Abstract
Plasma cell neoplasms are common, accounting for more than 1% of all malignancies. Its most common form is multiple myeloma, but others, such as extramedullary plasmacytoma (EMP), exist. Spinal cord compression secondary to these pathologies is not uncommon, however, adjacent bone involvement is usually present. Spinal cord compression caused by isolated epidural EMP is extremely rare, with only one case reported to date. We describe the case of a 75-year-old female patient that presented with paraparesis, due to an isolated epidural EMP associated with light chain amyloidosis. She was treated with surgical decompression and neoadjuvant chemotherapy, and is currently with a 15-month disease-free period.
Keywords: Plasma cell neoplasms , Plasmacytoma , Epidural neoplasms , Spinal cord compression , Immunoglobulin light-chain amyloidosis
Introduction
Multiple myeloma (MM) is the most common plasma cell neoplasm (PCN).
It is a hematologic disease characterized by the accumulation of monoclonal plasma cells (PCs) in the bone marrow, and represents 1% of all malignancies [1, 2].
Progression to extraosseous disease occurs in up to 70% of patients.
However, isolated extraosseous disease (also called extramedullary or extraosseous plasmacytoma (EMP)) is much less frequent than MM, amassing a cumulative incidence of only about 0.045/100,000 [3, 4, 5].
EMP is characterized by a single mass of PCs outside the bone marrow and with symptoms only derived from this lesion, with little or no bone marrow plasmacytosis [5].
The most frequent sites of EMP are the head and neck, gastrointestinal tract, upper airways and lungs, with epidural involvement being extremely rare [6, 7, 8].
Spinal cord compression by an epidural EMP has been reported only once in the literature [6].
Here, we describe the second case of spinal cord compression caused by an epidural EMP.
Case Report
A 75-year-old woman was referred for neurosurgical evaluation after experiencing lower limb strength loss, preventing walking.
The condition started 3 months earlier, with paresthesia in both feet, which progressed upwards, followed by progressive loss of strength associated with bladder sphincter dysfunction.
On physical examination, she had paraparesis, with grade 3 strength, and sensory blunting below the level of T10, in addition to patellar hyperreflexia, clonus and bilateral Babinski's sign.
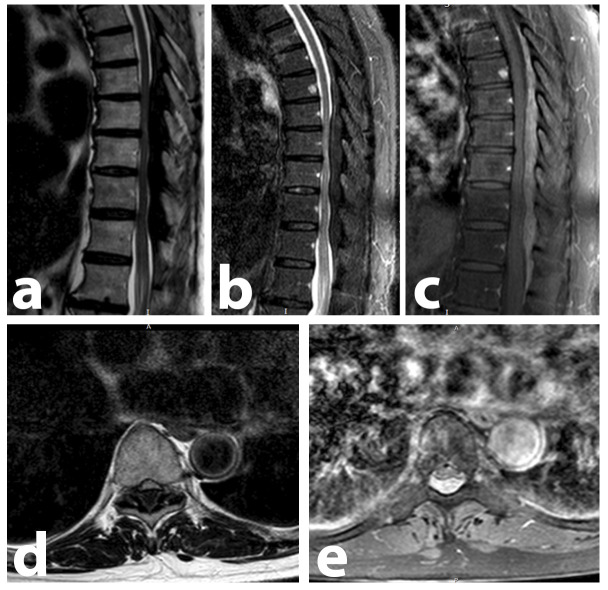
A Magnetic Resonance Imaging (MRI) of the thoracolumbar spine was performed (Figure 1), which revealed an extensive posterior extradural lesion, from T6 to L1 levels, with compressive effect (more severe between T8 to T11).
Figure 1.
: Thoracolumbar spine MRI performed at patient admission, demonstrating an extensive posterior extradural lesion, ranging from T6 to L1, causing spinal cord compression. Compression is most expressive between the T8-T11 levels. There are no bone lesions consistent with MM. (A) Sagittal T1-weighted image showing the hypointense lesion. (B) Sagittal STIR image showing the hypointense lesion. (C) Sagittal contrast-enhanced T1 SPIR image, where the lesion demonstrates a diffuse and homogeneous enhancement to the paramagnetic contrast medium. This is the image that most clearly demonstrates the full extent of the lesion. (D and E) These T9-level axial images, T1-weighted and T1-enhanced SPIR, demonstrate the extrinsic compression caused by the posterior epidural mass (hypo and hyperintense, respectively).
The lesion was hypointense on T1-weighted image and STIR, with diffuse contrast enhancement.
Hospitalization was carried out for surgical removal of the lesion and diagnostic investigation.
Alternate laminectomy from T8 to T12 was performed, coupled with complete resection of the lesion, confirmed by postoperative neuroimaging.
During surgery, an abscess-like mass of firm consistency was observed, which prompted the initiation of empirical antibiotic therapy with ceftriaxone, vancomycin, and metronidazole.
Anatomopathological examination showed a multifocal PC infiltrate associated with light chain amyloidosis (LCA) (seen under Congo red staining) (Figure 2).
Figure 2.
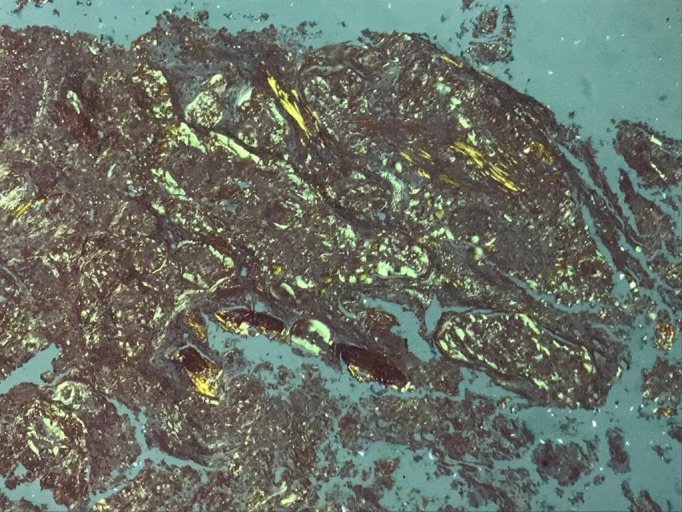
Microscopic view, 40x magnification, of the removed epidural mass. Amyloid deposits can be seen, greenish with Congo red, under polarized light, confirming the diagnosis of light-chain amyloidosis
These findings prompted a thorough investigation for PCNs.
The patient had microcytic and hypochromic anemia, but without loss of renal function or increase in serum calcium; MRI and CT of the thoracolumbar spine did not demonstrate bone lesions compatible with MM.
Blood protein electrophoresis showed a biclonal increase in gammaglobulins (IgM and IgG), and immunofixation of urinary proteins showed the presence of isolated kappa protein.
Bone marrow biopsy was performed, and showed 5% plasmacytosis, with immunophenotyping compatible with PC lymphoproliferative disease.
Due to the absence of bone involvement, the diagnosis of isolated extradural EMP with associated LCA was established.
After diagnosis, the patient underwent 8 cycles of chemotherapy with bortezomib, cyclophosphamide and dexamethasone (standard VCD regimen).
No side effects were noted.
At the end of chemotherapy, immunofixation of urine and serum showed the absence of monoclonal proteins, although there was still an increase in urinary light chains (lambda 1.680 and kappa 2.790mg/L).
After 15 months of follow-up, the patient’s lower limb strength and sphincter function were completely recovered, however, she still experiences mild paresthesia in the distal lower limbs.
An MRI performed at the 8th month showed no new expansive lesions (Figure 3).
Figure 3.
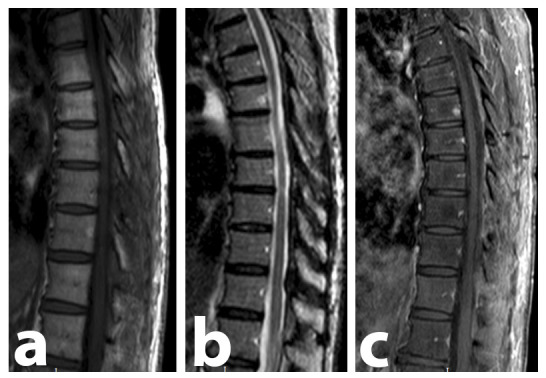
Thoracolumbar spine MRI performed 8 months after surgery. No lesion remains could be found, proving the complete resection of the lesion, as well as the absence of recurrence. There are no bone lesions consistent with MM. (A) Sagittal T1-weighted image. (B) Sagittal STIR-weighted image. (C) Sagittal contrast-enhanced T1-weighted SPIR image demonstrating the absence of contrast-enhancing lesions.
She performed a new bone marrow biopsy in the 12th month of follow-up, which showed plasmacytosis <5%, without immature or aberrant populations, and hematological remission was observed.
The patient is currently maintaining follow-up with the Hematology department due to LCA.
Written Informed Consent forms were acquired from the patient before the beginning of the production of the article.
Ethical approval was waived by the local Ethics Committee of Federal University of Fronteira Sul in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
The study was conducted in accordance with the declaration of Helsinki.
Discussions
Spinal cord compression is an infrequent finding in PCNs, present in 5% of patients [7, 8].
most cases, compression arises from fractures of the vertebral bodies consumed by MM [7].
Only 1 case of spinal cord compression caused by an isolated epidural EMP was found in our literature review (Table 1) [6].
Table 1.
Cases of isolated epidural EMP cases described in the literature until 2022
|
Author, year |
Sex, age (in years) |
Clinical presentation |
Lesion location |
Treatment |
Results and outcome |
|
Zeng et al., 2012 |
M, 35 |
Thoracic spine pain; paraparesis; urinary incontinence; sensory blunting below T5-T6 |
T2-T4 |
Surgical decompression (mass removal). Chemotherapy: 4 cycles of bortezomib, dexamethasone and thalidomide |
Complete remission of the plasmacytoma and total improvement of the motor deficit in 1 year of follow-up. Undetermined survival |
|
Present case |
F, 75 |
Paraparesis; sensory blunting below T10; urinary incontinence |
T6-L1 |
T8-T12 alternating decompressive laminectomy with mass removal. Chemotherapy: 8 cycles of bortezomib, dexamethasone and cyclophosphamide. |
Complete improvement of the motor and sphincter deficit and partial improvement of the sensory deficit in 15 months of follow-up. Patient still under follow-up. |
Note: Abbreviations: F = feminine; M = masculine; EMP = extramedullary plasmacytoma
The diagnostic evaluation of patients with signs of spinal cord compression is based on imaging tests, which seek to assess the cause and extent of compression.
An array of different masses can present in the epidural space, and it should be noted that, despite being extremely important for the evaluation and guidance of management, no imaging study can reliably differentiate PCNs from other masses that can affect the spine and related structures.
Epidural PCNs must be differentiated from meningiomas, neurinomas, lymphomas, metastases, epidural abscesses, solitary amyloidomas, and other benign epidural neoplasms, and for that histopathological and immunohistochemical evaluation are paramount for a reliable etiological diagnosis [5, 7, 8].
Macroscopically, plasmacytomas typically have a gray and friable appearance, being easily removed by surgical manipulation [7, 8].
Histopathological and immunohistochemical evaluation of EMP shows a homogeneous infiltrate of PCs, which usually express CD138 and CD38 [5].
To prove isolated extra-osseous disease, the absence of PCs infiltration of the bone marrow must be confirmed.
Unilateral bone marrow aspiration and trephine biopsy with immunophenotyping are recommended and should demonstrate less than 10% PCs infiltration.
Complete diagnostic criteria for EMP are described elsewhere [5].
Treatment of epidural EMPs follows the same principles of any other EMP, and may include chemotherapy, radiotherapy and/or surgical excision.
In the case reported by Zeng et al. [6], the treatment strategy was similar to ours, comprising immediate surgical decompression followed by chemotherapy.
Although chemotherapy regimens differed (VTD vs. VCD), both proved safe and effective. Radiotherapy is considered the mainstay of EMP therapy, as they are highly radiosensitive.
Nonetheless, failure to respond is frequent for bulky EMP of >5.0cm, which is considered to be a poor prognosis factor for survival [5].
Both in ours and in the case reported by Zeng et al., this was the reason for radiotherapy dismissal.
LCA accompanies 15% of MM cases, however, its association with EMP is still not well established [9, 10].
No case of isolated epidural disease found was associated with LCA, the present case being the first report of such association in the literature.
Even though they are extremely rare in the absence of adjacent bone involvement, PCNs should be considered in the differential diagnosis of masses in the epidural space.
This case provides evidence that the combination of surgical excision coupled with bortezomib, dexamethasone and cyclophosphamide chemotherapy may be a reliable, safe and effective alternative for treating epidural plasmacytomas.
We suggest that therapy be conducted by a multidisciplinary team, including spine surgeons and hematologists.
Funding
The authors did not receive support from any organization for the submitted work.
Conflict of interests
None to declare
References
- 1.van de, Pawlyn C, Yong KL. Multiple myeloma. The Lancet. 2021;397(10272):410–427. doi: 10.1016/S0140-6736(21)00135-5. [DOI] [PubMed] [Google Scholar]
- 2.Rosiñol L, Beksac M, Zamagni E, Van de, Anderson KC, Badros A, et al. Expert review on soft‐tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. British Journal of Haematology. 2021;194(3):496–507. doi: 10.1111/bjh.17338. [DOI] [PubMed] [Google Scholar]
- 3.Bladé J, Fernández de, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-Tissue Plasmacytomas in Multiple Myeloma: Incidence, Mechanisms of Extramedullary Spread, and Treatment Approach. Journal of Clinical Oncology. 2011;29(28):3805–3812. doi: 10.1200/JCO.2011.34.9290. [DOI] [PubMed] [Google Scholar]
- 4.Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Clinical course of solitary extramedullary plasmacytoma. Radiotherapy and Oncology. 1999;52(3):245–249. doi: 10.1016/s0167-8140(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 5.Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. Journal of Hematology & Oncology. 2018;11(1):1–10. doi: 10.1186/s13045-017-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ZENG Z, ZHENG L, LIN J, CHEN J. Successful bortezomib treatment in combination with dexamethasone and thalidomide for previously untreated epidural plasmacytoma. Oncology Letters. 2011;3(3):557–559. doi: 10.3892/ol.2011.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avadhani A, Shetty AP, Rajasekaran S. Isolated extraosseous epidural myeloma presenting with thoracic compressive myelopathy. The Spine Journal. 2010;10(4):e7–10. doi: 10.1016/j.spinee.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Okacha N, Chrif E, Brahim E, Ali A, Abderrahman E, Gazzaz M, et al. Extraosseous epidural multiple myeloma presenting with thoracic spine compression. Joint Bone Spine. 2008;75(1):70–72. doi: 10.1016/j.jbspin.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi G, Kumar S. Systemic Amyloidosis Due to Clonal Plasma Cell Diseases. Hematology/Oncology Clinics of North America. 2020;34(6):1009–1026. doi: 10.1016/j.hoc.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Wang M, Shen Y, Yan M, Xie W, Wang B, et al. Effects of Amyloid Light-Chain Amyloidosis on Clinical Characteristics and Prognosis in Multiple Myeloma: A Single-Center Retrospective Study. Cancer Management and Research. 2021;13:1343–1356. doi: 10.2147/CMAR.S287922. [DOI] [PMC free article] [PubMed] [Google Scholar]