Abstract
The antiviral activity of 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil (l-FMAU), a novel l-nucleoside analog of thymidine known to be an inhibitor of hepatitis B virus (HBV) replication in hepatoma cells (2.2.1.5 cell line), was evaluated in the duck HBV (DHBV) model. Short-term oral administration (5 days) of l-FMAU (40 mg/kg of body weight/day) to experimentally infected ducklings induced a significant decrease in the level of viremia. This antiviral effect was sustained in animals when therapy was prolonged for 8 days. The histological study showed no evidence of liver toxicity in the l-FMAU-treated group. By contrast, microvesicular steatosis was found in the livers of dideoxycytidine-treated animals. l-FMAU administration in primary duck hepatocyte cultures infected with DHBV induced a dose-dependent inhibition of both virion release in culture supernatants and intracellular viral DNA synthesis, without clearance of viral covalently closed circular DNA. By using a cell-free system for the expression of an enzymatically active DHBV reverse transcriptase, it was shown that l-FMAU triphosphate exhibits an inhibitory effect on the incorporation of dAMP in the viral DNA primer. Thus, our data demonstrate that l-FMAU inhibits DHBV replication in vitro and in vivo. Long-term administration of l-FMAU for the eradication of viral infection in animal models of HBV infection should be evaluated.
The development of new antiviral drugs for the therapy of chronic hepatitis B virus (HBV) infection remains a major problem since alpha interferon therapy is moderately active and its use is often limited because of dose-dependent side effects (14, 40). Therefore, the efficacies of nucleoside analogs, such as lamivudine and famciclovir, have been assessed in chronically HBV-infected patients to improve the response rate to antiviral therapy for chronic HBV infection. However, resistant viruses with mutations in the catalytic domain of the viral polymerase may be selected in 10 to 25% of the patients after 12 months of treatment, depending on the clinical setting (1, 21, 33). It is therefore important to continue research to design new nucleoside analogs which could provide the basis for the development of new antiviral strategies for combating the emergence of resistant mutants. Due to their high antiviral activities and very good selectivity indices, compounds which belong to the β-l-nucleoside analog family may represent potential candidates (2, 26). 2′-Fluoro-5-methyl-β-l-arabinofuranosyluracil (l-FMAU) is a novel β-l-nucleoside analog derived from thymidine. It was found to be a potent inhibitor of HBV replication in a stably transfected human hepatoma cell line (2.2.1.5) and to have a level of low cytotoxicity in vitro (6). In this cell line, it was further demonstrated that l-FMAU inhibits HBV without affecting the host DNA synthetic machinery (27). By contrast to d-FMAU and to 1-(2′-deoxy-2′-fluoro-1-β-d-arabinofuranosyl)-5-iodouracil (d-FIAU), l-FMAU did not decrease the mitochondrial DNA content, did not affect mitochondrial function, and was not incorporated into cellular DNA (27).
Considering its potent inhibitory activity against HBV DNA synthesis and its minimal inhibitory effect on the cellular machinery, l-FMAU has been further explored for development as a potential anti-HBV drug. Since 40 to 50 copies of viral covalently closed circular (CCC) DNA are maintained in the nuclei of infected cells and serve as templates for new viral DNA synthesis when antiviral therapy is withdrawn (13, 37), the ability of l-FMAU therapy to clear viral CCC DNA should be evaluated. Furthermore, because duck HBV (DHBV) reverse transcription is primed by the synthesis of a short DNA primer (GTAA) covalently linked to a conserved tyrosine residue of the amino-terminal domain of the viral polymerase (35, 39), the potential antipriming activity of l-FMAU should also be considered. Therefore, we have evaluated in more detail its anti-HBV activity in the DHBV model (23). This model provides relevant tools for the study of the modes of action of new anti-HBV agents. A primary duck hepatocyte culture system and studies with experimentally infected ducklings have been used to investigate the inhibition of viral DNA synthesis in hepatocytes, the clearance of CCC DNA from infected cells, and the toxicities of new antiviral agents (10, 13, 20, 29, 34, 38). In this study, we also used an in vitro assay for the expression of an enzymatically active viral reverse transcriptase which was first described by Wang and Seeger (35) and used the assay to study the mechanism of inhibition of DHBV reverse transcription by new anti-HBV compounds (9, 30, 35, 38, 39). Our results show that l-FMAU exhibits antiviral activity in vivo in experimentally infected ducklings and primary duck hepatocytes and that it has an inhibitory effect on the enzymatic activity of the DHBV reverse transcriptase.
MATERIALS AND METHODS
Drugs.
l-FMAU was synthesized in the Department of Medicinal Chemistry, University of Georgia, as described previously by Chu et al. (6). Its triphosphate form (l-FMAU-TP) and 2′,3′-dideoxy-β-l-5-fluorocytidine (β-l-F-ddC) were synthesized in the Department of Pharmacology, Yale University. Dideoxythymidine-triphosphate (ddTTP) and dideoxycytidine (ddC) were purchased from Boehringer Mannheim and Sigma, respectively.
An in vitro assay for the expression of enzymatically active DHBV reverse transcriptase.
The DHBV polymerase was expressed from plasmid pHP, which contains the DHBV polymerase gene under the control of the SP6 promoter and the sequence coding for the RNA template of reverse transcription, as described previously (35, 39). The polymerase gene was transcribed and translated in a coupled transcription-translation rabbit reticulocyte lysate system (TNT coupled reticulocyte system; Promega), and the reverse transcriptase reaction was performed as described previously (38).
To study the inhibitory effect of l-FMAU-TP on viral minus-strand DNA elongation as a result of TMP incorporation, the translation mixture was incubated at 30°C for 30 min with an equal volume of a reaction mixture containing 100 mM Tris-HCl (pH 7.5), 30 mM NaCl, 20 mM MgCl2, dGTP, dATP, and dCTP (200 μM each) and [α-32P]TTP (3,000 Ci/mmol, 0.60 μM). The inhibitors l-FMAU-TP and ddTTP were added at the concentrations indicated below.
To analyze the synthesis of the viral DNA primer, whose sequence is 5′-GTAA-3′, the DHBV polymerase was incubated with dGTP (final concentration, 100 μM), TTP or inhibitors (l-FMAU-TP or ddTTP) at increasing concentrations, and [α-32P]dATP (3,000 Ci/mmol; final concentration, 0.3 μM). The kinetics of [α-32P]dAMP incorporation into the viral DNA primer was also analyzed at different times after incubation of the polymerase mixture with dGTP, TTP (final concentration, 100 μM) or inhibitors (l-FMAU-TP or ddTTP; final concentration, 50 μM) and [α-32P]dATP (3,000 Ci/mmol; final concentration, 0.3 μM).
Radiolabelled viral DNA covalently attached to polymerase was subjected to electrophoresis through 0.1% sodium dodecyl sulfate (SDS)–10% polyacrylamide gels as described previously (35, 39). The dried gels were exposed to X-ray film, and the viral DNA was quantified by laser densitometry as previously described in detail (9, 30, 38).
Primary hepatocyte cultures.
Primary hepatocyte cultures were prepared from 4-week-old Pekin ducks chronically infected with DHBV. The procedures of liver perfusion and hepatocyte isolation and the culture conditions were described previously (34). Hepatocytes were seeded at confluence onto 35-mm petri dishes, and the serum-free growth medium was changed daily. The addition of the drugs to the culture medium at the indicated concentrations was carried out from day 3 to day 10 postseeding. Cellular toxicity was analyzed by daily examination with a light microscope, cellular DNA gel electrophoresis, and measurement of the lactic acid level in cell supernatants (Lactate PAP; Biomerieux, Marcy l’Etoile, France).
Experimental inoculation of ducklings.
Five-day-old ducklings were inoculated intravenously with a DHBV-positive serum specimen known to be infectious, and each duckling received 1.5 × 107 viral genome equivalents by following a protocol described by Lambert et al. (16). Ducklings were administered nucleoside analogs orally 3 days postinoculation according to the protocols described in Results. Animal weight and lactic acid levels were monitored daily during the study period.
Analysis of viral DNA.
DHBV DNA from experimentally infected ducklings and from hepatocyte culture supernatants was detected by a DNA spot hybridization assay at different time points, as indicated below, by using a Hybridot Manifold apparatus (Gibco BRL). Fifty microliters of serum or 800 μl of culture supernatant were spotted directly onto nitrocellulose filters (Schleicher & Schuell). After denaturation and neutralization, the filters were hybridized with a full-length DHBV genomic DNA probe labelled with 32P. The filters were autoradiographed, and the spots were counted in a scintillation counter (38).
DNA sequence analysis of the catalytic site of the reverse transcriptase domain of the DHBV polymerase gene was performed with circulating virions at the end of the 8-day administration of l-FMAU. One hundred microliters of serum was submitted to digestion with proteinase K and SDS (final concentrations, 1 mg/ml and 1%, respectively), and DNAs were extracted with phenol-chloroform. Viral DNA encompassing the catalytic site of the polymerase gene was amplified by PCR with primers p-pol-1 (nucleotide positions 171 [5′] to 185 [3′]) and p-pol-2 (nucleotide positions 1812 [3′] to 1833 [5′]). The PCR products were then directly sequenced with the Sequenase PCR product sequencing kit (United States Biochemicals) according to the manufacturer’s recommendations.
Intrahepatic viral DNA from experimentally inoculated ducklings was extracted by a procedure described in detail by Jilbert et al. (15). Liver samples were snap frozen in liquid nitrogen and were stored at −80°C and then analyzed for viral DNAs. One hundred milligrams of liver was homogenized in 0.01 M Tris-HCl (pH 7.5)–0.01 M EDTA, and the homogenate was divided into two parts, one for the isolation of total viral DNA and one for the isolation of non-protein-bound, CCC viral DNA. Five micrograms of the total DNA or the CCC DNA preparation was subjected to electrophoresis on 1.5% agarose gels. Southern blot analysis was carried out as described previously (15), and viral DNAs were detected by hybridization with a 32P-labelled probe representing the complete viral genome (15).
To analyze the viral DNA in primary hepatocytes, cells were rinsed with phosphate-buffered saline (PBS) and stored at −80°C for DNA isolation. Intracellular viral CCC DNA (non-protein-bound DNA) and replicative intermediates (protein-bound DNA) were isolated as described by Summers et al. (31). Viral DNA (corresponding to 0.5 μg of cellular DNA) was analyzed by electrophoresis through 1.5% agarose gels, transferred by blotting onto nylon membranes (Hybond N+; Amersham), and hybridized with a full-length DHBV genomic DNA probe labelled with 32P.
Anti-pre-S antibody detection in the serum of DHBV-infected ducklings.
Anti-pre-S antibody detection in the serum of infected ducklings was performed by an enzyme-linked immunosorbent assay (ELISA) with a recombinant pre-S protein as described previously (3).
Western blot analysis of intrahepatic viral proteins.
Fifty milligrams of duck liver was pulverized in liquid nitrogen and was resuspended in 10 volumes of lysis buffer (20 mM Tris [pH 7], 150 mM NaCl, 1 mM EDTA, 1 mM MgCl2, 5 mM KCl, 1% Nonidet P-40, 1 μg of leupeptin per ml, 1 μg of pepstatin A per ml, 2 mM phenylmethylsulfonyl fluoride) (8). The cell extracts were clarified by centrifugation (12,000 × g, 20 min, 4°C). One hundred fifty micrograms of proteins was subjected to electrophoresis through an SDS–15% polyacrylamide gel and was transferred to a polyvinylidene difluoride-Immobilon P transfer membrane (Immobilon P; Millipore). The membranes were soaked in 5% nonfat dry milk and 0.1% Tween 20 in PBS for 30 min and incubated for 1 h with anti-DHBV core rabbit polyclonal antibodies (final dilution, 1:5,000). Goat anti-rabbit immunoglobulin G antibodies conjugated to horseradish peroxidase (Dako) were then added for 30 min at room temperature. The membranes were washed (5% nonfat dry milk and 0.1% Tween 20 in PBS), and a chemiluminescence reaction was performed according to the manufacturer’s instructions (ECL kit, Amersham, France).
Analysis of liver histology in experimentally infected ducklings.
Coded, formalin-fixed liver tissue sections embedded in paraffin were sectioned at a thickness of 3 μM, stained with hematoxylin, eosin, and safran, and examined under a light microscope. The levels of hepatocyte necrosis (acidophilic bodies), portal tract inflammation, intralobular inflammation, steatosis, and ductular proliferation were assessed as described previously (5).
RESULTS
In vivo inhibition of DHBV replication in experimentally infected ducklings after oral administration of l-FMAU.
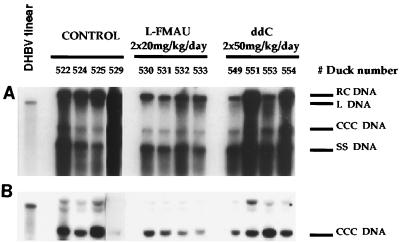
Ducklings were inoculated at 5 days of age by following a protocol which has already been described in detail (16). DHBV viremia was reproducibly detectable at day 4 postinoculation and reached a peak at day 6. This experimental model allowed us to study the inhibitory effect of l-FMAU therapy on DHBV replication in vivo and assess its toxicity and compare these with the effect and toxicity of ddC. In preliminary experiments, it was determined that oral administration of l-FMAU for 4 days at a dosage of 40 mg/kg of body weight once a day or 20 mg/kg twice a day gave similar antiviral effects but was followed by a rebound of viremia after drug withdrawal (data not shown). The results of Southern blot analysis of intrahepatic viral DNA at the end of therapy indicated that viral DNA synthesis was significantly decreased in l-FMAU-treated animals compared to that in control and ddC-treated animals (Fig. 1A). However, l-FMAU administration could not clear viral CCC DNA from the liver (Fig. 1B). Furthermore, a pulse therapy with three doses of 20 mg of l-FMAU per kg given orally every 12 h before intravenous inoculation of infectious serum resulted in a delay in the onset of viremia by 1 day in all five treated animals compared to the time of onset in five control animals (data not shown).
FIG. 1.
Oral administration of l-FMAU decreases viral DNA synthesis in the livers of experimentally infected ducklings but does not clear viral CCC DNA. Protein-bound (A) and protein-free (B) viral DNAs were extracted from the livers of experimentally infected birds and were subjected to Southern blot analysis at the cessation of therapy. Liver samples from four control animals, four animals that received l-FMAU at 20 mg/kg twice a day, per os, for 4 days, and four animals that were treated with ddC at 50 mg/kg twice a day, per os, for 4 days were available for analysis. The positions of relaxed circular (RC), linear (L), CCC, and single-stranded (SS) DNAs are indicated, as are the therapeutic protocols.
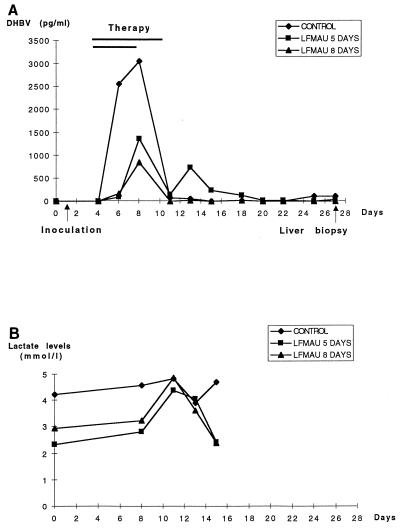
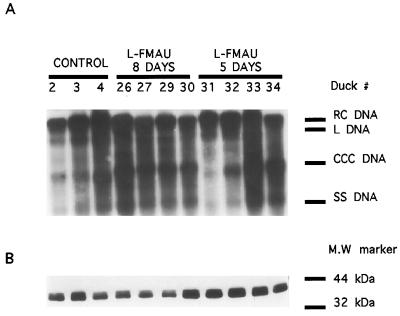
In the next experiment, we analyzed the antiviral efficacy of a more prolonged administration of l-FMAU in experimentally infected ducklings. Four animals received an oral dosage of 40 mg of l-FMAU per kg once a day for 5 days, four ducklings were given the same dosage for 8 days, and four birds served as controls. Figure 2 shows that administration of l-FMAU for 5 days was associated with a 55% inhibition of the peak of DHBV viremia, followed by a weak and transient rebound of viremia 5 days after drug withdrawal. Administration of l-FMAU for 8 days induced a 72% inhibition of the peak of DHBV viremia which was not followed by a rebound of viremia during the 2-week posttreatment follow-up (Fig. 2). In this experiment, the mean area under the curve for viremia, which reflects total virus production, was significantly lower for the group of l-FMAU-treated animals than for the control group (P = 0.0482; Wilcoxon-Mann Whitney test, Monte Carlo modification for small number; Stat-Xact-3 software). DNA sequence analysis of the DHBV polymerase gene was performed after amplification of circulating viral DNA by PCR at the end of the 8 days of administration of l-FMAU to all four treated animals. The results showed the absence of amino acid sequence variation in the catalytic site of the viral polymerase (data not shown). Determination of lactic acid levels in the plasma of the animals which received l-FMAU therapy for 8 days showed no significant increase compared with the levels in the control animals (Fig. 2). Then, after drug withdrawal we determined whether viral infection had been cleared from the liver. Analysis of viral DNA replicative intermediates by gel electrophoresis and Southern blot hybridization of the livers of infected ducklings 16 days after l-FMAU withdrawal showed the persistence of DHBV single-stranded and relaxed circular DNAs, as well as viral CCC DNA, indicating that short-term administration of l-FMAU is not able to clear viral infection from the liver, despite the dramatic drop in the level of viremia (Fig. 3A). Analysis of intrahepatic viral proteins by Western blot analysis of the same liver samples showed a similar expression of DHBV core proteins in l-FMAU-treated animals and in the control birds (Fig. 3B). Since in both groups of l-FMAU-treated animals and the control animals, the level of viremia dropped and DHBV DNA was detectable only by PCR assay, we asked whether an anti-DHBV antibody response could also be responsible for the clearance of circulating virus. However, detection of anti-pre-S antibody in the serum of these animals by an ELISA did not show any appearance of antibody (data not shown).
FIG. 2.
Oral administration of l-FMAU inhibits DHBV viremia in experimentally infected ducklings. Ducklings at 5 days of age were inoculated with a DHBV-positive serum specimen. l-FMAU was given orally at day 3 postinoculation by two different protocols. A group of four ducklings received l-FMAU at a dosage of 40 mg/kg once a day from day 3 to day 7 postinoculation (5 days of treatment). A second group of four ducklings was given l-FMAU at a dosage of 40 mg/kg once a day from day 3 postinoculation for 8 days. A third group of four ducklings served as controls. (A) Analysis of viremia levels. Viral DNA in serum was analyzed by a dot blot assay. The level of mean viral DNA in serum in each group of animals was plotted. (B) Analysis of lactic acid levels. The lactic acid levels in experimentally infected birds were determined before inoculation and at days 7, 10, 12, and 14 postinoculation. The mean serum lactic acid concentration in each group of animals was plotted.
FIG. 3.
DHBV is not cleared from the livers of experimentally infected ducklings after l-FMAU withdrawal. (A) Analysis of intrahepatic viral DNA. A preparation of enriched protein-bound viral DNAs, extracted from the livers of experimentally infected birds, was subjected to Southern blot analysis 16 days after the cessation of therapy. Liver samples from three control animals, four animals that received l-FMAU for 8 days, and four animals that were treated for 5 days were available for analysis. The positions of relaxed circular (RC), linear (L), CCC, and single-stranded (SS) DNAs are indicated, as are the therapeutic protocols (controls, oral administration of l-FMAU for 5 or 8 days). (B) Analysis of intrahepatic viral proteins. Viral core proteins were analyzed by Western blotting of the same liver samples, as described in Material and Methods. MW, molecular mass.
l-FMAU therapy of DHBV-infected ducklings is not toxic during short-term administration.
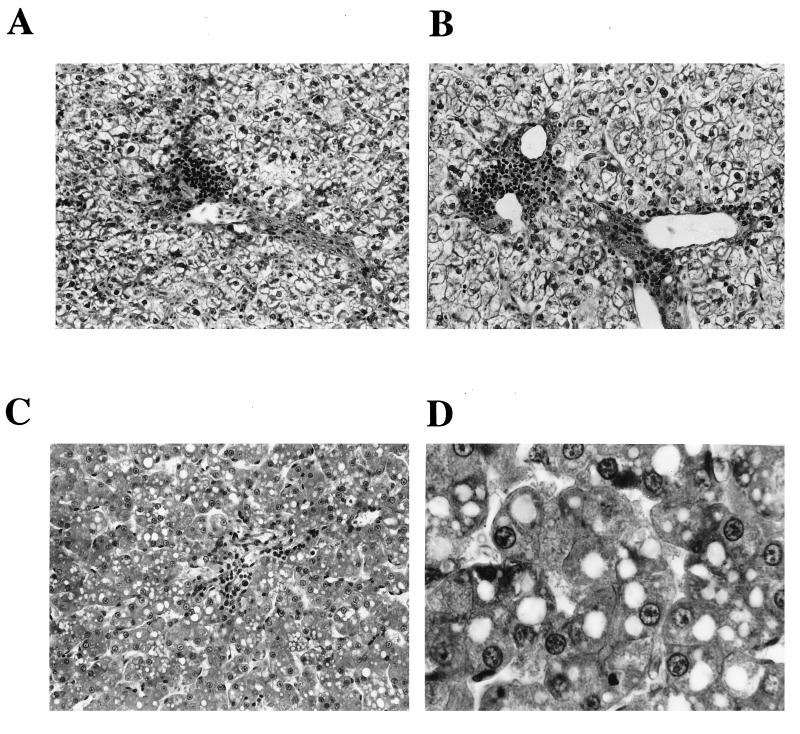
To analyze whether short-term l-FMAU administration may be toxic in vivo in ducklings, we examined the effect of l-FMAU treatment (20 mg/kg twice a day for 4 days, 40 mg/kg once a day for 4 days, or 80 mg/kg every other day for 4 days) on survival and liver histology and compared the effect with the effect of another oral dosage of ddC (50 mg/kg twice a day) given to another group of four animals from day 3 to day 6 postinoculation. This dosage of ddC was chosen since previous experiments had shown that this therapeutic regimen is associated with significant clinical toxicity and a negligible antiviral effect in ducklings (38). Analysis of intrahepatic viral DNA did not show any significant decrease in viral DNA synthesis in ddC-treated animals (Fig. 1). All four animals treated with ddC started to lose weight at the end of therapy and eventually died on days 4 (one animal), 6 (two animals), and 7 (one animal) posttreatment. By contrast, in comparison with the clinical condition of the control animals, none of the 12 animals treated with l-FMAU showed any clinical signs of toxicity. Liver histology was analyzed under code without knowing the treatment regimen that the animals received, and the results are summarized in Table 1 and Fig. 4. Microscopic examination of liver sections from the control animals showed a commonly observed pattern of mild viral hepatitis with ballooning degeneration (swollen hepatocytes), inflammatory infiltration of the portal tracts, and rare aspects of hepatocyte necrosis (Fig. 4A). For l-FMAU-treated animals, the histological aspect was similar to the one observed in the control group, regardless of the treatment protocol (Fig. 4B). The inflammation in the portal tracts and the lobules was somewhat weaker in l-FMAU-treated animals. In three of four ddC-treated animals, typical signs of liver injury characterized by microvesicular steatosis (accumulation of lipid droplets in the cytoplasms of hepatocytes) and acidophilic necrosis of hepatocytes were observed (Fig. 4C and D).
TABLE 1.
Analysis of liver histology in experimentally infected ducklings undergoing l-FMAU or ddC therapy
| Treatment protocol and duck no. | Hepatocyte aspecta | Portal inflammationb | Lobular inflammationb | Cell necrosis, Councilman bodiesb |
|---|---|---|---|---|
| Controls | Ballooning degeneration | |||
| 522 | + | ++ | ++ | + |
| 524 | + | ++ | ++ | ± |
| 525 | + | +++ | + | ± |
| 529 | + | ++ | + | − |
| ddC | Microvesicular steatosis | |||
| 549 | + | + | − | + |
| 551 | − (subnormal) | − | − | ± |
| 553 | + | ± | ++ | − |
| 554 | + | − | − | + |
| l-FMAU at 20 mg/kg twice a day | ||||
| 534 | Ballooning degeneration (±) | ++ | − | − |
| 537 | Macrovesicular steatosis | + | − | − |
| 538 | Subnormal | ± | − | +++ |
| 539 | Macrovesicular steatosis | ++ | + | − |
| l-FMAU at 40 mg/kg once a day | Ballooning degeneration | |||
| 530 | + | + | + | − |
| 531 | ± | ++ | + | − |
| 532 | + | ± | − | − |
| 533 | + | − | − | − |
| l-FMAU at 80 mg/kg every other day | ||||
| 540 | Macrovesicular steatosis | ++ | + | − |
| 541 | Ballooning degeneration | ++ | + | − |
| 543 | Ballooning degeneration | ++ | + | − |
| 546 | Ballooning degeneration | ++ | + | − |
For hepatocyte aspect (ballooning degeneration and microvesicular steatosis), plus signs indicate the presence of one given histological pattern and minus signs indicate its absence. ± indicates that the histological change was observed in only a few cells.
For portal inflammation, lobular inflammation, cell necrosis, and Councilman bodies, + indicates a minimal degree of the given histological lesion, ++ indicates a mild degree, and +++ indicates a moderate degree.
FIG. 4.
Analysis of liver histology revealing microvesicular steatosis in ddC-treated animals and no histological sign of toxicity during short-term administration of l-FMAU. (A) Liver histology for one control animal. Signs of viral hepatitis are observed: ballooning hepatocytes, portal tract infiltration, and lobular inflammation (obj., 25). (B) Liver histology for one l-FMAU (40 mg/kg every day)-treated animal. A pattern similar to that in the control animals is observed. (C) Liver histology for one ddC (50 mg/kg twice a day)-treated duckling. Typical signs of microvesicular steatosis (accumulation of lipid droplets in the cytoplasms of hepatocytes) and acidophilic necrosis of hepatocytes are shown. (D) Liver histology for the same ddC (50 mg/kg twice a day)-treated duckling but at a higher magnification (obj., 40), which shows intracytoplasmic lipid droplets.
l-FMAU inhibits DHBV DNA synthesis in primary duck hepatocyte cultures.
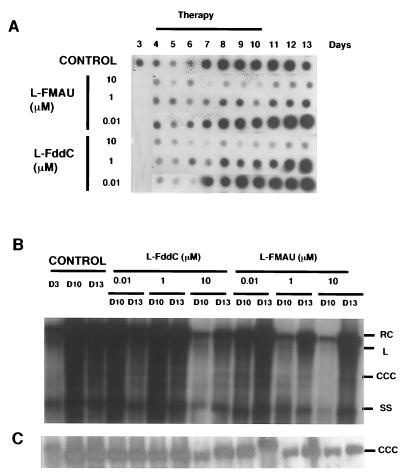
The inhibitory effect of l-FMAU was further investigated with cultures of primary hepatocytes from chronically infected ducklings. Different concentrations of l-FMAU and β-l-F-ddC (0.01 to 10 μM) were studied in parallel. Three days after plating, hepatocytes were incubated with nucleoside analogs which were renewed on a daily basis for 7 consecutive days. Quantification of the virion DNA released in the culture supernatant showed a reproducible and significant inhibitory effect of l-FMAU which was concentration dependent (Fig. 5A). The 50% inhibitory concentration (IC50) of l-FMAU for virion DNA release was 0.1 μM. At concentrations of 1 and 10 μM, the inhibitory effect reached a plateau (80 to 90% inhibition). We also confirmed our previous data showing that β-l-F-ddC inhibits DHBV replication in primary hepatocytes.
FIG. 5.
l-FMAU inhibits DHBV DNA synthesis in DHBV DNA-infected primary duck hepatocytes. l-FMAU or β-l-F-ddC was added at the indicated concentrations 3 days after plating for 6 days. Cells were harvested at the end of therapy (day 10 [D10]) and 3 days after drug release (day 13 [D13]). The viral DNA released in the supernatant of primary hepatocyte culture was analyzed by a dot blot assay (A). Intracellular protein-bound (B) and protein-free (C) viral DNAs were extracted and subjected to Southern blot analysis. The positions of relaxed circular (RC), linear (L), CCC, and single-stranded (SS) DNAs are indicated. The results of a typical experiment are shown.
Southern blot analysis of intracellular viral DNA showed a decrease in the intensity of replicative intermediates at the end of the treatment schedule for l-FMAU-treated cultures (Fig. 5B). At a concentration of 10 μM, l-FMAU induced a dramatic inhibition of viral DNA synthesis, as shown by the profound decrease in viral single-stranded DNA. However, viral CCC DNA was still detected at the end of therapy (Fig. 5C). These results were similar to those obtained with β-l-F-ddC.
Rebound of viral replication after drug release was analyzed in hepatocyte cultures. After l-FMAU and β-l-F-ddC were withdrawn, we observed a rebound in viral replication with an increase in the level of replicative intermediates, whose intensities remained weaker compared to those in the control cultures (Fig. 5B). This particular pattern was observed when l-FMAU or β-l-F-ddC was administered at concentrations of 10 μM.
No significant signs of cytotoxicity were observed during the daily microscopic examination of cultured cells. Testing of the culture supernatant for lactic acid levels showed no increase in lactic acid levels during cell culture, regardless of the therapeutic protocol (control cultures or β-l-F-ddC or l-FMAU administration).
Inhibitory effect of l-FMAU-TP on viral minus-strand DNA synthesis.
The inhibitory effect of the triphosphate form of l-FMAU on the synthesis of viral minus-strand DNA was analyzed by an in vitro assay for the expression of an enzymatically active DHBV reverse transcriptase. Viral DNA synthesis was studied by the incorporation of deoxynucleoside triphosphates (dGTP, dATP, dCTP) and radiolabelled [α-32P]TTP. Nascent viral DNA covalently linked to the DHBV polymerase was analyzed through 0.1% SDS–10% polyacrylamide gels. The incorporation of [α-32P]TMP in the presence of increasing concentrations of ddTTP (IC50 = 3 μM) was reproducibly inhibited. The level of inhibition of [α-32P]TMP incorporation in elongating viral minus-strand DNA by l-FMAU-TP was weaker since concentrations of 10 μM induced a 40% inhibition. These results suggest that, in vitro, l-FMAU-TP is a rather weak inhibitor of [α-32P]TMP incorporation in viral minus-strand DNA (data not shown).
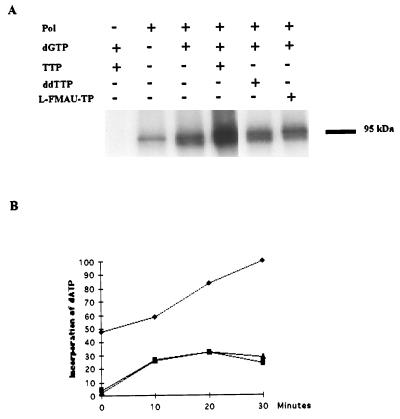
Then we asked whether l-FMAU-TP or ddTTP can terminate the synthesis of the short oligonucleotide primer for reverse transcription (POL-GTAA). The DHBV polymerase was therefore incubated with dGTP, TTP, or inhibitors (l-FMAU-TP or ddTTP) at increasing concentrations and [α-32P]dATP for 30 min. The amount of [α-32P]dAMP incorporated into the DNA primer was measured in the presence of the different thymidine analog triphosphates. The results obtained at 30 min showed an increasing level of incorporation of [α-32P]dAMP in the primer in the presence of increasing concentrations of TTP, reaching a plateau at a TTP concentration of 100 μM. In the presence of increasing concentrations of ddTTP or l-FMAU-TP, the incorporation of [α-32P]dAMP decreased to the background level (no TTP analog added). The results indicate that a complete inhibition of viral primer synthesis could not be obtained under our in vitro conditions. Figure 6A presents typical results obtained with 50 μM TTP analog.
FIG. 6.
Inhibitory effect of l-FMAU-TP on the synthesis of the viral DNA primer for DHBV reverse transcription. The sequence of the short DNA primer covalently linked to the DHBV polymerase is 5′-GTAA-3′. (A) The DHBV polymerase was incubated with different concentrations of thymidine-TP analogs (TTP or ddTTP or l-FMAU-TP) together with dGTP and [α-32P]dATP (3,000 Ci/mmol; final concentration, 0.3 μM) for 30 min. The incorporation of [α-32P]dAMP was also analyzed in control reactions without TTP, without dGTP and TTP, or without DHBV polymerase. Reactions were analyzed with a 0.1% SDS–10% polyacrylamide gel and autoradiographed. The figure shows the results obtained with the TTP analog at a concentration of 50 μM. (B) Kinetics of [α-32P]dAMP incorporation into primer DNA by the DHBV polymerase. The DHBV polymerase was incubated with thymidine-TP analogs at a concentration of 50 μM (⧫, TTP; ▪, ddTTP; ▴, l-FMAU-TP) together with dGTP (100 μM) and [α-32P]dATP (3,000 Ci/mmol; final concentration, 0.3 μM). The incorporation of [α-32P]dAMP in the viral primer was measured at different time points, as indicated. The activity at 30 min in the presence of TTP was arbitrarily defined as 100%.
The kinetics of [α-32P]dAMP incorporation into the viral DNA primer was also analyzed at different times after incubation of the polymerase mixture with cold dGTP, TTP (final concentration, 100 μM) or inhibitors (l-FMAU-TP or ddTTP; final concentration, 50 μM), and [α-32P]dATP (Fig. 6B). In the presence of TTP, the results showed an increasing level of incorporation of [α-32P]dAMP with time. A time-dependent inhibition of incorporation by ddTTP and l-FMAU-TP was observed, suggesting that ddTTP and l-FMAU-TP may terminate the synthesis of the short primer. Other experiments showed that l-FMAU-TP and ddTTP do not inhibit the incorporation of [α-32P]dGMP in viral minus-strand DNA (data not shown).
DISCUSSION
In this report, we present data on the mode of action of l-FMAU in the inhibition of hepadnavirus replication and on its antiviral activity in vivo in the DHBV model. l-FMAU was shown to inhibit HBV replication in the human hepatoma cell line 2.2.1.5, which permanently replicates the HBV genome. It was also shown to have a very good selectivity index (>2,000) in this cell line (6) and not to be incorporated into cellular DNA (27).
In this study, the in vivo administration of l-FMAU (40 mg/kg/day) by the oral route to experimentally infected ducklings showed a potent inhibition of viral replication which may support the therapeutic utility of this drug if its absence of in vivo toxicity is confirmed during long-term administration. The study of intrahepatic viral DNA at the end of 4 days of therapy showed that l-FMAU administration is associated with decreased viral DNA synthesis (Fig. 1). Administration for 5 days could inhibit viral replication significantly but was followed by a transient rebound of viremia 5 days after drug withdrawal (Fig. 2), as has also been observed with other drugs (2, 10, 13, 38). Interestingly, a more prolonged protocol with the administration of l-FMAU to ducklings for 8 days could prevent the rebound of viremia after drug withdrawal and was not associated with increased serum lactic acid levels (Fig. 2). Statistical analysis showed that, although the number of animals was small, there was a significant trend for decreased viral production in l-FMAU-treated animals compared with that in the controls (P < 0.05). Southern blot analysis of intrahepatic viral DNA 2 weeks after drug withdrawal showed the persistence of viral CCC DNA and replicative intermediates, as was also observed in tissue culture (Fig. 3 and 5), accompanied by the persistence of viral core protein expression, as determined by Western blot analysis (Fig. 3). This emphasizes the need for a prolonged therapeutic protocol in order to cure infected hepatocytes (10, 20, 22, 38). Although DHBV pol gene mutants were not selected during short-term administration of l-FMAU, it remains to be determined whether this phenomenon could be observed during long-term therapy. After drug withdrawal, the persistence of viral DNA and proteins was associated with a low level of viremia that was below the limit of detection of our dot blot assay. This may suggest either a decrease in viral particle secretion from infected hepatocytes or an enhanced clearance of viral particles from the serum. However, our attempts to detect antibodies against envelope proteins in the serum of DHBV-infected ducklings during the course of experimental infection did not show any antibody response. Analysis of the liver histology of infected ducklings showed the absence of significant signs of liver toxicity with a short-term treatment with l-FMAU (Fig. 4). With regard to toxicity, it is noteworthy that the drug was administered when the birds were rapidly growing and cell division, including hepatocytes, was occurring at a significant rate. In control animals as well as in l-FMAU-treated animals, a typical pattern of mild acute hepatitis characterized by portal tract inflammation and rare hepatocyte necrosis was observed. Since most studies have shown the absence of major liver damage during the natural course of experimental infection in ducklings (24), our observation may be related to the dose of the inoculum, as was recently shown by Jilbert’s group (14a). This further confirms the absence of toxicity of l-FMAU administration to mice for 30 days at a dosage of 25 mg/kg/day (4a). However, it will be necessary to confirm the absence of the in vivo toxicity of l-FMAU during long-term administration in ducks and woodchucks. Indeed, the administration of d-FMAU to woodchucks was associated with severe toxicity (11), which can now be explained by the inhibitory effect of d-FMAU on mitochondrial function (7, 19, 28). In our experiment, a typical pattern of liver toxicity as a result of ddC administration was observed: microvesicular steatosis and acidophilic necrosis, which are typical histological signs of mitochondrial toxicity (Fig. 4 and Table 1). These histological signs have been reported with the use of several nucleoside analogs such as fialuridine (7, 18, 25). Because it has been indicated that ddC interferes with mitochondrial DNA synthesis (4), the liver injury observed in these animals may be related to the inhibitory effect of ddC on mitochondrial DNA polymerase. This liver toxicity may have been responsible, at least in part, for the deaths of all ddC-treated birds.
To gain insight into the mechanism of action of l-FMAU, we have studied its antiviral activity in primary duck hepatocyte cultures chronically infected with DHBV. Our results demonstrated that l-FMAU is a strong inhibitor of viral DNA synthesis and virion DNA release (Fig. 5). The IC50 of l-FMAU on virion DNA release in primary duck hepatocytes (0.1 μM) was found to be similar to the one reported in human hepatoma cells permanently transfected with HBV (6, 27). Viral single-stranded DNA synthesis was significantly decreased, suggesting that l-FMAU inhibits the reverse transcriptase step of DHBV replication (Fig. 5). Furthermore, short-term therapy with l-FMAU could not clear viral CCC DNA from infected cells, as was also observed with other nucleoside analogs including β-l-F-ddC (38). Daily microscopic examination and determination of lactic acid levels in primary hepatocyte culture supernatants treated with l-FMAU did not show any significant sign of cellular toxicity, as has already been observed with human hepatoma cells (6, 27). This is in contrast to the observation made with the administration of d-FMAU, the dextrorotatory analog of FMAU, and d-FIAU (fialuridine), which proved to be toxic for mitochondrial functions and/or to be incorporated into cellular DNA (7, 25, 27). The antiviral efficacy of d-FMAU in the duck model was also evaluated by earlier studies with a dosage of 2 mg/kg/day given intraperitoneally for 5 days to adult ducks, but its toxic effect was not studied (12). It was further shown that d-FIAU is a more efficient substrate for mitochondrial thymidine kinase 2 than for cytosolic thymidine kinase 1 (36) and that d-FIAU-TP, as well as d-FMAU-TP, inhibits mitochondrial function through its incorporation into mitochondrial DNA by DNA polymerase-γ, leading to ultrastructural defects in the mitochondria and the accumulation of intracytoplasmic lipid droplets (19, 28). Although it was shown in previous studies and in the present work that l-FMAU does not significantly inhibit cellular functions at concentrations that inhibit HBV replication, it remains to be shown that its administration in hepatocyte culture does not induce any ultrastructural modifications of mitochondria. Moreover, results of recent studies have shown that administration of l-FMAU at a dosage of 10 mg/kg/day for 12 weeks is not toxic in woodchuck HBV-infected woodchucks (32).
Experiments performed in vitro with the DHBV polymerase expressed in a reticulocyte lysate system showed an inhibitory effect of l-FMAU-TP on the incorporation of radiolabelled TMP during viral minus-strand DNA synthesis, suggesting that l-FMAU-TP is indeed an inhibitor of DHBV reverse transcription. The study of l-FMAU metabolism in human hepatocytes showed that the l-FMAU-TP concentration may peak at 20 μM (27), which would already account for a 40% inhibition of viral reverse transcriptase. Under our in vitro conditions, the intracellular metabolism of the nucleoside analog is not taken into account. Depending on the half-life of the nucleoside analog triphosphate form, a greater inhibitory effect may be obtained in tissue culture. This hypothesis may be relevant to the case of l-FMAU, since its triphosphate metabolite was shown to have a long half-life in human hepatocytes (27). l-FMAU-TP was also shown to have a very potent activity against the DNA-dependent DNA polymerase activity of HBV polymerase (27). It may be hypothesized that l-FMAU-TP has a combined inhibitory effect on both the reverse transcriptase and the DNA polymerase activities of the hepadnavirus polymerase, which may result in a strong inhibition of viral replication in hepatocytes. By comparison with the results obtained by Staschke and Colacino (30), l-FMAU-TP appears to be a weaker inhibitor of TMP incorporation in viral minus-strand DNA than fialuridine triphosphate, suggesting that these drugs have a different mode of action. Interestingly, we could also show that l-FMAU-TP inhibits the synthesis of the DNA primer for reverse transcription in a time-dependent manner, suggesting that l-FMAU-TP may terminate the synthesis of the short primer (Fig. 6). The lack of complete inhibition of dAMP incorporation in the viral primer may be due to the fact that some of the viral polymerase polypeptides prime reverse transcription directly with dAMP in the first position, as was previously demonstrated by biochemical and genetic approaches (9, 17, 30). It has yet to be established that l-FMAU-TP is indeed incorporated in viral minus-strand DNA.
In conclusion, our results suggest that l-FMAU displays a strong inhibitory effect on hepadnavirus replication in primary hepatocytes and in vivo in ducklings. Long-term administration of l-FMAU, alone or in combination with other l-nucleosides, should be evaluated in the woodchuck model to study its toxicity and its ability to prevent the appearance of viral resistance and to eradicate viral infection.
ACKNOWLEDGMENTS
We thank L. Cova (INSERM Unit 271, Lyon, France) for critical review of the manuscript, P. Chossegros (INSERM Unit 271, Lyon, France) for statistical analysis, and Michael Nassal (University of Heidelberg, Heidelberg, Germany) for providing the anti-DHBV-core rabbit polyclonal antibody.
This work was supported in part by fundings from the Ligue Nationale Française Contre le Cancer and grants AI 33655 and AI 38204 from the National Institutes of Health. Stéphanie Aguesse-Germon was a recipient of a fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche, France.
REFERENCES
- 1.Bartholomew M, Jansen R, Jeffers L, Reddy K, Johnson L, Bunzendahl H, Condreay L, Tzakis A, Schiff A, Brown N. Hepatitis B virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet. 1997;349:20–22. doi: 10.1016/S0140-6736(96)02266-0. [DOI] [PubMed] [Google Scholar]
- 2.Bridges E, Cheng Y C. Use of novel β-l(−)-nucleoside analogues for treatment and prevention of chronic hepatitis B virus infection and hepatocellular carcinoma. Prog Liver Dis. 1995;13:231–245. [PubMed] [Google Scholar]
- 3.Chassot S, Lambert V, Kay A, Godinot C, Trépo C, Cova L. Identification of major antigenic domains of duck hepatitis B virus pre-S protein by peptide scanning. Virology. 1994;200:72–78. doi: 10.1006/viro.1994.1164. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Cheng Y C. Delayed cytoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2′,3′-dideoxycytidine. J Biol Chem. 1989;264:11934–11937. [PubMed] [Google Scholar]
- 4a.Cheng, Y.-C. Unpublished data.
- 5.Chevallier M, Guerret S, Chossegros P, Gérard F, Grimaud J A. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology. 1994;2:349–355. [PubMed] [Google Scholar]
- 6.Chu C, Ma T, Shanmuganathan K, Wang C, Xiang Y, Pai S, Yao G, Sommadossi J P, Cheng Y C. Use of 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil as a novel antiviral agent for hepatitis B virus and Epstein-Barr virus. Antimicrob Agents Chemother. 1995;39:979–981. doi: 10.1128/aac.39.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui L, Yoon S, Schinazi R, Sommadossi J P. Cellular and molecular events leading to mitochondrial toxicity of 1-(2-deoxy-2-fluoro-1-β-d-arabinofuranosyl)-5-iodouracil in human. J Clin Invest. 1995;95:555–563. doi: 10.1172/JCI117698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandri M, Schirmacher P, Rogler C. Woodchuck hepatitis virus X protein is present in chronically infected woodchuck liver and woodchuck hepatocellular carcinomas which are permissive for viral replication. J Virol. 1996;70:5246–5254. doi: 10.1128/jvi.70.8.5246-5254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannaoui E, Trépo C, Zoulim F. Inhibitory effect of penciclovir-triphosphate on duck hepatitis B virus reverse transcription. Antivir Chem Chemother. 1997;8:38–46. [Google Scholar]
- 10.Fourel I, Cullen J, Saputelli J, Aldrich C, Schaffer P, Averett D, Pugh J, Mason W. Evidence that hepatocyte turnover is required for rapid clearance of duck hepatitis B virus during antiviral therapy of chronically infected ducks. J Virol. 1994;68:8321–8330. doi: 10.1128/jvi.68.12.8321-8330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fourel I, Hantz O, Watanabe K, Jacquet C, Chomel B, Fox J, Trépo C. Inhibitory effects of 2′-fluorinated arabinosyl-pyrimidine nucleosides on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob Agents Chemother. 1990;34:473–475. doi: 10.1128/aac.34.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fourel I, Li J S, Hantz O, Jacquet C, Fox J J, Trépo C. Effects of 2′-fluorinated arabinosyl-pyrimidine nucleosides on duck hepatitis B virus DNA level in serum and in liver of chronically infected ducks. J Med Virol. 1992;37:122–126. doi: 10.1002/jmv.1890370209. [DOI] [PubMed] [Google Scholar]
- 13.Fourel I, Saputelli J, Schaffer P, Mason W. The carbocyclic analog of 2′-deoxyguanosine induces a prolonged inhibition of duck hepatitis B virus DNA synthesis in primary hepatocyte cultures and in the liver. J Virol. 1994;68:1059–1065. doi: 10.1128/jvi.68.2.1059-1065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoofnagle J H, Di Bisceglie A M. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 14a.Jilbert, A. Personal communications.
- 15.Jilbert A R, Wu T T, England J M, De La P, Hall M, Carp N Z, O’Connell A P, Mason W S. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992;66:1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert V, Chassot S, Kay A, Trépo C, Cova L. In vivo neutralization of duck hepatitis B virus by antibodies specific to the N-terminal portion of pre-S protein. Virology. 1991;185:446–450. doi: 10.1016/0042-6822(91)90796-e. [DOI] [PubMed] [Google Scholar]
- 17.Lanford R, Notvall L, Beames B. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J Virol. 1995;69:4431–4439. doi: 10.1128/jvi.69.7.4431-4439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee W. Drug-induced hepatotoxicity. N Engl J Med. 1995;333:1118–1127. doi: 10.1056/NEJM199510263331706. [DOI] [PubMed] [Google Scholar]
- 19.Lewis W, Levine E, Griniuvene B, Tankersley K, Colacino J, Sommadossi J P, Watanabe K, Perrino F. Fialuridine and its metabolites inhibit DNA polymerase gamma at sites of multiple adjacent analog incorporation, decrease mtDNA abundance, and cause mitochondrial structural defects in cultured hepatoblasts. Proc Natl Acad Sci USA. 1996;93:3592–3597. doi: 10.1073/pnas.93.8.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin E, Luscombe C, Wang Y, Shaw T, Locarnini S. The guanine nucleoside analog penciclovir is active against chronic duck hepatitis B virus infection in vivo. Antimicrob Agents Chemother. 1996;40:413–418. doi: 10.1128/aac.40.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling R, Mutimer D, Ahmed M, Boxall E H, Elias E, Dusheiko G M, Harrison T J. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711–713. doi: 10.1002/hep.510240339. [DOI] [PubMed] [Google Scholar]
- 22.Mason W, Cullen J, Saputelli J, Wu T, Liu C, London W, Lustbader E, Schaffer P, O’Connell A, Fourel I, Aldrich C, Jilbert A. Characterization of the antiviral activity of 2′-carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology. 1994;19:398–411. [PubMed] [Google Scholar]
- 23.Mason W S, Seal G, Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980;36:829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason W S, Taylor J M. Experimental systems for the study of hepadnavirus and hepatitis delta virus infections. Hepatology. 1989;9:635–645. doi: 10.1002/hep.1840090420. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie R, Fried M, Sallie R, Conjeevaram H, Di Bisceglie A, Park Y, Savarese B, Kleiner D, Toskos M, Luciano C, Pruett T, Stotka J, Strau S, Hoofnagle J. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med. 1995;333:1099–1105. doi: 10.1056/NEJM199510263331702. [DOI] [PubMed] [Google Scholar]
- 26.Nair V, Jahnke T. Antiviral activities of isomeric dideoxynucleosides of d- and l-related stereochemistry. Antimicrob Agents Chemother. 1995;39:1017–1029. doi: 10.1128/aac.39.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai S, Liu S, Zhu Y, Chu C K, Cheng Y C. Inhibition of hepatitis B virus by a novel l-nucleoside, 2′-fluoro-5-methyl-β-l-arabinofuranosyl uracil. Antimicrob Agents Chemother. 1996;40:380–386. doi: 10.1128/aac.40.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker W, Cheng Y C. Mitochondrial toxicity of antiviral nucleoside analogs. J NIH Res. 1994;6:57–61. [Google Scholar]
- 29.Shaw T, Amor P, Civitico G, Boyd M, Locarnini S. In vitro antiviral activity of penciclovir, a novel purine nucleoside, against duck hepatitis B virus. Antimicrob Agents Chemother. 1994;38:719–723. doi: 10.1128/aac.38.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staschke K, Colacino J. Priming of duck hepatitis B virus reverse transcription in vitro: premature termination of primer DNA induced by the 5′-triphosphate of fialuridine. J Virol. 1994;68:8265–8269. doi: 10.1128/jvi.68.12.8265-8269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Summers J, Smith P M, Horwich A L. Hepadnaviral envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990;64:2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tennant B, Jacob J, Graham L, Peek S, Korba B, Gerin J, Witcher J, Boudinot F, Du J, Chu C K. Pharmacokinetic and pharmacodynamic studies of 1-(2-fluoro-5-methyl-β-l-arabinofuranosyl)uracil (l-FMAU) in the woodchuck model of hepatitis B virus (HBV) infection. Antivir Res. 1997;34:52. (abstr. 36). [Google Scholar]
- 33.Tipples G A, Ma M M, Fischer K P, Bain V G, Kneteman N M, Tyrell D L. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;24:714–717. doi: 10.1002/hep.510240340. [DOI] [PubMed] [Google Scholar]
- 34.Tuttleman J S, Pugh J C, Summers J W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986;58:17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G H, Seeger C. The reverse transcriptase of hepatitis B virus acts a protein primer for viral DNA synthesis. Cell. 1992;71:633–640. doi: 10.1016/0092-8674(92)90599-8. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Eriksson S. Phosphorylation of the anti-hepatitis B nucleoside analog 1-(2′-deoxy-2′-fluoro-1-β-d-arabinofuranosyl)-5-iodouracil (FIAU) by human cytosolic and mitochondrial thymidine kinase and implications for cytotoxicity. Antimicrob Agents Chemother. 1996;40:1555–1557. doi: 10.1128/aac.40.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu T-T, Coates L, Aldrich C E, Summers J, Mason W S. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- 38.Zoulim F, Dannaoui E, Borel C, Hantz O, Lin T, Liu S, Trépo C, Cheng Y C. 2′,3′-Dideoxy-β-l-5-fluorocytidine inhibits duck hepatitis B virus reverse transcription and suppresses viral DNA synthesis in hepatocytes, both in vitro and in vivo. Antimicrob Agents Chemother. 1996;40:448–453. doi: 10.1128/aac.40.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoulim F, Seeger C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J Virol. 1994;68:6–13. doi: 10.1128/jvi.68.1.6-13.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoulim F, Trépo C. Nucleoside analogs in the treatment of chronic viral hepatitis. Efficiency and complications. J Hepatol. 1994;21:142–144. doi: 10.1016/s0168-8278(05)80386-1. [DOI] [PubMed] [Google Scholar]