Abstract
Honey bees are economically relevant pollinators experiencing population declines due to a number of threats. As in humans, the health of bees is influenced by their microbiome. The bacterium Snodgrassella alvi is a key member of the bee gut microbiome and has a role in excluding pathogens. Despite this importance, there are not currently any easy-to-use methods for modifying the S. alvi chromosome to study its genetics. To solve this problem, we developed a one-step procedure that uses electroporation and homologous recombination, which we term SnODIFY (Snodgrassella-specific One-step gene Deletion or Insertion to alter FunctionalitY). We used SnODIFY to create seven single-gene knockout mutants and recovered mutants for all constructs tested. Nearly all transformants had the designed genome modifications, indicating that SnODIFY is highly accurate. Mutant phenotypes were validated through knockout of Type 4 pilus genes, which led to reduced biofilm formation. We also used SnODIFY to insert heterologous sequences into the genome by integrating fluorescent protein-coding genes. Finally, we confirmed that genome modification is dependent on S. alvi’s endogenous RecA protein. Because it does not require expression of exogenous recombination machinery, SnODIFY is a straightforward, accurate, and lightweight method for genome editing in S. alvi. This workflow can be used to study the functions of S. alvi genes and to engineer this symbiont for applications including protection of honey bee health.
Introduction
Honey bees are critical pollinators in agriculture and are essential for global food security1–3. Both managed and wild bee populations are experiencing population declines due to a number of stressors that include viral and bacterial pathogens4–6. It has become increasingly clear that the health of bees is influenced by their microbiome7–12. One of the eight core gut microbiome constituents is Snodgrassella alvi (Neisseriaceae) which colonizes the wall of the hindgut ileum7,13. S. alvi is a keystone species within the gut community, as it depletes oxygen and thereby creates an anaerobic environment for other community members9. Experimental studies with gnotobiotic bees also show that S. alvi also protects against gut pathogens14–16. Further, S. alvi containing engineered plasmids have been used to induce RNAi to perform functional genomics in bees17,18, and to combat bee pathogens such as Nosema and deformed wing virus16,17,19. However, fundamental questions still remain concerning how S. alvi colonizes and interacts with its bee host.
To date, genetic studies of S. alvi have primarily been limited to -omics level investigations employing Tn-seq and RNA-seq7,14,20,21. Additionally, applications aimed at studying and improving bee health have been constrained to the use of wild type or plasmidbearing S. alvi16–18,22,23. As a result, research involving S. alvi has been hampered by the lack of streamlined tools for reverse genetics. Gene disruption in S. alvi was previously achieved through conjugation of a suicide vector containing an antibiotic resistance gene flanked by homology to the chromosome and a small guide RNA targeting cleavage of the integration site by Cas9 expressed from a second plasmid22. However, this conjugation-dependent method is technically complex and can lead to off-target single-crossover integrations, necessitating several selection and screening steps to isolate an on-target knockout. Therefore, there is a need for a more accurate and straightforward genome engineering workflow in S. alvi.
Homologous recombination-based gene knockout24–28 has long been employed for genome modification in bacteria. For this approach to be successful, one must deliver a sufficient quantity of DNA into cells, and cells must have native or exogenous recombination pathways that are efficient enough to reliably integrate this DNA into their genomes. Recombination with the native RecA pathway has been used for modifying the chromosomes of several species of Neisseria, which are close relatives of Snodgrassella species29. In these cases, delivery of the knockout DNA has typically relied on natural transformation30–32. S. alvi, however, appears to not be naturally competent. Its genome does not contain repeated DNA uptake-sequences characteristic of Neisseria species33, and it does not transform DNA in normal laboratory culture conditions. This has limited prior work using homologous recombination for genome modification to the delivery of DNA through conjugation, which is not as amenable to making fast and accurate genome modifications as is transforming linear double-stranded DNA fragments.
We have overcome the DNA delivery hurdle by demonstrating that S. alvi can be transformed by electroporation and find that S. alvi’s native RecA pathway is efficient enough to reliably use homologous recombination for genome modification. Here, we describe a streamlined, lightweight, and highly accurate method to modify the S. alvi genome, which we call SnODIFY (Snodgrassella-specific One-step gene Deletion or Insertion to alter FunctionalitY). SnODIFY will enable future studies of S.alvi-host interactions and applications that engineer this symbiont to protect bee health. Beyond S. alvi, the principles underlying this workflow may inform genome engineering technology development in other insect symbionts.
Results
S. alvi can be transformed by electroporation
To determine if S. alvi could be transformed by electroporation, we prepared electrocompetent S. alvi wkB229 cells in a manner similar to that used for Escherichia coli34. Cells grown in cell culture flasks containing Columbia broth were washed twice with dH2O, then resuspended in 10% glycerol. Electrocompetent cells were electroporated with 520 ng of the specR-containing plasmid pBTK800, which is capable of replicating in S. alvi and has previously been transferred to S. alvi by conjugation18. Transformed cells were recovered overnight, and plated on selective media. Cells that had received pBTK800 were able to grow on plates containing spectinomycin, whereas those that received no DNA could not (Fig. S1), indicating that growth is not due to spontaneous mutations in the chromosome that can yield spectinomycin resistance in other bacteria. These results demonstrate that S. alvi can be transformed with plasmids by electroporation.
Knockout of staA by transforming dsDNA
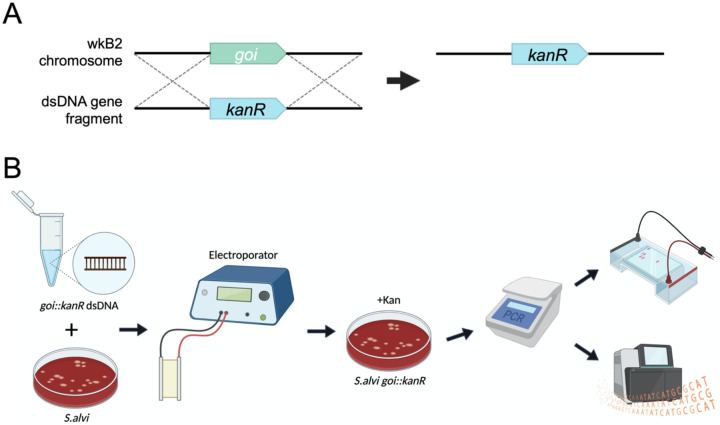
We next investigated whether electroporation of linear double-stranded DNA (dsDNA) could be leveraged to modify the S. alvi chromosome. We devised a strategy, SnODIFY, to knockout a gene of interest by homologous recombination (Fig. 1A). In the SnODIFY workflow, a construct is designed with homology arms to a gene of interest, flanking an antibiotic resistance cassette. Linear DNA constructs are chemically synthesized, assembled, and PCR amplified. Then, S. alvi wkB2 cells are electroporated with the dsDNA, recovered overnight, selected on antibiotic-containing media, and screened for proper insertion of the construct (Fig. 1B).
Fig 1. SnODIFY overview and workflow.
A. Cartoon depicting an overview of gene knockout by SnODIFY. An antibiotic resistance cassette, such as kanR, is designed to be flanked by arms with homology surrounding the deletion target. The targeted genomic locus is replaced by the antibiotic resistance cassette by single-step homologous recombination.
B. Diagram of SnODIFY workflow. 5 μg of linear or circular knockout construct DNA is electroporated into S. alvi wkB2. Following overnight liquid recovery with no antibiotics, transformants are selected for on media containing antibiotic. Transformants are isolated and the deleted region is screened via PCR. Amplicons are purified and visualized on a 1% agarose gel, then sent for amplicon/whole-genome sequencing and aligned to a reference sequence.
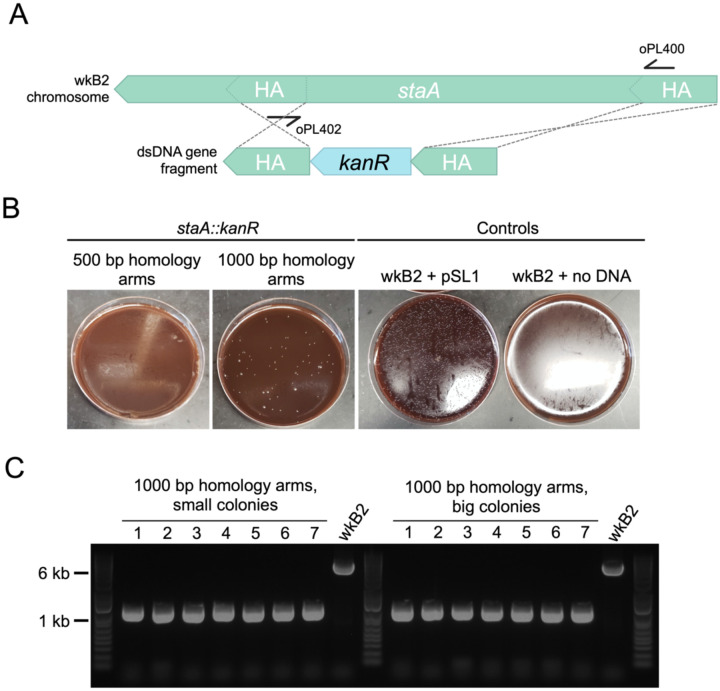
To pilot this method, we targeted staA for deletion. This gene is non-essential21 and was previously disrupted using the Cas9/suicide vector approach22. We designed kanR knockout constructs with homology arms inside of the staA gene that were either 500 or 1000 bp long (Fig 2A). Initial electroporations using approximately 150 ng and 300 ng of DNA did not yield any transformants. Increasing the concentration of DNA to 5 μg proved sufficient for isolating kanamycin resistant transformants (Fig 2B). We found that 1000 bp of homology yielded 85 transformants, whereas 500 bp of homology yielded none (Fig 2B; Table S1). From the plate transformed with 1000 bp homology arms, we picked seven large and seven small colonies. Colonies were screened via PCR for insertion of the kanR cassette using primers specific to sequences within the homology arms. All 14 of the screened colonies contained the kanR cassette inserted at the proper locus (Fig 2C; Table S1), indicating on-target editing accuracy of 100% in the screened colonies. Short-read whole-genome sequencing (WGS) of one of these staA::kanR strains confirmed that the targeted stretch of staA had been deleted and replaced with the kanR gene (Table S2).
Fig 2. Gene knockout in staA is highly accurate.
A. Gene deletion map depicting genomic staA and the corresponding deletion construct. The deletion construct is composed of a kanR cassette flanked by 500 or 1000 bp arms with homology internal to the staA gene. Primers oPL400 and oPL402 were designed for use in PCR screening for proper deletion.
B. Images of staA::kanR Columbia + 5% sheep’s blood (Col-B) agar plates + kanamycin transformation plates. wkB2 can be successfully transformed with a staA::kanR cassette containing 1000 bp (middle left), but not 500 bp (left), homology arms, indicating that 1000 bp of homology is needed to isolate colonies with the deletion. wkB2 cannot grow on kanamycin plates (right) without the presence of a kanamycin resistance cassette, as illustrated by control cells that received the kanR-containing pSL1 plasmid (middle right).
C. DNA gel of PCR screen for staA deletion. Large and small colonies of wkB2 + staA::kanR with 1000 bp homology arms or wkB2 were amplified via PCR using oPL400 and oPL402, and run on a 1% agarose gel. The ~6000 bp band observed in the wkB2 sample corresponds to the genomic region within staA, whereas the ~1000 bp band observed in experimental samples corresponds with the kanR cassette. 100% of both small and large colonies were found to contain a single 1000 bp band, indicating proper deletion of staA, regardless of colony size.
Genes can be reliably knocked out using SnODIFY
To further assess the reliability of SnODIFY, we designed and tested knockout constructs for six additional non-essential21 S.alvi genes: pilF, pilG, asr, asmE, recJ, and recA. Similar to the staA knockout construct, we designed kanamycin resistance cassettes flanked by 1000 bp homology arms (Fig. S2). However, this time, the homology arms were designed to match regions upstream and downstream of the CDS, with 10 bp of CDS left on 5’ and 3’ ends, so that nearly the whole gene would be deleted (Fig. S2). As before, dsDNA constructs were synthesized and electroporated into S. alvi wkB2. Then, cells were plated on antibiotic-containing media to select for integration of the cassette into the chromosome. The number of transformants ranged from ~20 to >100 colonies, depending on the gene targeted for knockout (Table S1). Because editing accuracy was found to be 100% for the staA knockout, in some cases, we screened fewer clones for successful deletion by PCR for these six additional knockouts. For five out of the six knockouts, editing accuracy (as determined by PCR or WGS) was 100% (Fig. S3; Table S1). For the remaining gene, asr, editing accuracy was 50% (Fig. S3; Table S1). (Inconclusive PCR screening of asr, evidenced by the presence of two bands instead of one (Fig. S3), may mask a higher editing accuracy). WGS confirmed each of these six genes was knocked out and successfully replaced by the kanamycin resistance cassette (Table S2). In some cases, point mutations or small (primarily ≤ 20 bp; in one case, 69 bp) deletions distant from the integration site were observed (Table S2; Table S3). In aggregate, these data demonstrate the feasibility of scaling up SnODIFY to construct multiple S. alvi knockout strains in parallel, with near-perfect on-target editing accuracy.
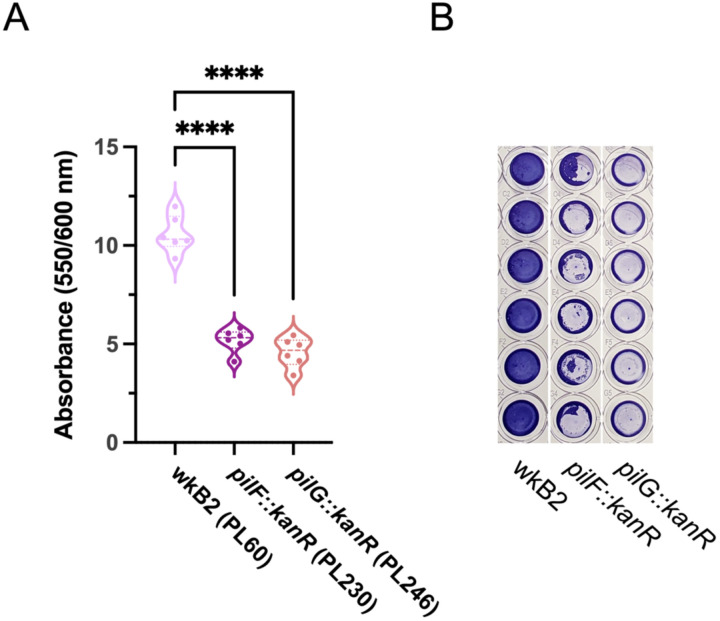
SnODIFY-generated type IV pilus mutants have impaired biofilm formation
Biofilm formation has previously been used as a phenotype for validating novel genome engineering tools35. Two of the S. alvi knockout strains we created had deletions of type 4 pilus genes, pilF and pilG. Biofilm formation was quantified for wild type wkB2 and the pilF::kanR and pilG::kanR mutants using a crystal violet assay, by assessing OD550/OD600 of washed cells grown in 96 well plates. We found that knockout of both pilF and pilG led to a significant and visually noticeable decrease in biofilm formation compared to the wild type strain (Fig 3; Fig S4), similar to that observed for mutant strains containing pilF and pilG genes disrupted by transposon insertions in a prior study21. This result indicates SnODIFY can be used to generate knockouts for downstream phenotype testing.
Fig 3. Phenotypic validation: Knockout of genes encoding adhesion proteins result in biofilm formation defect.
A. Plot of OD550/OD600 to quantify biofilm formation normalized to cell growth for biofilm knockout strains. Compared to WT, pilF::kanR and pilG::kanR have decreased biofilm formation relative to growth. Thick dotted line = median; thin dotted line = quartiles. N=6 for each condition. ****: P < 0.0001, as determined by One-way parametric ANOVA with Dunnett’s multiple comparison test.
B. Image of Crystal violet-stained 96 well plate columns demonstrating pilF::kanR and pilG::kanR mutants have reduced biofilm formation as compared to wkB2.
Heterologous genomic insertions can be made using circular plasmid DNA
We next sought to implement two additional features of SnODIFY: insertion of heterologous genes into the genome; and genome modification using circular DNA, as opposed to linear DNA. We approached these problems simultaneously through insertion of fluorescent protein-coding genes into the chromosome using electroporation of E. coli plasmids that do not replicate in S. alvi. We reasoned that recombination using plasmid DNA would allow for knockout cassettes to be constructed from part plasmids according to the bee microbiome toolkit (BTK) assembly scheme22, which would enable re-use of the same homology flanks to easily integrate different payloads into the genome.
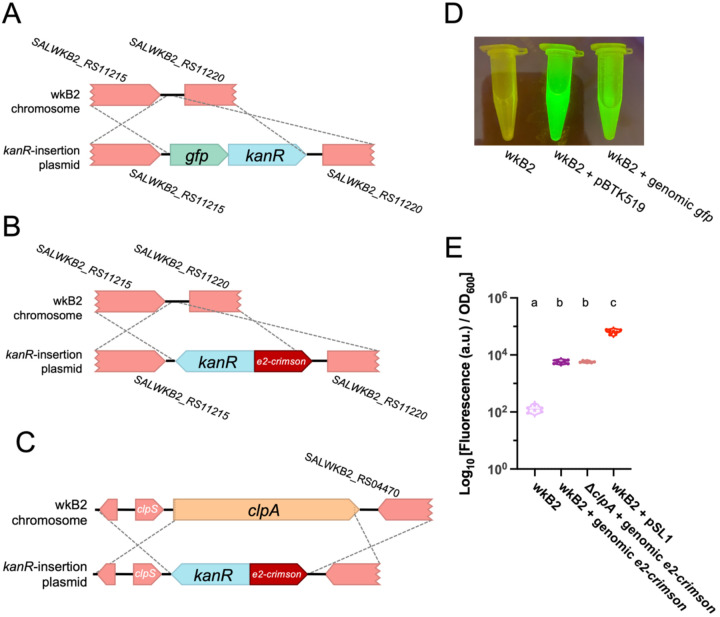
To pilot this approach, we identified an intergenic region of the wkB2 genome, situated between the non-essential genes SALWKB2_RS11215 and SALWKB2_RS1122021. We engineered part plasmids containing genes encoding one of two fluorescent proteins (GFP or E2-Crimson) and an antibiotic resistance gene, as well as homology arm part plasmids (Fig 4A, B; Fig S5; Fig S6). Parts were assembled into a single plasmid via Golden Gate cloning in E. coli22. The purified non-linearized plasmid was then electroporated into S. alvi followed by isolation of antibiotic resistant colonies (Fig S5; Fig S6). Insertion of the cassette into the proper genomic location was confirmed by WGS (Table S2).
Fig 4. Fluorescent protein-coding genes can be inserted into the S. alvi genome.
A. Gene insertion map depicting insertion of a gfp + kanR cassette into a non-essential, intergenic region of the genome.
B. Gene insertion map depicting insertion of a e2-crimson + kanR cassette into a non-essential, intergenic region of the genome.
C. Gene insertion map depicting deletion of the clpA gene via replacement insertion of a e2-crimson + kanR cassette.
D. Image of wkB2, wkB2 + pBTK519 (positive control), and wkB2 + genomic gfp cells in front of a blue light transilluminator (470 nm), demonstrating successful insertion of gfp as evidenced by green fluorescence that is not observed in WT cells.
E. Plot of quantification of e2-crimson fluorescence, demonstrating that wkB2 + genomic e2crimson cell pellets resuspended in PBS display significantly increased red fluorescence compared to wkB2, and only slightly less fluorescence than wkB2 + pSL1, which contains a multicopy plasmid expressing e2-crimson. Log10 of OD611 ex, 646 em/OD600 is shown for cell cultures that were pelleted, resuspended in PBS, and read in a 96 well plate. Solid line = median; thin dotted line = quartiles. N=6 for each condition. Dissimilar letters above each group indicate a significant difference in means (P < 0.0001, as determined by One-way parametric ANOVA with Tukey’s multiple comparison test).
Next, we validated the phenotype of the gfp and e2-crimson genomic insertion strains. S. alvi wkB2 and wkB2 + genomic gfp cells were grown in liquid culture and imaged on a blue light transilluminator. wkB2 + genomic gfp were fluorescent, in contrast to wkB2 control cells (Fig 4D), indicating genomically-encoded gfp is capable of being successfully transcribed and translated into protein with observable fluorescence. To test expression of e2-crimson, liquid cultures of wkB2, wkB2 + genomic e2-crimson, and wkB2 + pSL136 (which contains plasmid-encoded e2-crimson) were pelleted, resuspended in PBS, and quantified for E2-Crimson emission (622 nm excitation, 646 nm emission) normalized to cell growth (OD600). wkB2 + genomic e2-crimson had significantly higher fluorescence than wkB2 (Fig 4E), indicating successful production of genomically encoded E2-Crimson. Quantification of fluorescence in cultures prior to pelleting similarly indicated a significant increase in fluorescence in the mutant strains (Fig S8). As expected for one genomic copy versus a multicopy plasmid, wkB2 + genomic e2-crimson produced less fluorescence than did wkB2 + pSL1 (Fig 4E). These results indicate that exogenous genes can be successfully incorporated into the S. alvi genome.
Gene knockout in S. alvi can be coupled to functional genomic insertion
Next we demonstrated that a gene knockout could be made at the same time as a functional genomic insertion. To this end, we combined insertion of a fluorescent protein-coding gene with deletion targeting the non-essential clpA gene. We designed a construct containing e2-crimson and kanR, flanked by arms with homology upstream and downstream of clpA (Fig 4C; Fig S7). Similar to cells containing a fluorescent protein-coding gene inserted into an intergenic region, we found that ΔclpA + e2-crimson cells emitted fluorescence, with no significant difference between these strains (Fig 4E). This result confirms that fluorescent protein-coding gene insertion can be combined with gene deletion.
DNA integration is RecA-dependent
Having validated SnODIFY, we wanted to verify that genome integration was occurring through the expected RecA-mediated recombination mechanism that predominates in bacteria. To test this hypothesis, we generated a recA::specR strain and attempted to delete another gene, amsE, in this background using a kanR cassette. We found that, while recA::specR could still be transformed with a plasmid and amsE could be knocked out in wkB2, amsE could not similarly be knocked out in recA::specR cells (Fig. 5). To ensure that synthetic lethality between asmE and recA was not what accounted for lack of transformant, we repeated this experiment by trying to knock out recJ in a recA::specR background. As before, recJ could be knocked out in wild type S. alvi wkB2, but not in recA::specR cells (Fig. S9). This finding indicates that lack of double mutants in a recA::specR background is likely not due to synthetic lethality. Rather, these results demonstrate that gene knockout in S. alvi is RecA-dependent.
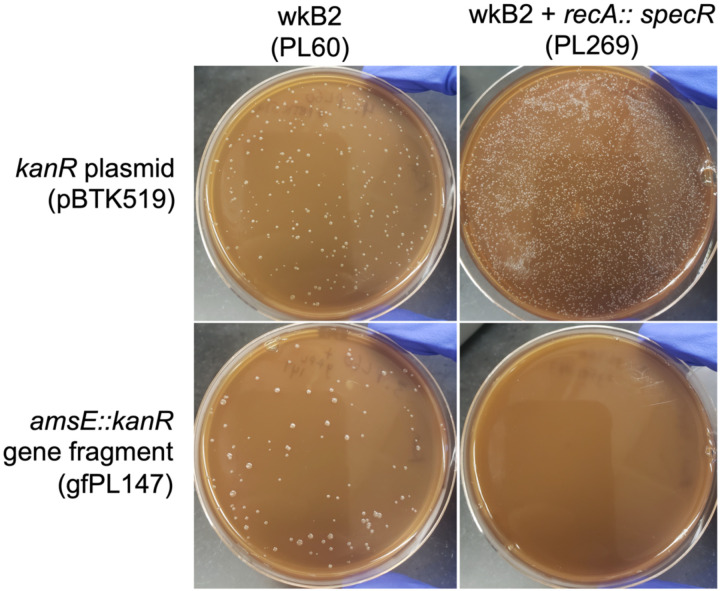
Fig 5. Gene knockout is recA-dependent.
Plates demonstrating that wkB2 (PL60; top left) and wkB2 + recA::specR (PL269; top right) are successfully electroporated with a control kanR plasmid (pBTK519). The knockout construct amsE::kanR (gfPL147) is incorporated into wkB2 (bottom left), but not wkB2 + recA::specR (bottom right).
Discussion
Here, we describe SnODIFY, a method for engineering the S. alvi chromosome using electroporation and homologous recombination. Strategies to genetically engineer other insect symbionts have had varied success37. While transposon mutagenesis (Tn5 and Himar1) has been described in a number of insect symbionts21,37–40, these strategies are not site-specific. Conversely, site-specific transposon mutagenesis via Tn7, while utilized in insect symbionts37, does not allow for gene deletion. Site-specific deletion has been demonstrated in a handful of insect symbionts, though these strategies have used expression of exogenous lambda Red machinery (BfO2, S. glossinidius)41,42, or use of a two-step suicide vector strategy (R. rhodnii, S. glossinidius, B. insecticola, S. alvi, B. apis, F. perrara)22,37,38,41,43,44, both of which add either complexity or time to knockout construction. Compared to symbiont engineering described in other insect systems, SnODIFY is highly accurate, straightforward, flexible, and reliable.
Integration of a circular plasmid into a genome is typically a two-step process45, with each crossover occurring sequentially. Previous modification of the S. alvi genome using conjugation of an R6K suicide vector resulted in mostly single-crossover integrations of the plasmid, and targeting Cas9 cleavage to the integration site was needed to increase the fraction of cells with double-crossover integration (i.e., just integration of the antibiotic resistance gene versus the entire plasmid)22. However, we observed only double-crossover integrants when we electroporated a ColE1 plasmid that cannot replicate in S. alvi, even when we did not linearize the plasmid. This discrepancy may be due to how conjugation delivers single-stranded DNA to a cell versus the dsDNA that was electroporated. Apparently, S. alvi recombination is efficient enough to favor the double-crossover integration, which makes linearization of the plasmid prior to electroporation unnecessary.
For each of the knockout strains we made, we observed what could be secondary mutations in the whole-genome sequencing results (Table S2; Table S3). While some of these mutations appear to already be present in our ancestral stock, others are found in only one or a few edited strains. Further work is needed to determine whether these mutations arose as suppressors to genes targeted for deletion, are common mutations in lab-cultured S. alvi, or were somehow selected for during the transformation/recombination process.
In its current form, SnODIFY provides immediate value to the bee microbiome field. This technique will facilitate study of S. alvi’s contribution to host-symbiont interactions by reverse genetics, allowing mechanistic investigation into host engraftment, immune stimulation, and host nutrient metabolism. SnODIFY will also enable investigations of how S. alvi interacts with other microbiome members and bee pathogens. Integration of fluorescent protein-coding genes into the genome of S. alvi allows it to be visualized in the bee host without needing to administer antibiotics to maintain a plasmid. Further, we foresee SnODIFY allowing for S. alvi engineering projects, ranging from migrating additional plasmid-based machinery to the genome, to construction of gain-of-function mutant strains that improve upon S. alvi’s probiotic capabilities23,46. Finally, the interoperability of SnODIFY and the bee microbiome toolkit (BTK)22 can expedite strain construction. Part plasmids constructed to contain homology arms can be mixed and matched with different payloads, allowing for streamlined knockout/insertion library construction.
With further development, new use cases and features could expand SnODIFY’s utility. Induction/depletion experiments (performed either with a gene knockout and complementation with a plasmid-based copy of a gene under control of an inducible promoter, or by placing an inducible promoter into the genome upstream of a gene) could be performed in vivo47, to study essential cellular processes on demand. Use of site-specific recombinases such as the Flp-FRT system for scarless gene deletion48 would remove confounders (such as the presence of antibiotic resistance cassettes) in attributing phenotypes to gene deletions and allow recycling antibiotic resistance cassettes to make multiple edits. Expression of exogenous recombineering systems (e.g., RecET or lambda Red) from a plasmid could improve recombination efficiency, as in E. coli or Acinetobacter baumanii, for example35,49,50. Finally, allelic exchange, facilitated by two-step recombination and use of counter-selectable markers51, would allow for study of loss-of-function or gain-of-function mutants. In short, we expect the SnODIFY platform presented in this paper to not only have immediate utility in reverse genetics and engineering applications in S. alvi, but to also serve as a springboard for future genome engineering toolsets.
Methods
Strains and DNA constructs
Strains, plasmids, oligos, and gene fragments are reported in Table S4.
Bacterial cell culture
S. alvi cells were grown on Columbia + 5% sheep’s blood (Col-B) agar plates (with or without antibiotics, as indicated) at 35°C, 5% CO2, with 2–3 day incubation time. S. alvi cells were grown in liquid culture (with or without antibiotics, as indicated) at 35°C, 5% CO2, with 2–3 day incubation time. Media and containers used for liquid culture depended on the application, as indicated below. E. coli cells were grown with antibiotics on LB + 1.5% agar plates or in liquid LB, at 37°C, incubating overnight. Where indicated, 25 μg/mL kanamycin (kan) was used in S. alvi cultures; 30 μg/mL of spectinomycin (spec) was used in S. alvi cultures and 60 μg/mL of spectinomycin was used in E. coli cultures. Strains were saved in 30% glycerol stocks and stored at −80°C. Plasmids were obtained from E. coli overnight cultures using a Monarch plasmid miniprep kit (New England Biolabs, USA) and concentration was quantified by NanoDrop (Thermo Scientific, USA).
Knockout construct design and synthesis
Putative genes identified for knockout were first determined to be non-essential in vitro by cross-referencing to the previously published S. alvi Tn-seq dataset21. Homology arms of 500 or 1000 bp either flanking or within the gene of interest were determined using NCBI gene, with wkB2 as a reference genome. Knockout constructs were then designed in Benchling’s molecular cloning tool52, by placing homology arms 5’ and 3’ to an antibiotic resistance cassette, either kanR or specR, flanked by a promoter and terminator. Constructs were commercially synthesized either as gBlocks (IDT, USA) or as plasmid inserts (Azenta, USA).
Fluorescent protein-coding gene insertion construct design and synthesis
Insertion sites in the S. alvi genome were identified by finding two regions of the genome deemed non-essential by the S. alvi Tn-seq experiment21. The first was a 378 base-pair intergenic region between the genes SALWKB2_RS11215 and SALWKB2_RS11220. The second was the non-essential gene clpA.
Golden Gate assembly part plasmids were made by cloning the parts into the cloning vector pBTK1001 using BsmBI-v2 (New England Biolabs, USA) (Fig S5; Fig S6). Flanking homology sequences of 1000 bp were PCR amplified from wkB2 genomic DNA with primers that added overhangs, so that they could be cloned into the entry vector pBTK1001 as Type 1 and Type 5 parts by BsmBI assembly, similar to previously described methods22. Integration cassettes were constructed as Type 2-3-4 parts in pBTK1001. Two versions of the Kanamycin resistance cassettes were amplified as Type 2 and Type 4 from pBTK1047. The E2-Crimson cassette was amplified as a Type 3–4 part from pLM70. The GFP cassette was amplified as Type 2–3 from pBTK503. Assembly reactions were heat-shock transformed into NEB 5-alpha E. coli cells (New England Biolabs, SUA) and plated on selective media. Transformants were cultured overnight. Part plasmids were extracted using a QIAprep Spin Miniprep Kit (Qiagen, Germany) and sequence verified by whole plasmid sequencing (Plasmidsaurus, USA). To create plasmids containing an integration cassette flanked by homology to the wkB2 genome, two homology parts and an integration cassette part were combined with the ColE1 origin-containing pYTK095 backbone53 using BsaI-HF v2 Golden Gate assembly (New England Biolabs, USA). As before, plasmids were transformed into E. coli, transformants selected for, and plasmid sequence verified.
PCR amplification of knockout constructs
Synthesized DNA constructs were amplified via PCR. Briefly, primers with homology to the 5’ and 3’ ends of the construct were designed. For each construct, several 50 μl PCR reactions were run using these oligos and Q5 or Phusion polymerase (New England Biolabs, USA). A sample was run on a 1% agarose gel and imaged via UV transilluminator, to confirm successful amplification. Amplicons were purified using Magbeads (Axygen, USA) or a DNA Clean & Concentrator kit (Zymo Research, USA), and concentration was measured via NanoDrop (Thermo Scientific, USA). Constructs were brought to a suitable concentration to allow for electroporation with around 5 μg of DNA.
S. alvi electroporation
Electrocompetent S. alvi cells were prepared prior to transformation in a manner similar to that commonly used for E. coli. Briefly, S. alvi was grown on solid media, as described above. After the appearance of colonies, cells were scraped and resuspended in a cell culture flask containing 50 ml Columbia broth. Cells were incubated without shaking at 35°C, 5% CO2 for 2–3 days. Working at room temperature, cells were then pelleted at approximately 5000 ×g for 10 minutes and supernatant decanted. Cells were washed twice by resuspending in 30 ml Ultrapure dH2O and pelleting, as above. After the third centrifugation, cells were resuspended in 1 ml filtered 10% glycerol in dH2O, dispensed into 100 μl aliquots, and stored at −80°C.
S. alvi cells were electroporated in a manner similar to that used for E. coli, with a few modifications. Briefly, electrocompetent cell preps were thawed on ice and incubated with approximately 500 ng of plasmid DNA or 5–10 μg of knockout/insertion construct DNA for 20 minutes (5 μg of DNA was used to construct most knockout strains; 10 μg of DNA was used to construct PL269 and PL271). The cell + construct mixture was added to either a 1 mm or 2 mm electroporation cuvette depending on the volume. Cells were electroporated with a Gene Pulser Xcell Electroporator (Bio-Rad, USA) using 1.8 kV (for 1 mm cuvettes) or 3 kV (for 2 mm cuvettes). Cells were resuspended in Columbia broth and allowed to recover without antibiotic overnight at 35°C, 5% CO2. The next day, cells were pelleted, resuspended in 100 μl of media, and the entirety plated on Columbia + 5% sheep’s blood agar plates with the respective antibiotic. Plates were incubated for 2–3 days at 35°C, 5% CO2, until the appearance of colonies. Multiple colonies were then isolated by passage onto a fresh Columbia + 5% sheep’s blood agar plate, which were allowed to form colonies.
PCR screen for knockout mutants
Colony PCR was performed to screen for proper genomic insertion of the knockout/insertion construct. Briefly, 2–14 colonies were picked from the transformation isolation plates and resuspended in 20 μl of dH2O. PCR was run using diluted template, oligos flanking the antibiotic resistance cassette, and Q5 polymerase. A control PCR was also run using WT wkB2. A portion of the PCR reaction was run on a 1% agarose gel and imaged using a UV transilluminator to visualize DNA band size. Amplicons of interest were prepared using magnetic beads (Axygen, USA) and concentration was quantified via NanoDrop (Thermo Scientific, USA). Amplicons were sent for Sanger or Next-gen amplicon sequencing. Sequences were aligned to the designed knockout construct reference using Benchling’s molecular cloning tool to confirm presence of the antibiotic resistance cassette.
Knockout/insertion confirmation by WGS
We sequenced strains with genome modifications using either short-read (Illumina) or long-read (Nanopore) technologies. For short-read sequencing, genomic DNA (gDNA) was purified from the relevant strain using a DNeasy Blood & Tissue kit (Qiagen, Germany). For longread sequencing, high molecular weight gDNA was isolated using the Quick-DNA HMW MagBead kit (Zymo Research, USA). gDNA concentrations were measured by Qubit (Invitrogen, USA) or NanoDrop (Thermo Scientific, USA).
gDNA was then sequenced in one of three ways: 1. Commercial library preparation and short-read sequencing (SeqCenter, USA); 2. In-house long-read sequencing; or 3. In-house library preparation and commercial short-read sequencing (Psomagen, USA). Commercial library preparation used the tagmentation DNA Prep Kit (Illumina, USA). For in-house short-read sequencing library preparation, 10 ng gDNA was inputted into the xGen DNA lib Prep EZ kit using the xGen Deceleration module (Integrated DNA Technologies, USA). All reactions were carried out at 20% of the manufacturer’s recommended volumes, with dual 6-bp indexes incorporated during a final 12 cycle PCR step. For long-read sequencing, 175 ng of gDNA was prepared using a Rapid Barcoding Sequencing Kit and sequenced on a R9.4.1 flow cell using a MinION MK1C instrument (Oxford Nanopore, UK). Basecalling was done using Guppy (v6.3.9) in fast mode. Raw FASTQ files are available from the NCBI Sequence Read Archive (PRJNA1017617).
Long-read FASTQ files were trimmed using Porechop (v0.2.4)54 with the option to discard reads with internal adaptors. Short-read FASTQ files were trimmed using fastp (v.0.23.4)55. Variant calling was performed on trimmed FASTQ files using breseq (v0.38.1)56 with the S. alvi wkB2 genome (Genbank: CP007446)33 and the insertion deletion/cassette sequences as references. Mutations, including the deletion/resistance cassette insertion and secondary mutations, were annotated. For analysis of Nanopore data, indels in homopolymers of 3+ bases were not reported.
Crystal violet biofilm formation assay
Biofilm formation in S. alvi was assayed using a Crystal violet assay, as previously described21,35. Briefly, S. alvi strains were inoculated directly from a freezer stock into an overnight culture in a 50 ml conical containing Insectagro DS2 (Corning, USA) + antibiotic, and incubated for 3 days at 35°C, 5% CO2. 1:40 dilutions of confluent cells (including resuspended biofilm) were made in Insectagro DS2 without antibiotics in a 96 well plate. (Note that outer wells were filled with either blank media or culture that would not be used in downstream analysis, due to differential growth resulting from differential oxygen availability). Plates were incubated for 2 days at 35°C, 5% CO2. After 2 days, OD600 was measured on a Spark 10M plate reader (Tecan, Switzerland) to quantify overall cell growth. Plates were washed 3 times by dunking in dH2O and stained in 0.1 % Crystal violet (in dH2O) for 10 minutes. Stain was then removed and plates were allowed to dry in a warm room (37°C) for 1–3 hours. Dried plates were then imaged. 30% acetic acid (in dH2O) was added to resolubilize the dried Crystal violet and incubated for 10 minutes. OD550 was then measured on a Spark 10M to quantify the amount of biofilm present.
Plate reading data were analyzed by first subtracting background (blank reading) in the OD600 and OD550 readings. Outer wells were omitted from analysis (see above) and OD600 values of less than 0.01 were removed. OD550 was then divided by OD600, and OD550/OD600 was plotted in Prism Graphpad for each strain. Only positive OD550/OD600 are displayed. A Shapiro-Wilk Normality test was run to determine if each sample was normally distributed and each condition passed. A One-way parametric ANOVA with Dunnett’s multiple comparison test (comparing to the WT control) was subsequently performed, and significance was indicated on plots.
Imaging and quantification of fluorescent strains
Fluorescent insertion strains were imaged and/or quantified for fluorescence. wkB2 + genomic gfp and wkB2 were grown for 2 days on Columbia agar + 5% Sheep’s blood + kanamycin media at 35 °C 5% CO2. After 2 days, cultures were scraped, resuspended in PBS in microcentrifuge tubes, and imaged in front of a blue light transilluminator (470 nm). For experiments involving E2-Crimson, wkB2 + genomic e2-crimson, wkB2 + pSL1, and wkB2 were grown for 2 days in liquid BHI media in a sealed bag maintaining a microaerobic environment (85% nitrogen; 10% CO2; 5% O2)57. After 2 days, cultures were pelleted and imaged. Pellets were then resuspended in PBS and transferred to a 96 well plate. Samples were read in a Spark 10M plate reader in triplicate, using 611 nm excitation and 646 nm emission58 and optimal gain was determined by the plate reader. Absorbance readings from technical replicates were averaged, then log-transformed and plotted in GraphPad Prism (Dotmatics, USA), with individual data points for each group representing biological replicates. A Shapiro-Wilk Normality test was run to determine if each sample was normally distributed, and each condition passed. A one-way parametric ANOVA with Tukey’s multiple comparison test was subsequently performed and significance indicated on plots.
Supplementary Material
Acknowledgements
We thank Daniel Deatherage for performing whole-genome sequencing. We also thank Eli Powell for technical advice, as well as members of the Moran and Barrick lab for discussions. Figures were made using BioRender. This work was supported by funding from the National Science Foundation (IOS-2103208), U.S. Army Research Office (W911NF-20-1-0195), National Institutes of Health (R35GM131738), and USDA NIFA (2023-67012-39356).
Footnotes
Competing interests
S.P.L., J.E.B., and N.A.M. have a pending patent (US20190015528A1) on the use of engineered symbionts to improve bee health. J.E.B. is the owner of Evolvomics LLC.
References
- 1.Bailes E. J., Ollerton J., Pattrick J. G. & Glover B. J. How can an understanding of plant–pollinator interactions contribute to global food security? Curr. Opin. Plant Biol. 26, 72–79 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Marshman J., Blay-Palmer A. & Landman K. Anthropocene Crisis: Climate Change, Pollinators, and Food Security. Environments 6, 22 (2019). [Google Scholar]
- 3.van der Sluijs J. P. & Vaage N. S. Pollinators and Global Food Security: the Need for Holistic Global Stewardship. Food Ethics 1, 75–91 (2016). [Google Scholar]
- 4.Genersch E. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 103, S10–S19 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Genersch E. Honey bee pathology: current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 87, 87–97 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Report on the national stakeholders conference on honey bee health. 9–68 (2013).
- 7.Kwong W. K. & Moran N. A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 14, 374–384 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymann K. & Moran N. A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 26, 97–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng H., Powell J. E., Steele M. I., Dietrich C. & Moran N. A. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl. Acad. Sci. U. S. A. 114, 4775–4780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raymann K., Shaffer Z. & Moran N. A. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol. 15, e2001861 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kešnerová L. et al. Disentangling metabolic functions of bacteria in the honey bee gut. PLoS Biol. 15, e2003467 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonilla-Rosso G. & Engel P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 43, 69–76 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Martinson V. G., Moy J. & Moran N. A. Establishment of characteristic gut bacteria during development of the honeybee worker. Appl. Environ. Microbiol. 78, 2830–2840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steele M. I., Motta E. V. S., Gattu T., Martinez D. & Moran N. A. The Gut Microbiota Protects Bees from Invasion by a Bacterial Pathogen. Microbiol. Spectr. 9, e0039421 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong W. K., Mancenido A. L. & Moran N. A. Immune system stimulation by the native gut microbiota of honey bees. R. Soc. Open Sci. 4, 170003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Q., Lariviere P. J., Powell J. E. & Moran N. A. Engineered gut symbiont inhibits microsporidian parasite and improves honey bee survival. Proc. Natl. Acad. Sci. 120, e2220922120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard S. P. et al. Engineered symbionts activate honey bee immunity and limit pathogens. Science 367, 573–576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lariviere P. J., Leonard S. P., Horak R. D., Powell J. E. & Barrick J. E. Honey bee functional genomics using symbiont-mediated RNAi. Nat. Protoc. 18, 902–928 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Lang H. et al. Engineered symbiotic bacteria interfering Nosema redox system inhibit microsporidia parasitism in honeybees. Nat. Commun. 14, 2778 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horak R. D., Leonard S. P. & Moran N. A. Symbionts shape host innate immunity in honeybees. Proc. Biol. Sci. 287, 20201184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell J. E., Leonard S. P., Kwong W. K., Engel P. & Moran N. A. Genome-wide screen identifies host colonization determinants in a bacterial gut symbiont. Proc. Natl. Acad. Sci. 113, 13887–13892 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard S. P. et al. Genetic engineering of bee gut microbiome bacteria with a toolkit for modular assembly of broad-host-range plasmids. ACS Synth. Biol. 7, 1279–1290 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell J. E., Carver Z., Leonard S. P. & Moran N. A. Field-Realistic Tylosin Exposure Impacts Honey Bee Microbiota and Pathogen Susceptibility, Which Is Ameliorated by Native Gut Probiotics. Microbiol. Spectr. 9, e0010321 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fels U., Gevaert K. & Van Damme P. Bacterial Genetic Engineering by Means of Recombineering for Reverse Genetics. Front. Microbiol. 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurushima J. & Tomita H. Advances of genetic engineering in streptococci and enterococci. Microbiol. Immunol. 66, 411–417 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Kormanec J. et al. Recent achievements in the generation of stable genome alterations/mutations in species of the genus Streptomyces. Appl. Microbiol. Biotechnol. 103, 5463–5482 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Sharan S. K., Thomason L. C., Kuznetsov S. G. & Court D. L. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Protoc. 4, 206–223 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Court D. L., Sawitzke J. A. & Thomason L. C. Genetic engineering using homologous recombination. Annu. Rev. Genet. 36, 361–388 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Kwong W. K. & Moran N. A. Cultivation and characterization of the gut symbionts of honey bees and bumble bees: Description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. Int. J. Syst. Evol. Microbiol. 63, 2008–2018 (2013). [DOI] [PubMed] [Google Scholar]
- 30.van Dam V. & Bos M. P. Generating knock-out and complementation strains of Neisseria meningitidis. in Neisseria meningitidis: Advanced Methods and Protocols (ed. Christodoulides M.) 55–72 (Humana Press, 2012). doi: 10.1007/978-1-61779-346-2_4. [DOI] [PubMed] [Google Scholar]
- 31.Aranda J. et al. Acinetobacter baumannii RecA protein in repair of DNA damage, antimicrobial resistance, general stress response, and virulence. J. Bacteriol. 193, 3740–3747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koomey M., Gotschlich E. C., Robbins K., Bergström S. & Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics 117, 391–398 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwong W. K., Engel P., Koch H. & Moran N. A. Genomics and host specialization of honey bee and bumble bee gut symbionts. Proc. Natl. Acad. Sci. 111, 11509–11514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzales M. F., Brooks T., Pukatzki S. U. & Provenzano D. Rapid protocol for preparation of electrocompetent Escherichia coli and Vibrio cholerae. J. Vis. Exp. JoVE 50684 (2013) doi: 10.3791/50684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tucker A. T. et al. Defining Gene-Phenotype Relationships in Acinetobacter baumannii through One-Step Chromosomal Gene Inactivation. mBio 5, e01313–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Leonard S. P., Powell J. E. & Moran N. A. Species divergence in gut-restricted bacteria of social bees. Proc. Natl. Acad. Sci. 119, e2115013119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elston K. M. et al. Engineering insects from the endosymbiont out. Trends Microbiol. 30, 79–96 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Kendra C. G., Keller C. M., Bruna R. E. & Pontes M. H. Conjugal DNA Transfer in Sodalis glossinidius, a Maternally Inherited Symbiont of Tsetse Flies. mSphere 5, 10.1128/msphere.00864-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S. et al. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 357, 1399–1402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damiani C. et al. Paternal transmission of symbiotic bacteria in malaria vectors. Curr. Biol. 18, R1087–R1088 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Whitten M. M. A. et al. Symbiont-mediated RNA interference in insects. Proc. Biol. Sci. 283, 20160042 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pontes M. H. & Dale C. Lambda red-mediated genetic modification of the insect endosymbiont Sodalis glossinidius. Appl. Environ. Microbiol. 77, 1918–1920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J. K. et al. Bacterial Cell Wall Synthesis Gene uppP Is Required for Burkholderia Colonization of the Stinkbug Gut. Appl. Environ. Microbiol. 79, 4879–4886 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt K. et al. Integration host factor regulates colonization factors in the bee gut symbiont Frischella perrara. eLife 12, e76182 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Aartsen J. J. & Rajakumar K. An optimized method for suicide vector-based allelic exchange in Klebsiella pneumoniae. J. Microbiol. Methods 86, 313–319 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Motta E. V. S., Powell J. E., Leonard S. P. & Moran N. A. Prospects for probiotics in social bees. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 377, 20210156 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chhun A. et al. Engineering a symbiont as a biosensor for the honey bee gut environment. 2023.02.02.526826 Preprint at 10.1101/2023.02.02.526826 (2023). [DOI] [Google Scholar]
- 48.Baba T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy K. C. λ Recombination and Recombineering. EcoSal Plus 7, 10.1128/ecosalplus.ESP0011-2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosberg J. A., Lajoie M. J. & Church G. M. Lambda Red Recombineering in Escherichia coli Occurs Through a Fully Single-Stranded Intermediate. Genetics 186, 791–799 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reyrat J. M., Pelicic V., Gicquel B. & Rappuoli R. Counterselectable markers: Untapped tools for bacterial genetics and pathogenesis. Infect. Immun. 66, 4011–4017 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benchling [Biology Software]. (2023).
- 53.Lee M. E., DeLoache W. C., Cervantes B. & Dueber J. E. A Highly Characterized Yeast Toolkit for Modular, Multipart Assembly. ACS Synth. Biol. 4, 975–986 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Wick R. R., Judd L. M., Gorrie C. L. & Holt K. E. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb. Genomics 3, e000132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen S., Zhou Y., Chen Y. & Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinforma. Oxf. Engl. 34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deatherage D. E. & Barrick J. E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 1151, 165–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stead C. M., Zhao J., Raetz C. R. H. & Trent M. S. Removal of the outer Kdo from Helicobacter pylori lipopolysaccharide and its impact on the bacterial surface. Mol. Microbiol. 78, 837–852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strack R. L. et al. A Rapidly Maturing Far-Red Derivative of DsRed-Express2 for Whole-Cell Labeling. Biochemistry 48, 8279–8281 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.