Abstract
Stroke is a major cause of death and disability worldwide. Between 1990 and 2010, its global burden increased notably with reference to the absolute number of incident events, number of deaths, and disability-adjusted life-years lost. Trazodone is a triazolopyridine derivative that was approved for more than 40 years as monotherapy or in combination with other antidepressant drugs for the treatment of major depressive disorder in adult patients. The aim was investigated if trazodone can improve behavioural outcome after stroke in a mice model of middle cerebral artery occlusion (MCAo) due to the potential neuroprotective and neurodegenerative effects by using three behavioural tests: adhesive tape test, beam test and hole board test. Trazodone administration show modest improvements regarding the motor-sensorial function after stroke especially in the acute post-stroke phase in aged and young animals. The antidepressant effect of the drug was observed in the post-stroke period in aged animals and to a lesser extent in young animals. Future research is needed to evaluate the effects of trazodone at the cellular level to be sure that it has no benefit in stroke patients who do not suffer from depression.
Keywords: Stroke , behavioral test , trazodone , drug repurposing
Introduction
Stroke is a major cause of death and disability worldwide.
Its global burden increased notably with more than 1 million individuals having a stroke each year, in Europe alone [1, 2].
Stroke involves multiple underlying pathological mechanisms, but ultimately it is caused by a sudden focal disruption of the cerebral blood flow, which is shortly fallowed by neurological deficits.
Approximately 80% of cases of cerebral infarction are attributed to ischemia, while the remaining 20% are classified as hemorrhagic [2].
At the cellular level, stroke primarily involves mechanisms such as hypoxia, edema, apoptosis, and infarction leading to cellular necrosis.
The pharmacological management of ischemic stroke, which accounts for 87% of all stroke cases, is currently inadequate.
Tissue-type plasminogen activator (tPA) is currently the sole authorized medication for stroke therapy.
However, its application is limited to only 3% of patients due to time constraints.
Furthermore, it does not possess any observable neuroprotective characteristics. [3, 4].
Given the increasing life expectancy and the fact that stroke predominantly impacts the elderly population, there exists a pressing demand for the discovery of neuroprotective agents that could aid post-stroke recovery.
Despite the intensification of research efforts in this area, no pharmacological agent has yet received approval [5].
Trazodone, a derivative of triazolopyridine, has been authorized for over four decades as a monotherapy or in conjunction with other antidepressants for managing major depressive disorder in adult individuals.
However, trazodone exhibits a unique dual mechanism of action, whereby it functions as both a serotonin transporter inhibitor and a serotonin type 2 (5-HT2) receptor antagonist (specifically targeting 5-HT2A and 5-HT2C receptors) (Figure 1).
Figure 1.

Trazodone mechanism of action. Inhibition of the serotonin transporter and serotonin type 2 (5-HT2) receptor antagonism (5-HT2A and 5-HT2C receptors); also acts as an antagonist on alpha 1-adrenergic receptors and histamine H1 receptors
With reports showing beneficial effects in treating post-stroke depression, this means that it may have a positive impact on rehabilitation outcomes by boosting patient motivation [6].
Another study also points out that trazodone administrated to individuals who have suffered a stroke and exhibit obstructive sleep apnea, reduces the severity of the condition without concomitantly increasing the occurrence of nocturnal hypoxia [7].
With that medication already given to stroke patients we wanted to investigate the potential neuroprotective effects of trazodone, as some reports have showered that it can reduce memory deficits, neuronal loss and hippocampal atrophy in prion-diseased and tauopathy mice [8].
Its neuroprotective effects were also proved in a cohort of Alzheimer’s disease patients that followed long term trazodone treatment, delaying their progressing neurological deficits [9].
Furthermore, other experimental work has shown that a unique dose of trazodone temporarily slows down motricity recovery in rats [10] and reducing catalase concentration and restoring mitochondrial activity ultimately leading to lower neuron toxicity and oxidative stress [11].
Here we aim to investigate, if trazodone can improve recovery after stroke in a mice model of middle cerebral artery occlusion (MCAo) by using powerful automatic tools to assess behavioural outcomes [12, 13].
Materials and Methods
The study was carried out within the Research Centre for Normal and Pathological Aging (ARES) of the University of Medicine and Pharmacy of Craiova.
The experiment received the approval of the Ethics Committee of the University of Medicine and Pharmacy of Craiova (No. 26/29.10.2020) and it was performed according with Directive 2010/63/EU, governing animal research in Europe (revising Directive 86/609/EEC).
Animals
We used 43 C57BL6 female mice, obtained from the Animal facility of the university.
Young (3 months, n=26) and aged animals (2 years, n=17) were taken out of their housing facilities and acclimated for 24 hours in the testing rooms.
The mice were randomly divided into groups and the behavioural assessment was done by the blinded investigators.
The animals received food and water ad libitum and were housed in rooms with the 12 hours day/night cycle from 07:00 to 19:00, the ambient temperature being 21°C and 60% air humidity.
Pharmacological treatment
Fresh trazodone solution was made every two days by dissolved trazodone hydrochloride (Sigma-Aldrich, Germany) in sterile saline solution.
The remaining solution was stored at 4°C, away from light.
Intraperitoneal injections of 36mg trazodone/kg body weight were made daily, starting 24 hours after MCAo.
Middle cerebral artery occlusion surgery
Before surgery, mice were fasted for 24h in order to reduce the blood glucose level.
MCAo was induced as previously described [14].
Briefly, after anaesthesia (1.5% isoflurane in a mixture of 75% nitrous oxide and 25% oxygen), both common carotid arteries were isolated and prepared for occlusion.
A small craniotomy above the middle cerebral artery was made and an electro-thermal instrument was used to coagulated the base of the MCAo, after the blood flow through the common carotid arteries was stopped.
The local changes in blood flow were monitored using a laser Doppler device (Perimed, Stockholm, Sweden).
A decrease in laser Doppler signals to<20% of control values was considered to be successful MCAo.
After 90 minutes, blood flow was restored through the common carotid arteries and the muscle, soft tissue and skin above the craniotomy were sutured.
Pain relief medication (buprenorphine) was given subcutaneous twice, every 6h after surgery at a dose of 0.3mg/kg.
Behavioural assessment
Adhesive tape removal test
This is the most relevant method for identifying sensory and motor deficit in animals.
The test involves the use of 0.3x0.4cm adhesive tapes on the front limbs of the mouse.
The adhesive tape is applied on the palmar face of the forelimbs with the same pressure on each member.
The animal is then placed in the test cage and 4 different values are registered (the time to contact and the time needed to remove the tape, for each member).
The animals have two minutes to complete the test [15].
Animals are given two days for training, meant to familiarize the animal with the test, to reduce the anxiety which leads to the decrease of the defecation and urination frequency during the test, important to achieve an optimal performance and not least to reduce the inter-individual variability, homogenizing the performance of animals.
The tactile response is defined as the time needed by the animal to feel the adhesive tape and is represented by a shaking of the limb or by the mouth touching the adhesive tape.
The complete removal of the adhesive tape represents the completion of the test or exceeding the 2 minutes allocated to this test.
Usually, the animals remove the adhesive tape by mouth or by grooming [15].
Beam test
This test is widely used in experimental studies in order to assess motor coordination and balance of the animal.
The equipment consisted of wooden bars with a length of 1 meter having a flat surface of 12mm and 6mm respectively.
Two lines were drawn on these, the first representing the start line and the second, at a distance of 80 centimeters, representing the stop line.
The beam is placed at a height of 50cm.
Every mouse received a two consecutive days training.
The training consisted of crossing each bar 3 times, encouraging the animals to move every time it stopped.
These trainings are meant to familiarize the animal with the test and make its behaviour during the test more stable and accurately, better reflecting motor coordination.
For each trial, the time needed to travel each of the two bars and the number of missteps were recorded.
A slip is defined as one or more limbs do not reach the beam.
At first, the mice had to cross the wide bar followed by the narrow bar, after a 10 minutes resting period.
Each time the animal stopped (to sniff or to look around) it was encouraged forward with a gentle push.
After each animal, the bar was cleaned with 70% ethanol to eliminate the odour of the previous mouse as through as possible [16].
Hole board test
The procedure is used to evaluate behavioural components in mice such as curiosity and the ability to explore.
These components provide information about anxiety or depression.
The equipment used for this test is automatic, represented by a 40x40cm grey plate with a thickness of 2.2cm.
It is provided with 16 holes with a diameter of 3cm each, in which detectors are located (recessed in each hole, 1cm from the surface of the plate).
The board was located at a distance of 15cm from the ground and connected to a source that records the number of head dives in each hole.
Each animal was placed in the centre of the plate and for 5 minutes the number of head dives in the holes on the plate was recorded; after each animal the plate was cleaned with 70% ethanol [17, 18].
Statistical Analysis
GraphPad 9.2 and Microsoft Excel 2016 were used for statistical analysis.
Differences in means among the groups were analysed using a two-stage step-up (Benjamini, Krieger, and Yekutieli) multiple unpaired t test, with a false discovery rate of 1%.
All figures show mean value and standard deviation (SD) and the statistical significance is displayed as follows: *, # p<0.05; **, ## p<0.01; ***, ### p<0.001 and ****, #### p<0.0001.
Results
Trazodone improves recovery outcome in acute and less in chronic post-stroke phase.
At the beginning of the adhesive tape test, both young and aged animals displayed similar detection and removal times for both tested front legs.
However, post-stroke, aged animals were slower in both detection and removal of the adhesive tape compared to their young counterparts (Figure 2).
Figure 2.

Adhesive tape test. A. Time needed by the animals to feel the tape on the left paw. B. Time needed by the animals to feel the tape on the right paw. C. Time needed by the animals to remove the tape on the left paw. D. Time needed by the animals to remove the tape on the right paw. Young Sham: young animals subjected to MCAo. Young Traz: young animals trated with trazodone, Aged Sham: aged animals subjected to MCAo. Aged Traz: aged animals trated with trazodone. * -statistical difference between the group of treated and control aged animals. # -statistical difference between the group of treated and control young animals. Data is presented as mean±SD. *, # p<0.05; **, ## p<0.01; ***, ### p<0.001 and ****, #### p 0.0001.
Trazodone administration to aged animals resulted in a significant reduction in the adhesive tape detection between day 9 to 12 post-stroke (Figure 2A).
On day 9, aged animals treated with trazodone feel the adhesive tape faster (20±5.07) compared with control (50.75±17.68), p<0.05.
The same is observed on day 12 post-stroke (18±3.89) vs. (50.75±11.87), p<0.05.
The observed dissimilarity between the detection times of untreated and treated aged animals was not sustained, as both groups began to display comparable detection times in the late acute and chronic phases (Figure 2A).
No difference was observed between control and treated animals, regardless of age when testing the ipsilateral (right) part of the body, regarding the detection time of the adhesive tape (Figure 2B).
Concerning the time needed to remove the tape, statistically significant differences were found from day 6 to day 15 post- (Figure 2C).
From day 6 to day 12 post-stroke, treated aged animals managed to remove the adhesive tape faster compared to aged controls, as follows: day 6 (76.83±42.25 vs. 108.75±19.92, p<0.05), day 9 (46.50±16.04 vs. 93.75±13.75, p<0.01), day 12 (43.62±32.91 vs. 88.75±7.32, p<0.01.
Regarding young animals, statistically significant differences were observed between young treated animals and young controls on day 6 (31.66±19.99 vs. 14.40±8.56, p<0.05), 12 (35.08±17.03 vs. 10.80±3.76, p<0.01) and 15 (33.33±9.56 vs. 9.80±1.48, p<0.05) post-stroke.
However, motor recovery seems to influence only acute recovery, as both young and aged treated animals lose their advantage starting 18 and respectively 15 days post-stroke (Figure 2C).
Also, no difference was observed between control and treated young animals, when testing the ipsilateral (right) part of the body, regarding the motor ability.
However, at day 3 and day 21 post-stroke, aged animals treated with trazodone showed superior motor skills compared to the untreated animals (Figure 2D).
Young control animals show a lack of dexterity when removing the tape on day 3 (9.52±5.52 vs. 23.66±26.33, p<0.05) and day 21 (5.20±1.92 vs. 19.83±23.31, p<0.05) post-stroke, compared with the group treated with trazodone (Figure 2D).
Young treated animals did not exhibit any significant improvement in their detection time compared to their control group, during both the acute and chronic phases of the experiment (Table 1).
Table 1.
Mean values of parameters obtained by adhesive tape test for all animal groups at each analysed time point.
Contact left / right: the time the animal felt the adhesive tape placed on the left / right paw, Remove left / right: the time the animal removed the
dhesive tape placed on the left / right paw, Young: young animals, Aged: aged animals, Control: control animals groups, Treated: trazodone
treated groups, Baseline: the test performed before the stroke, 1, 3, 6, 9, 12, 15, 18, 21, 24, 27: the days when the test was performed after the stroke.
Although trazodone appears to have enhanced the acute detection time solely in aged animals, it augmented dexterity, as evaluated by the duration required for adhesive tape removal, in both aged groups relative to their respective controls (Table 1).
A similar trend was observed when evaluating the differences between untreated and treatment animals using the beam crossing test (Table 2).
Table 2.
Mean values of parameters obtained by beam test for all animal groups at each analysed time point.
Velocity wide / narrow beam: the time that animals travelled the wide / narrow beam, Missteps wide / narrow beam: missteps that animals made
uring the crossing on the wide / narrow beam, Young: young animals, Aged: aged animals, Control: control animals groups, Treated: trazodone
treated groups, Baseline: the test performed before the stroke, 1, 3, 6, 9, 12, 15, 18, 21, 24, 27: the days when the test was performed after the stroke.
Regardless of the treatment administered, young animals exhibited a comparable frequency of errors in both the narrow and wide beam assessments (Figure 3 A,B).
Figure 3.

Beam test. A. Animals missteps on the narrow beam. B. Animals misstept on the wide beam. C. Crossing time on the narrow beam. D. Crossing time on the wide beam. Young Sham: young animals subjected to MCAo. Young Traz: young animals trated with trazodone, Aged Sham: aged animals subjected to MCAo. Aged Traz: aged animals trated with trazodone. * -statistical difference between the group of treated and control aged animals. # -statistical difference between the group of treated and control young animals. Data is presented as mean±SD. *, # p <0.05; **, ## p <0.01; ***, ### p <0.001 and ****, #### p <0.0001.
At the beginning of the experiment, aged treated animals tend to have less missteps compared with aged control animals on day 1 (25.86±11.33) vs. (47.0±17.26) p<0.0001, 3 (18.20±10.91) vs. (30.75±14.477) p<0.05 and day 6 (16.66±8.98) vs. (27.25±15.32) p>0.05, post-stroke (Figure 3A).
Trazodone administration had beneficial effect in aged animals in the acute post-stroke phase on day 1 (14.08±7.20) vs. (29.25±4.99), p<0.0001 and 3 (11.16±4.95) vs. (23.0±9.34) p<0.001 compared with aged controls (Figure 3B).
Regarding young animals, trazodone administration seems to have beneficial neuroprotective role more in the chronic post-stroke phases, statistically significant differences being observed on day 24 (1±1.67) vs. (3±2.55) p<0.05 and day 27 (0.83±1.60) vs. (3.2±3.95), p<0.05 (Figure 3B).
Aged trazodone treated animals had higher speeds between day 3 and 6 post-stroke, when crossing the narrow beam (Figure 3C).
However, for both tasks, these differences were not observed in late acute and chronic phases of stroke, as natural recovery of the untreated animals matched the accelerated recovery of treated animals (Figure 3 C,D).
Similarly, trazodone reduced the number of missteps of aged animals, in the acute post-stroke, with no impact on the chronic recovery.
The velocity in which aged treated animals were able to finish the crossing of the wide beam, in the first 21 days post-stroke, was significantly higher compared to their aged controls (Figure 3D).
Trazodone administration showed limited post-stroke antidepressant effect in aged animals.
Hole board test showed that trazodone had no post-stroke antidepressant effect in treated young animals (Table 3).
Table 3.
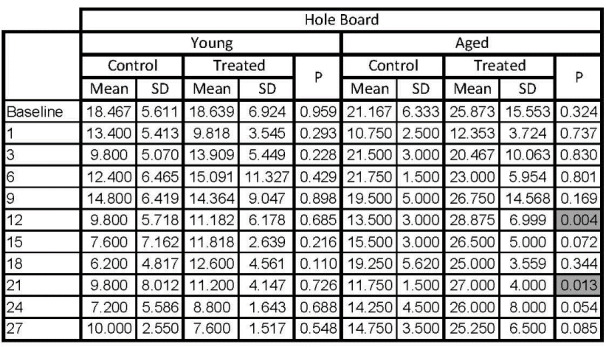
Mean values of parameters obtained by hole board test for all animal groups at each analysed time point
Hole Board: the number of head dippings, Young: young animals, Aged: aged animals, Control: control animals groups, Treated: trazodone
treated groups, Baseline: the test performed before the stroke, 1, 3, 6, 9, 12, 15, 18, 21, 24, 27: the days when the test was performed after the stroke.
Although, testing periods, the p value was close to significance, significance was only reached for day 12 and 21, with treated animals dipping their head around 28 times compared with only 13 times at 12 days post-stroke and 11.7 at 21 days post-stroke (Figure 4 and Table 3).
Figure 4.

Hole board test. Number of head dippings. Young Sham: young animals subjected to MCAo. Young Traz: young animals trated with trazodone, Aged Sham: aged animals subjected to MCAo. Aged Traz: aged animals treated with trazodone. * -statistical difference between the group of treated and control aged animals. # -statistical difference between the group of treated and control young animals. Data is presented as mean±SD. *, # p <0.05; **, ## p <0.01; ***, ### p <0.001 and ****, #### p <0.0001.
Discussions
The increasing longevity gives rise to emerging health issues that pertain to the unique requirements of older demographics.
Physiological aging is now widely acknowledged as encompassing all changes that transpire in various organs and systems throughout the aging process.
This phenomenon is distinguished by a diminishing capacity to cope with different forms of stress, disruption of homeostatic equilibrium, and susceptibility to diseases.
The process of brain ageing in particular, is characterized by a decline in both the structures and functions of neurons and glia.
This phenomenon is commonly known as neurodegeneration.
The phenomenon under consideration is a typical occurrence that has been observed to result in diminished communication abilities and impaired memory function [19].
Additionally, it has been found to be associated with suboptimal recovery following a stroke [20].
Neurodegeneration is a gradual phenomenon characterized by a reduction in the overall population of neurons.
This process is typically attributed to apoptosis and is accompanied by a deterioration in both the structural integrity and functional capabilities of cells in the cortex [21].
Here we investigated the neuroregenerative properties of trazodone in an animal stroke model.
Behavioural assessment showed that administration of trazodone in aged mice have limited beneficial effects in post-stroke recovery, with almost no impact or even a slight decrease in the motor recovery of young animals.
Adhesive tape test showed that trazodone had a limited positive impact regarding sensorial recovery after stroke in aged animals.
In the post-stroke period, trazodone treatment had a neuroprotective effect in aged animals while, regarding young animals, this seems to slow down the motor recovery.
Similar findings being highlighted in a study where trazodone in one single dose could temporarily slow down the motor rehabilitation in rat models of MCAo, even reinstating neurological deficits such as hemiparesis [10].
Regarding the right hemibody part, no statistical differences were found in the time needed to feel the tape, but when it comes to remove the tape, on day 3 and day 21 post-stroke, young control animals seem to have a greater deficit compared to the treated young group.
These outcomes are somewhat different compared to human trails where trazodone may have a better neuroprotective effect, proved in a cohort of Alzheimer’s disease patients, that showed a slowdown in cognitive degeneration [9].
These findings were correlated with animal models, where the administration of trazodone restored memory deficits, prevented neurodegeneration, and prolonged survival in tau-pathology [8] and prion induced pathology in mice [22].
Beam narrow test showed that trazodone administration had modest beneficial impact regarding the coordination of aged and young animals in post-stroke period.
At the beginning of the experiment, aged treated animals tend to have less missteps compared with aged control animals.
Regarding the wide beam, due to the fact that the thickness of the bar is greater, both aged and young animals tend to make fewer missteps.
Trazodone administration had beneficial effect in aged animals in the acute post-stroke phase on day 1 and compared with aged controls.
Regarding young animals, trazodone administration seems to have beneficial neuroprotective role more in the chronic post-stroke phases on day 24 and day 27.
Concerning the time needed to cross the narrow beam, from day 1 post-stroke to the end of the experiment, aged animals treated with trazodone need less time to cross the beam compared with aged controls, although human studies suggest that the trazodone may have negative side effects, particularly in older adults such as dizziness, weight gain and hypotension [23].
The same thing can be observed in young animals treated with trazodone for the first 3 days post-stroke, after that, control animals seem to cross the beam in a shorter time.
These findings are consistent with a study conducted on human subjects that showed that trazodone improved self-care function, but not motor function [24].
Regarding the crossing time on the wide beam, trazodone seems to have a neuroprotective effect only on aged animals.
Moreover, trazodone administration in young animals seems to slow down the crossing.
These findings are consistent with a study evaluating the psychomotor effects of trazodone in humans, which found that trazodone produced small but significant impairments in balance and arm muscle endurance [25].
The well-known antidepressant and anxiolytic effects of trazodone in humans [26] that have also been highlighted in dogs and cats [27] were also pointed out in the present study only on aged animals.
The effect was visible starting from day 9 to the end of the experiment.
This increased curiosity behaviour proved maybe a grade of neuroprotection in aged animals, similar to different human or animal models, which proved some neuroprotection in Alzheimer patients [9] or tau-pathology/prion [8] induced pathologies in mice.
This effect was highlighted to a lesser extent in young animals.
Conclusions
Trazodone did not show any improvements regarding the motor-sensorial function after stroke in young animals, and only transient acute effect in aged animals.
Interestingly, the antidepressant effect manifests itself in the post-stroke phases of aged animals, with young treated animals having no anti-depressant benefit when receiving trazodone compared to controls.
These results could be due to some limited neuroprotective effects of trazodone, but future research seems to be needed in order to fully understand trazodone’s cellular benefits in post-stroke patients who do not suffer from depression.
Source of funding
This work was supported by the grant POCU/993/6/13/153178,” Performanță în cercetare”-"Research performance" co-financed by the European Social Fund within the Sectorial Operational Program Human Capital 2014-2020.
Funding
The Article Processing Charges were funded by the Doctoral School of the University of Medicine and Pharmacy of Craiova, Romania
Conflict of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Thiele I, Linseisen J, Heier M, Holle R, Kirchberger I, Peters A, Thorand B, Meisinger C. Time Trends in Stroke incidence and in prevalence of risk factors in southern Germany, 1989 to 2008/09. Sci Reports. 2018;8(1):1–8. doi: 10.1038/s41598-018-30350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wajngarten M, Sampaio Silva. Hypertension and stroke: update on treatment. Eur Cardiol. 2019;14(2):111–115. doi: 10.15420/ecr.2019.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parrella E, Porrini V, Benarese M, Pizzi M. The role of mast cells in stroke. Cells. 2019;8(5):437–437. doi: 10.3390/cells8050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyravian N, Dikici E, Deo S, Toborek M, Daunert S. Opioid antagonists as potential therapeutics for ischemic stroke. Prog Neurobiol. 2019;182:101679–101679. doi: 10.1016/j.pneurobio.2019.101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houlton J, Abumaria N, Hinkley SFR, Clarkson undefined, A N. Therapeutic potential of neurotrophins for repair after brain injury: a helping hand from biomaterials. Front Neurosci. 2019;13:790–790. doi: 10.3389/fnins.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raffaele R, Rampello L, Vecchio I, Tornali C, Malaguarnera M. Trazodone therapy of the post-stroke depression. Arch Gerontol Geriatr. 1996;22(1):217–220. doi: 10.1016/0167-4943(96)86939-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen CY, Chen CL, Yu CC. Trazodone improves obstructive sleep apnea after ischemic stroke: a randomized, double-blind, placebo-controlled, crossover pilot study. J Neurol. 2021;268(8):2951–2960. doi: 10.1007/s00415-021-10480-2. [DOI] [PubMed] [Google Scholar]
- 8.Halliday M, Radford H, Zents KAM, Molloy C, Moreno JA, Verity NC, Smith E, Ortori CA, Barrett DA, Bushell M, Mallucci GR. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain. 2017;140(6):1768–1783. doi: 10.1093/brain/awx074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La AL, Walsh CM, Neylan TC, Vossel KA, Yaffe K, Krystal AD, Miller BL, Karageorgiou E. Long-term trazodone use and cognition: a potential therapeutic role for slow-wave sleep enhancers. J Alzheimers Dis. 2019;67(3):911–921–911–921. doi: 10.3233/JAD-181145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein LB. Potential effects of common drugs on stroke recovery. Arch Neurol. 1998;55(4):454–456. doi: 10.1001/archneur.55.4.454. [DOI] [PubMed] [Google Scholar]
- 11.Gaur V, Kumar A. Protective effect of desipramine, venlafaxine and trazodone against experimental animal model of transient global ischemia: possible involvement of NO–CGMP pathway. Brain Res. 2010;1353:204–212. doi: 10.1016/j.brainres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Boboc undefined, IKS undefined, Rotaru-Zavaleanu AD, Calina D, Albu CV, Catalin B, Turcu-Stiolica A. A preclinical systematic review and meta-analysis of behavior testing in mice models of ischemic stroke. Life. 2023;13(2):567–567. doi: 10.3390/life13020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reagan‐Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 14.Gresita A, Surugiu R, Catalin B, Doeppner TR, Pirici D, Calina D, Coman C, Hermann DM, Popa-Wagner A, Boboc IKS. Grafting of electrically stimulated subventricular neural stem cells embedded in a nutritional hydrogel into the stroke cavity improved cell survival and behavioural recovery in mice. Research Square. 2023 [Google Scholar]
- 15.Bouet V, Freret T. A master key to assess stroke consequences Across Species: the adhesive removal test. InTech. 2012 [Google Scholar]
- 16.Luong TN, Carlisle HJ, Southwell A, Patterson undefined, PH undefined. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. 2011;(49):2376–2376. doi: 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos P, Herrmann AP, Benvenutti R, Noetzold G, Giongo F, Gama CS, Piato AL, Elisabetsky E. Anxiolytic properties of N-acetylcysteine in mice. Behav Brain Res. 2017;317:461–469. doi: 10.1016/j.bbr.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 18.De Oliveira, ED undefined, Schallenberger C, Böhmer AE, Hansel G, Fagundes AC, Milman M, Silva MDP, Oses JP, Porciúncula LO, Portela LV, Elisabetsky E, Souza DO, Schmidt AP. Mechanisms involved in the antinociception induced by spinal administration of inosine or guanine in mice. Eur J Pharmacol. 2015;772:71–82. doi: 10.1016/j.ejphar.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Mitran SI, Catalin B, Sfredel V, Balseanu TA. Neuroregeneration and dementia: new treatment options. J Mol Psychiatry. 2013;1(1):12–12. doi: 10.1186/2049-9256-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balseanu AT, Buga AM, Catalin B, Wagner DC, Boltze J, Zagrean AM, Reymann K, Schaebitz W, Popa-Wagner A. Multimodal approaches for regenerative stroke therapies: combination of granulocyte colony-stimulating factor with bone marrow mesenchymal stem cells is not superior to G-CSF alone. Front Aging Neurosci. 2014;23(6):130–130. doi: 10.3389/fnagi.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cătălin B, Cupido A, Iancău M, Albu CV, Kirchhoff F. Microglia: first responders in the central nervous system. Rom J Morphol Embryol. 2013;54(3):467–72. [PubMed] [Google Scholar]
- 22.Ghemrawi R, Khair M. Endoplasmic reticulum stress and unfolded protein response In neurodegenerative diseases. IJMS. 2020;17(21):6127–6127. doi: 10.3390/ijms21176127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke SL, Hu T, Spadola CE, Li T, Naseh M, Burgess A, Cadet T. Mild cognitive impairment: associations with sleep disturbance, apolipoprotein e4, and sleep medications. Sleep med. 2018;52:168–176–168–176. doi: 10.1016/j.sleep.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyai I, Reding ME. Effects of antidepressants on functional recovery following stroke: a double-blind study. Neurorehabilitation and Neural Repair. 1998;1(12):5–13. [Google Scholar]
- 25.Roth AJ, McCall WV, Liguori A. Cognitive, psychomotor and polysomnographic effects of trazodone in primary insomniacs. J Sleep Res. 2011;4(20):552–558. doi: 10.1111/j.1365-2869.2011.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brogden RN, Heel RC, Speight TM, Avery GS. Trazodone: a review of its pharmacological properties and therapeutic use in depression and anxiety. Drugs. 1981;21(6):401–429–401–429. doi: 10.2165/00003495-198121060-00001. [DOI] [PubMed] [Google Scholar]
- 27.Chea B, Giorgi M. Trazodone: a review of its pharmacological properties and its off-label use in dogs and cats. Am J Anim Vet. 2017;4(12):188–194. [Google Scholar]




