-blinded fashion. Behavioral scores were recorded by using the following criteria: 5, alert, active, and eating; 4, alert, inactive, and eating; 3, depressed, inactive, and anorexic; 2, severely depressed and anorexic; and 1, recumbent. Beginning on PID 7, blood samples were collected from the jugular vein at approximately 7-day intervals until the conclusion of the study at 21 weeks.
Blood for complete blood cell counts, manual thrombocyte counts, and PCR analyses was collected in tubes containing EDTA. Serum for E. canis IFA testing and Western immunoblotting was separated from tubes not containing an anticoagulant.
Serology.
A microimmunofluorescence test was used to detect antibodies to E. canis Florida on 30-well Teflon-coated slides (19). Serial twofold dilutions of sera from the dogs were reacted with fluorescein isothiocyanate anti-canine immunoglobulin G conjugate (Organon Teknika, Westchester, Pa.). Endpoint titers were determined as the last dilution at which brightly staining organisms could be detected on a fluorescence microscope with exciter and barrier filters.
Documenting therapeutic elimination of E. canis.
Four techniques for documenting therapeutic elimination of E. canis were compared: tissue culture isolation, Western immunoblotting, PCR detection of E. canis DNA, and transfusion of blood into recipient dogs.
(i) Tissue culture isolation.
Isolation in tissue culture was performed by a modification of the method described by Iqbal and Rikihisa (11). For each of the 16 dogs, 6 ml of blood was collected aseptically from the jugular vein and placed into tubes containing EDTA anticoagulant. Whole blood was spun at 1,500 × g for 5 min, the erythrocyte fraction was discarded, and the plasma was spun again for 20 min. Leukocyte cell pellets were resuspended in phosphate-buffered saline (PBS)–bovine serum albumin. These suspensions were overlaid over 2 ml of Histopaque-1077 (Sigma, St. Louis, Mo.) and spun at 2,000 × g for 15 min. The resulting monocyte-rich cell fractions were inoculated onto cultures of 030 cells in 24-well plates in duplicate and fed RPMI 1640 (Gibco BRL, Gaithersburg, Md.) containing 20% fetal bovine serum (Hyclone, Logan, Utah), l-glutamine, and sodium bicarbonate. The plates were incubated at 35°C with 5% CO2 for 8 weeks. Cellular samples of culture supernatants were tested every 2 weeks by indirect immunofluorescence (with a monoclonal antibody provided by D. H. Walker, University of Texas Medical Branch, Galveston, Tex.) and direct immunofluorescence (with rabbit anti-ehrlichia fluorescein isothiocyanate-labeled conjugate obtained from J. E. Dawson, Centers for Disease Control and Prevention, Atlanta, Ga.) for the presence of morulae. Testing prior to 4 weeks can result in occasional nonspecific staining of monocytoid cells. Therefore, to be considered positive, morulae had to be detected on two occasions after 4 weeks in culture. Cultures were maintained for 8 weeks before they were considered negative. Failure of cells to persist in culture for at least 6 weeks was reported as an inconclusive result.
(ii) Western immunoblotting.
Antigen grown in 030 cells was purified by sucrose gradient centrifugation, and the protein concentration was determined. Dilutions made in final sample buffer at a protein concentration of 2.5 mg/ml were loaded at 10 μl per well and were electrophoresed on sodium dodecyl sulfate-polyacrylamide precast minigels (Jule, Inc., New Haven, Conn.). Proteins were electrotransferred to 0.45-μm-pore-size nitrocellulose paper. After blocking with 5% milk in PBS, proteins were reacted with canine serum samples at a 1:100 dilution followed by reaction with peroxidase-conjugated goat anti-canine immunoglobulin G at a 1:400 dilution in 1% milk in PBS. Bands were detected with the color reagent 4-chloro-1-naphthol. Serum from a dog experimentally infected with E. canis Florida with a reciprocal titer of 10,240 was used as a positive control. Serum from uninfected laboratory-raised dogs was reacted with E. canis and normal cell antigens to eliminate the possibility of nonspecific binding.
(iii) DNA extraction and nested PCR analysis.
With a commercially available QIAmp Blood Kit (Qiagen, Chatsworth, Calif.), DNA was extracted from 600-μl, EDTA-anticoagulated whole blood samples that had been stored frozen at −70°C. Cell culture-grown E. canis NCSU Jake was used as a positive control. A two-step seminested PCR method was established by using three primers (the primer sequences for the nested PCR used in this study were recommended by S.-M. Chen, University of Texas Medical Branch) derived from the 16S rRNA gene sequence of E. canis (8). The first PCR amplification was performed in a 50-μl reaction mixture containing 1 μg of DNA template; 200 μM (each) dATP, dTTP, dCTP, and dGTP; 10 pmol of primers designated GE2f (5′-GTTAGTGGCATACGGGTGAAT-3′) and EC19 (5′-AAGGATCCACTCATCGTTTACAGCGTGG-3′); 2 mM MgCl2; and 1.25 U of Taq DNA polymerase (Promega, Madison, Wis.) in a 1× reaction buffer (50 mM KCl, 10 mM Tris HCl [pH 8.3]). All reactions were carried out in sterile HotStart tubes (Molecular Bio-Products, San Diego, Calif.), which allow separation of the first and second PCR steps by a wax bead. Amplification cycles included denaturation at 94°C for 30 s, annealing at 52°C for 1 min, and chain extension at 72°C for 2 min. The PCR cycle was repeated for 30 cycles, with a final extension of 3 min at 72°C. For the second round of PCR, a 50-μl reaction mixture similar to that described above was used, except that no DNA template was added, primer EC19 was replaced with 50 pmol of GE7r (5′-CCGTATCTCAGTTCCAGTGTG-3′), and 50 more pmol of primer GE2f was added. The 50-μl mixture was added on top of the wax bead to separate the first and second reactions. An initial cycle of 94°C for 5 min melted the wax, denatured the DNA, and initiated the subsequent reactions. Amplification cycles were then run at 94°C for 30 s, annealing at 60°C for 1 min, and chain extension at 72°C for 2 min. The PCR cycle was repeated for 30 cycles, with a final extension of 3 min at 72°C. The PCR products were electrophoresed through 1% agarose gels in Tris-boric acid-EDTA buffer, and the DNA fragments were visualized by ethidium bromide staining under UV fluorescence.
(iv) Secondary transfusion to recipient dogs.
To further document the sensitivity of culture and PCR as modalities for the detection of ehrlichiemia, blood was collected from all infected dogs and shipped to Fort Dodge Laboratories in Fort Dodge, Iowa. There, 20 ml of blood from each infected control dog in group II was transfused into a new recipient (n = 4). A 40-ml pooled sample from four dogs in group III receiving a homologous challenge (10 ml from each dog) was transfused to two recipients (20 ml each). The same approach was used for dogs in group IV receiving a heterologous challenge.
Statistics.
Treatment and challenge inoculation effects were analyzed by multivariate repeated-measures analysis of variance (14) with PROC GLM software (version 6-09) from SAS Inc. (Cary, N.C.). A separate analysis was performed for each physiologic parameter. A Student-Newman-Keuls comparison procedure at an alpha level of 0.05 was carried out each time to differentiate significant effects among the groups. The analysis was done separately for times prior to infection, prior to treatment, following treatment and prior to challenge, and following challenge. Profile contrasts were used to detect significant differences in the shape of the parameter response curves among the experimental groups. The nonparametric Kruskal-Wallis test was performed on attitudinal scores due to the nonnormality of the data. Proc NPARIWAY software (version 6.09) from SAS Inc. was used.
RESULTS
Initial infection.
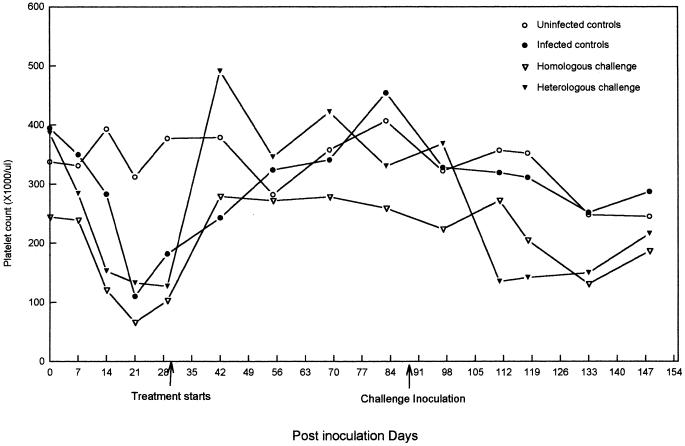
Prior to infection there were no significant differences in attitudinal scores, rectal temperature measurements, or neutrophil or platelet counts among the four groups of dogs. Despite randomization, prior to and throughout the study there was a tendency for dogs in groups I and III to have higher mean packed cell volumes (PCV), which at various time points was significantly (P < 0.05) lower than those for dogs in groups II and IV. On the basis of the development of consistent clinical and hematologic abnormalities (Fig. 1), seroconversion to E. canis antigens (Fig. 2), positive tissue culture results (Table 1), and PCR detection of E. canis DNA (Table 1; Fig. 3), all 12 dogs in groups II, III, and IV became infected following the initial inoculation with E. canis Florida. Fever was detected in the dogs in these three groups between days 14 and 21, and behavioral scores decreased from 5 to 4 between PIDs 13 and 32. During the pretreatment period (PIDs 1 to 30), there was a mild decrease in PCVs and neutrophil counts and a significant decrease in platelet counts (P = 0.02) among the infected dogs. The platelet count nadir for the dogs in the three inoculated groups occurred on PID 21 (Fig. 1). There was no significant difference (P > 0.05) in mean PCV, neutrophil count, or platelet count among the dogs in the three infection groups prior to the initiation of doxycycline treatment. Morulae were not observed on Wright’s-stained blood smears at any time during the study. E. canis-specific antibodies were detectable in all 12 dogs on PID 14 (reciprocal titers ranged from 20 to 640). Western immunoblotting detected a genus-specific protein of approximately 30 kDa by PID 29 in all but one dog (dog 150, group II). Full recognition of ehrlichial proteins, as exemplified by a positive control serum sample obtained from a chronically infected experimental dog, was not detected in most dogs until after PID 90. Control sera consistently recognized 28- through 30-, 32-, 40-, 53-, and 60-kDa proteins, with variable recognition of 24-, 26-, 27-, and 64-kDa proteins. Tissue culture isolation of E. canis was successful in samples from 3 dogs on PID 7, from 10 dogs on PID 14, from all 12 infected dogs on PID 21, and from 7 dogs on PID 29 (Table 1). PCR results were concordant with tissue culture isolation results except on four occasions when PCR was negative and three occasions when PCR was positive (Table 1).
FIG. 1.
Mean platelet counts for dogs in the uninfected control, infected control, homologous challenge, and heterologous challenge groups after transfusion of E. canis-infected blood (day 0), after initiation of doxycycline treatment (day 31), and after challenge inoculation (day 90).
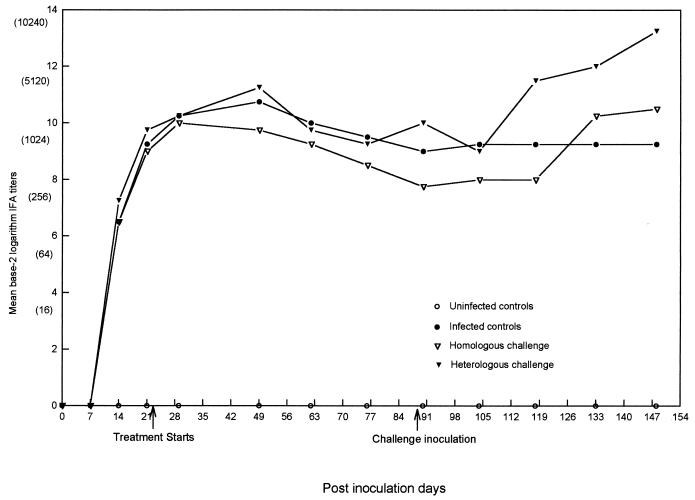
FIG. 2.
Mean microimmunofluorescence antibody (IFA) titers for dogs in the uninfected control (no seroconversion), infected control, homologous challenge, and heterologous challenge groups after transfusion of E. canis-infected blood (day 0), after the initiation of doxycycline treatment (day 30), and after challenge inoculation (day 90). The equivalent reciprocal IFA titers are given in parentheses.
TABLE 1.
Tissue culture and PCR results for control and experimental infection groups
| Experimental group | Dog no. | Tissue culture result/PCR result at the following timesa:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection period
|
Posttreatment period (PID)
|
Postchallenge period
|
|||||||||||
| 7 | 14 | 21 | 29 | 49 | 62 | 76 | 90 | 111 | 118 | 133 | 148 | ||
| Uninoculated control group | 151 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | */− | −/− | −/− | −/− | −/− |
| 161 | − | − | − | −/− | −/− | −/− | −/− | */− | − | − | − | − | |
| 162 | − | −/− | − | −/− | −/− | −/− | */− | */− | − | − | − | − | |
| 164 | − | − | − | −/− | −/− | */− | */− | */− | − | − | − | − | |
| Infected control group | 150 | −/+ | −/+ | +/+ | +/+ | +/+ | −/− | */− | */− | − | − | − | − |
| 152 | +/− | +/− | +/+ | +/+ | −/+ | −/+ | */− | */− | − | * | + | − | |
| 153 | −/+ | +/+ | +/+ | +/− | −/− | +/+ | −/− | */+ | +/+ | −/+ | −/+ | −/+ | |
| 163 | − | +/+ | +/+ | − | −/+ | −/− | −/− | */− | − | − | − | − | |
| Homologous challenge group | 155 | − | + | +/− | +/+ | −/− | −/− | */− | */− | + | − | − | − |
| 156 | − | + | +/+ | */+ | −/− | −/− | */− | */− | − | − | + | − | |
| 157 | − | + | + | +/+ | −/− | −/− | −/− | */− | + | + | + | + | |
| 158 | − | + | + | +/+ | −/− | −/− | */− | */− | − | + | − | − | |
| Heterologous challenge group | 149 | + | −/− | +/− | −/− | −/− | −/− | −/− | */− | − | + | + | − |
| 154 | + | +/+ | +/+ | +/+ | −/− | −/− | */− | */− | + | + | + | + | |
| 159 | − | +/+ | + | −/− | −/− | −/− | −/− | */− | − | + | − | − | |
| 160 | − | +/+ | + | − | −/− | −/− | */− | */− | + | − | − | − | |
If only a single result is provided, the results reflect tissue culture findings only. Tissue culture supernatants were examined at 4, 6, and 8 weeks after cultures were established. Positive (+) results reflect detection of organisms on at least two occasions. Negative (−) results indicate that no organisms were observed. Asterisk indicate that cultures failed to persist for at least 6 weeks; therefore, the results were considered inconclusive. For dogs in the homologous challenge and heterologous challenge groups, treatment was from PIDs 31 to 44 and the challenge inoculation was given on PID 90.
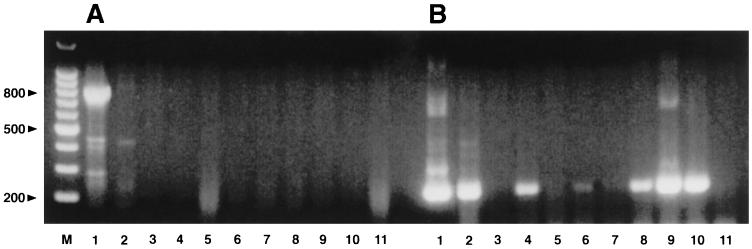
FIG. 3.
Results of PCR for E. canis detection demonstrating enhanced sensitivity of the seminested method. (A) By using first-step primers, a 780-bp PCR product can be visualized in lane 1 (positive control, cultured E. canis) but not in lanes 2 to 11 (DNA extracted from EDTA-anticoagulated blood samples from dogs 2 to 11, respectively). (B) A seminested PCR product yielding a 220-bp band can be identified for the positive control (lane 1) and blood samples from dogs 2, 4, 6, 8, 9, and 10 (the same samples tested in panel A). Lane M, 100-bp DNA ladder (Promega). Numbers on the left are in base pairs.
Doxycycline treatment.
Immediately prior to treatment, the behavioral score was 5 (alert, active, and eating) for all 16 dogs, and all dogs were afebrile. During the period of treatment, the behavioral score remained 5 for all 16 dogs. There were no significant differences in rectal temperature measurements, mean hematocrit values, absolute neutrophil counts, or platelet counts.
Documenting therapeutic elimination of E. canis.
Following treatment with doxycycline, tissue culture isolation attempts and PCR results were negative during the 45-day posttreatment period (Table 1). During this time period, reciprocal IFA titers decreased gradually (greater than or equal to a twofold dilution) in 11 of 12 dogs (groups II to IV); 1 dog (dog 149, group IV) maintained a stable titer. Sera from untreated infected control dogs (group II) developed protein recognition patterns on Western immunoblots that were similar to those seen for sera from control dogs by PID 90, with recognition of 24-, 26-, 28- through 30-, 32-, 40-, and 60-kDa proteins, whereas similar patterns of protein recognition were delayed in the dogs in the two treatment groups until after challenge (PID 118).
Challenge inoculation and postchallenge period.
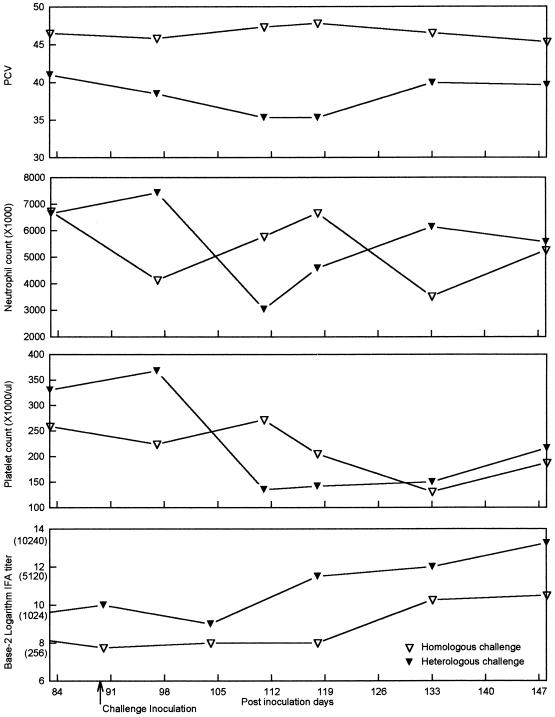
With the exception that the mean platelet counts for dogs in group II were significantly higher (P = 0.05) than those for dogs in group III or IV immediately prior to challenge inoculation, there were no differences among the groups. Following challenge inoculation on PID 90, both challenge groups (groups III and IV) became E. canis culture positive (Table 1). Compared to homologous challenge, heterologous challenge resulted in an enhanced severity of fever, anemia, neutropenia, and thrombocytopenia and an increased anamnestic serologic response (Fig. 4). During this period (PIDs 90 to 148), E. canis was detected by tissue culture on two occasions (dog 153 on PID 111 and dog 152 on PID 133) and PCR on four occasions (dog 153) among the dogs in the infected control group. There were no discernible differences in the Western immunoblot patterns among the three infection groups during the postchallenge period (PIDs 90 to 148). Despite an anamnestic IFA serologic response in the groups receiving homologous and heterologous challenge, no discernible change in the staining intensity or the number of protein bands was detected by Western immunoblotting in samples from those groups compared to the staining intensity and number of protein bands in samples from the infected control group.
FIG. 4.
Mean PCV, neutrophil and platelet counts, and IFA titers for dogs in the homologous challenge and heterologous challenge groups following challenge inoculation (day 90). The equivalent reciprocal IFA titers are given in parentheses.
Blood obtained on PID 148 from each of the infected control dogs was transfused into four healthy recipient dogs. These dogs did not develop clinical abnormalities or thrombocytopenia or demonstrate seroconversion to E. canis antigens. Blood obtained on PID 148 from dogs in both the homologous challenge and the heterologous challenge groups was similarly transfused into eight healthy recipient dogs. Resulting compatible clinical abnormalities included fever (rectal temperatures, greater than 39.8°C), thrombocytopenia (platelet counts, below 100,000/μl), and seroconversion to E. canis antigens (reciprocal titers, 5,120). Although it was expensive and of limited practical utility, administration of blood to recipient dogs served to support the tissue culture and PCR results derived during the final stage of this project.
Summarization of the culture and PCR data in Table 1 indicates test agreement for 56 datum points and test disagreement for 12 datum points. Agreement between culture and PCR results was found for 56 negative and 17 positive datum points, respectively; culture was positive and PCR was negative in five instances, whereas culture was negative and PCR was positive in seven instances.
DISCUSSION
Doxycycline treatment and the possibility of natural immunity.
When untreated, E. canis infection generally persists in the presence of extremely high concentrations of antibody to the organism (3, 9). Therefore, humoral immunity and cell-mediated immunity following natural infection do not usually confer protection against chronic infection. In nature, many dogs remain infected, presumably for years, without developing obvious abnormalities as a result of the disease (5). Since we are unable to find definitive supportive evidence of spontaneous resolution of E. canis infection in the literature, it is perhaps of importance that all four untreated infected control dogs appeared to eliminate the infection or, alternatively, suppress the degree of ehrlichiemia to a level that was not detectable by tissue culture isolation or PCR or by the transfusion of blood into recipient dogs. This finding may have important implications, namely, that an E. canis vaccine, once developed, might potentially confer protective immunity against the organism.
In a previous study (11), three of five dogs treated for 7 days with doxycycline (10 mg/kg once daily) remained tissue culture positive following treatment, and E. canis DNA could be amplified from several tissues, including blood, kidney, lymph node, liver, and spleen, that were collected when the dogs were killed 54 to 59 days following treatment. In contrast, following doxycycline administration at a similar dosage for 14 days in this study, all eight dogs became culture and PCR negative during the posttreatment period. Although tissue culture isolation and/or PCR detection of E. canis DNA was intermittent for the untreated infected control group during this same time period, doxycycline appears to have been uniformly effective in eliminating E. canis infection from the eight treated dogs.
Utility of various techniques for detecting the presence or absence of E. canis.
During recent years, there has been increasing interest in the evolving role of Ehrlichia species as human and veterinary pathogens. Historical difficulties associated with the isolation of Ehrlichia species in tissue culture have compromised the design of therapeutic trials and have limited the number of E. canis isolates available for comparative microbiologic or immunopathogenic studies. Since tissue culture isolation of E. canis can require up to 60 days (11), the technique is of limited clinical utility. Similarly, tissue culture isolation of E. chaffeensis, the cause of human monocytic ehrlichiosis, is a difficult, laborious, and time-consuming process, known to require as many as 35 (7) or 60 (8) days for the detection of E. chaffeensis morulae.
In an effort to circumvent the difficulties associated with tissue culture isolation, recent attempts to document E. canis infection in dogs have incorporated antigen detection by enzyme-linked immunosorbent assay (22) or DNA amplification by PCR (12, 13). Unfortunately, antigen assays may have limited utility because of variability in antigen detection during the early stages of experimental E. canis infection (22). In this study, we attempted to examine the diagnostic utility of seminested PCR analysis, using EDTA-anticoagulated blood samples that would be comparable to the type of sample routinely obtained from sick dogs for the purpose of hematologic examination. As depicted in Fig. 3, the seminested PCR proved to be considerably more sensitive than the single-step PCR. Recent modifications to the procedure, such as eliminating the necessity of opening the tube to add reagents for the second step, decreases problems associated with PCR contamination. In this study there was generally good agreement between tissue culture and PCR results. In no instance was an uninfected dog found to be culture or PCR positive. Similar to a previous study (12, 13), tissue culture appeared to be slightly more sensitive than seminested PCR for the detection of E. canis, particularly during the acute infection period.
Similar to one of the objectives of this study, Iqbal and colleagues (11–13) compared the sensitivity of PCR with those of tissue culture isolation, IFA testing, and Western immunoblotting with experimentally infected German shepherd-mixed breed dogs. Unlike this study, however, Iqbal and colleagues (12, 13) used single-step PCR analysis and large quantities of blood, potentially as much as 30 ml, for the extraction of E. canis DNA. The important influence of variables such as inoculum size and route of administration and the potentially detrimental effects of tissue culture passage of inoculum on the course of experimentally induced canine ehrlichiosis have been emphasized in two recent reports (9, 16). Therefore, differences in several experimental variables, including age, breed, E. canis strains, source of inocula, timing and duration of treatment, and the type of samples used for various tests, make direct comparison of the results of these two studies somewhat difficult.
In our experience, Western immunoblotting is useful for confirmation of acute versus chronic E. canis exposure or for clarification of questionable IFA serologic test results (10). In this study, treatment appeared to alter the pattern of antigenic protein recognition, with fewer epitopes identified in treated dogs than in infected control dogs. However, posttreatment immunoblot patterns did not identify predictable changes that would facilitate confirmation of the therapeutic elimination of the organism or identify subsequent infection following reexposure to E. canis. In light of our experience with clinical canine ehrlichiosis and given the relatively short time span encompassed by this study, Western immunoblotting for E. canis detection appears to have limited applicability as a diagnostic modality other than to confirm the specificity of an IFA test result (18).
Severity of disease manifestations after homologous or heterologous challenge.
Experimental studies performed nearly three decades ago indicated that prior infection induced minimal protection to homologous E. canis challenge (3). Therefore, conventional wisdom has suggested that following therapeutic elimination of the organism, reinfection will result in recurrent but milder disease. In this study, which used more sensitive culture and molecular detection techniques, dogs that were infected and subsequently cleared of their infection after treatment with doxycycline were found to develop ehrlichiemia and disease manifestations following both homologous challenge and heterologous challenge. Of potential importance, heterologous challenge resulted in increased disease severity. Although other factors may have influenced these results, it is possible that (i) the heterotypic immune response caused enhancement of disease manifestations or (ii) the NCSU Jake strain of E. canis is more virulent than the historical Florida strain. These findings also suggest the possibility that late-stage disease manifestations in naturally infected dogs may develop only in those instances when the host is repeatedly exposed to E. canis or when a chronically infected dog is subsequently exposed to other intracellular pathogens. Regardless of the interpretation, these results indicate that variability among E. canis strains, similar to the experience with E. chaffeensis and E. risticii, may be of importance when comparing the results of experimental infection studies or when developing vaccination strategies for the prevention of monocytic ehrlichiosis in dogs. Because prevention of canine monocytic ehrlichiosis through vaccination would provide a beneficial approach to the prevention of this disease, additional efforts to characterize differences in virulence among E. canis strains appear to be justified.
ACKNOWLEDGMENTS
This work was supported by the state of North Carolina and by a grant from Fort Dodge Laboratories.
We thank Kendall Wills Sterling for editorial assistance; David Wilson for statistical analysis of the data; Cynthia Babineau, Julie Bradley, and David Corns for technical assistance; Dale Brown for careful monitoring of the dogs; and the Laboratory Animal Resources staff for the care of these dogs.
REFERENCES
- 1.Adawa D A Y, Hassan A Z, Abdullah S U, Ogunkoya A B, Adeyanju J B, Okoro J E. Clinical trial of long-acting oxytetracycline and piroxicam in the treatment of canine ehrlichiosis. Vet Q. 1992;15:118–120. doi: 10.1080/01652176.1992.9694345. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch R C, Greene R T. Post-therapy antibody titers in dogs with ehrlichiosis: follow-up study on 68 patients treated primarily with tetracycline and/or doxycycline. J Vet Intern Med. 1996;10:271–274. doi: 10.1111/j.1939-1676.1996.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 3.Buhles W C, Huxsoll D L, Ristic M. Tropical canine pancytopenia: clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J Infect Dis. 1974;130:357–367. doi: 10.1093/infdis/130.4.357. [DOI] [PubMed] [Google Scholar]
- 4.Chaichanasiriwithaya W, Rikihisa Y, Yamamoto S, Reed S, Crawford T B, Perryman L E, Palmer G H. Antigenic, morphologic and molecular characterization of new Ehrlichia risticii isolates. J Clin Microbiol. 1994;38:3026–3033. doi: 10.1128/jcm.32.12.3026-3033.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Codner E C, Farris-Smith L L. Characterization of the subclinical phase of ehrlichiosis in dogs. J Am Vet Med Assoc. 1986;189:47–50. [PubMed] [Google Scholar]
- 6.Davidson D E, Dill G S, Tingpalapong M, Premabutra S, Nguen P L, Stephenson E H, Ristic M. Prophylactic and therapeutic use of tetracycline during an epizootic of ehrlichiosis among military dogs. J Am Vet Med Assoc. 1978;172:697–700. [PubMed] [Google Scholar]
- 7.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumler J S, Chen S-M, Asanovich K, Trigiani E, Popov V L, Walker D H. Isolation and characterization of a new strain of Ehrlichia chaffeensis from a patient with nearly fatal monocytic ehrlichiosis. J Clin Microbiol. 1995;33:1704–1711. doi: 10.1128/jcm.33.7.1704-1711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaunt S D, Corstvet R E, Berry C M, Brennan B. Isolation of Ehrlichia canis from dogs following subcutaneous inoculation. J Clin Microbiol. 1996;34:1429–1432. doi: 10.1128/jcm.34.6.1429-1432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegarty B C, Levy M G, Gager R F, Breitschwerdt E B. Immunoblot analysis of the immunoglobulin G response to Ehrlichia canis in dogs: an international survey. J Vet Diagn Invest. 1997;9:32–38. doi: 10.1177/104063879700900106. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal Z, Rikihisa Y. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J Clin Microbiol. 1994;32:1644–1649. doi: 10.1128/jcm.32.7.1644-1649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iqbal Z, Chaichanasiriwithaya W, Rikihisa Y. Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J Clin Microbiol. 1994;32:1658–1662. doi: 10.1128/jcm.32.7.1658-1662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal Z, Rikihisa Y. Application of the polymerase chain reaction for the detection of Ehrlichia canis in tissues of dogs. Vet Microbiol. 1994;42:281–287. doi: 10.1016/0378-1135(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 14.Johnson T A, Wichern D W. Applied multivariate statistical analysis. 2nd ed. Englewood Cliffs, N.J: Prentice-Hall, Inc.; 1988. p. 607. [Google Scholar]
- 15.Levy M G, Gebhard D, Gager R, Breitschwerdt E B. Proceedings of the International Union of Immunology Society, 4th International Veterinary Immunology Symposium. 1995. A newly described canine monocytoid cell line capable of supporting growth of the rickettsial parasite Ehrlichia canis, and useful for examination of host:parasite interactions, abstr; p. 274. [Google Scholar]
- 16.Mathew J S, Ewing S A, Barker R W, Fox J C, Dawson J E, Warner C K, Murphy G L, Kocan K M. Attempted transmission of Ehrlichia canis by Rhipicephalus sanguineus after passage in cell culture. Am J Vet Res. 1996;57:1594–1598. [PubMed] [Google Scholar]
- 17.Price J E, Dolan T T. A comparison of the efficacy of imidocarb dipropionate and tetracycline hydrochloride in the treatment of canine ehrlichiosis. Vet Rec. 1980;107:275–277. doi: 10.1136/vr.107.12.275. [DOI] [PubMed] [Google Scholar]
- 18.Rikihisa Y, Ewing S A, Fox J C. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J Clin Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ristic M, Huxsoll D L, Weisiger R M, Hildebrandt P K, Nyindo M B A. Serological diagnosis of tropical canine pancytopenia by indirect immunofluorescence. Infect Immun. 1972;6:226–231. doi: 10.1128/iai.6.3.226-231.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Heerden J, Immelman A. The use of doxycycline in the treatment of canine ehrlichiosis. J S Afr Vet Assoc. 1979;50:241–244. [PubMed] [Google Scholar]
- 21.Vemulapalli R, Biswas B, Dutta S K. Pathogenic, immunologic, and molecular differences between two Ehrlichia risticii strains. J Clin Microbiol. 1995;33:2987–2992. doi: 10.1128/jcm.33.11.2987-2993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waner T, Rosner M, Harrus S, Naveh A, Zass R, Keysary A. Detection of ehrlichia antigen in plasma of beagle dogs with experimental acute Ehrlichia canis infection. Vet Parasitol. 1996;63:331–335. doi: 10.1016/0304-4017(95)00902-7. [DOI] [PubMed] [Google Scholar]