Abstract
Background:
Treatments for inflammatory bowel diseases (IBD) have evolved in the era of biologics. However, the real-world data on their usage patterns and sequencing are still limited.
Objectives:
We aimed to investigate treatment persistence and dose intensification of first- and second-line biologics in patients with IBD.
Design:
In this retrospective, cohort study using nationwide claims data, 13,087 patients with IBD initiating biologic therapy between 2010 and 2020 were identified.
Methods:
Treatment persistence and dose intensification during the first 2 years and switching patterns of biologics were analysed while identifying predictors of non-persistence.
Results:
As a first-line treatment of Crohn’s disease (CD), ustekinumab had a lower risk for non-persistence compared to infliximab [adjusted hazard ratio (aHR), 0.69, p = 0.048]. Second-line ustekinumab and vedolizumab showed the highest and lowest persistence (79.2% and 54.9%), respectively. As a first-line treatment of ulcerative colitis (UC), golimumab had a higher risk for non-persistence compared to infliximab (aHR, 1.68, p < 0.001). Second-line golimumab also showed a significantly lower persistence rate than adalimumab and vedolizumab. The risk of non-persistence was higher in UC than in CD (first line: aHR, 1.97; second line: aHR, 1.39; p < 0.001), and in the second-line treatment than in the first-line treatment for CD (aHR, 1.55; p < 0.001). The cumulative rate of dose intensification was highest with ustekinumab for CD (first line, 43.3%, second line, 69.1%) and adalimumab for second-line UC (40.7%). It was significantly increased in second-line therapy in CD, but not in UC. Among switchers of first-line anti-tumour necrosis factor-α inhibitor therapy, after all biologics were approved, 69% of CD patients and 78.4% of UC patients switched to other classes of second-line treatment.
Conclusion:
Ustekinumab had higher persistence in the first-line treatment of CD, while golimumab had lower persistence for first- and second-line treatments of UC. Dose intensification rates varied, with the highest cumulative rates observed for ustekinumab in CD and adalimumab in second-line UC.
Keywords: biologics, Crohn’s disease, dose intensification, inflammatory bowel disease, persistence, real-world treatment, ulcerative colitis
Introduction
Inflammatory bowel diseases (IBDs), comprising Crohn’s disease (CD) and ulcerative colitis (UC), are chronic immune-related diseases characterized by inflammation of the gastrointestinal tract. 1 Currently, the global prevalence of IBD is 90 cases per 100,000 people; this has increased by 31% from 1990 to 2017.2,3 In South Korea, the prevalence of IBD also increased from 2010 to 2019, more than doubling in the span of 10 years, ranging from 15.1 to 36.9 per 100,000 for CD and 31.4 to 65.7 per 100,000 for UC. 4
Over the past decades, various therapeutics, including biologics and small molecules, have been developed, bringing significant clinical improvements and benefits compared to existing treatments.5,6 The agents approved for the treatment of IBD by the Ministry of Food and Drug Safety (MFDS) in South Korea are as follows: anti-tumour necrosis factor (TNF)-α (infliximab, adalimumab and golimumab), anti-α4β7-integrin (vedolizumab), anti-interleukin-12/23 p40 antibody (ustekinumab) and Janus kinase inhibitor (tofacitinib) (Supplemental Figure S1).
Despite the efficacy of biologics in inducing and maintaining remission in IBD, 10–30% and 12–22% of patients with CD and UC, respectively, do not initially respond to biologics (primary non-response). Moreover, 23–64% of CD patients and 49–59% of UC patients eventually lose their responses.7,8 In addition, it has been reported that the response rate is lower in biologics-experienced patients (second line or above) with IBD than in biologic-naïve patients. 9 Although the primary response of biologics is an important goal in the treatment of IBD, it is also important to clinically guarantee long-term outcomes without switching or discontinuing them due to lack of efficacy or tolerability issues, as advanced therapeutic options are limited in IBD. Therefore, it is important to consider the persistence of biologics when selecting therapeutic agents. Several studies have reported the persistence of anti-TNF-α agents,10–13 but few have examined recently approved biological agents, such as vedolizumab and ustekinumab. Additionally, dose intensification (increase in dosage or in the frequency of treatment administration) of biologics is one option for patients with secondary loss of response 14 ; yet, there are few real-world data on dose intensification, especially in Asia.
This study aimed to examine the treatment patterns of first- and second-line biologics in patients with IBD using a nationwide database in South Korea. The primary objective of this study was to assess the persistence of different therapies at 2 years, while the secondary objectives were to compare the cumulative incidence of dose intensification and switching patterns between index biologics, as well as identify predictors of non-persistence and dose intensification.
Methods
Data source
This study was based on an anonymized dataset spanning a 12-year period from January 2008 through July 2021 from the National Health Insurance database of the Health Insurance Review and Assessment Service (HIRA). The HIRA contains almost the entire population of South Korea; the dataset includes approximately 51 million insured individuals in the country, which reflects 98% of the South Korean population insured by the National Health Insurance system. 15 Thus, these data reliably present the overall treatment of the South Korean population. 15 The dataset covered a wide range of healthcare information, encompassing outpatients, inpatients and emergency visits. It includes demographic characteristics, patients’ diagnoses, surgeries, hospitalizations and comorbidities. Furthermore, the dataset contains prescription details, including daily dosages, dates of prescription, days of supply and quantities of supply.
Study design and population
A retrospective, longitudinal cohort study was conducted using data from patients with IBD who initiated biologic therapy during the index period (from 1 January 2010 to 31 December 2020). The index date for each patient was defined as the date of occurrence of the first claim for administration of any biologic during the index period. We defined the 2-year period after the index date as the follow-up period and the 1-year period before the index date as the pre-index period (Supplemental Figure S2).
This study included consecutive patients who (1) had at least one insurance claim for IBD using International Classification of Diseases, 10th Revision (ICD-10) codes (K50.X for CD and K51.X for UC) and the codes for Rare and Intractable Diseases registration program (V130 for CD and V131 for UC) during the index period; (2) had at least one claim for biologics (infliximab, adalimumab, golimumab, vedolizumab and ustekinumab) during the index period; and (3) were aged ⩾18 years at the index date. Patients were excluded if they (1) had a claim with a diagnostic code for other indications of biologics; (2) had both CD and UC diagnosis codes in one claim; and (3) had a claim for any biologics or tofacitinib before the index period (pre-index period). Patients with multiple recorded K50 and K51 ICD-10 diagnoses were assigned to either the CD or UC group, according to their final diagnosis. Since claims data were extracted based on first-line biologic users, data on first-line tofacitinib before using any biologics were excluded from the analysis. All patients were individually followed up for 24 months after the index date. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement. 16
Study outcomes
Patient demographics and clinical characteristics were assessed using first-line index biologics. The Charlson Comorbidity Index (CCI) and IBD-related surgery and hospitalization at baseline were evaluated during the 1-year pre-index period for each patient; IBD-related surgery – including bowel resection and operation for perianal disease – was identified using the procedural code for related surgery, and IBD-related hospitalization was defined as admission for ⩾1 day in the Department of Emergency and Gastroenterology. Concomitant medications were defined as conventional treatments prescribed within 60 days of the index date. Conventional treatment types were classified as 5-aminosalicylic acids (5-ASAs), corticosteroids and immunomodulators such as azathioprine, mercaptopurine and methotrexate.
Persistence was defined as the proportion of patients who remained on the index treatment without a gap of >180 days or who switched to a different biologic or tofacitinib within 90 days after the last prescription date. This definition of persistence aligns with previous claims data studies in IBD.11,12 For the analysis of switching patterns, patients who were sequentially switched from index biologic to another biologic or tofacitinib up to third lines of treatment within the follow-up period were assessed, considering multiple transitions in treatment regimens.
Dose intensification was defined as two consecutive doses, each at least 50% higher than the recommended weekly maintenance standard dose (intensified dosage criteria: infliximab: ⩾7.5 mg/kg/8 weeks; adalimumab: ⩾60 mg/2 weeks; ustekinumab: ⩾135 mg/12 weeks; vedolizumab: ⩾450 mg/8 weeks); the maintenance standard dose of each biologic was based on the product label approved by the MFDS of South Korea. The weekly dose of each agent was calculated as the prescribed dosage divided by the time gap between two consecutive prescriptions and translated into weekly doses. For infliximab, because dosing is weight based and the weight information was not available from the claims data, the first loading dosage (week 0) of each patient was used as the weekly maintenance dose. Dose intensification was evaluated only among patients with at least one administration of their index therapy during maintenance, and this maintenance period varied according to the efficacy evaluation period for insurance coverage [infliximab: after 2 weeks (CD), and after 14 weeks (UC); adalimumab: after 4 weeks (CD), and after 8 weeks (UC); vedolizumab: after 14 weeks (CD and UC); ustekinumab: after 20 weeks (CD and UC); golimumab: after 14 weeks (UC)]. Then, the time to dose intensification was calculated as the number of months from the index date to the date of the first dose intensification. Only the data from adalimumab and vedolizumab in UC patients were analysed for dose intensification because infliximab and golimumab were not approved for dose intensification in UC patients in South Korea. Additionally, the analysis excluded the data from ustekinumab and subcutaneous infliximab users due to their small sample size.
Statistical analysis
A descriptive statistical analysis of the baseline demographic and clinical characteristics of first-line biologics was performed. Data were assessed for normality with Shapiro–Wilk testing for relevant continuous variables and non-normality was assumed for all analyses. Continuous variables were summarized using the mean, standard deviation, median and interquartile range. Categorical variables were summarized using frequencies and percentages. To show the persistence of different index biological groups, Kaplan–Meier analyses and log-rank tests were used. In addition to index biologics, subgroups of CD versus UC and between treatment lines were analysed. These analyses were corrected for multiple comparison testing using the Benjamini–Hochberg method with adjusted p values; data were censored if a patient withdrew or the observation period ended. Moreover, a Cox proportional hazards model was used to estimate the adjusted hazard ratios (HRs) of discontinuation rates and dose intensification rates among each group. Clinical factors, including age, sex, index biologic, CCI score, the use of concomitant medication, history of hospitalization and history of surgery, were adjusted as covariates. Furthermore, Kaplan–Meier curves and log-rank tests were also used to investigate the cumulative incidence of dose intensification and time to dose intensification. Additionally, biological switching patterns in subsequent treatment lines were evaluated using a Sankey diagram. Statistical significance was set at a p value <0.05. All statistical analyses and data preparation were conducted using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
We identified 13,087 biologic-naïve patients (CD: 8283; UC: 4804) who were newly treated with a biologic at least once between 2010 and 2020 (Figure 1). The most prescribed first-line biologics were infliximab (CD: 5429, 65.5%; UC: 3019, 62.8%) and adalimumab (CD: 2459, 29.7%; UC: 1204, 25.1%) (Table 1). The proportion of females in the patient population was higher in UC (36.9%) than in CD (28.5%) (p < 0.05). Furthermore, the majority of CD patients (83.4%) belonged to the youngest age group (18–39 years) upon their first biologic administration, while patients with UC had a higher proportion in the older age groups (40–59 years: 38.9% or ⩾60 years: 16.9%) (p < 0.05). Additionally, among CD patients aged ⩾60 years, there was a numerically higher proportion of ustekinumab (6.5%) and vedolizumab (14.3%) users compared to infliximab (2.3%) and adalimumab (1.9%) users. The mean CCI score was significantly higher in UC (mean CCI: 1.4) than in CD (mean CCI: 0.9) (p < 0.01), while it remained consistent across index biologics within both CD and UC groups (Table 1).
Figure 1.
Flow chart of the study.
CD, Crohn’s disease; IBD, inflammatory bowel disease; ICD-10, International Classification of Diseases, 10th revision; UC, ulcerative colitis.
Table 1.
Baseline demographic and clinical characteristics of first-line biologics.
| Crohn’s disease (n = 8283) | Ulcerative colitis (n = 4804) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 8283) | IFX (n = 5429) | ADA (n = 2459) | UST (n = 325) | VED (n = 70) | p Value | Total (n = 4804) | IFX (n = 3019) | ADA (n = 1204) | GOL (n = 417) | VED (n = 164) | p Value | |
| Sex | 0.760 | 0.630 | ||||||||||
| Male, n (%) | 5919 (71.5%) | 3879 (71.4%) | 1761 (71.6%) | 233 (71.7%) | 46 (65.7%) | 3030 (63.1%) | 1896 (62.8%) | 763 (63.4%) | 260 (62.4%) | 111 (67.7%) | ||
| Female, n (%) | 2364 (28.5%) | 1550 (28.6%) | 698 (28.4%) | 92 (28.3%) | 24 (34.3%) | 1774 (36.9%) | 1123 (37.2%) | 441 (36.6%) | 157 (37.6%) | 53 (32.3%) | ||
| Age at index date, years | <0.05 | 0.005 | ||||||||||
| 18–39, n (%) | 6907 (83.4%) | 4608 (84.9%) | 2017 (82.0%) | 235 (72.3%) | 47 (67.1%) | 2124 (44.2%) | 1350 (44.7%) | 537 (44.6%) | 165 (39.6%) | 72 (43.9%) | ||
| 40–59, n (%) | 1172 (14.1%) | 695 (12.8%) | 395 (16.1%) | 69 (21.2%) | 13 (18.6%) | 1870 (38.9%) | 1176 (39.0%) | 479 (39.8%) | 165 (39.6%) | 50 (30.5%) | ||
| ⩾60, n (%) | 204 (2.5%) | 126 (2.3%) | 47 (1.9%) | 21 (6.5%) | 10 (14.3%) | 810 (16.9%) | 493 (16.3%) | 188 (15.6%) | 87 (20.9%) | 42 (25.6%) | ||
| CCI score, mean ± SD | 0.9 ± 1.1 | 0.9 ± 1.1 | 0.9 ± 1.1 | 0.9 ± 1.1 | 1.0 ± 1.1 | 0.979 | 1.4 ± 1.3 | 1.4 ± 1.3 | 1.4 ± 1.3 | 1.3 ± 1.3 | 1.3 ± 1.3 | 0.487 |
| Categorical CCI score | 0.861 | 0.251 | ||||||||||
| 0, n (%) | 3554 (42.9%) | 2346 (43.2%) | 1038 (42.2%) | 141 (43.4%) | 29 (41.4%) | 1444 (30.1%) | 902 (29.9%) | 345 (28.7%) | 141 (33.8%) | 56 (34.1%) | ||
| 1, n (%) | 2698 (32.6%) | 1742 (32.1%) | 831 (33.8%) | 102 (31.4%) | 23 (32.9%) | 1518 (31.6%) | 959 (31.8%) | 396 (32.9%) | 122 (29.3%) | 41 (25.0%) | ||
| ⩾2, n (%) | 2031 (24.5%) | 1341 (24.7%) | 590 (24.0%) | 82 (25.2%) | 18 (25.7%) | 1842 (38.3%) | 1158 (38.4%) | 463 (38.5%) | 154 (36.9%) | 67 (40.9%) | ||
| Any IBD-related hospitalization within 1 year pre-index, n (%) | 3090 (37.3%) | 2141 (39.4%) | 810 (32.9%) | 116 (35.7%) | 23 (32.9%) | <0.05 | 1656 (34.5%) | 1121 (37.1%) | 402 (33.4%) | 83 (19.9%) | 50 (30.5%) | <0.05 |
| Any IBD-related surgery within 1 year pre-index, n (%) | 826 (10.0%) | 647 (11.9%) | 152 (6.2%) | 20 (6.1%) | 7 (10.0%) | <0.05 | – | – | – | – | – | |
| Bowel surgery | 14 (0.2%) | 8 (0.1%) | 3 (0.1%) | 1 (0.3%) | 2 (2.9%) | – | – | – | – | – | ||
| Perianal surgery | 812 (9.8%) | 639 (11.8%) | 149 (6.1%) | 19 (5.8%) | 5 (7.1%) | – | – | – | – | – | ||
| Concomitant medication at first biologics used, n (%) | ||||||||||||
| 5-ASA | 2489 (30.0%) | 1810 (33.3%) | 616 (25.1%) | 51 (15.7%) | 12 (17.1%) | <0.05 | 3736 (77.8%) | 2359 (78.1%) | 911 (75.7%) | 336 (80.6%) | 130 (79.3%) | 0.143 |
| Corticosteroid | 2657 (32.1%) | 1910 (35.2%) | 642 (26.1%) | 87 (26.8%) | 18 (25.7%) | <0.05 | 2915 (60.7%) | 1981 (65.6%) | 657 (54.6%) | 212 (50.8%) | 65 (39.6%) | <0.05 |
| Immunomodulator | 5567 (67.2%) | 3634 (66.9%) | 1710 (69.5%) | 185 (56.9%) | 38 (54.3%) | <0.05 | 2535 (52.8%) | 1687 (55.9%) | 611 (50.7%) | 186 (44.6%) | 51 (31.1%) | <0.05 |
Statistically significant results are highlighted in bold.
ADA, adalimumab; 5-ASA, 5-aminosalicylic acid; CCI, Charlson Comorbidity Index; GOL, golimumab; IBD, inflammatory bowel disease; IFX, infliximab; SD, standard deviation; UST, ustekinumab; VED, vedolizumab.
Persistence of biologics
Figure 2 shows the survival curve for persistence by biologic agents in the first- and second-line treatments during the 2-year follow-up period. The cumulative probability of persistence in CD patients who started their first-line index biologics was highest for ustekinumab (88.6%), followed by adalimumab (81.6%), infliximab (80.8%) and vedolizumab (72.2%) [Figure 2(a)]. However, there was no significant difference in persistence between first-line biologics. Amongst second-line biologics, the cumulative probability of persistence was also highest for ustekinumab (79.2%), followed by adalimumab (73.6%), infliximab (70.9%) and vedolizumab (54.9%). For second-line therapy, ustekinumab showed a significantly higher persistence rate (p < 0.01), whereas vedolizumab showed a significantly lower persistence rate than all other second-line biologics (p < 0.01).
Figure 2.
Kaplan–Meier curve for the persistence of first- and second-line biologics among patients with (a) Crohn’s disease (CD) and (b) ulcerative colitis (UC).
ADA, adalimumab; GOL, golimumab; IFX, infliximab; TOF, tofacitinib; UST, ustekinumab; VED, vedolizumab.
In UC, first-line vedolizumab displayed the highest persistence rate (82.9%), followed by infliximab (69%) and adalimumab (68.9%), while the lowest was golimumab (59.3%) [Figure 2(b)]; first-line golimumab showed a significantly lower persistence rate compared to all other biologics (p < 0.001). Conversely, for second-line UC patients, persistence rates were highest for tofacitinib (80%), adalimumab (67.7%), vedolizumab (63.5%), infliximab (59.4%) and lowest for golimumab (47.1%); second-line golimumab also showed a significantly lower persistence rate than adalimumab (p < 0.001), vedolizumab (p < 0.001) and tofacitinib (p < 0.001).
Risk factors for non-persistence of biologics
The risk of non-persistence was higher in UC than in CD (first line: HR, 1.97; second line: HR, 1.39; both p < 0.001) and also in second-line treatment than in first-line treatment for CD (HR, 1.55; p < 0.001). However, no significant difference in persistence was observed between the treatment lines for UC (HR, 1.07; p = 0.498). Furthermore, the risk factors associated with the non-persistence of first-line biologics in CD and UC are shown in Tables 2 and 3, respectively. In CD, the presence of comorbidities was associated with a higher risk of non-persistence [CCI: 1 (HR, 1.28; 95% CI, 1.13–1.46), ⩾2 (HR, 1.54; 95% CI, 1.34–1.77)], while first-line ustekinumab use had a lower risk of non-persistence compared to first-line infliximab use (HR, 0.69; 95% CI, 0.47–1.00). Moreover, concomitant 5-ASA and steroid use in patients with first-line CD tended to be associated with an increased risk of non-persistence. In UC, female sex (HR, 0.86; 95% CI, 0.76–0.96) and age ⩾60 years (HR, 0.78; 95% CI, 0.66–0.93) were associated with a decreased risk of non-persistence, whereas the presence of comorbidities [CCI: 1 (HR, 1.19; 95% CI, 1.02–1.38), ⩾2 (HR, 1.61; 95% CI, 1.39–1.86)], first-line golimumab use (HR, 1.68; 95% CI, 1.40–2.01), and concomitant use of 5-ASA (HR, 1.29; 95% CI, 1.12–1.50), immunomodulators (HR, 1.14; 95% CI, 1.02–1.27) and steroids (HR, 1.55; 95% CI, 1.37–1.75) were associated with a higher risk of non-persistence.
Table 2.
Cox proportional hazards regression for the risk of non-persistence of first-line biologics in patients with Crohn’s disease.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.14 (1.01–1.28) | 0.028 | 1.10 (0.98–1.24) | 0.099 |
| Age group (years) | ||||
| 18–39 | Reference | Reference | ||
| 40–59 | 1.06 (0.91–1.24) | 0.432 | 1.01 (0.86–1.18) | 0.924 |
| ⩾60 | 1.19 (0.84–1.69) | 0.332 | 0.96 (0.67–1.37) | 0.814 |
| History of recent hospitalization | 1.09 (0.98–1.21) | 0.124 | 0.96 (0.86–1.07) | 0.454 |
| History of recent surgery | ||||
| Bowel | 1.28 (0.32–5.11) | 0.730 | 1.08 (0.27–4.32) | 0.919 |
| Perianal | 0.99 (0.83–1.19) | 0.941 | 1.02 (0.85–1.22) | 0.818 |
| CCI score | ||||
| 0 | Reference | Reference | ||
| 1 | 1.31 (1.15–1.49) | <0.001 | 1.28 (1.13–1.46) | <0.001 |
| ⩾2 | 1.67 (1.47–1.91) | <0.001 | 1.54 (1.34–1.77) | <0.001 |
| Index biologics | ||||
| Infliximab | Reference | Reference | ||
| Adalimumab | 0.97 (0.86–1.09) | 0.594 | 1.05 (0.93–1.18) | 0.434 |
| Ustekinumab | 0.63 (0.43–0.91) | 0.013 | 0.69 (0.47–1.00) | 0.048 |
| Vedolizumab | 0.99 (0.44–2.21) | 0.983 | 1.08 (0.48–2.43) | 0.845 |
| Concomitant medication | ||||
| 5-ASA | 1.52 (1.36–1.70) | <0.001 | 1.43 (1.28–1.60) | <0.001 |
| Immunomodulator | 1.00 (0.89–1.12) | 0.953 | 0.93 (0.83–1.04) | 0.222 |
| Corticosteroid | 1.83 (1.65–2.04) | <0.001 | 1.74 (1.56–1.94) | <0.001 |
Statistically significant results are highlighted in bold.
5-ASA, 5-aminosalicylic acid; CCI, Charlson Comorbidity Index; CI, confidence interval; HR, hazard ratio.
Table 3.
Cox proportional hazards regression for the risk of non-persistence of first-line biologics in patients with ulcerative colitis.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.87 (0.78–0.98) | 0.023 | 0.86(0.76–0.96) | 0.010 |
| Age group (years) | ||||
| 18–39 | Reference | Reference | ||
| 40–59 | 1.11 (0.99–1.25) | 0.081 | 1.01 (0.89–1.14) | 0.876 |
| ⩾60 | 0.97 (0.82–1.15) | 0.717 | 0.78 (0.66–0.93) | 0.006 |
| History of recent hospitalization | 1.07 (0.96–1.20) | 0.231 | 0.99 (0.88–1.11) | 0.824 |
| CCI score | ||||
| 0 | Reference | Reference | ||
| 1 | 1.20 (1.03–1.39) | 0.019 | 1.19 (1.02–1.38) | 0.024 |
| ⩾2 | 1.59 (1.39–1.83) | <0.001 | 1.61 (1.39–1.86) | <0.001 |
| Index biologics | ||||
| Infliximab | Reference | Reference | ||
| Adalimumab | 1.06 (0.93–1.21) | 0.369 | 1.13 (0.99–1.28) | 0.075 |
| Golimumab | 1.52 (1.27–1.81) | <0.001 | 1.68 (1.40–2.01) | <0.001 |
| Vedolizumab | 0.75 (0.50–1.13) | 0.173 | 0.89 (0.59–1.34) | 0.567 |
| Concomitant medication | ||||
| 5-ASA | 1.42 (1.22–1.64) | <0.001 | 1.29 (1.12–1.50) | 0.001 |
| Immunomodulator | 1.16 (1.04–1.30) | 0.008 | 1.14 (1.02–1.27) | 0.024 |
| Corticosteroid | 1.56 (1.39–1.76) | <0.001 | 1.55 (1.37–1.75) | <0.001 |
Statistically significant results are highlighted in bold.
5-ASA, 5-aminosalicylic acid; CI, confidence interval; CCI, Charlson Comorbidity Index; HR, hazard ratio.
Dose intensifications of biologics
Among patients who continued biologic treatment after the induction phase, dose intensification occurred in 1448 (18%) and 576 (38.1%) patients with CD during first- and second-line treatments, respectively, and in 233 (19.8%) and 235 (28%) patients with UC during first- and second-line treatments, respectively; this occurred during the 2-year follow-up period (Supplemental Table S1). The cumulative incidence of dose intensification in patients with CD was highest for ustekinumab (first line, 43.3%; second line, 69.1%) in both treatment lines [Figure 3(a)]. In CD, the cumulative incidence of dose intensification for first-line ustekinumab was significantly higher than that for infliximab and adalimumab. Furthermore, for second-line ustekinumab, the cumulative incidence of dose intensification was significantly higher than that of all other second-line biologics (p < 0.001). In UC, no difference was observed between adalimumab and vedolizumab during first-line treatment, but the cumulative incidence of dose intensification with adalimumab (40.7%) was significantly higher than vedolizumab (33.6%) during second-line treatment (p = 0.01) [Figure 3(b)]. In first-line CD, female sex (HR, 1.42; 95% CI, 1.28–1.59) and the concomitant use of steroids (HR, 1.21; 95% CI, 1.08–1.35), as well as patients with a higher CCI, were associated with a higher risk of dose intensification of biologics (CCI ⩾ 2 versus CCI = 0; HR, 1.29; 95% CI, 1.13–1.47) (Supplemental Figure S3A). However, no significant association between CCI score and the risk of dose intensification was observed in first-line UC (Supplemental Figure S3B).
Figure 3.
Cumulative dose intensification rates by index biologics: (a) CD and (b) UC.
ADA, adalimumab; CD, Crohn’s disease; IFX, infliximab; UC, ulcerative colitis; UST, ustekinumab; VED, vedolizumab.
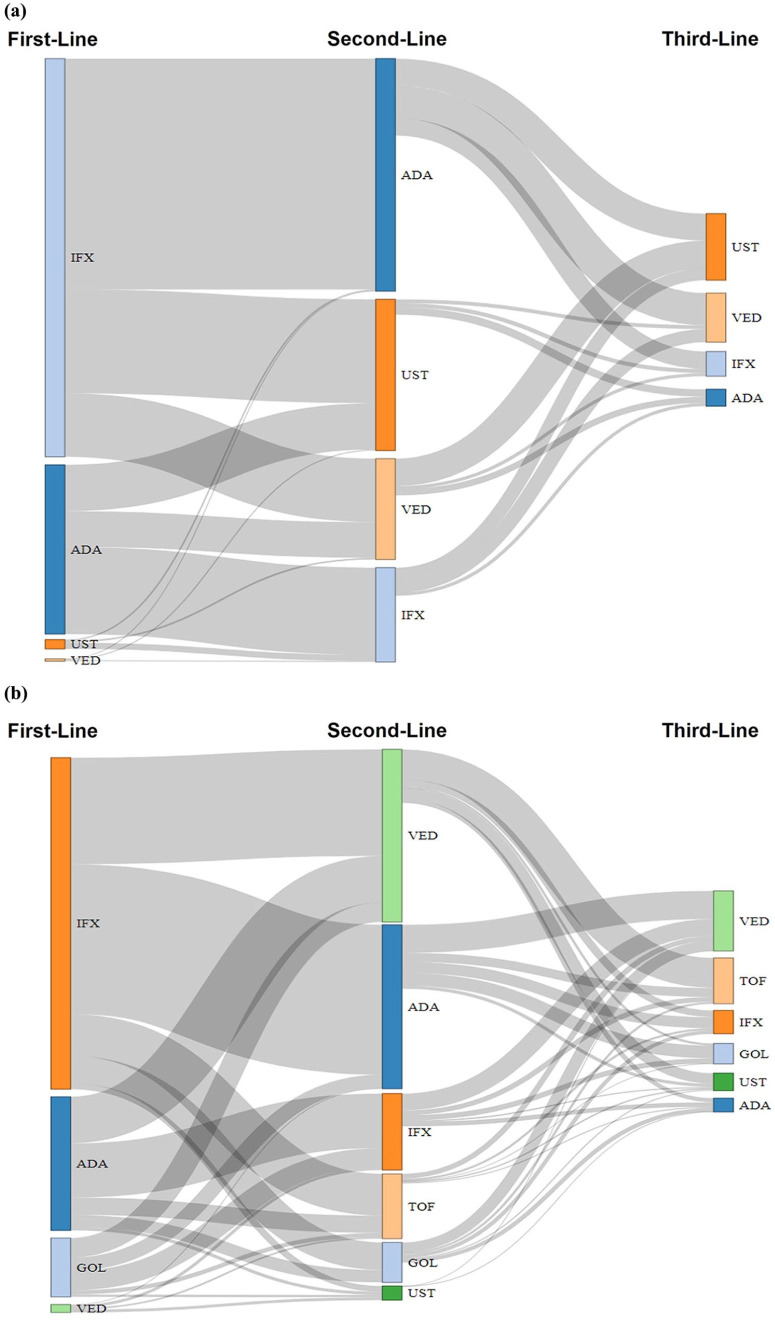
Switching patterns of biologics
Figure 4 depicts the switching patterns of first- to second-line biologics and second- to third-line biologics. Overall, 1688 (20.4%) and 1546 (32.2%) patients with CD and UC, respectively, received second-line biologic treatment after first-line treatment, while the remaining patients continued their first biologic or simply discontinued it and did not receive a second-line treatment. To assess the patterns of biologic switching from the year when all index biologics were approved for each indication, a sensitivity analysis was performed (from 2018 for CD and from 2019 for UC). Among switchers of first-line TNF inhibitor (infliximab, adalimumab or golimumab) therapy, 198 out of 287 (69%) patients with CD and 243 out of 310 (78.4%) patients with UC switched to other classes of second-line biologics (vedolizumab, ustekinumab or tofacitinib); meanwhile, 89 out of 287 (31%) patients with CD and 67 out of 310 (21.6%) patients with UC switched to same class second-line biologics (Supplemental Figure S4). Temporal changes in prescription patterns of biologics and tofacitinib were found in index biologics as first-, second-, or third-line treatment (Supplemental Figure S5). After 2019, in patients with CD, ustekinumab became the most administrated drug at the second-line treatment [345 out of 682 (50.6%)] and third-line treatment [153 out of 249 (61.4%)]. Conversely, for patients with UC, vedolizumab and tofacitinib were the most frequently administered drugs at the second-line treatment [vedolizumab: 299 out of 608 (49.2%), tofacitinib: 149 out of 608 (24.5%)], and third-line treatment [tofacitinib: 109 out of 388 (28.1%), vedolizumab: 95 out of 388 (24.5%)], respectively.
Figure 4.
Sankey diagram of biologic switching patterns over the follow-up period: (a) CD and (b) UC.
ADA, adalimumab; CD, Crohn’s disease; GOL, golimumab; IFX, infliximab; TOF, tofacitinib; UC, ulcerative colitis; UST, ustekinumab; VED, vedolizumab.
Discussion
This study showed that the risk of non-persistence and dose intensification differed among biologics according to lines of treatment and types of IBD. Regarding the first-line treatment of CD, ustekinumab showed a lower risk of non-persistence than other biologics in a multivariate analysis. This result aligns with recent real-world registry data from Australia that showed superior treatment persistence with ustekinumab in CD compared with other biologics. 17 On the other hand, recent meta-analyses have suggested that TNF-α inhibitors, such as infliximab and adalimumab, are better than ustekinumab as a first-line treatment for CD,18,19 but these mainly focused on the induction of remission. Because keeping the same biologic without loss of response or adverse events during the maintenance phase is also important in IBD treatment, we should consider the high persistence rate of ustekinumab when selecting a biologic as a first-line treatment for CD.
Meanwhile, ustekinumab also showed a significantly higher cumulative incidence of dose intensification than TNF-α inhibitors as a first-line treatment of CD. The 2-year cumulative incidence of dose intensification was 43.3% and 69.1% in the first- and second-line treatments of CD, respectively. A recent systematic literature review supported our findings by indicating that ustekinumab with an initial regimen of 90 mg every 12 weeks had a dose intensification rate of 43% in patients with CD. 20 Furthermore, dose intensifications in CD patients were reported as 19% for first-line treatment and 37% for second-line treatment with increasing rates observed according to the line of treatment. 21 The consistency between our study results and the literature review underscores the robustness of the observed dose intensification patterns in the treatment of CD using ustekinumab.
It is unclear whether it is better to administer ustekinumab every 8 weeks from the beginning of treatment or if it is sufficient to try dose intensification when loss of response occurs during maintenance treatment. The labelling recommendations for ustekinumab in CD and UC differ between the United States and the European Medicines Agency (EMA), as well as in Asian countries like South Korea and Japan. In the EMA label, the recommended initial maintenance dosage is 90 mg every 12 weeks, with dose intensification to 90 mg every 8 weeks permitted if patients experience a loss of response to the initial lower dose regimen. On the other hand, in the United States, the approved maintenance dosage is 90 mg every 8 weeks from the start. Although data in the clinical trial of ustekinumab in CD suggested that the long-term clinical remission rate was not different between the 8- and 12-week interval dosing groups, 22 a recent meta-analysis using real-world data reported that nearly one-third of patients with CD who started ustekinumab required dose intensification. 23
Taken together, ustekinumab has benefits in terms of persistence compared to other biologics as a first-line treatment for CD, but the administration at 8-week intervals rather than 12-week intervals may be more appropriate. Some patients prefer ustekinumab because it is known to have the longest administration interval among biologics, but clinicians should keep in mind that even in second-line treatment, the risk of dose intensification with ustekinumab is higher than that with other biologics. Thus, information regarding the risk of dose intensification in ustekinumab should be provided to patients, and shared decision-making should be performed during drug selection.
Because the drug used as the first-line treatment can influence the selection of the second-line treatment, multivariate analysis was not possible. However, in the survival curve, ustekinumab showed a higher persistence rate than anti-TNF agents, whereas vedolizumab showed a lower one, as a second-line treatment for CD. These results support previous meta-analyses, suggesting that ustekinumab is generally a better option than vedolizumab for the second-line treatment of CD, and it is particularly superior to vedolizumab in maintenance therapy.18,24 Furthermore, as shown in the Sankey diagram of biologic switching patterns in this study, the majority of patients with CD who started these two drugs as second-line treatment used TNF-α inhibitors as the first line. Therefore, TNF-α inhibitor exposure in patients with CD should preferentially consider ustekinumab over vedolizumab.
Regarding first-line treatment of UC, golimumab showed a significantly higher risk of non-persistence than other biologics in multivariate analysis. In second-line treatment, the persistence rate of golimumab was also significantly lower than that of adalimumab and vedolizumab in the survival curve. These results correspond well with those of previous studies.11,25 Furthermore, the use of golimumab monotherapy in the treatment of UC seems to be gradually narrowing, and the significantly higher dose intensification rate of adalimumab compared with vedolizumab in the second-line treatment of UC should also be considered in drug selection.
The risk of non-persistence was higher in patients with UC than in patients with CD in both first- and second-line treatments, which was also reported in another study using South Korean claims data. 11 Interestingly, in contrast to CD, the risk of non-persistence did not differ between first- and second-line treatments in patients with UC, which may be due to differences in disease characteristics of UC and CD. The accumulation of bowel injury over time is more prominent and difficult to reverse in CD than in UC. 26 Therefore, active intervention using biologics in early CD has been emphasized, as well as choosing a first-line biologic that can function for an extended period. By contrast, it is unclear whether there is an additional benefit of early intervention for UC.27,28
In both UC and CD, the higher the CCI score, the higher the risk of non-persistence. This may be related to more frequent complications, such as infection during biological treatment in patients with comorbidities. Interestingly, the risk of non-persistence was lower in UC patients aged ⩾60 years than in younger patients, suggesting that the patient’s biological age should be greater than the absolute age when choosing biologics for IBD. For both CD and UC, the concomitant use of other drugs was associated with an increased risk of non-persistence, but this result requires cautious interpretation. It is more plausible that patients who received these drugs had stronger disease severity than those who received combination therapy, which was less effective.
This study has several strengths. First, its initial data extraction was based on a large claims database of approximately >17,000 individuals containing complete and detailed information on prescriptions and outpatient and inpatient treatment. Second, the current study included a wide range of biologics for IBD treatment, including new ones that were recently approved in South Korea. Therefore, it provides real-world data on new drugs and can be helpful in choosing biologics for IBD. Third, unlike previous studies on the persistence of biologics in IBD, we also compared the risk of dose intensification.
However, there are also some limitations to this claims data analysis. First, we were unable to capture clinical information regarding disease severity such as serum C-reactive protein levels, fecal calprotectin levels and disease activity index. To compensate for this, we compared the risk of non-persistence with biologics by adjusting for major clinical variables related to prognosis, such as history of hospitalization and bowel surgery. Second, we were unable to compare the persistence patterns of biologics before and after the availability of all biologics due to the varying approved timelines for each biologic. To address this limitation, we conducted an analysis focusing on assessing the patterns of biologic switching starting from the year when all index biologics were approved for each indication. Third, because vedolizumab has been approved as a first-line biologic in patients with IBD since August 2020, the number of first-line vedolizumab users with IBD during the study period was small. Likewise, due to the recent approval of ustekinumab as a first-line biologic for patients with UC since September 2022 and the prescription of subcutaneous infliximab in patients with IBD since January 2021, we could not collect this data. Future studies comparing recently approved treatments with various formulation types, such as subcutaneous and oral formulations, may provide more insights into the effectiveness and safety of advanced treatments in the real-world setting. Finally, while combination therapy of immunomodulators is known to potentially offer a better treatment persistence rate and lower intensification rate than monotherapy, our study did not specifically analyse this aspect. We focused on the baseline data of concomitant conventional medications, including immunomodulator usage within 60 days of the index date but did not perform a subgroup analysis comparing combination therapy to monotherapy in terms of treatment persistence rates and dose intensification rates.
Conclusions
In conclusion, our nationwide cohort study found that ustekinumab showed a significantly lower risk of non-persistence but a higher risk of dose intensification than other biologics for the first-line treatment of CD. Furthermore, golimumab showed a significantly higher risk of non-persistence for first- and second-line treatments of UC, and adalimumab showed a higher cumulative risk of dose intensification than vedolizumab in the second-line treatment of patients with UC. Switching to second-line biologics was common, with most switchers opting for a different class of treatment. However, the absence of clinical disease activity assessment and biochemical data remains a substantial gap, requiring further prospective study to support future research. Our study findings may provide insights into the real-world treatment patterns of biologics in patients with IBD and potentially suggest the importance of personalized treatment approaches to optimize long-term outcomes.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231201728 for 10 years of biologic use patterns in patients with inflammatory bowel disease: treatment persistence, switching and dose intensification – a nationwide population-based study by Hee Moon Koo, Yu Kyung Jun, Yonghoon Choi, Cheol Min Shin, Young Soo Park, Nayoung Kim, Dong Ho Lee, Young Kee Shin and Hyuk Yoon in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iD: Hyuk Yoon  https://orcid.org/0000-0002-2657-0349
https://orcid.org/0000-0002-2657-0349
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hee Moon Koo, Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, South Korea.
Yu Kyung Jun, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, South Korea.
Yonghoon Choi, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea.
Cheol Min Shin, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, South Korea.
Young Soo Park, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea.
Nayoung Kim, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, South Korea.
Dong Ho Lee, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, South Korea.
Young Kee Shin, Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, South Korea.
Hyuk Yoon, Department of Internal Medicine, Seoul National University Bundang Hospital, 82 Gumi-ro 173 Beon-gil, Bundang-gu, Seongnam, Gyeonggi-do 13620, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, South Korea.
Declarations
Ethics approval and consent to participate: The study was approved by the Seoul National University Institutional Review Board (IRB) (IRB approval No.: E2202/004-003, approved on 2 February 2022), Seoul National University Bundang Hospital (IRB approval No.: X-2203-745-901, approved on 9 March 2022) and HIRA (No.: M20220311869, approved on 16 June 2022), which waived the requirements for informed consent since the data are anonymized and all patient details are de-identified.
Consent for publication: Not applicable.
Author contributions: Hee Moon Koo: Conceptualization; Data curation; Formal analysis; Software; Writing – original draft.
Yu Kyung Jun: Writing – review & editing.
Yonghoon Choi: Writing – review & editing.
Cheol Min Shin: Writing – review & editing.
Young Soo Park: Writing – review & editing.
Nayoung Kim: Writing – review & editing.
Dong Ho Lee: Writing – review & editing.
Young Kee Shin: Supervision; Writing – review & editing.
Hyuk Yoon: Conceptualization; Formal analysis; Methodology; Project administration; Resources; Software; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: H.M.K. is currently an employee of Celltrion Pharm Inc. The authors declare that they have no conflicts of interest regarding the content of this article.
Availability of data and materials: The datasets generated and analysed during the current study are not publicly available because they were used pursuant to a data use agreement. The data are available through requests made directly to HIRA.
References
- 1. Jairath V, Feagan BG. Global burden of inflammatory bowel disease. Lancet Gastroenterol Hepatol 2020; 5: 2–3. [DOI] [PubMed] [Google Scholar]
- 2. Kofla-Dlubacz A, Pytrus T, Akutko K, et al. Etiology of IBD – is it still a mystery? Int J Mol Sci 2022; 23: 12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piovani D, Danese S, Peyrin-Biroulet L, et al. Inflammatory bowel disease: estimates from the global burden of disease 2017 study. Aliment Pharmacol Ther 2020; 51: 261–270. [DOI] [PubMed] [Google Scholar]
- 4. Lee JW, Eun CS. Inflammatory bowel disease in Korea: epidemiology and pathophysiology. Korean J Intern Med 2022; 37: 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barberio B, Gracie DJ, Black CJ, et al. Efficacy of biological therapies and small molecules in induction and maintenance of remission in luminal Crohn’s disease: systematic review and network meta-analysis. Gut 2023; 72: 264–274. [DOI] [PubMed] [Google Scholar]
- 6. Burr NE, Gracie DJ, Black CJ, et al. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut 2022; 71: 1976–1987. [DOI] [PubMed] [Google Scholar]
- 7. Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016; 7: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamamoto-Furusho JK, Al Harbi O, Armuzzi A, et al. Incidence of suboptimal response to tumor necrosis factor antagonist therapy in inflammatory bowel disease in newly industrialised countries: the EXPLORE study. Dig Liver Dis 2020; 52: 869–877. [DOI] [PubMed] [Google Scholar]
- 9. Gisbert JP, Chaparro M. Primary failure to an anti-TNF agent in inflammatory bowel disease: switch (to a second anti-TNF agent) or swap (for another mechanism of action)? J Clin Med 2021; 10: 5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis 2019; 25: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 11. Jung YS, Han M, Park S, et al. Biologic use patterns and predictors for non-persistence and switching of biologics in patients with inflammatory bowel disease: a nationwide population-based study. Dig Dis Sci 2020; 65: 1436–1444. [DOI] [PubMed] [Google Scholar]
- 12. Shin JY, Park HM, Lee MY, et al. Real-world incidence of suboptimal response to anti-tumor necrosis factor therapy for ulcerative colitis: a nationwide population-based study. Gut Liver 2021; 15: 867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gil-Candel M, Gascon-Canovas JJ, Urbieta-Sanz E, et al. Comparison of drug survival between infliximab and adalimumab in inflammatory bowel disease. Int J Clin Pharm 2020; 42: 500–507. [DOI] [PubMed] [Google Scholar]
- 14. Dalal SR, Cohen RD. What to do when biologic agents are not working in inflammatory bowel disease patients. Gastroenterol Hepatol (NY) 2015; 11: 657–665. [PMC free article] [PubMed] [Google Scholar]
- 15. Kim HJ, Choi HE, Jang HJ, et al. Current status and trends of pulmonary rehabilitation in South Korea: national level data analysis using Health Insurance Review and Assessment Service (HIRA) database from 2016 to 2018. Medicine (Baltimore) 2022; 101: e31085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 17. Ko Y, Paramsothy S, Yau Y, et al. Superior treatment persistence with ustekinumab in Crohn’s disease and vedolizumab in ulcerative colitis compared with anti-TNF biological agents: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study. Aliment Pharmacol Ther 2021; 54: 292–301. [DOI] [PubMed] [Google Scholar]
- 18. Singh S. Network meta-analysis to inform positioning of biologics in patients with Crohn’s disease: promise and perils. Best Pract Res Clin Gastroenterol 2019; 38–39: 101614. [DOI] [PubMed] [Google Scholar]
- 19. Singh S, Murad MH, Fumery M, et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2021; 6: 1002–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panaccione R, Lee WJ, Clark R, et al. Dose escalation patterns of advanced therapies in Crohn’s disease and ulcerative colitis: a systematic literature review. Adv Ther 2023; 40: 2051–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Einarson TR, Bereza BG, Ying Lee X, et al. Dose escalation of biologics in Crohn’s disease: critical review of observational studies. Curr Med Res Opin 2017; 33: 1433–1449. [DOI] [PubMed] [Google Scholar]
- 22. Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: Three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohns Colitis 2020; 14: 23–32. [DOI] [PubMed] [Google Scholar]
- 23. Rubin de Celix C, Chaparro M, Gisbert JP. Real-world evidence of the effectiveness and safety of ustekinumab for the treatment of Crohn’s disease: systematic review and meta-analysis of observational studies. J Clin Med 2022; 11: 4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parrot L, Dong C, Carbonnel F, et al. Systematic review with meta-analysis: the effectiveness of either ustekinumab or vedolizumab in patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther 2022; 55: 380–388. [DOI] [PubMed] [Google Scholar]
- 25. Macaluso FS, Ventimiglia M, Fries W, et al. A propensity score weighted comparison of Vedolizumab, Adalimumab, and Golimumab in patients with ulcerative colitis. Dig Liver Dis 2020; 52: 1461–1466. [DOI] [PubMed] [Google Scholar]
- 26. Koh SJ, Hong SN, Park SK, et al. Korean clinical practice guidelines on biologics for moderate to severe Crohn’s disease. Intest Res 2023; 21: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solitano V, D’Amico F, Zacharopoulou E, et al. Early intervention in ulcerative colitis: ready for prime time? J Clin Med 2020; 9: 2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Na SY, Choi CH, Song EM, et al. Korean clinical practice guidelines on biologics and small molecules for moderate-to-severe ulcerative colitis. Intest Res 2023; 21: 61–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231201728 for 10 years of biologic use patterns in patients with inflammatory bowel disease: treatment persistence, switching and dose intensification – a nationwide population-based study by Hee Moon Koo, Yu Kyung Jun, Yonghoon Choi, Cheol Min Shin, Young Soo Park, Nayoung Kim, Dong Ho Lee, Young Kee Shin and Hyuk Yoon in Therapeutic Advances in Gastroenterology