Abstract
Background
The impact of insulin resistance on the prognosis of heart failure with preserved ejection fraction (HFpEF) remains unknown. This study aimed to investigate the association between the triglyceride-glucose (TyG) index, an easily calculated marker of insulin resistance, and the long-term prognosis of HFpEF.
Methods
A total of 823 patients with HFpEF were enrolled in the study. The TyG index was determined using the formula ln(fasting triglycerides [mg/dL] × fasting glucose [mg/dL]/2). The primary endpoint was all-cause death. The secondary endpoints were cardiovascular (CV) death and heart failure (HF) rehospitalization. Restricted cubic spline, multivariate Cox proportional hazard models, and competing risk models were used for analyses.
Results
During a median follow-up period of 3.16 years, 147 (17.8%) all-cause deaths, 139 (16.8%) CV deaths, and 222 (27.0%) HF rehospitalizations occurred. Restricted cubic spline analysis revealed a J-shaped association between the TyG index and the mortality and rehospitalization rates. In the multivariate Cox proportional hazard models, compared with those in the lowest TyG index tertile, patients in the highest tertile exhibited the greatest susceptibility to all-cause death (HR 1.53, 95% CI 1.19–1.98) and CV death (HR 1.52, 95% CI 1.19–1.96). In the competing risk model, a significant association between the TyG index and HF rehospitalization was observed (HR 1.31, 95% CI, 1.07–1.61).
Conclusion
A high TyG index is associated with an increased risk of mortality and rehospitalization in patients with HFpEF. The TyG index may serve as a promising prognostic marker for patients with HFpEF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-02001-4.
Keywords: Heart failure with preserved ejection fraction, Triglyceride-glucose index, Insulin resistance, Mortality
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a prevalent and challenging clinical syndrome characterized by diastolic dysfunction and elevated filling pressures [1]. Despite advances in treatment, patients with HFpEF continue to experience high mortality rates and frequent rehospitalization, leading to a substantial healthcare burden [2]. Identification of novel risk factors associated with adverse outcomes in HFpEF is essential for improving patient care and prognosis.
Metabolic disturbances, including insulin resistance (IR), dyslipidemia, and abnormal fatty acid metabolism, have been implicated in HFpEF pathogenesis and progression [3, 4]. The triglyceride-glucose (TyG) index, a surrogate marker of IR, has emerged as a potential predictor of adverse cardiovascular (CV) outcomes in various populations [5–9]. The TyG index is calculated as the logarithm of the product of fasting plasma triglyceride (TG) and fasting plasma glucose (FPG) levels, reflecting the interplay between lipid and glucose metabolisms [5].
While previous studies have examined the association between the TyG index and CV outcomes in various populations, limited data are available regarding its impact on long-term prognosis, specifically in individuals with HFpEF. Furthermore, exploring the relationship between the TyG index and HFpEF established risk scores, such as the HFA-PEFF [10] and H2FPEF scores [11], could provide valuable insights into the prognostic utility of this metabolic marker within the existing risk assessment frameworks.
Therefore, this cohort study aimed to investigate the impact of the TyG index on the mortality and rehospitalization rates in patients diagnosed with HFpEF over a 3-year follow-up period. Additionally, we examined the relationship between TyG index across and HFA-PEFF and H2FPEF scores to provide valuable insights into the potential utility of the TyG index as a risk-stratification tool.
Methods
Study design and population
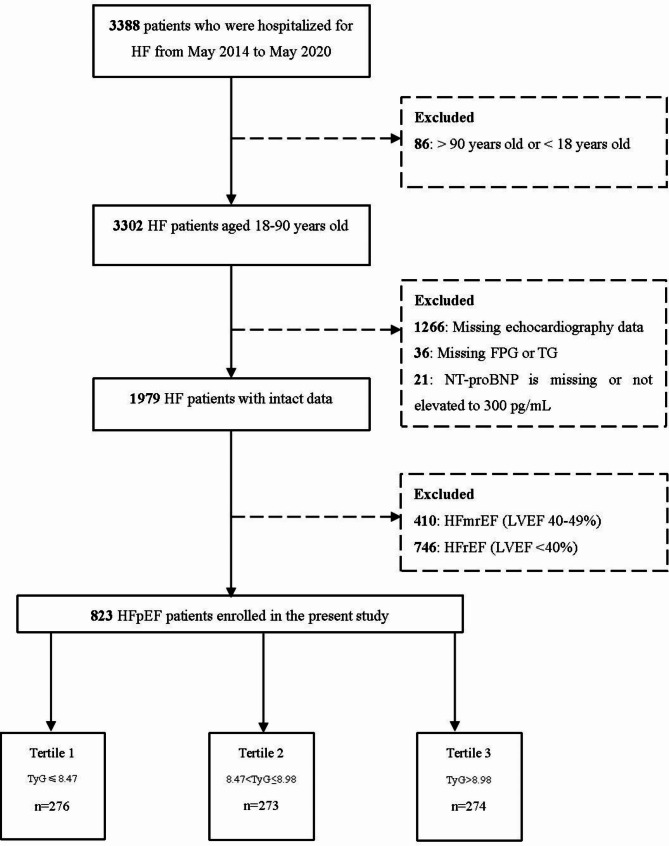
This prospective study was conducted at the Peking University Third Hospital. A total of 3,388 patients hospitalized for acute heart failure (HF) between 2014 and 2020 were screened consecutively for eligibility. The diagnostic criteria of HFpEF were as follows: (1) left ventricular ejection fraction (LVEF) ≥ 50%; (2) typical heart failure symptoms and signs: breathlessness, coughing up pink frothy sputum, lower limb swelling or jugular venous distension; (3) N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels exceeding 300 pg/mL; (4) relevant structural heart changes suggested by echocardiography (meeting any one of the following criteria): (a) left ventricular mass index > 115 g/m2 for males or > 95 g/m2 for females. (b)left atrial (LA) diameter > 40 mm for males or > 38 mm for females. (c) E/e’ ratio ≥ 9. The inclusion criteria were: (1) diagnosed with HFpEF; (2) aged 18–90 years; The exclusion criteria were as follows: (1) aged > 90 years or < 18 years; (2) missing echocardiography, FPG, TG, or NT-proBNP data. The detailed population enrollment process is presented in Fig. 1. During the study, patients were followed-up every six months after discharge through telephone calls or outpatient clinical visits. The patients were then categorized into tertiles based on their admission TyG index levels: the 1st tertile consisted of 276 patients with a TyG index ≤ 8.47, the 2nd tertile included 273 patients with a TyG index ranging 8.48–8.98, and the 3rd tertile comprised 274 patients with a TyG index > 8.98. The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Review Committee of Peking University Third Hospital. Informed consent was obtained from all the participants.
Fig. 1.
Flowchart of study population enrollment. Abbreviations: HF, heart failure; FPG, fasting plasma glucose; TG, triglyceride; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVEF, left ventricular ejection fraction; TyG, triglyceride-glucose; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction
Data collection and endpoint definitions
Baseline demographic and clinical data, including age, sex, BMI, smoking history, laboratory tests, vital signs, echocardiographic data, comorbidities, and medication history, were retrieved from the electronic medical recording system. Peripheral venous blood samples were obtained from the participants within 24 h of hospital admission after an overnight fasting period of > 8 h. Laboratory tests included measurements of FPG, HbA1c, LDL-cholesterol (LDL-C), total cholesterol (TC), HDL-cholesterol (HDL-C), TG, NT-proBNP, creatinine (Cr), Hb, troponin T, and thyroid function. Medication history was also recorded, including the use of insulin, metformin, sodium-glucose cotransporter-2 inhibitors (SGLT2i), other hypoglycemic drugs, ACE inhibitors (ACEI), angiotensin receptor blockers (ARB), angiotensin receptor II blocker-neprilysin inhibitor (ARNI), calcium channel blockers (CCB), aldosterone receptor antagonists (MRA), and loop and thiazide diuretics.
BMI was defined as weight (kg) divided by height squared (m2). Diabetes was defined by fasting plasma glucose ≥ 126 mg/dL, a 2-hour oral glucose tolerance test value ≥ 200 mg/dL, HbA1c ≥ 6.5%, or random plasma glucose ≥ 200 mg/dL with classic symptoms of hyperglycemia. Hypertension was identified by a systolic blood pressure (SBP) ≥ 140 mmHg or a diastolic blood pressure (DBP) ≥ 90 mmHg, and/or the current use of antihypertensive medication. Hyperlipidemia was diagnosed when patients had LDL-C levels ≥ 140 mg/dL or a TG level ≥ 150 mg/dL.
The primary endpoint of this study was all-cause mortality. The secondary endpoints included CV death and rehospitalization due to HF.
TyG index formula
The TyG index was calculated using the following formula: TyG index = ln(fasting TG [mg/dL] × FPG [mg/dL]/2).
Statistical analyses
Continuous variables with a normal distribution were reported as mean ± standard deviation, while non-normally distributed variables were presented as median and interquartile range (IQR). Categorical variables were expressed as frequencies and percentages. Differences in continuous variables were assessed using analysis of variance (ANOVA) or the Kruskal–Wallis H test, whereas differences in categorical variables were evaluated using the chi-square test or Fisher’s exact test. We compared the baseline characteristics between the three TyG index groups of patients with HFpEF. We also compared the TyG index levels in patients with HFpEF (LVEF ≥ 50%) with those with heart failure with reduced ejection fraction (HFrEF) (LVEF < 40%) and those with heart failure with mid-range ejection fraction (HFmrEF) (LVEF 40–49%). In addition, we conducted a partial correlation analysis to assess the relationship between the TyG index and HFA-PEFF as well as H2FPEF scores, while controlling for age, smoking and coronary heart disease (CHD).
To explore the differences in event-free survival among the different TyG index groups in patients with HFpEF, Kaplan–Meier (K–M) survival analyses were performed. The time to event in this study was defined as the number of days that elapsed from the date of the blood draw to the occurrence of the endpoint or the censored date. Multivariate Cox regression analysis was conducted to examine the association between the TyG index and the incidence rates of the three outcomes. Variables that displayed notable differences among the TyG index tertiles, along with those of significant clinical relevance, were chosen as potential covariates for inclusion in the multivariable Cox regression model. A correlation matrix for these variables was generated, and a variance inflation factor (VIF) analysis was carried out. Variables with correlation coefficients greater than 0.6 or VIF values surpassing 10 were omitted from the Cox regression models. Finally, three models were used for adjustment: model 1 adjusted for age and sex; model 2 adjusted for model 1 variables plus BMI, SBP, HbA1c, HDL-C, Cr, Hb, cTnT,NT-proBNP, free thyroxine (FT4), diabetes, hyperlipidemia, and atrial fibrillation; and model 3 adjusted for model 2 variables plus LVEF, septal e’, pulmonary artery systolic pressure (PASP), tricuspid regurgitation (TR) velocity, LA diameter, LA pressure, right ventricular (RV) diameter and usage of insulin, metformin, SGLT2i and ACEI/ARB/ARNI. Tests for trends were performed by including the TyG index tertiles as ordinal variables in the statistical model, and significance was assessed using the Wald statistic. Several BMI values were missing. To effectively handle this issue, we employed the multiple imputation by chained equations (MICE) method, utilizing the “mice” function in R software. A competing risk analysis for HF rehospitalization was conducted, with all-cause death considered as the competing event. The Fine & Gray model was performed for this analysis. Furthermore, restricted cubic spline (RCS) analyses with four knots were conducted to examine the relationship between the TyG index and endpoints. Subgroup analyses were performed based on various factors, including age (< 65 or ≥ 65 years old), sex, BMI (< 25 or ≥ 25 kg/m²), cause of HFpEF (coronary heart disease [CHD] or others), diabetes, hyperlipidemia, renal dysfunction and LVEF (> 60% or 50–60%). To assess potential interactions, the regression models included cross-product terms between the TyG index and each potential effect modifier, and likelihood ratio tests were used to determine the significance of these interactions. To evaluate predictive performance, we utilized receiver operating characteristic (ROC) curves and calculated the area under the curve (AUC), comparing the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score [12] to the composite score of MAGGIC and the TyG index. The composite score of MAGGIC and TyG index = MAGGIC score + TyG index×2.
Statistical analyses were performed using SPSS Statistics (version 26; SPSS, Chicago, IL, USA) and R software (version 4.2.0). A two-tailed P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 823 patients diagnosed with HFpEF met the inclusion criteria and were enrolled in the study. The population had a mean age of 73.0 ± 12.7 years, comprising 427 (51.9%) female patients. Table 1 presents the baseline characteristics of the patients stratified by TyG index tertiles. A higher TyG index was associated with younger age; female sex; higher BMI; elevated Cr levels and SBP; a higher prevalence of diabetes, hyperlipidemia, and hypoglycemic drug use; and a lower proportion of atrial fibrillation. As shown in Supplemental Fig. 1, the TyG index was significantly lower in patients with HFpEF than in those with HFmrEF and HFrEF (P = 0.0005 and 0.0036, respectively). Among the patients with HFpEF, the partial correlation analysis revealed that, after controlling for age, smoking and CHD, the correlation between TyG index and HFA-PEFF score were insignificant (TyG index and HFA-PEFF score: correlation coefficient, 0.009, P = 0.801), as did the correlation between TyG index and H2FPEF score (TyG index and H2FPEF score: correlation coefficient, 0.002, P = 0.960).
Table 1.
Baseline characteristics of the HFpEF patients according to TyG tertiles
| Characteristic | Total | Tertile 1 | Tertile 2 | Tertile 3 | P value |
|---|---|---|---|---|---|
| n = 823 | n = 276 | n = 273 | n = 274 | ||
| TyG ≤ 8.47 | 8.47 < TyG ≤ 8.98 | TyG > 8.98 | |||
| age, years | 73.0 ± 12.7 | 75.2 ± 12.3 | 72.7 ± 12.5 | 71.1 ± 13.0 | 0.001* |
| Sex, female, n (%) | 427 (51.9) | 129 (46.7) | 139 (50.9) | 159 (58.0) | 0.028* |
| NYHA class, n (%) | |||||
| I | 81 (9.84) | 24 (8.70) | 26 (9.52) | 31 (11.3) | 0.387 |
| II | 398 (48.4) | 136 (49.3) | 127 (46.5) | 135 (49.3) | |
| III | 237 (28.8) | 77 (27.9) | 91 (33.3) | 69 (25.2) | |
| IV | 107 (13.0) | 39 (14.1) | 29 (10.6) | 39 (14.2) | |
| BMI, kg/m2 | 25.5 ± 4.7 | 24.6 ± 4.6 | 25.4 ± 4.9 | 26.5 ± 4.5 | < 0.001* |
| smoking, n (%) | 188 (22.9) | 58 (21.1) | 60 (22.0) | 70 (25.6) | 0.406 |
| Laboratory test | |||||
| FPG, mmol/L | 6.20 [5.20, 7.90] | 5.20 [4.60, 5.90] | 6.10 [5.40, 7.20] | 8.30 [6.60, 11.2] | < 0.001* |
| HbA1c, % | 6.30 [5.80, 7.10] | 6.00 [5.70, 6.40] | 6.30 [5.80, 6.90] | 7.00 [6.20, 8.20] | < 0.001* |
| NT-proBNP, pg/ml | 1811 [840, 3704] | 1888 [934, 3898] | 1733 [828, 3637] | 1794 [766, 3558] | 0.493 |
| LDL-C, mmol/L | 2.22 ± 0.83 | 1.97 ± 0.72 | 2.25 ± 0.81 | 2.43 ± 0.89 | < 0.001* |
| TC, mmol/L | 3.75 ± 1.04 | 3.43 ± 0.85 | 3.72 ± 0.99 | 4.10 ± 1.16 | < 0.001* |
| HDL-C, mmol/L | 0.97 ± 0.29 | 1.06 ± 0.31 | 0.97 ± 0.27 | 0.88 ± 0.26 | < 0.001* |
| TG, mmol/L | 1.13 [0.84, 1.56] | 0.77 [0.65, 0.92] | 1.19 [1.01, 1.36] | 1.81 [1.38, 2.29] | < 0.001* |
| Cr, µmol/L | 98.0 [79.0, 134.0] | 93.5 [76.0, 125.0] | 97.0 [77.0, 126.0] | 105.0 [83.0, 150.0] | 0.002* |
| HGB, g/L | 118 ± 23 | 116 ± 22 | 121 ± 24 | 118 ± 23 | 0.042* |
| cTnT, ng/mL | 0.03 [0.02, 0.06] | 0.03 [0.01, 0.05] | 0.03 [0.02, 0.05] | 0.04 [0.02, 0.09] | 0.008* |
| TSH, mIU/L | 1.87 [1.13, 3.16] | 1.81 [1.12, 3.32] | 1.82 [1.18, 3.13] | 1.91 [1.04, 3.11] | 0.673 |
| FT3, pg/mL | 2.62 ± 0.81 | 2.66 ± 1.07 | 2.65 ± 0.64 | 2.54 ± 0.64 | 0.175 |
| FT4, ng/dL | 1.25 [1.11, 1.43] | 1.24 [1.11, 1.39] | 1.30 [1.13, 1.47] | 1.22 [1.07, 1.37] | 0.004* |
| Vital signs | |||||
| Heart rate, beats/min | 86 [76, 102] | 84 [73, 98] | 88 [76, 102] | 89.5 [80, 110] | 0.051 |
| SBP, mmHg | 138 ± 22 | 134 ± 22 | 139 ± 20 | 141 ± 23 | < 0.001* |
| DBP, mmHg | 73 ± 13 | 72 ± 13 | 74 ± 13 | 74 ± 13 | 0.057 |
| Comorbidity | |||||
| Diabetes, n (%) | 346 (42.0) | 67 (24.3) | 102 (37.4) | 177 (64.6) | < 0.001* |
| Hypertension, n (%) | 640 (77.8) | 204 (73.9) | 217 (79.5) | 219 (79.9) | 0.167 |
| Coronary heart disease, n (%) | 414 (50.3) | 128 (46.4) | 134 (49.1) | 152 (55.5) | 0.091 |
| Atrial fibrillation, n (%) | 381 (46.3) | 152 (55.1) | 128 (46.9) | 101 (36.9) | < 0.001* |
| Hyperlipidemia, n (%) | 266 (32.3) | 67 (24.3) | 91 (33.3) | 108 (39.4) | 0.001* |
| Hyperthyroid, n (%) | 49 (6.19) | 18 (6.79) | 15 (5.77) | 16 (5.99) | 0.877 |
| Renal dysfunction, n (%) | 116 (14.1) | 30 (10.9) | 42 (15.4) | 44 (16.1) | 0.164 |
| Medication use | |||||
| Insulin, n (%) | 169 (20.5) | 31 (11.2) | 44 (16.1) | 94 (34.3) | < 0.001* |
| SGLT2i, n (%) | 116 (14.1) | 21 (7.61) | 42 (15.4) | 53 (19.3) | < 0.001* |
| Metformin, n (%) | 77 (9.36) | 13 (4.71) | 23 (8.42) | 41 (15.0) | < 0.001* |
| ACEI/ARB/ARNI, n (%) | 358 (43.5) | 98 (35.5) | 133 (48.7) | 127 (46.4) | 0.004* |
| Beta blocker, n (%) | 435 (52.9) | 141 (51.1) | 148 (54.2) | 146 (53.3) | 0.753 |
| MRA, n (%) | 119 (14.5) | 47 (17.0) | 37 (13.6) | 35 (12.8) | 0.319 |
| Loop diuretics, n (%) | 485 (58.9) | 170 (61.6) | 161 (59.0) | 154 (56.2) | 0.438 |
| Echocardiography | |||||
| LVEF, % | 64.6 ± 7.7 | 64.9 ± 7.4 | 64.8 ± 8.0 | 64.0 ± 7.7 | 0.331 |
| Septal E/e’ ratio | 10.0 [8.0, 14.0] | 11.0 [8.0, 14.0] | 10.0 [8.0, 15.0] | 10.0 [8.0, 14.0] | 0.104 |
| Septal e’, cm/s | 8.85 ± 2.98 | 9.37 ± 3.30 | 8.91 ± 2.90 | 8.26 ± 2.61 | < 0.001* |
| LV mass index, g/m2 | 100.0 ± 36.7 | 98.6 ± 36.9 | 102.0 ± 37.8 | 101.0 ± 35.4 | 0.534 |
| LV wall thickness, mm | 8.92 ± 1.60 | 8.90 ± 1.45 | 8.98 ± 1.67 | 8.89 ± 1.67 | 0.782 |
| LVEDD, mm | 48.9 ± 6.5 | 48.9 ± 7.1 | 48.9 ± 6.2 | 48.9 ± 6.3 | 0.999 |
| TR velocity, m/s | 2.78 ± 0.54 | 2.86 ± 0.55 | 2.82 ± 0.53 | 2.62 ± 0.52 | < 0.001* |
| RWT | 0.37 ± 0.08 | 0.37 ± 0.08 | 0.37 ± 0.08 | 0.37 ± 0.08 | 0.913 |
| PASP, mmHg | 38.0 ± 13.9 | 40.3 ± 15.1 | 38.6 ± 13.2 | 34.0 ± 12.0 | < 0.001* |
| LA diameter, mm | 41.3 ± 7.16 | 43.8 ± 8.59 | 40.7 ± 6.46 | 39.4 ± 5.45 | < 0.001* |
| LA pressure, mmHg | 15.0 ± 5.99 | 15.2 ± 5.52 | 15.0 ± 6.73 | 14.8 ± 5.66 | 0.855 |
| RV diameter, mm | 21.2 ± 4.17 | 22.6 ± 5.25 | 20.9 ± 3.58 | 20.3 ± 3.11 | < 0.001* |
Data are expressed as mean ± standard deviation, median [interquartile range] or n (%)
* refers to p < 0.05
ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; ARNI: angiotensin receptor II blocker - neprilysin inhibitor; BMI: body mass index; CCB: calcium channel blockers; Cr: creatinine; cTnT: troponin T; DBP: diastolic blood pressure; FPG, fasting plasma glucose; FT3: free triiodothyronine; FT4: free thyroxine; HDL-C: high-density lipoprotein cholesterol; HGB: hemoglobin; LA, left atrial; LDL-C: low-density lipoprotein cholesterol; LVEDD: left ventricular end-diastolic dimension; LV, left ventricular; LVEF: left ventricle ejection fraction; MRA: aldosterone receptor antagonist; NT-proBNP: N-terminal pro-B-type natriuretic peptide;NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; RV, right ventricular; RWT, relative wall thickness; SBP: systolic blood pressure; SGLT2i: sodium-glucose cotransporter-2 inhibitors; TC: total cholesterol; TG: triglycerides; TR, tricuspid regurgitation; TSH: thyroid stimulating hormone
TyG index and outcomes
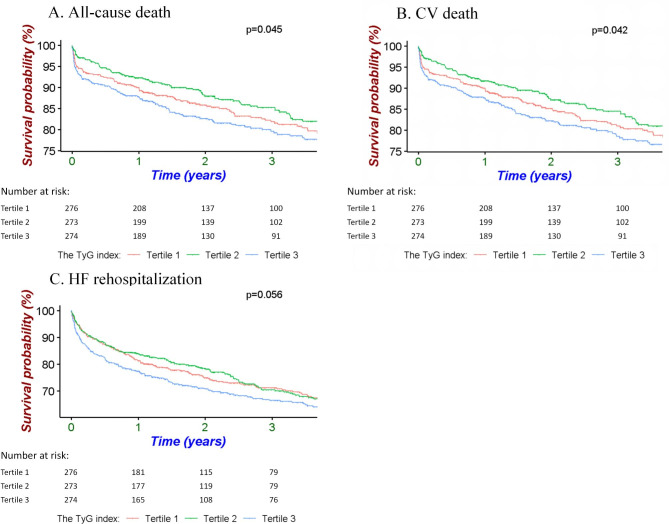
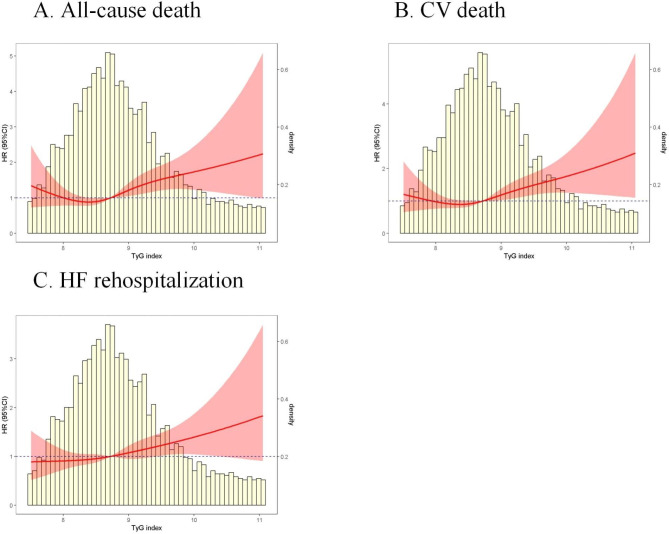
During a median follow-up of 3.16 years (IQR: 2.87–3.45 years), a total of 147 deaths (17.9% of the cohort) were recorded. K–M curves demonstrated that patients in the 3rd tertile had the lowest survival rate (p = 0.045; Fig. 2A). RCS analysis (Fig. 3A) revealed a J-shaped association between the TyG index and the risk of all-cause death. Table 2 presents the three multivariate Cox regression models used to assess the correlations between the TyG index and outcomes. In all three models, the highest TyG index tertile was associated with a higher incidence of all-cause death (model 1: HR 1.44, 95% CI 1.10–1.90, P = 0.009; model 2: HR 1.45, 95% CI 1.12–1.86, P = 0.004; model 3: HR 1.53, 95% CI 1.19–1.98, P < 0.001). The TyG index as a continuous variable also had significant association with all-cause death (Model 1: HR 1.41, 95% CI 1.06–1.88, P = 0.018; Model 2: HR 1.45, 95% CI 1.10–1.92, P = 0.008; Model 3: HR 1.46, 95% CI 1.10–1.96, P = 0.009). In the subgroup analyses, the TyG index was associated with a high incidence of all-cause mortality, which was consistent across subgroups including age, gender, BMI, CHD, diabetes, hyperlipidemia, renal dysfunction and LVEF (Supplemental Table 1). Meanwhile, there were no interactions between the TyG index and the variables in subgroup analyses (All P values for interaction > 0.05). Additionally, the ROC analysis demonstrated that the composite score of MAGGIC and TyG index (AUC 0.647, 95% CI 0.598–0.695) displayed superior predictive accuracy compared to MAGGIC alone (AUC 0.633, 95% CI 0.596–0.694, Supplemental Fig. 2). The net reclassification index (NRI) was 0.250 (95% CI 0.054–0.446, P = 0.012).
Fig. 2.
Kaplan–Meier analyses for different endpoints among the TyG index tertiles. A: all-cause death. B: CV death. C: HF rehospitalization. TyG, triglyceride-glucose; CV, cardiovascular; HF, heart failure
Fig. 3.
Nonlinear associations of the TyG index with different outcomes in the HFpEF patients. A: all-cause death. B: CV death. C: HF rehospitalization. TyG, triglyceride-glucose; HFpEF, heart failure with preserved ejection fraction; CV, cardiovascular; HF, heart failure
Table 2.
Hazard ratios of triglyceride-glucose index tertiles for outcomes in the study participants
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Variables | HR | 95%CI | P-value | HR | 95%CI | P-value | HR | 95%CI | P-value | ||
| All-cause death | TyG index* | 1.41 | 1.06–1.88 | 0.018 | 1.45 | 1.10–1.92 | 0.008 | 1.46 | 1.10–1.96 | 0.009 | ||
| Tertile 1 | Reference | Reference | Reference | |||||||||
| Tertile 2 | 0.90 | 0.68–1.19 | 0.452 | 0.92 | 0.71–1.20 | 0.550 | 1.00 | 0.77–1.30 | 0.969 | |||
| Tertile 3 | 1.44 | 1.10–1.90 | 0.009 | 1.45 | 1.12–1.86 | 0.004 | 1.53 | 1.19–1.98 | < 0.001 | |||
| P for trend | 0.011 | 0.029 | 0.038 | |||||||||
| CV death | TyG index* | 1.43 | 1.08–1.89 | 0.010 | 1.43 | 1.07–1.90 | 0.016 | 1.49 | 1.13–1.97 | 0.005 | ||
| Tertile 1 | Reference | Reference | Reference | |||||||||
| Tertile 2 | 0.92 | 0.7–1.22 | 0.576 | 0.94 | 0.72–1.21 | 0.612 | 1.01 | 0.79–1.32 | 0.880 | |||
| Tertile 3 | 1.45 | 1.10–1.90 | 0.007 | 1.44 | 1.12–1.85 | 0.004 | 1.52 | 1.19–1.96 | < 0.001 | |||
| P for trend | 0.009 | 0.042 | 0.045 | |||||||||
| HF rehospitalization | TyG index* | 1.30 | 1.03–1.63 | 0.021 | 1.28 | 1.02–1.61 | 0.031 | 1.29 | 0.97–1.72 | 0.071 | ||
| Tertile 1 | Reference | Reference | Reference | |||||||||
| Tertile 2 | 1.00 | 0.79–1.26 | 0.981 | 0.95 | 0.77–1.18 | 0.681 | 1.07 | 0.77–1.49 | 0.698 | |||
| Tertile 3 | 1.34 | 1.06–1.70 | 0.013 | 1.34 | 1.09–1.65 | 0.004 | 1.36 | 0.98–1.90 | 0.069 | |||
| P for trend | 0.013 | 0.041 | 0.077 |
HR, hazard ratio; CI, confidence interval; TyG, triglyceride glucose index; * TyG index as a continuous variable
Model 1 adjusted for age and sex
Model 2 adjusted for model 1 variables plus BMI, SBP, HbA1c, HDL-C, Cr, Hb, cTnT,NT-proBNP, FT4, diabetes, hyperlipidemia, and atrial fibrillation
Model 3 adjusted for model 2 variables plus LVEF, septal e’, PASP, TR velocity, LA diameter, LA pressure, RV diameter, and usage of insulin, metformin, SGLT2i and ACEI/ARB/ARNI
For CV death, a total of 139 deaths were recorded. The K–M curve also demonstrated that the 3rd tertile had a significantly lower event-free survival rate (Fig. 2B). Figure 3B shows a clear J-shaped association between the TyG index and CV death. In comparison to patients in the 1st tertile, those in the 3rd tertile exhibited a significantly higher risk of CV death (model 1: HR 1.45, 95% CI 1.10–1.90, P = 0.007; model 2: HR 1.44, 95% CI 1.12–1.85, P = 0.004; model 3: HR 1.52, 95% CI 1.19–1.96, P = 0.001). When treated as a continuous variable, the TyG index also demonstrated a significant association with CV mortality (Model 1: HR 1.43, 95% CI 1.08–1.89, P = 0.010; Model 2: HR 1.43, 95% CI 1.07–1.90, P = 0.016; Model 3: HR 1.49, 95% CI 1.13–1.97, P = 0.005). The subgroup analysis showed that the associations of TyG index with the risk of CV mortality was consistent across subgroups of age, sex, BMI, CHD, diabetes, renal dysfunction and LVEF. However, this association became insignificant in patients with dyslipidemia (Supplemental Table 2).
Regarding HF rehospitalization, 222 cases were recorded, accounting for 27.0% of the cohort. The K–M curve indicated that the 3rd tertile had the lowest event-free survival rate, whereas the curves for the 1st and 2nd tertiles exhibited some overlap (Fig. 2C). The RCS curve initially remained constant, and then rapidly increased (Fig. 3C). In the multivariable Cox regression analysis, compared to patients in the 1st tertile of the TyG index, those in the 3rd tertile had a significantly higher rate of HF rehospitalization in models 1 and 2, but this relationship became insignificant in model 3 (model 1: HR 1.34, 95% CI 1.06–1.70, P = 0.013; model 2: HR 1.34, 95% CI 1.09–1.65, P = 0.004; model 3: HR 1.36, 95% CI 0.98–1.90, P = 0.069). Similar results were observed when using the TyG index as a continuous value (Model 1: HR 1.30, 95% CI 1.03–1.63, P = 0.021; Model 2: HR 1.28, 95% CI 1.02–1.61, P = 0.031; Model 3: HR 1.29, 95% CI 0.97–1.72, P = 0.071). In the competing risk model shown in Table 3, a significant association between the TyG index and HF rehospitalization was observed. Specifically, when considering TyG as a continuous variable, the sub-distribution hazard ratio (SHR) was 1.17 (95% CI, 1.02–1.34, P = 0.031). When comparing TyG index tertile 3 to tertile 1, the SHR was 1.31 (95% CI, 1.07–1.61, P = 0.008). Subgroup analyses indicated that sex, BMI, CHD, hyperlipidemia, and renal dysfunction influenced this association. The association remained significant in the male, BMI ≥ 25 kg/m², CHD, non-hyperlipidemia, and non-renal dysfunction patients (Supplemental Table 3). The number of events in each tertile were presented in Supplemental Table 4.
Table 3.
Regression coefficients in competing risk model for heart failure rehospitalization
| Variables | β | P | SHR | 95% CI |
|---|---|---|---|---|
| TyG index* | 0.154 | 0.031 | 1.17 | 1.02–1.34 |
| Tertile 1 | Reference | |||
| Tertile 2 | -0.049 | 0.650 | 0.95 | 0.77–1.17 |
| Tertile 3 | 0.272 | 0.008 | 1.31 | 1.07–1.61 |
SHR, subdistribution hazard ratio; * TyG index as a continuous variable
The results were adjusted for age, sex, BMI, SBP, HbA1c, HDL-C, Cr, Hb, cTnT, NT-proBNP, FT4, diabetes, hyperlipidemia, atrial fibrillation, LVEF, septal e’, PASP, TR velocity, LA diameter, LA pressure, RV diameter and usage of insulin, metformin, SGLT2i and ACEI/ARB/ARNI
Discussion
To the best of our knowledge, this is the first study to investigate the relationship between TyG index and long-term prognosis in patients with HFpEF. Our findings demonstrate that an increased TyG index is an independent predictor of higher mortality and rehospitalization rates in patients with HFpEF.
Previous studies have examined the association between IR and HF. A prospective cohort study revealed that IR assessed using the hyperinsulinemic-euglycemic clamp technique can independently predict HF development [13]. However, that technique, regarded as the gold standard for evaluating insulin sensitivity, is invasive and costly, making it impractical in routine clinical practice. As an alternative, the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) has been widely used to assess IR, using fasting glucose and insulin levels. A meta-analysis reported that higher levels of IR assessed by HOMA-IR are associated with an increased risk of developing HF, even after accounting for traditional risk factors [14]. This association has also been observed in studies involving individuals with and without diabetes mellitus [14]. Another study demonstrated an inverse correlation between HOMA2-IR (an updated version of HOMA-IR) and improvements in ejection fraction in patients with HFrEF [15]. Other methods for assessing IR include the Quantitative Insulin Sensitivity Check Index (QUICKI) and Fasting Insulin Resistance Index (FIRI). In a study that used HOMA-IR, FIRI, and QUICKI to evaluate insulin sensitivity, all three assessment methods indicated impaired insulin sensitivity in patients with chronic HF compared with healthy controls [16].
However, owing to the complex mathematical calculations and the need for insulin concentration measurements, the clinical adoption of QUICKI, FIRI, and HOMA-IR for evaluating IR remains challenging. In the past decade, the TyG index has emerged as a simple and reliable surrogate marker of IR [17]. Previous studies have demonstrated a strong correlation between the TyG index and the gold-standard hyperinsulinemic-euglycemic clamp technique in individuals with and without diabetes. Moreover, compared to HOMA-IR, TyG index showed better evaluation efficiency. An advantage of the TyG index is that it relies on routine and low-cost biochemical tests, including serum triglycerides and glucose, making it easily accessible in any clinical setting. Recent studies have highlighted the association between the TyG index and risk of HF. A study based on two large cohorts and Mendelian randomization analysis reported that participants in the highest quartile of the TyG index had the highest risk of incident HF in both cohorts [18]. The results of the Mendelian randomization study revealed a causal relationship between a high TyG index and an increased risk of HF. Analyses of the Atherosclerosis Risk in Communities (ARIC) study have also shown that individuals in the highest quartile of the TyG index have a greater risk of incident HF and are more likely to experience adverse left ventricular remodeling and dysfunction [19]. Furthermore, several studies conducted in 2022 provided evidence to support the predictive value of the TyG index in patients with HF. Han et al. [20] found a significant association between an increased TyG index and in-hospital mortality among patients with HF, and Huang et al. [21]. reported that an elevated TyG index at admission was associated with higher mortality and major adverse cardiovascular and cerebrovascular event rates during a 478-day follow-up period in patients with HF. In the context of HFpEF, a recent cross-sectional study demonstrated a positive correlation between the TyG index and the risk of subclinical HFpEF in patients with type 2 diabetes mellitus [22]. Patients with a TyG index ≥ 9.47 showed an increased risk of metabolic syndrome and diastolic dysfunction. These studies collectively support the association between the TyG index and the incidence and prognosis of HF. However, data regarding the association between the TyG index and the long-term prognosis of HFpEF are still lacking, and the relationship between the TyG index and diastolic dysfunction remains unknown. Therefore, our study aimed to investigate the impact of IR, assessed using the TyG index, on the long-term prognosis of HFpEF.
Throughout the follow-up period, we observed a significant association between a high TyG index and an increased mortality rate. Additionally, in the competing risk model, which accounts for all-cause death as the competing event, we detected a higher rate of HF rehospitalization among individuals in the high TyG index tertiles. These findings imply that as time advanced during the three-year follow-up, patients with elevated TyG index levels experienced a heightened risk of mortality and more pronounced HF symptoms. Subgroup analyses revealed that this association was particularly prominent in patients with high BMI, CHD, and diabetes. This highlights the potential of the TyG index as an indicator of IR, especially in patients with metabolic syndromes. Furthermore, we compared the TyG index at baseline among patients with HFpEF, HFmrEF, and HFrEF and found that the TyG index was significantly lower in HFpEF patients than in those with HFmrEF and HFrEF. This finding suggests a positive association between the TyG index and severity of left ventricular systolic dysfunction. Additionally, we explored the relationship between the TyG index and the HFA-PEFF and H2FPEF scores among the 823 patients with HFpEF included in our study. We found that there was no significant association between the TyG index with HFA-PEFF and H2FPEF scores. In our study, patients with a high TyG index were typically younger, had higher BMI, were more likely to be smokers, and exhibited elevated levels of blood glucose and lipids. Additionally, a large proportion of these patients had CHD. These factors might elevate their risk of experiencing fatal myocardial infarction and stroke in the future. This may offer an explanation as to why individuals with a high TyG index experienced a poorer prognosis during the 3-year follow-up period.
The association between IR assessed using the TyG index and the prognosis of HFpEF can be explained by several mechanisms. First, a higher TyG index indicates IR, which is associated with metabolic abnormalities, such as dyslipidemia, hypertension, and obesity. These metabolic abnormalities can directly cause cardiovascular stiffening and contribute to the progression of HFpEF [23–25]. Secondly, the TyG index has been linked to endothelial dysfunction [26] and microvascular damage [27] characterized by impaired vasodilation and increased vascular resistance in small arteries and arterioles. In HFpEF, microvascular dysfunction plays a critical role in impairing the diastolic function by reducing myocardial perfusion and promoting myocardial fibrosis [28, 29]. Third, IR and metabolic dysfunction can trigger systemic inflammation, leading to increased levels of proinflammatory cytokines and activation of inflammatory pathways [30]. Inflammation then contributes to myocardial fibrosis, which is a hallmark of HFpEF [31].
Our findings revealed a significant association between higher TyG index levels and increased mortality and rehospitalization rates in patients with HFpEF over a 3-year follow-up period. The TyG index has been validated against established measures of IR, such as the hyperinsulinemic-euglycemic clamp [17] and HOMA-IR [32, 33]. Notably, it only requires fasting triglyceride and glucose measurements, which are readily accessible. Therefore, incorporating the TyG index into risk stratification protocols may facilitate the identification of individuals with HFpEF at high risk of death. Moreover, certain therapeutic interventions have demonstrated effectiveness in improving IR and reducing cardiac remodeling in patients with HF. For example, SGLT2i has shown promise for enhancing the cardiac prognosis of patients with type 2 diabetes mellitus, partly by alleviating cardiac IR [34]. Additionally, metformin has been found to significantly reduce left ventricular hypertrophy in patients with IR [35]. Hence, utilizing the TyG index to identify IR may enable early identification of high-risk individuals who could benefit from targeted interventions.
Moreover, IR is closely related to obesity. Clinical studies have presented evidence that obesity is linked to an elevated risk of mortality in patients with HFpEF [36]. Patients with obesity often exhibit hypertriglyceridemia and elevated plasma levels of fatty acids. These lipids are absorbed and stored within lipid droplets in the heart [37]. When intramyocardial lipid accumulation exceeds the heart’s capacity for storage and oxidation, it can become lipotoxic, leading to the development of nonischemic and nonhypertensive cardiomyopathy, commonly referred to as diabetic cardiomyopathy or lipotoxic cardiomyopathy. The clinical characteristics of diabetic cardiomyopathy typically include cardiac hypertrophy and diastolic dysfunction, which culminate in HF, particularly HFpEF [38].
Nevertheless, our study has some limitations. First, owing to the unavailability of fasting insulin levels for most subjects, we were unable to compare the predictive power of different methods for assessing IR in our observational study. Second, we only measured the TyG index at baseline, precluding the assessment of its fluctuations during the follow-up period. Third, despite our best efforts to include a wide range of covariates and potential confounding factors in our analysis, there might still be unmeasured variables such as genetic factors, dietary patterns, psychosocial factors and health care disparities. Fourth, we were unable to capture long-term changes in diastolic function or monitor the incidence of myocardial infarction and stroke. Additionally, we were unable to monitor changes in the NYHA classification and BNP levels during the follow-up period.
Conclusions
Our study demonstrated that increased IR, as indicated by the TyG index, is a significant predictor of all-cause and CV mortality in patients with HFpEF. The TyG index can serve as a valuable tool for identifying early insulin-resistant patients with HFpEF, enabling improved risk stratification and guiding subsequent interventions. These findings underscore the importance of considering IR in the management and prognosis of HFpEF.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the investigators and subjects who participated in this project.
Abbreviations
- ACEI
Angiotensin-converting enzyme inhibitors
- ARB
Angiotensin receptor blockers
- ARNI
Angiotensin receptor II blocker - neprilysin inhibitor
- BMI
Body mass index
- CCB
Calcium channel blockers
- CHD
Coronary heart disease
- Cr
Creatinine
- cTnT
Troponin T
- CV
Cardiovascular
- DBP
Diastolic blood pressure
- E/e’
The Ratio of early diastolic mitral inflow velocity to septal mitral annulus tissue relaxation velocity in early diastole
- FPG
Fasting plasma glucose
- FT3
Free triiodothyronine
- FT4
Free thyroxine
- HDL-C
High-density lipoprotein cholesterol
- HF
Heart failure
- HFmrEF
Heart failure with mid-range ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- HGB
Hemoglobin
- LDL-C
Low-density lipoprotein cholesterol
- LVEDD
Left ventricular end-diastolic dimension
- LVEF
Left ventricle ejection fraction
- MRA
Aldosterone receptor antagonist
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- SBP
Systolic blood pressure
- SGLT2i
Sodium-glucose cotransporter-2 inhibitors
- TC
Total cholesterol
- TG
Triglycerides
- TSH
Thyroid stimulating hormone
- TyG
Triglyceride-glucose index
Authors’ contributions
The study design and data collection were conducted by Q.Z., J.Y., H.T., K.Z. and X.H. Statistical analyses were performed by Q.Z., C.S. X.M., and W.W. Q. Z., Z.G., W.D., Y.W. and H.T. wrote the initial draft of the manuscript. All authors participated in the review and approval of the final version of the manuscript. Y.-D.T. reviewed and edited the manuscript, and served as the guarantor of the study, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by National Natural Science Foundation of China (81825003, 82270376), Beijing Nova Program (Z201100006820002) from Beijing Municipal Science & Technology Commission.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was authorized by the Peking University Third Hospital Ethics Review Committee. Informed consent was obtained from all participants before they were enrolled in the study.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest relevant to this article were reported.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing Zhou, Jie Yang and Hongyi Tang contributed equally to this work and shared first authorship.
Contributor Information
Xinwei Hua, Email: xhua@bjmu.edu.cn.
Yi-Da Tang, Email: tangyida@bjmu.edu.cn.
References
- 1.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–73. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 2.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 3.Abudureyimu M, Luo X, Wang X, Sowers JR, Wang W, Ge J, et al. Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: from pathophysiology to therapeutics. J Mol Cell Biol. 2022;14:mjac028. doi: 10.1093/jmcb/mjac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn VS, Petucci C, Kim MS, Bedi KC, Wang H, Mishra S, et al. Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation. 2023;147:1147–61. doi: 10.1161/CIRCULATIONAHA.122.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42:1569–73. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 6.Alizargar J, Bai CH, Hsieh NC, Wu SFV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19:8. doi: 10.1186/s12933-019-0982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J, Fan H, Wang T, Yu B, Mao S, Wang X, et al. TyG index is positively associated with risk of CHD and coronary atherosclerosis severity among NAFLD patients. Cardiovasc Diabetol. 2022;21:123. doi: 10.1186/s12933-022-01548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20:76. doi: 10.1186/s12933-021-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the heart failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 11.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–70. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Meta-analysis Global Group in Chronic Heart failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–13. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 13.Ingelsson E, Sundström J, Ärnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 14.Erqou S, Adler AI, Challa AA, Fonarow GC, Echouffo-Tcheugui JB. Insulin resistance and incident heart failure: a meta-analysis. Eur J Heart Fail. 2022;24:1139–41. doi: 10.1002/ejhf.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CD, Pan WQ, Feng S, Quan JW, Chen JW, Shu XY, et al. Insulin resistance is associated with heart failure with recovered ejection fraction in patients without diabetes. J Am Heart Assoc. 2022;11:e026184. doi: 10.1161/JAHA.122.026184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo T, von Haehling S, Habedank D, Rauchhaus M, Lainscak M, Sandek A, et al. Usefulness of minimal modelling to assess impaired insulin sensitivity in patients with chronic heart failure. Int J Cardiol. 2011;147:47–51. doi: 10.1016/j.ijcard.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Chan JSK, Guan B, Peng S, Wu X, Lu X, et al. Triglyceride-glucose index and the risk of heart failure: evidence from two large cohorts and a mendelian randomization analysis. Cardiovasc Diabetol. 2022;21:229. doi: 10.1186/s12933-022-01658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang R, Lin Y, Ye X, Zhong X, Xie P, Li M, et al. Triglyceride–glucose index in the development of heart failure and left ventricular dysfunction: analysis of the ARIC study. Eur J Prev Cardiol. 2022;29:1531–41. doi: 10.1093/eurjpc/zwac058. [DOI] [PubMed] [Google Scholar]
- 20.Han S, Wang C, Tong F, Li Y, Li Z, Sun Z, et al. Triglyceride glucose index and its combination with the get with the Guidelines-Heart failure score in predicting the prognosis in patients with heart failure. Front Nutr. 2022;9:950338. doi: 10.3389/fnut.2022.950338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21:88. doi: 10.1186/s12933-022-01507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Xu J, Zhang H, Tao L, Huang X. Triglyceride-glucose index for the detection of subclinical heart failure with preserved ejection fraction in patients with type 2 diabetes. Front Cardiovasc Med. 2023;10:1086978. doi: 10.3389/fcvm.2023.1086978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sajdeya O, Beran A, Mhanna M, Alharbi A, Burmeister C, Abuhelwa Z, et al. Triglyceride glucose index for the prediction of subclinical atherosclerosis and arterial stiffness: a meta-analysis of 37,780 individuals. Curr Probl Cardiol. 2022;47:101390. doi: 10.1016/j.cpcardiol.2022.101390. [DOI] [PubMed] [Google Scholar]
- 24.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864–86. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 25.Lau ES, Panah LG, Zern EK, Liu EE, Farrell R, Schoenike MW, et al. Arterial stiffness and vascular load in HFpEF: differences among women and men. J Card Fail. 2022;28:202–11. doi: 10.1016/j.cardfail.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med. 2007;262:173–83. doi: 10.1111/j.1365-2796.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18:95. doi: 10.1186/s12933-019-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–9. doi: 10.1093/eurheartj/ehx721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R-S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439–50. doi: 10.1093/eurheartj/ehy531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 31.Xiao H, Li H, Wang JJ, Zhang JS, Shen J, An XB, et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur Heart J. 2018;39:60–9. doi: 10.1093/eurheartj/ehx261. [DOI] [PubMed] [Google Scholar]
- 32.Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32:596–604. doi: 10.1016/j.numecd.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Wang M, Mei L, Jin A, Cai X, Jing J, Wang S, et al. Association between triglyceride glucose index and atherosclerotic plaques and Burden: findings from a community-based study. Cardiovasc Diabetol. 2022;21:204. doi: 10.1186/s12933-022-01638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Ni J, Guo R, Li L, Su J, He F, et al. SGLT2 inhibitors break the vicious circle between heart failure and insulin resistance: targeting energy metabolism. Heart Fail Rev. 2021;27:961–80. doi: 10.1007/s10741-021-10096-8. [DOI] [PubMed] [Google Scholar]
- 35.Mohan M, Al-Talabany S, McKinnie A, Mordi IR, Singh JSS, Gandy SJ, et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Heart J. 2019;40:3409–17. doi: 10.1093/eurheartj/ehz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsujimoto T, Kajio H. Abdominal obesity is Associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;5;70;2739-49. [DOI] [PubMed]
- 37.Paul A, Chan L, Bickel PE. The PAT family of lipid droplet proteins in heart and vascular cells. Curr Hypertens Rep. 2008;10;461-6. [DOI] [PMC free article] [PubMed]
- 38.Nakamura M, Sadoshima J. Cardiomyopathy in obesity, insulin resistance and diabetes. J Physiol. 2020;598:2977–93. doi: 10.1113/JP276747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.