Abstract
Purpose
To evaluate the efficacy of topical losartan after blast injury-simulating irregular phototherapeutic keratectomy (PTK) in rabbits.
Methods
Twelve NZW rabbits underwent 100 pulse 6.5 mm diameter PTK over a metal screen to generate severe surface irregularity and inhibit epithelial basement membrane regeneration. Corneas were treated with 0.8 mg/mL losartan in balanced salt solution (BSS) or BSS 50 µL six times per day for six weeks after PTK. All corneas had slit lamp photography, with and without 1% fluorescein at two, four, and six weeks after PTK, and were analyzed using immunohistochemistry for the myofibroblast marker α-smooth muscle actin (α-SMA), keratocyte marker keratocan, mesenchymal cell marker vimentin, transforming growth factor (TGF)–β1, and collagen type IV.
Results
Topical 0.8 mg/mL losartan six times a day significantly decreased anterior stromal α-SMA intensity units compared to BSS at six weeks after anterior stromal irregularity-inducing screened PTK (P = 0.009). Central corneal opacity, however, was not significantly different between the two groups. Keratocan, vimentin, TGF-β1, or collagen type IV levels in the anterior stroma were not significantly different between the two groups.
Conclusions
Topical losartan effectively decreased myofibroblast generation after surface blast simulation irregular PTK. However, these results suggest initial masking-smoothing PTK, along with adjuvant topical losartan therapy, may be needed to decrease corneal stromal opacity after traumatic injuries that produce severe surface irregularity.
Translational Relevance
Topical losartan decreased scar-producing stromal myofibroblasts after irregular PTK over a metal screen but early smoothing of irregularity would also likely be needed to significantly decrease corneal opacity.
Keywords: losartan, cornea, fibrosis, scarring, myofibroblasts, corneal fibroblasts, basement membranes, perlecan, collagen type IV, TGF-β1, persistent epithelial defects, PTK
Introduction
Topical losartan, an angiotensin II receptor blocker with known efficacy in blocking ERK-modulated transforming growth factor (TGF)-β signaling involved in myofibroblast development from precursor cells,1 has been shown to decrease myofibroblast generation and corneal stromal opacity after posterior fibrosis-producing Descemetorhexis,2 full-thickness corneal fibrosis-producing alkali burns,3 and anterior stromal scarring fibrosis-producing photorefractive keratectomy4 in rabbits. A case report in a human also found that topical losartan was effective in treating stromal scarring after laser in situ keratomileusis complicated by a displaced flap and severe diffuse lamellar keratitis.5 The epithelial basement membrane (EBM), and its components collagen type IV and perlecan, have been shown to have a critical role in modulating activated TGF-β1 or -β2 entry into the anterior stroma after corneal injury.6,7
Trauma to the cornea and some diseases of the cornea, as well as some surgical complications, may produce severe corneal surface irregularity and vision loss. Corneal surface irregularity interferes mechanically and directly with EBM regeneration and thereby facilitates TGF-β entry into the stroma to promote the development of myofibroblasts from precursor cells.6,8 “Irregular” excimer laser phototherapeutic keratectomy (PTK) applied through a metal screen can be used to produce precise and reproducible levels of stromal surface irregularity, depending on the number of pulses of laser delivered to the corneal surface and the impediments, such as a screen, that are placed in the path of the laser beam.8 Because myofibroblast development is a major component of corneal opacity, it was hypothesized that topical losartan treatment could itself improve the visual performance in these eyes without the need to resort to surgical approaches, such as phototherapeutic keratectomy with masking smoothing, lamellar corneal transplantation, or penetrating keratoplasty. The results of this study show that topical losartan alone would not likely be beneficial in such corneas. Rather, the results suggest that initial smoothing of the surface would probably be an important first step, even if a medication, such as topical losartan,2–5 that decreased myofibroblast development and promoted apoptosis of stromal myofibroblasts and their precursor cells, was subsequently used in treatment.
Methods
Animals and Irregular PTK Surgery
Rabbit surgeries and procedures were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic Foundation. All animals were treated in accordance with the tenets of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Twelve female 12- to 15-week-old New Zealand white rabbits weighing 2.5 to 3 kg each were included in this study. Twenty-four hours before irregular PTK, and continuing at least five days after PRK, all rabbits received 60 mL children's liquid acetaminophen (Johnson & Johnson, Ft. Washington, PA, USA) in each liter of drinking water. One eye of each rabbit was randomly selected to have irregular PTK and received two drops of topical 1% proparacaine hydrochloride (Alcon, Fort Worth, TX, USA) before the surgery. The rabbit was placed under general anesthesia with 30 mg/kg ketamine hydrochloride and 5 mg/kg xylazine by intramuscular injection.
In the treated eye, an 8 to 9 mm manual epithelial debridement was performed with a no. 6400 Beaver blade (MedexSupply, Passaic, NJ, USA). PTK with a 6.5 mm optical zone centered on the entrance pupil was performed with a VISX (Santa Clara, CA, USA) S4 IR excimer laser by holding a metal screen (individual rectangles in the screen approximately 2 mm × 1.5 mm) stationary against the intended ablation zone and applying a total of 100 pulses (each pulse removes approximately 0.25 µm of corneal stroma, and therefore the screen pattern ablated onto the central cornea had a depth of 25 µm into the anterior stroma (Fig. 1).8 No mitomycin C or corticosteroids were administered after surgery, but all eyes that had irregular PTK were treated with one drop of topical ciprofloxacin three times a day until the epithelium had closed (typically five days after surface ablation surgery).6,7
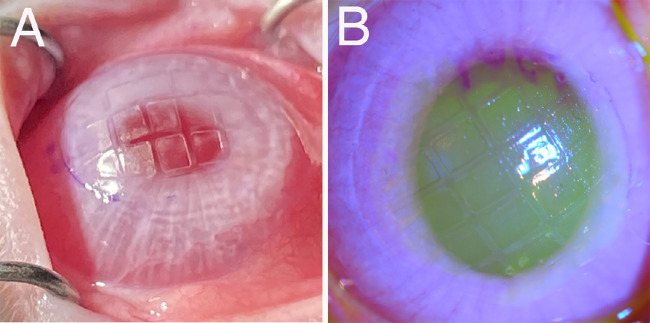
Figure 1.
Corneal surface irregularity produced by PTK through a screen (approximate 2.0 × 1.5 mm rectangles) in a rabbit. (A) Image taken with iPhone 12 (Apple, Cupertino, CA, USA) without fluorescein. (B) Slit lamp image after 30 µL of topical 1% fluorescein. In both images the screen pattern can be seen imprinted on the corneal stromal surface. Magnification × 10.
Monitoring Corneas for Closure of Corneal Epithelial Defects
All corneas were monitored for epithelial defects with 1% fluorescein drops (Bausch & Lomb, Rochester, NY, USA) and a cobalt blue filter at two weeks, four weeks, and six weeks after irregular PTK surgery. All pupils were dilated with two drops of 1% tropicamide (Akorn Co., Lake Forest, IL, USA) for 30 minutes, and slit-lamp photos were obtained, without and with 1% fluorescein drops. Slit lamp photos were taken at a standardized illumination level, angle of illumination and imaging angle, with magnification ×10, using a Topcon (Oakland, NJ, USA) SL-D7 slit-lamp photography system with the rabbit under ketamine-xylazine general anesthesia.
Corneal Cryofixation and Sectioning
At six weeks after PRK injury and treatment with topical 0.8 mg/mL losartan or topical BSS vehicle, each rabbit was euthanized while under ketamine-xylazine general anesthesia with 100 mg/kg Beuthanasia (Shering-Plough, Kenilworth, NJ, USA) by intravenous injection, followed by bilateral pneumothorax. Corneoscleral rims were removed with sharp Westcott scissors (Fairfield, CT, USA) and 0.12 forceps (Storz, St. Louis, MO, USA) without touching the corneal surfaces. Corneoscleral rims were centered in a 24-mm × 24-mm × 5-mm mold (Fisher Scientific, Pittsburgh, PA, USA) that was subsequently filled with optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA). The mold and cornea were then quick frozen on dry ice and stored at −80°C until sectioning was performed.
Blocks were bisected in the center of the original ablated zone of each cornea and 10-µm–thick transverse sections were cut from the central cornea using a cryostat (HM 505M; Micron GmbH, Walldorf, Germany). Three to four sections from each cornea were placed on each 25-mm × 75-mm × 1-mm Superfrost Plus microscope slide (Fisher Scientific). Slides with sections were maintained at −20°C before immunohistochemistry (IHC).
IHC for α-SMA, Keratocan, Vimentin, TGF-β1, and Collagen Type IV
Triplex IHC was performed for α-SMA (myofibroblast marker, red), vimentin (mesenchymal cell marker, at a concentration where all corneal fibroblasts and myofibroblasts, but only rare keratocytes were positive, yellow),9,10 and keratocan (marker for keratocytes, green), using previously described methods6 and primary antibodies confirmed by Western blotting and IHC to recognize rabbit antigens or isotypic non-specific control antibodies (ThermoFisher Scientific, Waltham, MA, USA), and previously described secondary fluorescent tagged antibodies (Table).6 IHC was also performed for TGF-β1 and collagen type IV with 4′,6-diamidino-2-phenylindole staining of nuclei using previously characterized primary and secondary antibodies (Table).6 The TGF-β1 antibody used in this study does not bind TGF-β2 or TGF-β3.6 The collagen type IV antibody (cat. AB769; Millipore, Temecula, CA, USA) used in this study was generated against purified human and bovine collagen type IV and affinity purified with human and bovine collagen type IV crosslinked to agarose and cross-absorbed by the manufacturer with human and bovine collagens type I, II, III, V, and VI to eliminate cross-reactivity. This collagen type IV antibody was shown previously to bind rabbit collagen IV in IHC7 and binds the α1/α2 chains but not the α3 to α6 chains. Winston Kao, Ph.D., graciously provided the keratocyte-specific keratocan antibody raised against peptide H2N-LRLDGNEIKPPIPIDLVAC-OH.6,7 Images were obtained at total magnification ×200 on a Leica DM6B upright microscope equipped with an automated stage and Leica 7000 T camera using the LASX software (Leica Microsystems, GmbH, Wetzlar, Germany).
Table.
Antibodies Used in Immunohistochemistry
| Antigen | Source | Species Source | Ab Isotype | Catalog Number | Dilution |
|---|---|---|---|---|---|
| Primary antibodies | |||||
| α-SMA | DAKO | Mouse | IgG2a | M0851 | 1:400 |
| Vimentin | Abcam | Chicken | IgY | ab24525 | 1:2000 |
| Keratocan | Winston Kao, Ph.D. | Goat | IgG | — | 1:200 |
| TGF-β1 | GeneTex | Mouse | IgG1 | GTX21279 | 1:100 |
| Collagen type IV | EDM Millipore | Goat | IgG1 | AB769 | 1:2000 |
| Secondary antibodies | |||||
| Alexa Fluor 568 anti-mouse | Thermo Fisher Scientific | Donkey | IgG | A10037 | 1:200 |
| Alexa Fluor 488 anti-goat | Thermo Fisher Scientific | Donkey | IgG | A11055 | 1:200 |
| Alexa Fluor 488 anti-mouse | Thermo Fisher Scientific | Donkey | IgG | A21202 | 1:200 |
| Alexa Fluor 568 anti-goat | Thermo Fisher Scientific | Donkey | IgG | A11057 | 1:200 |
| Alexa Fluor 647 anti-chicken | Jackson ImmunoResearch Inc. | Donkey | IgY | 703-605-155 | 1:400 |
All images were converted to 900 pixel-width × 673 pixel-height 300 DPI images with Photoshop 23.5.2 (Adobe, San Jose, CA, USA) to prepare composite image figures and images for α-SMA or TGF-β1 quantitation in the anterior stroma using ImageJ 1.53a analysis software (https://imagej.net).6,7 The mean pixels of stromal keratocan, vimentin, α-SMA, collagen type IV, and TGF-β1 were determined using image panels showing only the antigen of interest in a 200 pixel-wide × 75 pixel-high rectangle with the anterior side tangent to the anterior stromal surface with ImageJ.
Statistics
Statistical analyses were performed using the non-parametric Mann Whitney U test (https://www.statskingdom.com/kruskal-wallis-calculator.html), and P < 0.05 was considered statistically significant.
Results
An epithelial defect beyond the three to seven days normally required for initial epithelial closure after surface ablation procedures was only detected in a single cornea (cornea no. 2 in the losartan-treated group) at four weeks after irregular PTK (Supplementary Figure), but the epithelium in this cornea was subsequently healed at the six-week time point. Therefore that epithelial defect was present between two and six weeks after injury, but the precise duration is unknown. All other corneas had healed epithelium without fluorescein staining at two weeks, four weeks, and six weeks after PRK and treatment.
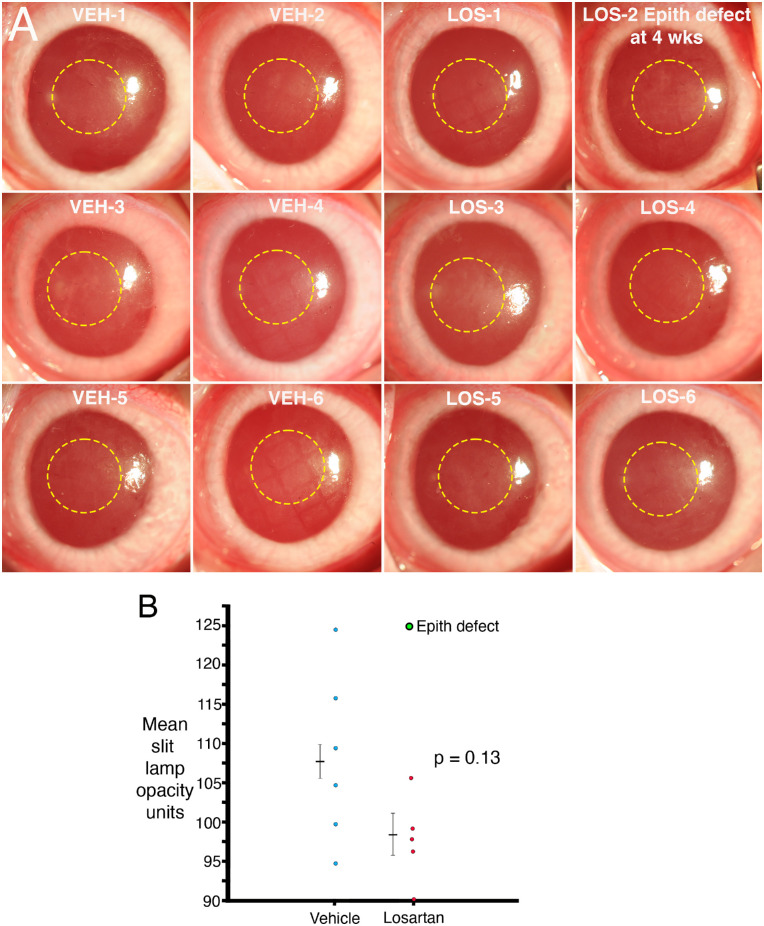
Figure 2A shows the standardized slit lamp images of each cornea after irregular PTK and six weeks of treatment with topical BSS vehicle (VEH) or topical 0.8 mg/mL losartan (LOS) six times per day. The yellow dotted circles indicate the locations where ImageJ was used to measure total stromal opacity pixel units. The LOS-2 cornea had an epithelial defect detected at four weeks that was subsequently found to have healed with topical fluorescein staining (not shown) by the six-week examination (Supplementary Figure). Figure 2B provides the mean slit lamp opacity units in the central ablated cornea quantitated with ImageJ for each cornea. The cornea LOS-2 that developed an epithelial defect at four weeks is shown as a green data point in Figure 2B because it was considered an outlier. The difference in mean slit lamp opacity trended toward a difference but did not reach statistical significance (P = 0.13), even when the outlier was not included in the analysis.
Figure 2.
Slit lamp opacity. (A) Standardized slit lamp photos of corneas at six weeks after irregular PTK and treatment with 50 µL of BSS vehicle (VEH) or 0.8 mg/mL LOS six times per day for six weeks. Yellow dashed circles are 3 mm diameter ImageJ opacity quantitation areas. The LOS cornea that developed an epithelial defect (likely from rabbits fighting or perhaps the animal scratching an eye on the water bottle or cage) is labeled. (B) Mean slit lamp opacity quantitation with ImageJ. The losartan cornea that developed an epithelial defect at four weeks after irregular PTK is indicated as green. Although the overall opacity trended toward less in the losartan-treated group, the result did not reach statistical significance, with or without the outlier included in the analysis.
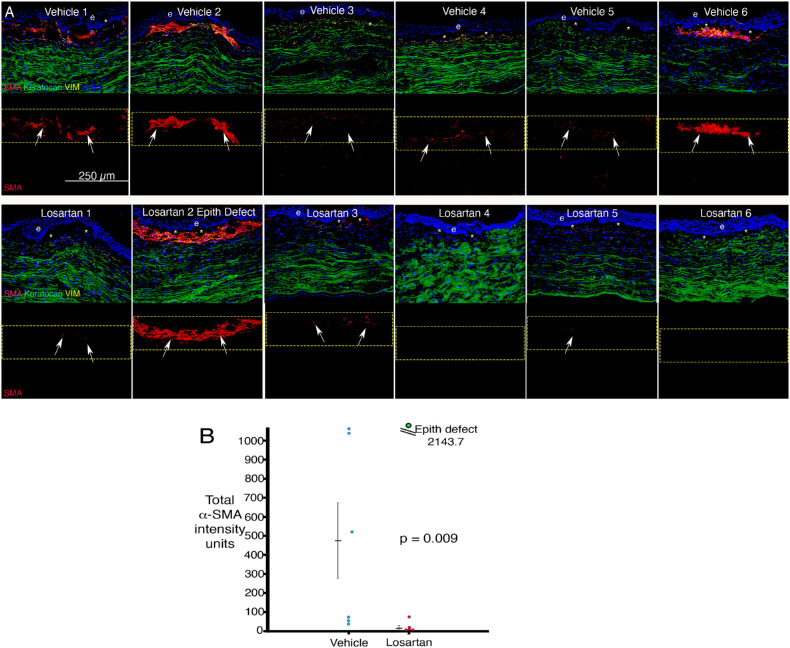
IHC for α-SMA (SMA, myofibroblast marker, red), keratocan (keratocyte marker, green) and vimentin (VIM, mesenchymal cell marker, yellow) after irregular PTK and six weeks of topical vehicle BSS or topical 0.8 mg/mL losartan in BSS six times per day is shown in Figure 3A. The only losartan-treated cornea that developed high density of myofibroblasts was the losartan no. 2 cornea that had developed an epithelial defect at four weeks after irregular PTK and topical losartan treatment (Supplementary Figure). Total α-SMA intensity measured with ImageJ in anterior stromal 200 pixel-wide × 75 pixel-quantitation boxes, with one side approximately tangent to the anterior stromal surface, is shown in Figure 3B. The difference between the two groups in anterior stromal myofibroblast α-SMA intensity was significant (P = 0.009). The cornea that developed an epithelial defect was considered an outlier and was not included in the statistical analysis, but its α-SMA intensity is shown in green in Figure 3B. Total keratocan and vimentin intensity measured with ImageJ in anterior stromal 200 pixel-wide × 75 pixel-quantitation boxes with one side approximately tangent to the anterior stromal surface were also determined using three IHC slides for each cornea and no difference was found for either marker between the two groups for either keratocan (P = 0.23) or vimentin (P = 0.74). The keratocan marker would be specific for keratocytes, but the vimentin-positive cells would include corneal fibroblasts, myofibroblasts, fibrocytes, and some keratocytes, and distinguishing between the different vimentin-positive cells would only be possible with simultaneous multiplex IHC, including several more markers, including CD34 and CD45, for example, to distinguish fibrocytes.
Figure 3.
Triplex IHC for α-SMA (marker for myofibroblasts, red), vimentin (marker for mesenchymal cells, yellow), and keratocan (marker for keratocytes, green) in corneas at six weeks after irregular PTK and treatment with topical vehicle or losartan six times per day. (A) α-SMA staining tended to be greater in vehicle-treated corneas than LOS-treated corneas at six weeks after irregular PTK and treatment. The outlier no. 2 LOS cornea that formed an epithelial defect by four weeks developed far more myofibroblasts than other corneas in the losartan group. e is epithelium. Asterisk is artifactual dislocation of the epithelium that occurs during sectioning in many corneas after this injury. (B) Mean α-SMA intensity units measured in 200 pixel-width × 75 pixel-height rectangles on 300 DPI images. The difference between the two groups was highly significant if the outlier (green) losartan-treated cornea with an epithelial defect was excluded from the analysis and trended toward significance (P = 0.09) even if that cornea was included.
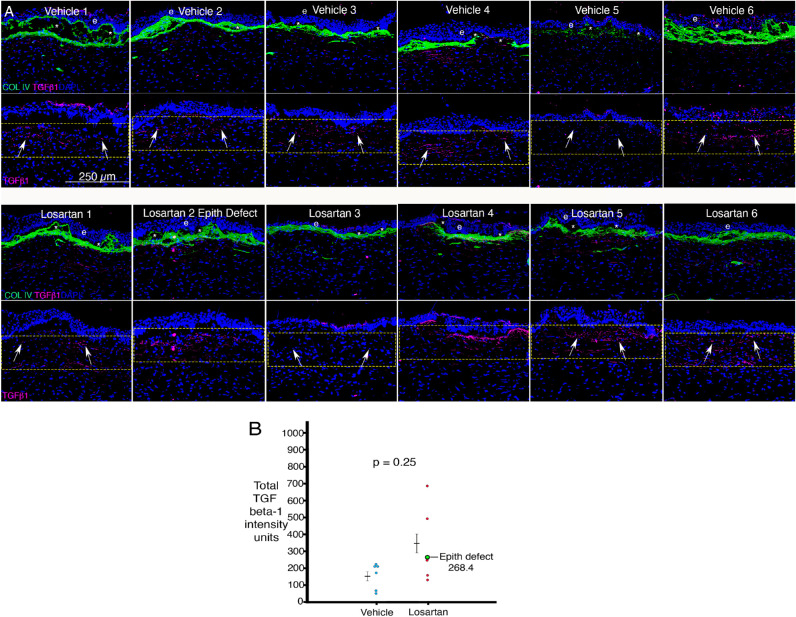
Representative IHC for TGF-β1 and collagen type IV at six weeks after irregular PTK and treatment with topical vehicle (VEH) BSS or topical LOS is shown for each cornea in Figure 4A. Total TGF-β1 intensity measured with ImageJ in anterior stromal 200 pixel-wide × 75 pixel-height quantitation rectangles with one side approximately tangent to the anterior stromal surface is shown in Figure 4B. The difference between the two groups in anterior stromal TGF-β1 intensity was not significant (P = 0.25). The cornea that developed an epithelial defect was considered an outlier and was not included in the statistical analysis, but its TGF-β1 intensity is shown in green on the graph. Although anterior stromal TGF-β1 levels appeared to be relatively high in some losartan treated corneas (Fig. 4B), the means for anterior stromal TGF-β1 in the losartan- and vehicle BSS-treated groups were not significantly different. Similarly, no difference in anterior stromal collagen type IV immunohistochemistry staining intensity (Fig. 4A) was found between the two groups (P = 0.65).
Figure 4.
IHC for TGF β-1 and collagen type IV in corneas at six months after irregular PTK and treatment with topical BSS vehicle or topical losartan six times per day. (A) Representative IHC images from the center of each cornea. TGF-β1 was variably localized in the apical epithelium and consistently throughout the anterior stroma in each cornea of both groups. Collagen type IV (green) was deposited at high levels in the anterior stroma in all corneas in both groups. e is epithelium. Asterisk indicates artifactual dislocations of epithelium from stroma that occur during sectioning. The yellow rectangles are 200-pixel × 75-pixel ImageJ quantitation areas. (B) Although the mean anterior stromal TGF-β1 trended higher in losartan-treated corneas, there was no statistically significant difference, whether or not the cornea that developed an epithelial defect (LOS no. 2) was included in the analysis.
Discussion
Topical losartan has shown efficacy and safety in decreasing corneal myofibroblast development and opacity in rabbits after posterior Descemetorhexis injury,2 full-thickness alkali burns,3 and anterior photorefractive keratectomy injury.4 Topical losartan has also been found to be effective in a human case report after complicated refractive surgery.5 It has been hypothesized that topical losartan or losartan released intraocularly by drug delivery devices could be of benefit for a variety of anterior segment and posterior segment ocular fibrotic disorders.9,10
In the present study, topical losartan was evaluated in a particularly challenging anterior corneal scarring injury where a metallic screen is placed over the ablated cornea during the excimer laser PTK ablation to generate a stromal grid patten on the corneal surface (Fig. 1). This produces surface irregularity which mechanically interferes with regeneration of the EBM.6,7 The resulting corneal injury is reproducible and similar to what might occur with severe blast trauma to the cornea in an accident or military combat. Although the topical losartan was effective in decreasing stromal myofibroblast density, as it was in prior studies,2–4 the results of this study demonstrated that at six weeks after this injury, the central corneal opacity in the losartan and vehicle groups was not significantly different. Thus other factors such as the surface irregularity itself or the presence of other cells not affected by losartan, such as corneal fibroblasts, and the disordered extracellular matrix those cells produce, remain critical determinants of the overall corneal opacity in both groups.11 The central corneal opacity might have continued to diminish in these corneas with further topical losartan treatment beyond six weeks because the wound healing response was ongoing. However, this study suggests a better approach in corneas with severe surface irregularity would be to perform a surgical treatment, like PTK with a masking-smoothing agent,12 to regularize the stromal surface as soon as possible after the injury, and thereby facilitate regeneration of normal EBM. This treatment could include a course of antifibrotic treatment with topical 0.8 mg/mL losartan six times per day and continuing for at least six months, and perhaps longer if improvement is noted without complete resolution. In the early stages after such a severe injury, it is likely that topical corticosteroids would also be helpful because of the severe keratitis associated with these injuries.13
After surface ablation procedures in normal corneas, the EBM lamina lucida-lamina densa pattern is usually regenerated by eight to 10 days after injury12 and results in a marked decrease in TGF-β1 and TGF-β2 entry into the anterior stroma from the epithelium and tears.6,7 When a cornea subsequently develops an epithelial defect, either spontaneously or by trauma to the eye, then the passage of TGF-β into the stroma at high levels resumes and once again stimulates the development of anterior stromal myofibroblasts from corneal fibroblast and fibrocyte precursors.6,7 The effect of an EBM abnormality can be seen in cornea no. 2 in the losartan-treated group, where a dense layer of myofibroblasts was present at six weeks after injury (Fig. 3A). The grid pattern produced on the corneal surface by irregular PTK likely prolonged EBM regeneration in all of the corneas in both groups of this study by at least a few weeks. However, abrasion or spontaneous epithelial breakdown likely further delayed this EBM regeneration and led to the development of greater numbers of α-SMA myofibroblasts and overall stromal opacity in the no. 2 losartan-treated cornea that developed a late epithelial defect. This cornea therefore was considered an outlier and was not included in statistical comparisons between the two groups in this study.
This study reinforces the observations that visually significant corneal opacity is produced by a combination of factors that include (1) ocular surface irregularity that causes disruptions in the tear film, (2) the development of relatively opaque cells compared to keratocytes (including corneal fibroblasts and myofibroblasts) that have downregulated production of intracellular crystallins,11 and (3) the production of disordered extracellular matrix components, including collagen type I, collagen type III, and collagen type IV, by corneal fibroblasts and myofibroblasts, that disrupt the normal morphology and organization of the corneal stromal fibrils associated with transparency.2–4,6,7,14 Thus topical losartan alone is not likely to be effective in reducing overall opacity in corneas with severe surface irregularity.
This study also highlights the complexities of the overall corneal wound healing response that control corneal opacity versus transparency. Some of these factors include (1) the critical importance of timely and persistent healing of the epithelium (because no EBM regeneration is possible until the epithelium closes over a particular area of the cornea and opacity will occur after late breakdown of the epithelium),15,16 (2) the regeneration of the EBM through the coordinated production of components by both the epithelial cells and the corneal fibroblasts/keratocytes that include laminins, perlecan, and collagen type IV is a key to transparency of the cornea,17–19 (3) the downregulation of TGF-β effects on myofibroblasts/corneal fibroblasts because of decreased stromal passage of active TGF-β or inhibition of TGF-β effects,6,7 (4) apoptosis or reversion to precursor cells by corneal fibroblasts and myofibroblasts,6,7 (5) return of a normal cellularity with the affected stroma repopulated mostly with keratocytes,6,7 and (6) normal keratocytes functioning over time to reabsorb/reorganize corneal stromal ECM and return the cornea to transparency.14 A failure to return to normal in more than one of these factors, and likely others not well understood, is often involved in the development and persistent of vision compromising opacity.
The levels and localization of TGF-β1 and collagen type IV in the anterior stroma did not differ between the two groups (Fig. 4A), although there was higher anterior stromal TGF-β1 in two corneas treated with losartan (Fig. 4B). It is likely this represented the normal variations that can occur in stromal TGF-β1 levels between corneas that have identical injuries and treatments. The collagen type IV that localized to the anterior stroma in all corneas in this study was likely produced by corneal fibroblasts that coordinate with the epithelium to regenerate the mature EBM, and also to modulate TGF-β-driven effects on corneal stromal cells.2,20 Topical losartan treatment did not affect the stromal collagen type IV levels in this study.
In conclusion, topical losartan was effective in decreasing stromal opacity-producing myofibroblast generation after reproducible corneal surface irregularity produced by PTK ablation through a metal screen. This study suggests that efforts to decrease surface irregularity after injury, such as masking-smoothing excimer laser PTK, would likely be an important first treatment step before the use of adjuvant topical losartan treatments, because smoothing of the ocular surface would promote regeneration of the EBM and subsequent restoration of the regulation of tear and epithelial TGF-β1 and TGF-β2 localization to the corneal stroma, while at the same time decreasing corneal opacity that is directly related to the corneal surface irregularity itself. Experiments testing that hypothesis are planned for the future.
Supplementary Material
Acknowledgments
The authors thank Winston Kao, PhD, Cinncinnati, OH for providing the keratocan antibody used in this study.
Supported in part by Department of Defense grant VR210001 (SEW) and P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD, and Research to Prevent Blindness, New York, NY, USA, and the Cleveland Eye Bank Foundation, Cleveland, OH, USA.
Disclosure: L.P. Sampaio, None; V. Villabona-Martinez, None; T.M. Shiju, None; M.R. Santhiago, None; S.E. Wilson, Cleveland Clinic submitted a patent on the use of topical losartan and other angiotensin II receptor blockers (ARBs) to prevent and treat corneal scarring fibrosis
References
- 1. Habashi JP, Doyle JJ, Holm TM, et al.. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011; 332: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sampaio LP, Hilgert GSL, Shiju TM, Murillo SE, Santhiago MR, Wilson SE.. Topical losartan inhibits corneal scarring fibrosis and collagen type IV deposition after Descemet's membrane-endothelial excision in rabbits. Exp Eye Res. 2022; 216: 108940. [DOI] [PubMed] [Google Scholar]
- 3. Sampaio LP, Hilgert GSL, Shiju TM, Santhiago MR, Wilson SE.. Topical losartan and corticosteroid additively inhibit corneal stromal myofibroblast generation and scarring fibrosis after alkali burn injury. Transl Vis Sci Technol. 2022; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sampaio LP, Hilgert GSL, Shiju TM, Santhiago MR, Wilson SE.. Losartan inhibition of myofibroblast generation and late haze (scarring fibrosis) after PRK in rabbits. J Ref Surg. 2022; 38: 820–829. [DOI] [PubMed] [Google Scholar]
- 5. Souza ALP, Ambrosio R Jr., Bandeira F, Salomão MQ, Lima AS, Wilson SE. Topical losartan for treating corneal fibrosis (haze): first clinical experience. J Ref Surg. 2022; 38: 741–746. [DOI] [PubMed] [Google Scholar]
- 6. de Oliveira RC, Tye G, Sampaio LP, et al.. TGFβ1 and TGFβ2 proteins in corneas with and without stromal fibrosis: delayed regeneration of epithelial barrier function and the epithelial basement membrane in corneas with stromal fibrosis. Exp Eye Res. 2021; 202: 108325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Oliveira RC, Sampaio LP, Shiju TM, Santhiago MR, Wilson SE.. Epithelial basement membrane regeneration after PRK-induced epithelial-stromal injury in rabbits: fibrotic vs. non-fibrotic corneal healing. J Ref Surg. 2022; 38: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohan RR, Stapleton WM, Sinha S, Netto MV, Wilson SE.. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser. Exp. Eye Res. 2008; 86: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson SE . Magic Bullets: the coming age of meaningful pharmacological control of the corneal responses to injury and disease. J Ocul Pharmacol Ther. 2022; 38: 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson SE. Topical losartan: practical guidance for clinical trials in the prevention and treatment of corneal scarring fibrosis and other eye diseases and disorders. J Ocular Pharm Ther. 2023; 39: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jester JV, Moller-Pedersen T, Huang J, et al.. The cellular basis of corneal transparency: evidence for “corneal crystallins.” J Cell Sci. 1999; 112(Pt 5): 613–622. [DOI] [PubMed] [Google Scholar]
- 12. Wilson SE, Marino G, Medeiros C, Santhiago MR.. Phototherapeutic keratectomy (PTK): science and art. J Ref Surg. 2017; 33: 203–210. [DOI] [PubMed] [Google Scholar]
- 13. Santhanam A, Marino GK, Torricelli AAM, Wilson SE.. EBM regeneration and changes in EBM component mRNA expression in the anterior stroma after corneal injury. Mol Vision. 2017; 23: 39–51. [PMC free article] [PubMed] [Google Scholar]
- 14. Hassell JR, Birk DE.. The molecular basis of corneal transparency. Exp Eye Res. 2010; 91: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson SE, Medeiros CS, Santhiago MR.. Pathophysiology of corneal scarring in persistent epithelial defects after PRK and other corneal injuries. J Ref Surg. 2018; 34: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sampaio LP, Villabona-Martinez V, Shiju TM, Hilgert GSL, Santhiago MS, Wilson SE.. Cell biology of spontaneous persistent epithelial defects after photorefractive keratectomy in rabbits. Trans Vis Sci Tech. 2023; 12: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson SE. Defective perlecan-associated basement membrane regeneration and altered modulation of transforming growth factor beta in fibrosis. Cell and Molec Life Sci. 2022; 79: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson SE. Fibrosis is a basement membrane-related disease in the cornea: injury and defective regeneration of basement membranes may underlie fibrosis in other organs. Cells. 2022; 11: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson SE. Interleukin-1 and transforming growth factor beta: commonly opposing, but sometimes supporting, master regulators of the corneal wound healing response to injury. Invest Ophth Vis Sci. 2021; 62: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson SE. The yin and yang of mesenchymal cells in the corneal stromal fibrosis response to injury: the cornea as a model of fibrosis in other organs. Biomolecules. 2022; 13: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.