Abstract
Introduction
Penconazole (PEN) is a widely applied triazole fungicide. This study sought to define the efficacy of N-acetyl-l-cysteine (NAC) in mitigating PEN-triggered hepatorenal toxicity in rats.
Material and Methods
Twenty-eight adult male albino Wistar rats were assigned to four groups: a normal control (NC), a PEN group, a NAC group and a PEN+NAC group. Administration of PEN (50 mg/kg body weight (b.w.) every 2 days) and NAC (150 mg/kg b.w., daily) took place via oral gavage for 10 days.
Results
Effective amelioration by NAC of PEN-induced liver and kidney dysfunction was indicated by a significant reduction in the circulating liver and kidney markers (aspartate aminotransferase, alanine aminotransferase, urea and creatinine). Attenuation of PEN-induced oxidative stress and lipid peroxidation in liver and kidney tissues was evident in a significant reduction in malondialdehyde and enhanced total antioxidant capacity. Moreover, NAC significantly reduced the histopathological alterations and the expression of tumour necrosis factor α in liver and kidney tissue. Furthermore, NAC maintained the messenger RNA levels of nuclear factor erythroid 2-related factor 2 (Nrf2), haem oxygenase 1, and Kelch-like erythroid cell-derived protein 1 and prevented nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) protein upregulation caused by PEN.
Conclusion
N-acetyl-1-cysteine protected against PEN-induced hepatorenal oxidative damage and inflammatory response via activation of Nrf2 and inhibition of NF-κB pathways.
Keywords: penconazole, Nrf2, HO-1, Keap1, NF-κB
Introduction
The widespread utilisation of fungicides in the agricultural sector and their harmful effects on environmental safety and non-target organisms’ health emphasise the need to investigate their hazardous impact (31). Penconazole (1-(2-(2,4-dichlorophenyl)-pentyl)-1H-1,2,4-triazole – PEN) is a typical systemic triazole fungicide intensively used in the agricultural sector to control powdery mildew on fruits and vegetables (36). Residues of PEN remain in soil and wastewater and on crops, and consequently impact human and animal health adversely (1, 9, 25, 26, 30). Detection of PEN and its major metabolic derivatives (PEN-OH and PEN-COOH) in human urine was previously reported (25). Indeed, PEN exposure has been associated with various toxicological effects including reproductive and endocrine disorders and neurotoxicity (8–11, 14, 26). This compound is metabolised in the liver and its metabolites are predominately released by the kidney, which may induce hepatorenal alterations at the cellular level (10, 24).
Penconazole has been reported to significantly affect the antioxidant system and disrupt the redox status of various tissues in different species (9, 19, 26). In addition, PEN was found to disrupt the metabolic functions of the liver (24). Despite the high risk of prolonged exposure to PEN, studies on its hepatorenal toxicity in mammals are limited.
Given the crucial role of oxidative stress in the mechanism of PEN-induced toxic effects through generation of reactive oxygen species (ROS) and alteration in the redox balance, the potential usefulness of antioxidants to ameliorate the organ damage associated with PEN intoxication has been explored, and studies have reported the beneficial effect of compounds with antioxidant activity when used for this purpose (10, 26).
Oxidative stress is propagated when an imbalance between the pro- and antioxidant systems occurs. Accumulation of free radicals with reduction of the total antioxidant capacity (TAC) results in oxidative damage to the major cellular biomolecules (32). Lipid peroxidation is one of the typical outcomes of oxidative stress, when ROS attack the polyunsaturated lipid components of biological membranes and make highly bioactive by-products. These by-products, such as malondialdehyde (MDA), contribute to a series of oxidative changes resulting in various physiopathological effects related to cell aging and diseases (16). Oxidative stress affects various redox-sensitive transcription factors and their signalling pathways (32). Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcription modular protein that controls both the basal and stress-inducible activation of many cytoprotective genes including glutathione (GSH), thioredoxin (TXN) and haem oxygenase 1 (HO-1). Through this control, Nrf2 acts in different cellular processes, such as the cellular response to xenobiotic and oxidative stress and the maintenance of redox homeostasis (6, 27). Another transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), is involved in the control of inflammatory response through regulation of a vast array of target genes linked to cytokine release and ROS generation (23, 28). Therefore, targeting Nrf2 and NF-B may be a beneficial therapeutic strategy for management of hepatorenal toxicity.
N-acetyl-l-cysteine (NAC), the acetylated derivative of the amino acid L-cysteine, is widely accepted clinically as an antioxidant therapy with good reported safety. The cysteine derivative serves as a donor of L-cysteine, which is the building block and the limiting substrate for de novo synthesis of GSH (22). A large body of evidence has shown that NAC is potentially useful in treatment of various liver and kidney injuries (2, 7, 13, 17, 20, 35).
Considering the safety of NAC as a drug and its potential to interfere with an array of biochemical pathways connecting oxidative stress to inflammatory response (20), the goal of the current study was to investigate the protective effects of NAC against hepatorenal toxicity induced by PEN intoxication in an experimental rat model, and to determine whether those effects were associated with regulation of the Nrf2 and NF-κB pathways. The study comprises biochemical, immunohistochemical and gene expression evaluations, as well as histopathological assessment.
Material and Methods
Chemicals and reagents
Penconazole was bought from Advanced Agrochemicals & Veterinary Products Industrial Co. (Chemvet, Amman, Jordan). N-acetyl-1-cysteine (catalogue no. 66246-88-6) was bought from Sigma-Aldrich (Waltham, MA, USA) and prepared freshly in distilled water before use. All kits for biochemical analyses were bought from Biodiagnostic Co. (Cairo, Egypt). All other reagents were of high analytical grade.
Experimental animals and study protocol
Twenty-eight adult male albino Wistar rats weighing 180 ± 20 g were obtained from the Faculty of Veterinary Medicine, Cairo University, Egypt. All animals were kept at 25 ± 2°C in a 12/12 h light/dark cycle and allowed free access to water and commercial pellet feed. The protocol used for animal experimentation was approved by the Cairo University Institutional Animal Care and Use Committee (approval code: Vet Cu 2009 2022487). After a week of preadaptation to feeding, the rats were allocated randomly into the following four groups of 7:
Group I (Normal Control group; NC), which received 1 mL of distilled water by oral gavage for 10 days;
Group II (PEN group), which was administered 50 mg/kg body weight (b.w.) of PEN (equivalent to 1/40 of the median lethal dose) by oral gavage every two days for 10 days (13);
Group III (PEN+NAC group), which was administered daily doses of 150 mg/kg b.w. of NAC and 50 mg/kg b.w. of PEN by oral gavage every two days for 10 days;
Group IV (NAC group), which was administered 150 mg/kg b.w. of NAC by oral gavage daily for 10 days. The dosage of NAC was selected based on previous reports of the hepato- and reno-protective doses (17).
Twenty-four hours after the end of the experiment, all rats were euthanised via cervical dislocation followed by decapitation, this method having been adopted to avoid the effects of euthanasia chemicals on the tissue samples. Serum samples were taken, and liver and kidney tissue samples were harvested, rinsed in normal saline, blotted, and stored at –80°C for subsequent analyses. For histological assessments, tissues samples were washed and fixed in neutral buffered formalin for at least 48 h. Thereafter, liver and kidney homogenates were prepared in cold phosphate-buffered saline for measurement of the oxidative stress biomarkers they contained.
Serum biochemical parameters
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, and creatinine levels were evaluated using a spectrophotometer (UNICO Instruments, Dayton, NJ, USA) and commercial liver function, kidney function, and Berthelot method urea measurement kits (Biodiagnostic Co., Cairo, Egypt) according to the instructions provided by the manufacturer.
Tissue oxidative stress parameters
Products of lipid peroxidation were estimated by determining MDA content in the liver and kidney in the form of thiobarbituric acid reactive substances according to the method described by Tanideh et al. (34) with slight modifications. Briefly, a reaction mixture containing 200 μL of tissue homogenate, 200 μL of sodium dodecyl sulphate (8.1%), 1,500 μL of acetic acid (20%), 1,500 μL of thiobarbituric acid (0.8%), 0.0034 mM of butylated hydroxytoluene in ethanol, and 540 μL of deionised distilled water was boiled for 1 h and centrifuged at 2,000 rpm for 10 min. The upper layer, which was reddish-pink in colour, had its absorbance measured spectrophotometrically at 532 nm. Tetramethoxypropane was used as the standard. The MDA concentration was expressed in mmol/mg protein.
The total antioxidant capacity (TAC) was spectrophotometrically assessed in tissue homogenates according to the method described by Bartosz (4) using the same kits used for measuring serum biochemical parameters, following the manufacturer’s protocol.
Real-time quantitative polymerase chain reaction (qPCR)
Liver and kidney total RNA extraction was performed using an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Residual DNA digestion was carried out using a DNAase kit (Invitrogen, Carlsbad, CA, USA), then RNA integrity and concentration were assessed by a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Complementary DNA was synthesised using a SuperScript IV VILO reverse transcriptase kit (Invitrogen). Relative messenger RNA (mRNA) expression levels of Nrf2, Kelch-like erythroid cell-derived protein 1 (Keap-1), and HO-1 were quantified by qPCR using specific primers. The PCR primer sets had the following sequences: Nrf2 sense primer 5’-CACATCCAGACAGAC ACCAGT-3’ and antisense primer 5’-CTACAAATGGGAATG TCTCTGC-3’ (GenBank accession No. XM_006234398.3), Keapl sense primer 5’-AACTCGGCAGAATGTTACTACCC-3’ and antisense primer 5’-CTACGAAAGTCCAGGTCTCTG TCTC-3’ (GenBank accession No. NM_017000.3), HO-1 sense primer 5’-ACAGGGTGACAGAAGAGGCTAA-3’ and antisense primer 5’-CTGTGAGGGACTCTGGTCTTTG-3’ (GenBank accession No. NM_012580.2), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sense primer 5’-ACCACAGTCCATGCCATCAC-3’ and antisense primer 5’-TCCACCACCCTGTTGCTGTA-3’ (GenBank accession No. NC_005103.4). Luminaris Color HiGreen Low ROX SYBR Green Master mix (Thermo Scientific) was used for qPCR amplification. The specificity of the amplified PCR products was confirmed through melting-curve analysis and agarose gel electrophoresis. Each cDNA sample was analysed in triplicate with a minus template negative control. The relative mRNA expression of the target genes was calculated as fold change of the normal control after normalisation to a GAPDH reference transcript, using a 2-ΔΔCT method as described previously (26).
Histopathological examinations of liver and kidney
Liver and kidney tissue specimens were preserved in 10% neutral buffered formalin and processed by a conventional method using ascending grades of alcohol and xylene, then embedded in paraffin wax, microsectioned at 4.5μm, stained with haematoxylin and eosin, and examined under a light microscope (Olympus, Tokyo, Japan) to record any histological alterations in different groups (3).
Seven microscopic fields per five sections representing five rats per group were assessed to record the degree of severity of the pathological lesions in both the liver and kidneys using the technique established by Hassanen et al. (18). The parameters used to assess hepatorenal alterations were cellular degeneration and necrosis, congestion, haemorrhaging, intracellular eosinophilic bodies (Mallory bodies, renal droplets and cast), and inflammatory cell infiltrations. The microscopic lesions were graded and scored as slight, mild, moderate and severe, and on a scale from 0 to 4. Diffuse lesions were graded as 0 for normal histology, 1 for < 25% of tissue affected, 2 for 25–50%, 3 for 50–75%, or 4 for >75% of tissue affected. Focal lesions were graded as 0 for no foci apparent, 1 for <3 foci, 2 for 3–6 foci, 3 for 7–12 foci, or 4 for >12 foci apparent (18).
Immunohistochemical studies and morphometric analysis
TNF-α and NF-κB (markers for inflammation) were detected on paraffin-embedded formalin-fixed liver and kidney tissue sections. Briefly, slides were incubated with primary antibodies for different immune markers (Abcam, Cambridge, UK) at 1 :200 dilutions, then incubated with Peroxidase Block (Sakura Finetek, Alphen aan den Rijn, the Netherlands) and the reagent required for measuring the antigen antibody complex (Power-Stain 1.0 Poly HRP DAP Kit, Sakura Finetek). The sections were treated with diaminobenzidine chromogen substrate for 10 min and counterstained with haematoxylin.
Statistical analysis
The Shapiro–Wilk test for normality was performed, and its results indicated normal distribution of the data. Therefore, we carried out the statistical analysis of the obtained results using one-way analysis of variance followed by Duncan’s multiple-range test to compare the means of all data between groups, where a P-value < 0.05 was considered significant. Spearman’s rank correlation was applied to recognise the relationships between TAC and MDA content in both hepatic and renal tissue. Data were analysed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). All data were expressed as means ± standard error of the mean (SEM).
Results
Effects of NAC on liver and kidney functions against PEN-induced hepatorenal toxicity
Liver function was assessed through the determination of serum AST and ALT concentrations. Table 1 reveals that PEN significantly elevated AST and ALT levels compared to those in control group samples (P < 0.05). Co-treatment with NAC (150 mg/kg b.w.) significantly reduced the levels of these hepatocellular enzymes in the sera (P < 0.05) compared with those of the PEN group (Table 1). The group exposed to the fungicide without NAC showed significantly higher levels of urea and creatinine in serum compared to the control group (P < 0.05) and the PEN+NAC–treated group (P < 0.05) (Table 1).
Table 1. Effects of N-acetyl-1-cysteine (NAC) on liver and kidney function tests in adult male albino Wistar rats exposed to penconazole (PEN).
| Group | AST (U/L) | ALT (U/L) | Creatinine (mg/dL) | Urea (mg/dL) |
|---|---|---|---|---|
| NC | 69.66 ± 1.16# | 24.70 ± 0.82# | 0.72 ± 0.01# | 25.18 ± 0.56 |
| PEN | 114.05 ± 4.79* | 51.35 ± 1.46* | 1.62 ± 0.02* | 57.36 ±0.60* |
| PEN+NAC | 86.20 ± 2.15*# | 38.24 ± 1.55*# | 0.85 ± 0.01# | 38.51 ± 0.45*# |
| NAC | 71.23 ± 1.52# | 27.12 ± 0.67# | 0.68 ± 0.01# | 27.94 ± 0.35# |
Data are presented as mean ± standard error of the mean (n = 6)
significantly different to control group
significantly different to PEN group; AST – aspartate aminotransferase; ALT – alanine aminotransferase; NC – normal control group
Effects of NAC against PEN-induced hepatorenal toxicity – effects on MDA and TAC in the liver and kidneys
As shown in Fig. 1A, marked increases in MDA contents were detected in both the liver and kidneys of the PEN group as compared to these tissues of the NC group. Supplementation with NAC at a dose of 150 mg/kg b.w. significantly suppressed the by-production of MDA in the liver and kidney tissues from PEN-induced lipid peroxidation. Total antioxidant content was found to be significantly lower in the liver and kidney tissues of rats administered PEN than in these tissues of NC rats (P < 0.05). Acetylcysteine also significantly prevented the PEN-induced reduction in TAC in the liver and kidneys (P < 0.05) (Fig. 1B).
Fig. 1.
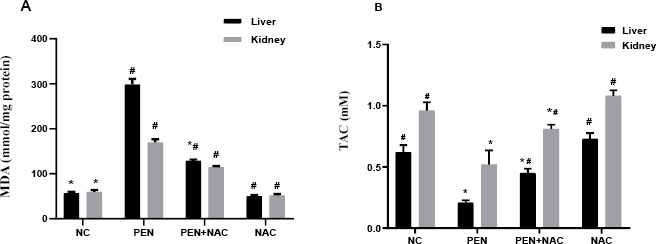
Effects of NAC on (A) malondialdehyde and (B) TAC in the liver and kidneys of adult male albino Wistar PEN-exposed rats. Data are presented as mean ± standard error of the mean (whiskers) (n = 6)
# – significantly different to NC group; * – significantly different to PEN group; NC – normal control; PEN – penconazole; NAC – N-acetyl-1-cysteine; TAC – total antioxidant capacity
There was a significant negative correlation between MDA content and TAC in both liver and kidney tissue. The Spearman’s rank rs values were -0.972 and -0755 (P < 0.01) respectively in liver and kidney tissue.
Effects of NAC on Nrf2, Keap1 and HO-1 mRNA expression in PEN-intoxicated rats
As shown in Figs 2 and 3, both the hepatic and renal mRNA expression of Nrf2 and HO-1 showed significant downregulation in the PEN-intoxicated group compared to NC group expression levels. On the other hand, PEN significantly increased Keap1 mRNA expression in liver and kidney tissues (Figs 2B and 3B). Administration of NAC concurrently with PEN effectively reversed the effects on these genes, as evident in the significantly elevated mRNA levels of Nrf2 and HO-1 and the downregulated expression of Keap1 in the liver and kidneys of the PEN+NAC group compared to these indicators’ levels in the PEN group.
Fig. 2.
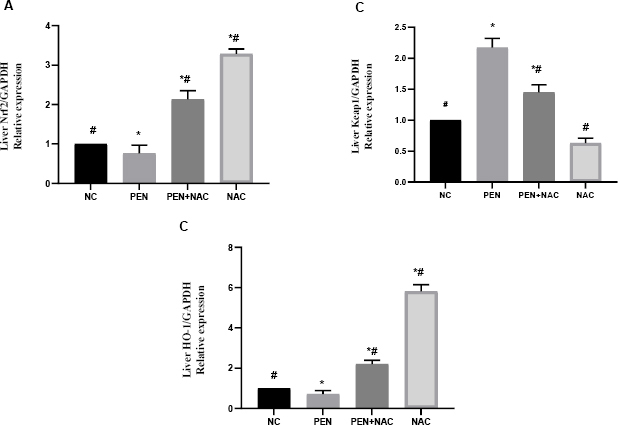
Effect of N-acetyl-1-cysteine (NAC) in adult male albino Wistar rats against penconazole (PEN)-induced alterations on hepatic messenger RNA expression of nuclear factor erythroid 2-related factor 2 (Nrf2) (A), Kelch-like erythroid cell-derived protein 1 (Keap1) (B) and haem oxygenase 1 (HO-1) (C). Data are presented as mean ± standard error of the mean (whiskers) (n = 6)
GAPDH – glyceraldehyde 3-phosphate dehydrogenase; # – significantly different to normal control (NC) group; * – significantly different to PEN group
Fig. 3.
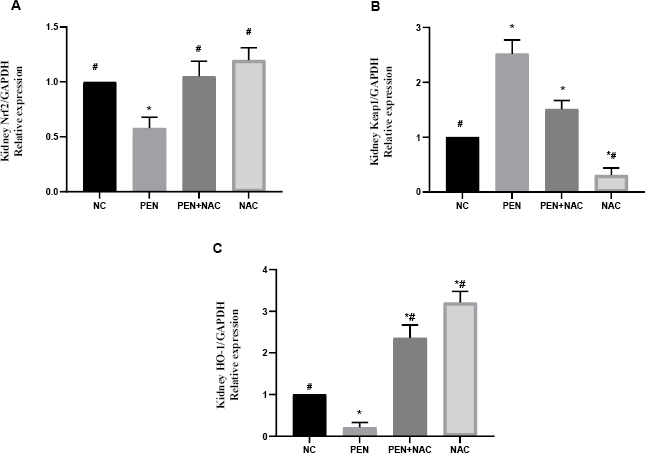
Effect of N-acetyl-1-cysteine (NAC) in adult male albino Wistar rats against penconazole (PEN)-induced alterations on renal mRNA expression of nuclear factor erythroid 2-related factor 2 (Nrf2) (A), Kelch-like erythroid cell-derived protein 1 (Keap1) (B) and haem oxygenase 1 (HO-1) (C). Data are presented as mean ± standard error of the mean (whiskers) (n = 6)
GAPDH – glyceraldehyde 3-phosphate dehydrogenase; # – significantly different to normal control (NC) group; * – significantly different to PEN group
Liver histopathology
Liver tissue sections obtained from rats in the NC group and from those which had received NAC showed normal histological architectures (Fig. 4A). On the other hand, liver sections obtained from PEN-receiving rats showed extensive severe histopathological changes. Some hepatocytes had granular and vacuolar swelling, while others were necrotic and apoptotic (Fig. 4B). Focal areas of hepatocellular coagulative necrosis were noted and these were infiltrated by mononuclear inflammatory cells (Fig. 4C). The necrotic hepatocytes appeared swollen with eosinophilic cytoplasm and different necrobiotic changes. Fatty changes were observed in most sections and characterised by swelling of cells with large clear fat globules in the cytoplasm and translocation of the nucleus to the periphery (Fig. 4D). Large focal to coalescent areas of hepatic haemorrhaging were noticed in some sections (Fig. 4E). The portal triad showed congestion, oedema, fibroplasia and severe mononuclear inflammatory cell infiltration (Fig. 4F). The bile ducts were enlarged, and the ducts’ epithelial linings were hyperplastic. The liver sections of the PEN+NAC group showed neither necrosis nor inflammation (Fig. 4G). Mild hepatocellular degenerations were observed in a few sections and the portal area presented infiltration by low numbers of inflammatory cells (Fig. 4H).
Fig. 4.
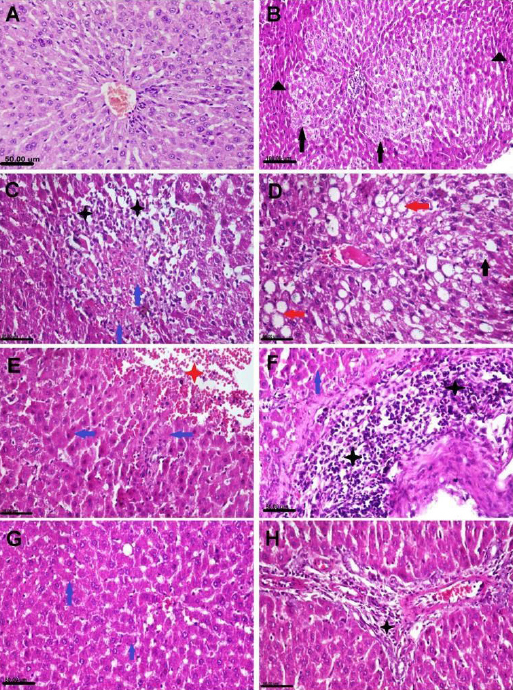
Photomicrograph of adult male albino Wistar rat liver tissue sections stained with haematoxylin and eosin representing the control group with normal histological structure (A), the penconazole (PEN)-receiving group showing hepatocellular vacuolisation (black arrows), necrosis (blue arrows), atrophy (black triangles), macrovesicular steatosis (red arrows), haemorrhaging (red star) and inflammatory cells infiltration (black stars) (B–F) and the PEN+N-acetyl-1-cysteine–receiving group showing sparse cell necrosis (blue arrows) and slight portal inflammation (black star) (G and H)
Kidney histopathology
Kidney tissue sections obtained from rats in the NC group and those which had received NAC likewise showed normal histological structures (Fig. 5A). In contrast, kidney sections obtained from rats in the PEN-receiving group showed severe pathological alterations. Some glomeruli were congested, degenerated and atrophied. Most renal tubules had degeneration and necrosis in the epithelial lining associated with either intracellular or intraluminal renal cast (Fig. 5B and 5C). Some sections displayed vascular congestion with haemorrhaging between renal tubules (Fig. 5D). Renal capsules presented a moderate degree of fibrosis in some sections (Fig. 5E). The kidney sections of the PEN+NAC group exhibited normal histological architecture (Fig. 5F).
Fig. 5.
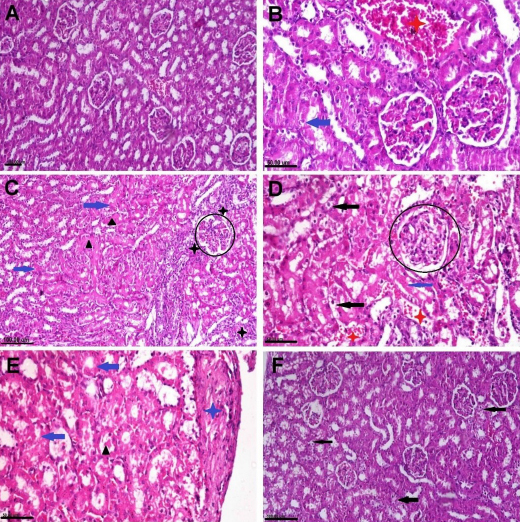
Photomicrograph of adult male albino Wistar rat kidney tissue sections stained with haematoxylin and eosin representing the control group with normal histological structure (A), the penconazole (PEN)-receiving group showing cytoplasmic vacuolisation (black arrows), necrosis (blue arrows) in the renal epithelium, intraluminal hyaline cast and droplets (black triangles), vascular congestion and haemorrhaging (red stars), inflammatory cells infiltration (black stars), capsular fibrosis (blue star) and glomerular degeneration (black circles) (B–E) and the PEN+N-acetyl-1-cysteine–receiving group showing mild degenerative changes in the tubular epithelium and some glomeruli (black arrows) (F)
The results of the microscopic lesion scoring are summarised in Table 2. The pathological parameter’s scores of the liver and kidney sections of PEN-receiving rats exceeded those of the other groups. The lesion scores were significantly lower in NAC co-treated rats than in PEN group rats but were nevertheless still higher than in NC group animals. There were no significant differences in lesion scores between the control group and the group receiving only NAC.
Table 2. The microscopic lesion scoring in adult male albino Wistar rats unexposed to penconazole (PEN), exposed to it, and exposed to it with N-acetyl-1 cysteine (NAC) coadministration.
| NC | NAC | PEN | PEN+NAC | |
|---|---|---|---|---|
| Hepatic lesion scoring | ||||
| HCD | 0 a | 1 b | 4 c | 2 d |
| HCN | 0 a | 1 b | 2 c | 1 b |
| ICB | 0 a | 0 a | 4 b | 1 c |
| Congestion | 0 a | 1 b | 3 c | 1 b |
| Haemorrhage | 0 a | 0 a | 2 b | 0 a |
| Inflammation | 0 a | 0 a | 2 b | 1 c |
| Renal lesion scoring | ||||
| RTD | 0 a | 1 b | 4 c | 2 d |
| RTN | 0 a | 1 b | 4 c | 2 d |
| ICB | 0 a | 0 a | 4 b | 1 c |
| Congestion | 0 a | 1 b | 4 c | 2 d |
| Haemorrhage | 0 a | 0 a | 2 b | 0 a |
| Inflammation | 0 a | 0 a | 4 b | 1 c |
Values are presented as medians from five sections representing five rats/group. Values with different letters in the same column are significantly different at P ≤ 0.05
NC – normal control; HCD – hepatocellular degeneration; HCN – hepatocellular necrosis; ICB–intracellular eosinophilic bodies; RTD – renal tubular degeneration; RTN – renal tubular necrosis
Immunohistochemical assessments
Strong TNF-α and NF-κB protein expression along with high numbers of NF-κB immunostained nuclei were observed in the liver and kidney sections of the PEN group. The expression of TNF-α was evident in the cytoplasm of degenerated hepatocytes, epithelial lining bile ducts, epithelial lining renal tubules, and within the inflammatory cells, while NF-κB expression was observed in both the nuclei and cytoplasm of degenerated cells. The group treated with NAC developed mild to moderate immunostaining reactions for these immune markers (Figs 6 and 7).
Fig. 6.
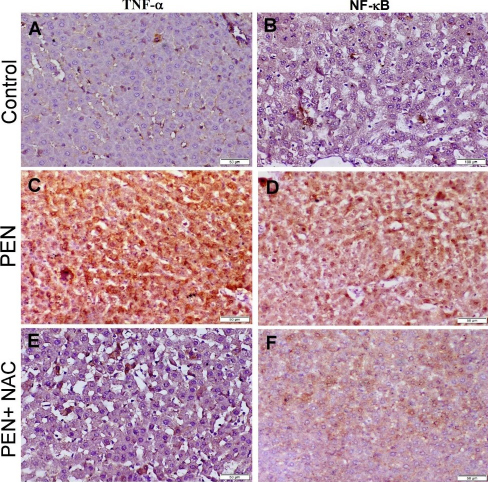
Photomicrograph of adult male albino Wistar rat liver tissue sections stained by immunoperoxidase to localise TNF-α and NF-κB. The control group with normal TNF-α and NF-κB cytoplasmic expression (A and B), PEN group showing strong positive expression of both immune markers (C and D) and the PEN+NAC–receiving group showing weak positive expression of TNF-α and NF-κB (E and F)
Fig. 7.
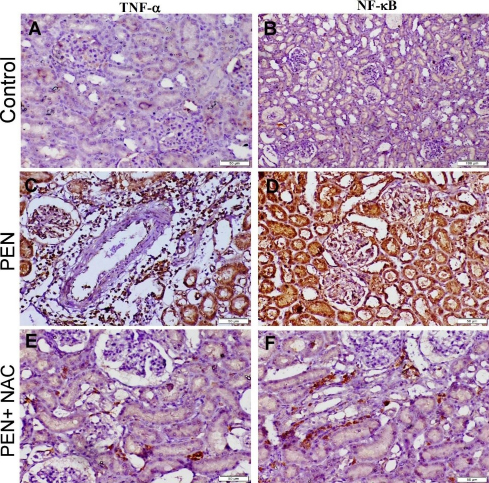
Photomicrograph of adult male albino Wistar rat kidney tissue sections stained by immunoperoxidase to localise TNF-α and NF-κB. The control group with normal TNF-α and NF-κB cytoplasmic expression (A and B), PEN group showing strong positive expression of both immune markers (C and D) and the PEN+NAC–receiving group showing weak positive expression of TNF-α and NF-κB (E and F)
Discussion
Oxidative damage mediated by ROS is a manifestation of PEN-induced toxicity (10, 26). Therefore, intensive efforts have been directed to learning the potential of antioxidant therapies against PEN intoxication. The presented approach took advantage of the safety, affordability and availability of NAC as a well-established anti-oxidation and anti-inflammation agent against PEN-induced hepatorenal injuries.
Biotransformation of PEN in the liver takes place in three phases (35). Phase I detoxification of PEN involves cleavage or oxidation of the triazole ring and oxidation of the alkane chain forming the respective carboxylic (PEN-COOH), monohydroxylic and dihydroxylic derivatives (PEN-OH). Phase II involves secondary metabolic reactions of the carboxylic acid derivatives forming an α-keto carboxylic derivative and 3- or 4-keto derivatives and finally conjugation of the resulting alkanol metabolites with glucuronic acid. In phase III, these metabolic derivatives are exported from the cells through an ATP-binding cassette transporter/ multidrug-resistance protein–dependent mechanism.
In the present study, the hepatotoxic effect of PEN was evidenced by the significant elevation in serum AST and ALT levels. One of the potential mechanisms reported for PEN-induced toxicity is the disruption of sterol biosynthesis through inhibition of lanosterol 14α-demethylase (CYP51), leading to progressive liver injury. Penconazole blocks the CYP51 active site by forming water-bridging interactions or van der Waals bonds with its hydrophobic amino acid pocket, or by binding with its haem moiety (19). These effects cause hepatocyte damage that eventually increases serum liver enzyme levels because of membrane integrity loss and cellular leakage (11). In addition, the levels of serum creatinine and urea, the critical indices for assessing renal function, were significantly increased in PEN-intoxicated rats when compared to those of the control counterparts. Increased creatinine and urea levels were sensitive indicators for kidney dysfunction and correlated particularly with the reduction of glomerular filtration rate (5). These findings are in line with the report of Chaâbane et al. (10), who demonstrated that PEN disrupted the functional integrity of the kidney and increased the membrane permeability, which might be due to oxidative damage.
Accumulated evidence suggested the involvement of oxidative stress and lipid peroxidation in PEN-induced cellular damage (10, 11, 26). In the present study, the PEN group exhibited a significant elevation in both liver and kidney MDA levels. These changes could be attributed to the excessive ROS generated by PEN, which deplete the endogenous antioxidant pool, resulting in oxidative stress in the liver and kidney cells. The oxidant/antioxidant status was further evaluated by measuring the TAC, which showed a significant reduction in the PEN group. This finding was consistent with those of previous studies, where PEN induced a significant increase in MDA and ROS levels and a marked reduction in antioxidant enzyme activity in the liver and kidneys of rats (10, 11). Moreover, a recent study by Jiang et al. (21) showed that PEN treatment in zebrafish downregulated mRNA expression of cat and sod1 genes encoding CAT and SOD enzymes, respectively, as a compensatory effect to their enzyme inhibition, which was associated with an irreversible ROS overproduction. Overall, these reports provided evidence that that oxidative stress contributed to the PEN-triggered hepatorenal toxicity observed in our study.
Furthermore, these biochemical findings reflecting PEN-induced hepatorenal dysfunction were verified through histopathological observations. In the current study, the PEN-receiving group showed extensive hepatic and renal histopathological alterations including cellular degeneration and necrosis accompanied by interstitial inflammation indicated by inflammatory leucocyte infiltration. These injuries could be due to the accumulation of ROS and lipid peroxidation in both hepatic and renal tissues of PEN-administered rats. It has been suggested that because of its lipophilic nature, PEN could easily penetrate the cellular membrane to reach the mitochondria causing mitochondrial dysfunction with excessive ROS production (9). Similar histopathological findings have been reported previously (10, 11).
Interestingly, the present study demonstrated that NAC administration effectively ameliorated all the adverse impacts on the liver and kidneys of PEN exposure. The obtained findings showed that NAC significantly preserved liver and kidney functions, inhibited lipid peroxidation, maintained TAC and limited histopathological alterations. Several lines of evidence demonstrated that NAC was a potent antioxidant and anti-inflammatory agent and exerted a potential therapeutic effect in oxidative stress-related disorders. N-acetyl-1-cysteine has been noted for its boosting role for endogenous GSH, the key determinant of redox signalling. Glutathione acts as scavenger for intracellular reactive species and is an essential molecule for the regeneration of other antioxidative agents (15). In addition, NAC exerts GSH-independent antioxidant properties mediated through promoting detoxification and disulphide bond reduction (12). A direct ROS-scavenging activity can also be demonstrated by NAC through a direct reaction of its free thiol group with free radicals (37). Moreover, as a cysteine (Cys) pro-drug, NAC slowly delivers Cys to cells, which modestly elevates endogenous H2S and other sulphane sulphur species. A recent study proposed Cys pro-drug activity as a new mechanism of action for NAC, mediating the cytoprotective activities which could not be explained by the mechanisms previously attributed to it: the provision of protection by the sustained production of Cys-derived H2S as protection independent of GSH replenishment (29).
To further understand the molecular mechanism including the signalling pathway that contributes to the protective effects of NAC against PEN-induced hepatorenal injuries, the relative expression levels of genes involved in the Nrf2 signalling pathway were quantified. Nuclear factor erythroid 2-related factor 2 is an inducible transcription factor involved in the regulation of redox balance through targeting multiple stress response proteins and detoxifying enzymes. It also contributes either directly or indirectly to the regulation of a battery of genes involved in inflammation, apoptosis, cell growth and differentiation, among other processes (6). The transactivation of Nrf2 is negatively regulated by its intracellular inhibitor, Keap1, via cytoplasmic ubiquitination and degradation of Nrf2. Upon activation, the Keap1 –Nrf2 interaction is resolved and Nrf2 translocates freely to the nucleus, heterodimerises, and recognises the antioxidant response element in the regulatory region of Nrf2 downstream target genes (6, 27). Mounting evidence reveals that the Keap1/Nrf2 pathway mediates various cytoprotective responses to counteract oxidative stress and inflammation caused by various oxidative and chemical insults (23, 28). One of the Nrf2 downstream genes is HO-1, an inducible enzyme known for its pivotal role in maintaining antioxidant/oxidant homeostasis in various tissues (6).
Our findings confirm that PEN intoxication triggered oxidative stress by modulating the Keap1/Nrf2 pathway. This was evidenced by the downregulated mRNA expression of Nrf2 and its downstream gene HO-1 and the upregulated mRNA expression of Keap1 in both liver and kidney tissue (Figs 2 and 3). A large body of evidence indicates the role of Nrf2 activation in mediating the hepatoprotective and renoprotective effects of NAC (7, 28, 36). The present study accorded with this evidence, demonstrating that treatment with NAC also contributed to the activation of the Nrf2 pathway. As compared to their levels in control rats, both Nrf2 and HO-1 mRNA levels were significantly increased in rats exposed to PEN+NAC simultaneously, while Keap1 mRNA expression was significantly reduced. This finding was further verified by the results of TAC and MDA level determination in the PEN+NAC group, which together indicated that upregulation of Nrf2/Keap1 may be the mechanism by which NAC restores the redox status and decreases the lipid peroxidation induced by PEN.
Nuclear factor kappa-light-chain-enhancer of activated B cells is a redox-sensitive transcription factor contributing to immune/inflammatory responses induced by many stimuli (23). Activation of the NF-κB signalling pathway can prompt tissue inflammation via activation of several proinflammatory cytokines including TNF-α (33). To clarify whether PEN induces a hepatic or renal inflammatory response via the NF-κB cascade, the protein expression of NF-κB and its downstream target protein TNF-α were determined. The obtained results revealed that in the PEN-exposed group samples, expression of NF-κB was significantly upregulated in the liver and kidneys. In addition, TNF-α protein expression was also upregulated by PEN intoxication. These data implied the involvement of the NF-κB pathway in the inflammatory response induced by PEN. Concurrent administration of NAC attenuated the upregulation of NF-κB and TNF-α. These results are similar to those of a previous report in which NAC was demonstrated to exert its anti-inflammatory effect through the suppression of NF-κB (28).
Noteworthily, negative crosstalk between Nrf2 and NF-κB was established (23). In this context, pharmacological activation of Nrf2 reduces the inflammatory response via stimulation of antioxidants and inhibition of NF-κB. Conversely, Nrf2 downregulation is associated with NF-κB activation with subsequent stimulation of the pro-inflammatory cytokine TNF-α. This suggests that Nrf2 downregulation induced by PEN may inflame the liver and kidneys via NF-κB activation. Our data provide the first convincing evidence that PEN inhibits the Nrf2 antioxidant pathway and causes ROS-driven upregulation of the NF-κB pathway.
In conclusion, this study substantiates the protective potential of NAC against PEN-induced hepatorenal injuries. The protective effects of NAC are mediated, at least in part, through its antioxidant and anti-inflammatory mechanisms. Based on the obtained results, NAC prevented hepatorenal oxidative damage and inflammation in PEN-exposed rats through a mechanism involving crosstalk between the Nrf2/HO-1 and NF-κB pathways. This study supports the notion that control of the Nrf2 pathway through the enhancement of endogenous antioxidants by the hepatorenal protective agent NAC is a therapeutic strategy against PEN-associated hepatorenal toxicity.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work.
Footnotes
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This study was supported by the Deanship of Scientific Research at King Khalid University through the large group Research Project under grant number RGP2/93/44.
Animal Rights Statement: The authors declare that all animal experiments were performed according to the Institutional Animal Care and Use Committee of Cairo University’s laws and regulations regarding the care and use of laboratory animals (Vet Cu 2009 2022487).
Contributor Information
Hanan A. Ogaly, Email: ohanan@kku.edu.sa.
Marwa A. Ibrahim, Email: marwaibrahim@cu.edu.eg.
References
- 1.Aksakal F.I., Ciltas A. Developmental toxicity of penconazole in Zebrfish (Danio rerio) embryos Chemosphere. 2018;200:8. doi: 10.1016/j.chemosphere.2018.02.094. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 2.Badr A.M., Al-Kharashi L.A., Attia H., Alshehri S., Alajami H.N., Ali R.A., Mahran Y.F. TLR4/Inflammasomes Cross-Talk and Pyroptosis Contribute to N-Acetyl Cysteine and Chlorogenic Acid Protection against Cisplatin-Induced Nephrotoxicity Pharmaceuticals. 2023;16:337. doi: 10.3390/ph16030337. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancroft J., Stevens A., Turner D. Theory and Practice of Histological Techniques. Fourth Edition Vol. 20 Churchill Living Stone; New York: 1996. : , , , , . [Google Scholar]
- 4.Bartosz G. Total antioxidant capacity Advances in Clinical Chemistry. 2003;37:219. doi: 10.1016/S0065-2423(03)37010-6. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 5.Basile D.P, Yoder M.C. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury Cardiovasc Hematol Disord Drug Targets. 2014;14:3. doi: 10.2174/1871529x1401140724093505. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress Biochim Biophys Acta (BBA)-Molecular Cell Res. 2018;1865:721. doi: 10.1016/j.bbamcr.2018.02.010. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 7.Cai Z., Lou Q., Wang F., Li E., Sun J., Fang H., Xi J., Ju L. N-acetylcysteine protects against liver injure induced by carbon tetrachloride via activation of the Nrf2/HO-1 pathway Int J Clin Exp Pathol. 2015;8:8655. : . , , –. . [PMC free article] [PubMed] [Google Scholar]
- 8.Chaâbane M., Elwej A., Ghorbel I., Chelly S., Mnif H., Boudawara T., Chaabouni S.E., Zeghal N., Soudani N. Penconazole alters redox status, cholinergic function and lung’s histoarchitecture of adult rats: Reversal effect of vitamin E Biomed Pharmacother. 2018;102:645. doi: 10.1016/j.biopha.2018.03.113. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 9.Chaâbane M., Ghorbel I., Elwej A., Mnif H., Boudawara T., Chaâbouni S.E., Zeghal N., Soudani N. Penconazole alters redox status, cholinergic function, and membrane-bound ATPases in the cerebrum and cerebellum of adult rats Hum Exp Toxicol. 2017;36:854. doi: 10.1177/0960327116672911. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 10.Chaâbane M., Koubaa M., Soudani N., Elwej A., Grati M., Jamoussi K., Boudawara T., Ellouze Chaabouni S., Zeghal N. Nitraria retusa fruit prevents penconazole-induced kidney injury in adult rats through modulation of oxidative stress and histopathological changes Pharm Biol. 2017;55:1061. doi: 10.1080/13880209.2016.1278455. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaâbane M., Soudani N., Benjeddou K., Turki M., Ayadi Makni F., Boudawara T., Zeghal N., Ellouze Ghorbel R. The protective potential of Nitraria retusa on penconazole-induced hepatic injury in adult rats Toxicol Environ Chem. 2015;97:1253. doi: 10.1080/02772248.2015.1093633. : . , , –. , doi: . [DOI] [Google Scholar]
- 12.Dodd S., Dean O., Copolov D.L., Malhi G.S., Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility Expert Opin Biol Ther. 2008;8:1955. doi: 10.1517/14728220802517901. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 13.Elsayed A., Elkomy A., Elkammar R., Youssef G., Abdelhiee E.Y., Abdo W., Fadl S.E., Soliman A., Aboubakr M. Synergistic protective effects of lycopene and N-acetylcysteine against cisplatin-induced hepatorenal toxicity in rats Sci Rep. 2021;11:1. doi: 10.1038/s41598-021-93196-7. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Sharkawy E.E., El-Nisr N.A. Testicular dysfunction induced by penconazole fungicide on male albino rats Comp Clin Path. 2013;22:475. doi: 10.1007/s00580-012-1435-4. : . , , –. , doi: . [DOI] [Google Scholar]
- 15.Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis Mol Aspects Med. 2009;30:1. doi: 10.1016/j.mam.2008.08.006. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death Biochem Biophys Res Commun. 2017;482:419. doi: 10.1016/j.bbrc.2016.10.086. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hang W, Shu H, Wen Z, Liu J, Jin Z, Shi Z, Chen C, Wang D.W. N-Acetyl Cysteine Ameliorates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease and Intracellular Triglyceride Accumulation by Preserving Mitochondrial Function Front Pharmacol. 2021;12:636204. doi: 10.3389/fphar.2021.636204. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassanen E.I., Korany R.M.S., Bakeer A.M. Cisplatin-conjugated gold nanoparticles-based drug delivery system for targeting hepatic tumors J Biochem Mol Toxicol. 2021;35:e22722. doi: 10.1002/jbt.22722. : . , , , doi: . [DOI] [PubMed] [Google Scholar]
- 19.Husak V.V., Mosiichuk N.M., Storey J.M., Storey K.B., Lushchak V.I. Acute exposure to the penconazole-containing fungicide Topas partially augments antioxidant potential in goldfish tissues Comp Biochem Physiol C Toxicol Pharmacol. 2017;193:1. doi: 10.1016/j.cbpc.2016.12.003. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 20.Jawaid H., Ali M.M., Khan M.U., Sami S., Shaikh M.A. Efficacy and safety of N-acetylcysteine for the treatment of nonacetaminophen-induced acute liver failure: an updated systematic review and meta-analysis Clin Exp Hepatol. 2021;7:156. doi: 10.5114/ceh.2021.107171. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X., Wang J., Liu J., Zhu H., Hu J., Sun X., Zhou W. Resveratrol ameliorates penconazole-induced cardiotoxicity by inhibition of oxidative stress and apoptosis in zebrafish larvae Ecotoxicol Environ Saf. 2023;256:114865. doi: 10.1016/j.ecoenv.2023.114865. : . , , , doi: . [DOI] [PubMed] [Google Scholar]
- 22.Lu S.C. Regulation of glutathione synthesis Mol Aspects Med. 2009;30:42. doi: 10.1016/j.mam.2008.05.005. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmoud A.M., Hussein O.E., Abd El-Twab S.M., Hozayen W.G. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis Food Funct. 2019;10:4593. doi: 10.1039/C9FO00114J. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 24.Meng Z., Liu L., Xi Y., Jia M., Yan S., Tian S., Sun W., Zhu W., Li X., Zhou Z. Different effects of exposure to penconazole and its enantiomers on hepatic glycolipid metabolism of male mice Environ Pollut. 2020;257:113555. doi: 10.1016/j.envpol.2019.113555. : . , , , doi: . [DOI] [PubMed] [Google Scholar]
- 25.Mercadante R., Polledri E., Scurati S., Moretto A., Fustinoni S. Identification of metabolites of the fungicide penconazole in human urine Chem Res Toxicol. 2016;29:1179. doi: 10.1021/acs.chemrestox.6b00149. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 26.Morgan A.M., Hassanen E.I., Ogaly H.A., Al Dulmani S.A., Al-Zahrani F.A.M., Galal M.K., Kamel S., Rashad M.M., Ibrahim M.A., Hussien A.M. The ameliorative effect of N-acetylcysteine against penconazole induced neurodegenerative and neuroinflammatory disorders in rats J Biochem Mol Toxicol. 2021;35:e22884. doi: 10.1002/jbt.22884. : . , , , doi: . [DOI] [PubMed] [Google Scholar]
- 27.Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress J Biol Chem. 2009;284:13291. doi: 10.1074/jbc.r900010200. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan X., Wu X., Yan D., Peng C., Rao C., Yan H. Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs Toxicol Lett. 2018;288:55. doi: 10.1016/j.toxlet.2018.02.002. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 29.Pedre B., Barayeu U., Ezeriņa D., Dick T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species Pharmacol Ther. 2021;228:107916. doi: 10.1016/j.pharmthera.2021.107916. : . , , , doi: . [DOI] [PubMed] [Google Scholar]
- 30.Polledri E., Mercadante R., Fustinoni S. Determination of tebuconazole and penconazole fungicides in rat and human hair by liquid chromatography/tandem mass spectrometry Rapid Comm Mass Spectrom. 2018;32:1243. doi: 10.1002/rcm.8153. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 31.Roman D.L., Voiculescu D.I., Matica M.A., Baerle V., Filimon M.N., Ostafe V., Isvoran A. Assessment of the Effects of Triticonazole on Soil and Human Health Molecules. 2022;27:6554. doi: 10.3390/molecules27196554. : . , , , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sies H., Berndt C., Jones D.P. Oxidative stress Annu Rev Biochem. 2017;86:715. doi: 10.1146/annurev-biochem-061516-045037. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 33.Sun S.C., Chang J.H., Jin J. Regulation of nuclear factor-κB in autoimmunity Trends Immunol. 2013;34:282. doi: 10.1016/j.it.2013.01.004. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanideh N., Sadeghi F., Amanat S., Firoozi D., Noorafshan A., Iraji A., Koohi-Hosseinabadi O. Protection by pure and genistein fortified extra virgin olive oil, canola oil, and rice bran oil against acetic acid-induced ulcerative colitis in rats Food Funct 2020;11:860. doi: 10.1039/C9FO01951K. : . . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 35.Zhang L., Zhu Z., Liu J., Zhu Z., Hu Z. Protective effect of N-acetylcysteine (NAC) on renal ischemia/reperfusion injury through Nrf2 signaling pathway J Recept Signal Transduct Res. 2014;34:396. doi: 10.3109/10799893.2014.908916. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., Wang X., Luo F., Sheng H., Zhou L., Zhong Q., Lou Z., Sun H., Yang M., Cui X., Chen Z. Application and enantioselective residue determination of chiral pesticide penconazole in grape, tea, aquatic vegetables and soil by ultra performance liquid chromatography-tandem mass spectrometry Ecotoxicol Environ Saf. 2019;172:530. doi: 10.1016/j.ecoenv.2019.01.103. : . , , –. , doi: . [DOI] [PubMed] [Google Scholar]
- 37.Zhitkovich A. N-acetylcysteine: antioxidant, aldehyde scavenger, and more Chem Res Toxicol. 2019;32:1318. doi: 10.1021/acs.chemrestox.9b00152. : . , , –. , doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]


