Abstract
Background:
Little is known about the stability of adjustable-loop devices (ALDs) for anterior cruciate ligament (ACL) reconstruction (ACLR).
Purpose:
To evaluate the stabilization behavior of 3 different types of ALDs for all-inside ACLR in a full-construct surgical technique-based manner.
Study Design:
Controlled laboratory study.
Methods:
The femoral and tibial devices of Ultrabutton (Smith & Nephew), Infinity (Conmed), and TightRope II (Arthrex) were applied to quadrupled bovine tendon grafts (n = 8 each) with tibial-sided traction applied (350 N) for graft tensioning in a simulated fully extended knee. Knotless femoral graft fixation was based on either a suture-locking device (SLD; Ultrabutton), button-locking device (BLD; Infinity), or dual-locking device (DLD; TightRope II). All constructs were progressively loaded (50 N/500 cycles) from 50 to 300 N for 3000 cycles (0.75 Hz), including complete unloading situations and pull to failure (50 mm/min). Construct elongation, stiffness, and ultimate load were analyzed.
Results:
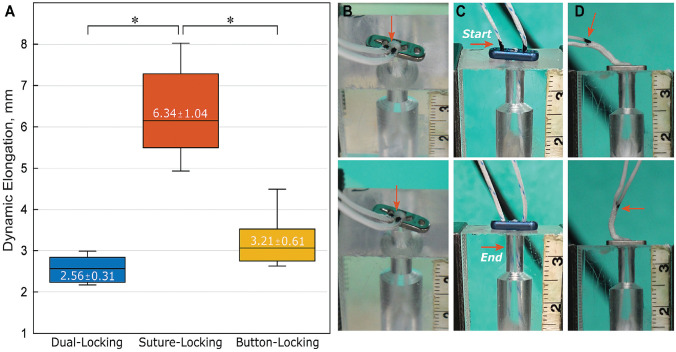
BLD showed significantly greater initial elongation (-2.69 ± 0.15 mm) than DLD (-3.19 ± 0.21 mm; P < .001) but behaved similarly to SLD (-2.93 ± 0.23 mm). While DLD and SLD had the smallest initial elongation at the same significance level, they behaved opposite to each other with gradually increasing peak loading. At the end of testing, DLD had the lowest (-0.64 ± 0.32 mm) and SLD the highest (3.41 ± 1.01 mm) total elongation (P < .003 for both). SLD displayed significantly higher dynamic elongation (6.34 ± 0.23 mm) than BLD (3.21 ± 0.61 mm) and DLD (2.56 ± 0.31 mm) (P < .001 for both). The failure load of BLD (865.0 ± 183.8 N) was significantly lower (P < .026) compared with SLD and DLD (>1000 N). The predominant failure mode was suture rupture and tibial bone breakage with button subsidence (SLD, n = 4). No significant difference in stiffness between constructs was found.
Conclusion:
While DLD successfully restricted critical construct elongation, BLD partially and SLD completely exceeded the clinical failure threshold (>3 mm) of plastic elongation with loop lengthening during increasing cyclic peak loading with complete unloading. Higher failure loads of SLD and DLD implants (>1000 N) were achieved at similar construct stiffness to BLD.
Clinical Relevance:
A detailed biomechanical understanding of the stabilization potential is pertinent to the continued evolution of ALDs to improve clinical outcomes.
Keywords: adjustable loop length device, all-inside ACL, graft tensioning
All-inside anterior cruciate ligament (ACL) reconstruction (ACLR) with adjustable loop device (ALD) fixation of a quadrupled hamstring tendon graft has become a routine procedure with good clinical outcomes.6,8,12,38 Potential benefits of using ALDs in a less invasive all-inside ACLR procedure includes bone-saving anatomic tunnel preparation and decreased graft length requirements, as well as incremental graft tension adjustments with maximized graft-bone interface and ultimate failure strength.5,6,13,33
Primary fixation of ACLR grafts is important in eliminating initial knee laxity and securing the graft in place within the bone tunnel for healing.3,10 Adjustable tensioning was shown in controlled laboratory studies to significantly increase the graft force at the time of fixation and reduce the overall elongation in ACLR grafts.31,33 Currently, there is no consensus on an optimal primary graft force, with ALD graft tensioning in a fully extended knee position reducing the risk of overconstraining the knee.29,34 The retention mechanism of various available ALDs relies on suture-locking below the button surface within a loaded loop suture (the so-called “Chinese finger”) and suture-locking by a loaded loop suture on the button surface (hereafter, “button-locking”) or recently of a combination of both (hereafter, “dual-locking”).17,18,44 Although clinical results with ALDs have generally good clinical outcomes,6,8,12,38 recent biomechanical studies including complete unloading situations raised concerns about gradual loop lengthening.2,17 Knee laxity of more than 3 mm is generally considered the threshold for clinical failure. 14 To date, the performance of the newly released ALDs used in our study for soft tissue graft suspension in all-inside ACLR has not yet been tested in a full-construct model, including complete unloading situations. A native ACL function reference model, which was established in a previous ACLR-related study, 4 served as reference to quantify and qualify the stabilization potential of the all-inside ACLR groups.
The primary purpose of this study was to evaluate and compare the stability behavior of 3 different ALDs for all-inside ACLR during increasingly cyclic testing at 6 peak load levels, including complete unloading situations with construct elongation and ultimate strength measured in a full-construct surgical technique-based manner. We hypothesized that (1) the ALD construct with a knotless femoral suture-locking device (SLD) would lengthen more than the constructs with fixation devices including a button-locking retention mechanism, and (2) all ALDs would show similar construct stiffness.
Methods
Testing Groups
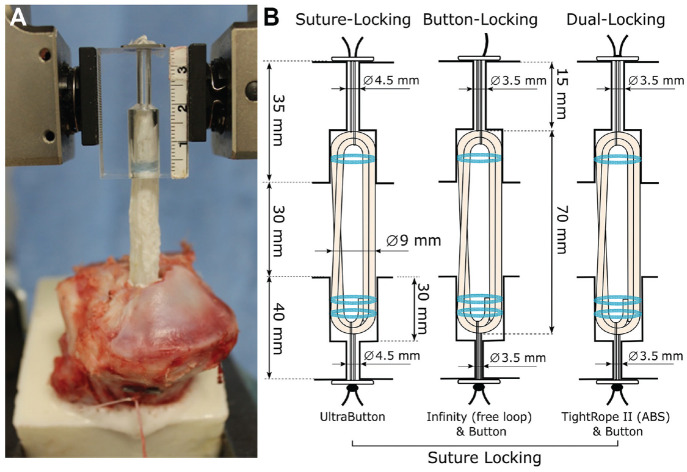
Three construct groups with ALD suspensory devices (n = 8 per group, total = 24) were prepared according to surgical technique guide for all-inside ACLR. For testing, 3 different types of adjustable fixation were used (Figure 1), including femoral and tibial devices of Ultrabutton (Smith & Nephew), Infinity (Conmed), and TightRope II (Arthrex). The retention mechanism of all tibial devices was based on suture-locking, whereas the femoral fixation differed between groups having either an SLD (Ultrabutton), button-locking device (BLD, Infinity) or dual-locking device (DLD, TightRope II). Adjustable loop shortening of all-inside fixation devices was performed in this study according to surgical technique recommendations.
Figure 1.
(A) Experimental setup. (B) Schematic illustration of the all-inside groups with femoral SLD, BLD, and DLD, with bone tunnel- and graft-related definitions. ABS, attachable button system; BLD, button-locking device; DLD, dual-locking device; SLD, suture-locking device.
Specimen Preparation
Fresh porcine tibias (age, 6-8 months) and bovine flexor tendons from adult bovine hind limbs (age, 20-24 months) were obtained from a local slaughterhouse. Porcine tibias were previously reported to have structural properties similar to those of young adult human tissue. 21 All soft tissues were removed, and the porcine tibias were cut 14 cm distal to the joint line. Embedding using a bicomponent material (RenCast, Huntsman Advanced Materials) was carried out in line with the tibial tunnel axis until 2 cm distal to the predetermined exit of the tibial tunnel axis to have sufficient space for adjustable loop tensioning. The lateral plateau of the tibia was cut with a bone saw to ensure a constant tunnel length of 40 mm. Tibias were prepared with a 9 mm–diameter graft tunnel and 30 mm in length using a cannulated drill over an implant specific guide pin, leaving a 10 mm bone-bridge (Figure 1B). Acrylic blocks with a total length of 35 mm and implant-specific tunnel preparations according to the clinical setting of femoral-sided ACLR with 20 mm of graft insertion were used in place of the femoral bone stock to allow intratunnel visualization during graft insertion and cyclic loading.
For all testing constructs, tendons were cut to a single-stranded length of 290 mm and trimmed in line with the fiber orientation to achieve a 9 mm quadrupled graft diameter and an overall length of 70 mm measured with a graft-sizing block (Arthrex). The all-inside ACL graft was prepared by quadrupling the tendon through the femoral and tibial-sided ALDs. The 2 free tendon ends were sutured together with No. 0 FiberWire (Arthrex) with the sutured part positioned inside of the construct near the tibial end. 14 The ALD on each end was used to fix the construct in a graft preparation station with a spring-loaded tensioning device by suturing all graft limbs together on the tibial side using No. 2 FiberWire (Arthrex) at 20 N of tension. For finalization of the graft construct, the tension was increased to 80 N, and a further circumferential stitch was added to each graft end.
All tissues were stored at -20°C. The embedded bones were thawed at room temperature overnight and the grafts for 2 hours before biomechanical testing. All specimens were kept moist with physiological saline solution during specimen preparation and testing.
Graft Fixation
All constructs were subjected to preloading of 80 N weightbearing for 5 minutes before device insertion and mechanical testing to allow stress relaxation of the graft.26,39 The tibia bone and femoral-sided acrylic block were secured to the base plate and actuator of a dynamic testing machine (ElectroPuls E10000, Instron) using custom clamps (Figure 1A). A dynamic load cell (2 kN, Instron) with a resolution of 0.01 N was used for testing.
Femoral passing and tensioning sutures were shuttled through the acrylic block with the device button flipped and the passing suture removed. Femoral graft insertion until tunnel docking was performed by adjustable loop shortening with the ALD remaining knotless. Tibial-sided ALD passing and tensioning sutures were shuttled through the tibia tunnel. The grafts were introduced into the tibial tunnel by pulling on the adjustable loop. Implant-specific tibial buttons were attached to free loop ALDs. A caliper was used to ensure the acrylic block and tibia were separated by a joint space of 30 mm in every test, which simulated an intra-articular distance of a fully extended knee and served as a reference position for later elongation analysis. In vivo kinematic data have shown that the native ACL experiences consistent length decreases of 1 and 3 mm at 30° and 90°, respectively, during weightbearing knee flexion activity starting from full extension. 45 In reference to the current graft fixation position (joint space of 30 mm), it can be assumed that a 29-mm joint space represents 30° of flexion and a joint space of 27 mm a knee in 90° of flexion.
Tibial-sided graft tensioning was performed according to surgical recommendations by manually pulling on the ALD shortening strand in line with the actuator axis. Graft tensioning was performed using tensioning handles (Arthrex) with a traction of 350 N measured with the load cell. Thereafter, all tibial ALD were knotted with 4 half-hitch suture knots, and the femoral loop length was indicated visually by a stripe with a colored marker on the available tensioning suture reaching the button surface. The ultimate traction level of 350 N was defined based on pretests to achieve a resulting graft tension of about 200 N for all constructs after traction release without tearing the traction suture. 44
Mechanical Testing
Cyclic peak loading was performed in force-control mode and started at 50 N over 500 cycles with a test frequency of 0.75 Hz. Peak loading was then increased in 50 N increments every 500 cycles up to 300 N for a total of 3000 cycles (Figure 2). An incremental increasing loading protocol every 500 load cycles were used in multiple previous ACLR studies.5,17,20,43 The peak load range up to 300 N should cover the range of daily activity loading during the time of early and late rehabilitation and the load spectrum used in other related literature over at least 2500 load cycles.5,17,18,34,40
Figure 2.
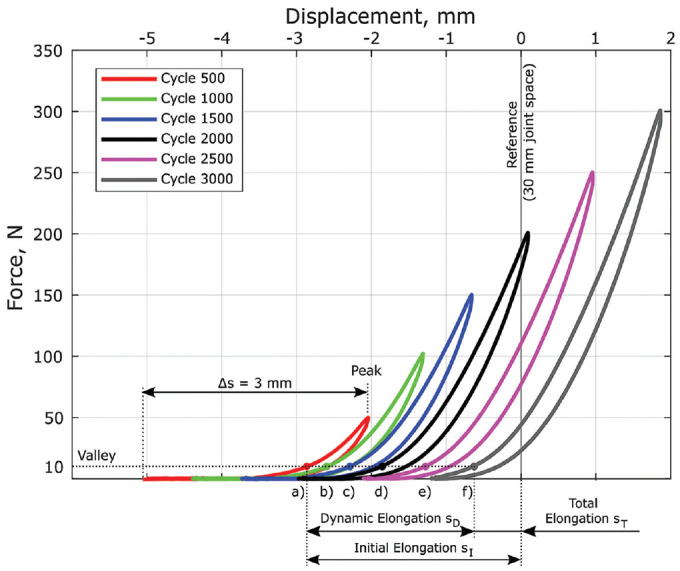
Selected hysteresis curves at the end of each peak load with complete unloading situation. Metrics for comparison included construct valley elongation (points a-f) with initial elongation (sI, a), dynamic elongation (sD, Δaf), and total elongation (sT, f) analyzed.
The valley elongation of each load cycle was defined in position control mode. Actuator translation of 3 mm (Δs) relative to the peak elongation provided for a complete unloading-loading situation at all load levels and represented the most suitable mechanical testing conditions to prove the fixation stability of the ALD retention mechanism. Relative unloading in the range of 3 mm correlates with ACL length changes during weightbearing knee flexion in relation to a fully extended knee. 28 Metrics for comparison included initial elongation (sI), dynamic elongation (sD), and total elongation (sT). Finally, grafts underwent a pull to failure at 50 mm/minute.6,7,8,14
Note that the obtained valley elongation at the end of each load level quantified the loading situation at 10 N (Figure 2, points a-f). Negative and positive elongation values indicated a tight or slack graft in reference to the graft fixation position (simulated fully extended knee). Ultimate load and stiffness were determined during pull to failure with the mechanism of failure noted. Stiffness was calculated within the linear load-elongation portion between 300 and 450 N. Load-displacement data during cycling and pull to failure were recorded using WaveMatrix software (Instron) with a sampling rate of 750 Hz and a translational accuracy of test machine actuator below 0.01 mm.
Statistical Analysis
Power analysis (power, 0.8; alpha, 0.05) with a detectable difference of 0.3 mm, which represents 10% of the clinical laxity limit,19,23 indicated a sample size requirement of 5 per construct for valid comparisons. With regard to other related studies,31,33 this number was increased to 8 to accommodate possible errors. In this study, the initial elongation, dynamic elongation, and total elongation, as well as ultimate load and stiffness, were defined as primary outcome variables. Statistical analysis was performed using Sigma Plot Statistics for Windows, Version 13.0 (Systat Software). Data analysis was performed with MATLAB, Version R2019a (MathWorks). For significant pairwise analysis of all primary outcome variables, we conducted a 1-way analysis of variance (ANOVA) with Tukey post hoc test for statistical analysis. Significance was defined as P ≤ .05, and the desired power level was set at 0.8. The Shapiro-Wilks test was used to confirm each dataset followed a normal distribution. A nonparametric test, the Kruskal-Wallis, was used for datasets that failed this test. For Kruskal-Wallis tests that found significance, a Tukey post hoc test was conducted to further analyze the differences. The observed post hoc average power values of all 1-way ANOVA tests were much higher than the desired power level of 0.8, leading us to conclude that our sample size was sufficient.
Results
Stability Testing
Valley elongation at the end of each load level was used for statistical comparison of the various constructs using the ANOVA (Figure 3). The BLD showed significantly greater (P < .001) initial elongation than DLD but behaved similar to the SLD. While DLD and SLD had the smallest initial elongation on the same significance level, they behaved opposite to each other with gradually increasing peak loading. DLD showed significantly lowest and SLD the highest total elongation at the end of testing (each P < .001).
Figure 3.
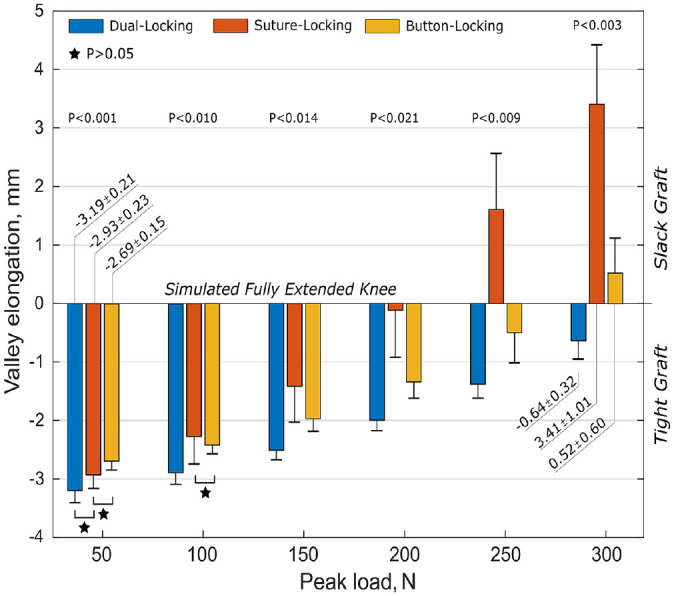
Test results (mean ± SD values) with comparative statistical analysis of the construct elongation at various load levels indicating tight (negative values) and slack (positive values) graft state in relation to the reference position (simulated fully extended knee). Stars represent statistical nonsignificance between constructs as indicated (P > .05). BLD, button-locking device; DLD, dual-locking device; SLD, suture-locking device.
SLD showed considerably higher dynamic elongation (Figure 4A) than the other constructs and demonstrated progressive loop lengthening with increasing cycling loading, as indicated by the migration of the colored stripe into the tunnel (Figure 4C).
Figure 4.
(A) Boxplot of dynamic elongation with mean ± SD values included. The plots represent median (horizontal line), interquartile range (box), and 95% CI (whiskers). Asterisks represent statistically significant difference between constructs (P < .001). (B-D) Representative colored marker position on the available tensioning suture (red arrow) of the femoral (B) DLD, (C) SLD, and (D) BLD before (upper row) and at the end of (bottom row) cyclic loading, indicating loop lengthening. BLD, button-locking device; CI, confidence interval; DLD, dual-locking device; SLD, suture-locking device.
A representative hysteresis curve of each group at the end of testing is shown in Figure 5 and referenced to the native ACL function reference model, which was established in a previous ACLR-related study. 4 The final loading situation of the group’s coincidence either completely (DLD) or partially (BLD) with the native ACL function zone, with only the SLD group showing a complete loose state.
Figure 5.
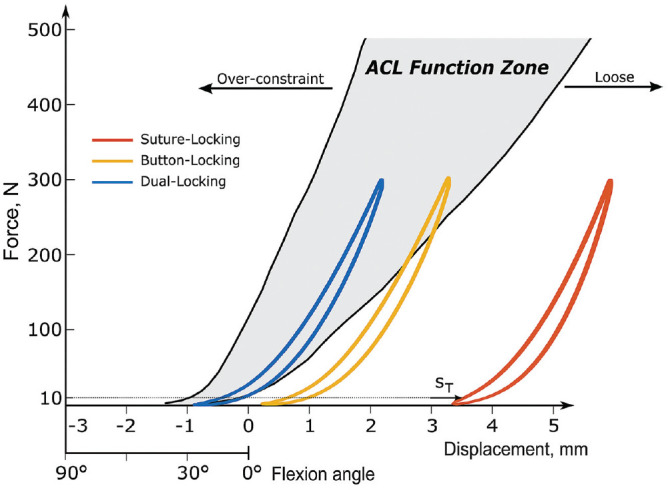
Representative hysteresis curve of each construct with total elongation (sT) as indicator for the final valley loading situation (10 N) in reference to the native functional ACL model. 4 ACL, anterior cruciate ligament.
Pull to Failure
All constructs reached the regular test end and were pulled to failure. The devices showed a high variance with significantly lower failure loads of BLD compared with SLD and DLD (Figure 6). The predominant failure mode was suture rupture and tibial bone breakage with button subsidence (SLD, n = 4). Overall, no significant difference was found in the ultimate stiffness between the constructs.
Figure 6.
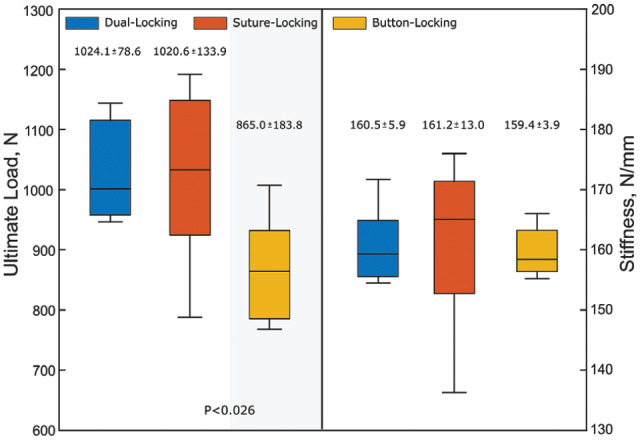
Boxplot of the ultimate failure data with mean ± SD values. The plots represent median (horizontal line), interquartile range (box), and 95% CI (whiskers). Different background shadings indicate statistically significant differences between constructs (test power, P = .99). BLD, button-locking device; CI, confidence interval; DLD, dual-locking device; SLD, suture-locking device.
Discussion
The most important finding of this full-construct surgical technique-based study was that biomechanical outcomes between 3 different types of adjustable loop fixation for all-inside ACLR with graft tensioning in a simulated fully extended knee position revealed significant differences. While DLD successfully restricted critical construct elongation, BLD partially and SLD completely exceeded the clinical failure threshold (>3 mm) of plastic elongation, with loop lengthening during increasing cyclic peak loading with complete unloading.2,5 Higher failure loads of SLD and DLD implants (>1000 N) were achieved at similar construct stiffness to BLD.
Stability of the soft tissue graft in the bone tunnel is essential for graft incorporation in the early postoperative period.7,10,41 Insufficient primary graft tension and stability during daily activities could lead to micromotion with increased knee laxity and compromised healing. 26 The primary tension of an ACL graft at the time of fixation is not linked conclusively to its final clinical outcome, 16 but ALDs provide an important intervention for increased initial graft tension to eliminate laxity and settling effects in the graft placement process as well as to prepare the ACLR construct for the later loading situation.31,33 Adjustable tensioning and suture knot tying have been shown to reduce the overall construct displacement with ALD fixation when compared with fixed loop devices or reference groups.31,33,34 Although multiple biomechanical studies are available on the stability of ALDs,5,9,17,18,34,40 the primary goal of this study was to quantitatively evaluate the stabilization behavior of different commercially available ALDs with femoral and tibial graft fixation in a full-construct clinically relevant model for all-inside ACLR.
In clinical practice, there is currently a wide range of tensioning force applied on variable graft types, depending mainly on the surgeon’s experience without consensus on the optimal graft tension or correlation with the clinical outcome.25,32,37 Although a relatively wide safe window for initial graft tension seems to exist, a higher tensioning force in laboratory studies was shown to better compensate graft tension loss due to the time-dependent viscoelastic behavior of the soft tissue graft.24,27,36 In the current study, femoral-sided graft insertion within an adequate intratunnel graft portion until tunnel docking replicated an optimized clinical condition for graft ingrowth until final healing. Femoral-sided ALD suture knot tying was avoided in this study in accordance with the clinical requirements for simplified intraoperative workflow and reduced danger of postoperative knot irritation of surrounding soft tissue. Tibial-sided adjustable loop shortening was performed in a simulated fully extended knee position with the greatest ACL length to achieve approximately 200 N tension on the graft. 45 Adjustable ACLR graft tensioning in higher knee flexion angles may “overtension” the graft. 29 Overtensioned grafts are associated with limited range of motion, higher contact stress with cartilage degeneration, pain, and early clinical failure after ACLR.32,46 Tibial-sided adjustable graft tensioning may reduce suture-related settling effects in all-inside ACLR constructs with femoral ALDs including suture button-locking fixation, which has been shown to limit the magnitude of graft force after tension release. 44 Distal load applied on the graft confirms a proper button seating on the femoral cortex in clinical practice and transfers the femoral-sided loop sutures into its final state for prompt button-locking function.
Reconstruction of the ACL aims to restore the native ligament structural properties to provide for appropriate knee laxity and stability over normal range of motion. During daily activities, the native ACL is loaded up to 450 N within a load range of approximately 20% of the native actual failure capacity of the ACL,30,35 and has a stiffness of about 182 N/mm to resist anterior-posterior motion.35,47 Current applied peak loads up to 300 N should cover loading from daily activities during the time of early and late rehabilitation and were used in multiple other femoral fixation device studies.5,17,18,34,40 According to previous biomechanical studies,9,21,22,39,40,42 the results of the current study demonstrated predominantly the necessary time-zero biomechanical properties of ALDs with regard to ultimate failure strength, displacement, and stiffness for soft tissue graft fixation in ACLR. In agreement with latest biomechanical studies, including repetitive complete unloading situation,2,17,18 the all-inside ACLR construct with a knotless femoral SLD raised concerns about gradual loop lengthening.2,5 Although it is unclear what amount of ACLR construct lengthening indicates clinical failure, we used the clinical reported side-to-side difference of >3 mm in anterior tibial translation as the failure threshold for plastic elongation.
While all samples of SLD and a few samples of BLD (5 of 8 specimens) exceeded dynamic elongation more than 3 mm, DLD restricted dynamic elongation to less than 3 mm and was able to maintain a tight graft in relation to the reference primary fixation position (simulated fully extended knee). Differences in the elongation behavior of the study constructs may have occurred because of (1) slightly different graft tension levels established by manual loop shortening before cycling and (2) differences in the implant stability during cyclic complete unloading-loading situations with various suture loop configuration and locking mechanism. Although the role of complete unloading situation of ALD for graft fixation on the clinical significance is unclear, ALDs with higher fixation strength tested in more extreme testing conditions (including an unloading situation) could be considered to be the choice devices for clinical use in ACLR with early range of motion and accelerated rehabilitation.
Limitations
This study has certain limitations. Bovine tendon and porcine bone were used as substitutes for human tissue because of their reported similar biomechanical properties and common use in other related biomechanical research.1,15,33,34,44 Porcine tibia was found to have higher bone mineral density and may lead to increased ultimate failure loads. 1 Load was applied along the device and graft long-axes to achieve worse-case testing conditions for ACLR. Discrepancies between the biomechanical and clinical outcomes may be explained by the distinct in vivo load transfer with angled bone tunnels and the graft bathed in the synovial fluid within a dynamic knee joint. Clinical results with ALD have shown generally good postoperative knee stability and failure rate without significance to FLD with tibial screw fixation. 11 This is a time-zero, in vitro biomechanical study focusing on the stabilization potential of available ALD for femoral fixation in all-inside ACLR. Further short- and long-term translational animal and clinical follow-up studies are required to assess adequate fixation strength of variable ALD for graft stabilization throughout graft healing and maturation.
Conclusion
While DLD successfully restricted critical construct elongation, BLD partially and SLD completely exceeded the clinical failure threshold (>3 mm) of plastic elongation with loop lengthening during increasing cyclic peak loading with complete unloading. Higher failure loads of SLD and DLD implants (>1000 N) were achieved at construct stiffness similar to that of BLD.
Footnotes
Final revision submitted April 28, 2023; accepted May 19, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: research support for this study was provided by Arthrex. S.B. and C.A.W. are employees of Arthrex. P.A.W. has received education payments from Elite Orthopedics and United Orthopedics; consulting fees from Arthrex; nonconsulting fees from Arthrex, Kairos Surgical, and Medical Device Business Services; and royalties from Arthrex. R.M.F. has received education payments from Arthrex, consulting and nonconsulting fees from Arthrex, and hospitality payments from JRF Ortho, Smith & Nephew, and Stryker. E.G.M. has received education payments and nonconsulting fees from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663-670. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad SS, Hirschmann MT, Voumard B, et al. Adjustable loop ACL suspension devices demonstrate less reliability in terms of reproducibility and irreversible displacement. Knee Surg Sports Traumatol Arthrosc. 2018;26(5):1392-1398. [DOI] [PubMed] [Google Scholar]
- 3. Arneja S, McConkey MO, Mulpuri K, et al. Graft tensioning in anterior cruciate ligament reconstruction: a systematic review of randomized controlled trials. Arthroscopy. 2009;25(2):200-207. [DOI] [PubMed] [Google Scholar]
- 4. Bachmaier S, Smith PA, Bley J, Wijdicks CA. Independent suture tape reinforcement of small and standard diameter grafts for anterior cruciate ligament reconstruction: a biomechanical full construct model. Arthroscopy. 2018;34(2):490-499. [DOI] [PubMed] [Google Scholar]
- 5. Barrow AE, Pilia M, Guda T, Kadrmas WR, Burns TC. Femoral suspension devices for anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(2):343-349. [DOI] [PubMed] [Google Scholar]
- 6. Boyle MJ, Vovos TJ, Walker CG, Stabile KJ, Roth JM, Garrett WE., Jr. Does adjustable-loop femoral cortical suspension loosen after anterior cruciate ligament reconstruction? A retrospective comparative study. Knee. 2015;22(4):304-308. [DOI] [PubMed] [Google Scholar]
- 7. Brand J, Jr, Weiler A, Caborn DNM, Brown CH, Jr, Johnson DL. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28(5):761-774. [DOI] [PubMed] [Google Scholar]
- 8. Browning WM, III, Kluczynski MA, Curatolo C, Marzo JM. Suspensory versus aperture fixation of a quadrupled hamstring tendon autograft in anterior cruciate ligament reconstruction: a meta-analysis. Am J Sports Med. 2017;45(10):2418-2427. [DOI] [PubMed] [Google Scholar]
- 9. Chapman G, Hannah J, Vij N, Liu JN, Morrison MJ, Amin N. Biomechanical comparison of adjustable-loop femoral cortical suspension devices for soft tissue ACL reconstruction. Orthop J Sports Med. 2023;11(2):23259671221146788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Cooley V, Rosenberg T. ACL reconstruction with hamstring tendon. Orthop Clin North Am. 2003;34(1):9-18. [DOI] [PubMed] [Google Scholar]
- 11. Choi HG, Jeong HW, Park SB, Shim SJ, Lee YS. Additional tying on the adjustable-loop device improves the outcomes of anterior cruciate ligament reconstruction using hamstring autograft. Knee Surg Sports Traumatol Arthrosc. 2022;30:3673-3680. [DOI] [PubMed] [Google Scholar]
- 12. Choi N, Yang B, Victoroff B. Clinical and radiological outcomes after hamstring anterior cruciate ligament reconstructions: comparison between fixed-loop and adjustable-loop cortical suspension devices. Am J Sports Med. 2017;45(4):826-831. [DOI] [PubMed] [Google Scholar]
- 13. Connaughton AJ, Geeslin AG, Uggen CW. All-inside ACL reconstruction: how does it compare to standard ACL reconstruction techniques? J Orthop. 2017;14(2):241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401-407. [DOI] [PubMed] [Google Scholar]
- 15. Domnick C, Wieskötter B, Raschke MJ, et al. Evaluation of biomechanical properties: are porcine flexor tendons and bovine extensor tendons eligible surrogates for human tendons in in vitro studies? Arch Orthop Trauma Surg. 2016;136(10):1465-1471. [DOI] [PubMed] [Google Scholar]
- 16. Fleming BC, Fadale PD, Hulstyn MJ, et al. The effect of initial graft tension after anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(1):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glasbrenner J, Domnick C, Raschke MJ, et al. Adjustable buttons for ACL graft cortical fixation partially fail with cyclic loading and unloading. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2530-2536. [DOI] [PubMed] [Google Scholar]
- 18. Götschi T, Rosenberg G, Li X, et al. Biomechanical evaluation of a novel loop retention mechanism for cortical graft fixation in ACL reconstruction. Orthop J Sports Med. 2020;8(2):2325967120904322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heijne A, Fleming BC, Renstrom PA, Peura GD, Beynnon BD, Werner S. Strain on the anterior cruciate ligament during closed kinetic chain exercises. Med Sci Sports Exerc. 2004;36(6):935-941. [DOI] [PubMed] [Google Scholar]
- 20. Iuchi R, Mae T, Tachibana Y, et al. Mechanical properties of an adjustable-loop cortical suspension device for anterior cruciate ligament reconstruction. Orthop J Sports Med. 2018;6(8):2325967118791183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson JS, Smith SD, LaPrade CM, Turnbull TL, LaPrade RF, Wijdicks CA. A biomechanical comparison of femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction under high loads. Am J Sports Med. 2015;43(1):154-160. [DOI] [PubMed] [Google Scholar]
- 22. Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C. Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy. 2009;25(7):767-776. [DOI] [PubMed] [Google Scholar]
- 23. Kawakami H, Shino K, Hamada M, et al. Graft healing in a bone tunnel: bone-attached graft with screw fixation versus bone-free graft with extra-articular suture fixation. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):384-390. [DOI] [PubMed] [Google Scholar]
- 24. Kayaalp ME, Collette R, Kruppa P, et al. A higher initial tensioning force of an ACL graft results in a higher graft force after screw fixation irrespective of the screw diameter: a biomechanical study. Am J Sports Med. 2021;49(14):3825-3832. [DOI] [PubMed] [Google Scholar]
- 25. Kirwan GW, Bourke MG, Chipchase L, Dalton PA, Russell TG. Graft tensioning practices in anterior cruciate ligament reconstruction amongst orthopaedic surgeons in Australia: a national survey. Arch Orthop Trauma Surg. 2015;135(12):1733-1741. [DOI] [PubMed] [Google Scholar]
- 26. Kousa P, Järvinen TLN, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: part i: femoral site. Am J Sports Med. 2003;31(2):174-181. [DOI] [PubMed] [Google Scholar]
- 27. Kruppa P, Flies A, Wulsten D, et al. Significant loss of ACL graft force with tibial-sided soft tissue interference screw fixation over 24 hours: a biomechanical study. Orthop J Sports Med. 2020;8(5):2325967120916437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li G, DeFrate LE, Rubash HE, Gill TJ. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23(2):340-344. [DOI] [PubMed] [Google Scholar]
- 29. Lubowitz JH. Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1190-1195. [DOI] [PubMed] [Google Scholar]
- 30. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med 1999;27:35-43. [DOI] [PubMed] [Google Scholar]
- 31. Monaco E BS, Fabbri M, Lanzetti RM, Wijdicks CA, Ferretti A. Intraoperative workflow for all-inside anterior cruciate ligament reconstruction: an in vitro biomechanical evaluation of preconditioning and knot tying. Arthroscopy. 2018;34(2):538-545. [DOI] [PubMed] [Google Scholar]
- 32. Nicholas SJ, D’Amato MJ, Mullaney MJ, Tyler TF, Kolstad K, McHugh MP. A prospectively randomized double-blind study on the effect of initial graft tension on knee stability after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(8):1881-1886. [DOI] [PubMed] [Google Scholar]
- 33. Noonan BC, Bachmaier S, Wijdicks CA, Bedi A. Intraoperative preconditioning of fixed and adjustable loop suspensory anterior cruciate ligament reconstruction with tibial screw fixation – an in vitro biomechanical evaluation using a porcine model. Arthroscopy. 2018;34(9):2668-2674. [DOI] [PubMed] [Google Scholar]
- 34. Noonan BC, Dines JS, Allen AA, Altchek DW, Bedi A. Biomechanical evaluation of an adjustable loop suspensory anterior cruciate ligament reconstruction fixation device: the value of retensioning and knot tying. Arthroscopy. 2016;32(10):2050-2059. [DOI] [PubMed] [Google Scholar]
- 35. Noyes FR, Butler D, Grood E, Zernicke R, Hefzy M. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66(3):344-352. [PubMed] [Google Scholar]
- 36. Nurmi JT, Kannus P, Sievänen H, Järvelä T, Järvinen M, Järvinen TLN. Interference screw fixation of soft tissue grafts in anterior cruciate ligament reconstruction: part 2: effect of preconditioning on graft tension during and after screw insertion. Am J Sports Med. 2004;32(2):418-424. [DOI] [PubMed] [Google Scholar]
- 37. O’Neill BJ, Byrne FJ, Hirpara KM, Brennan WF, McHugh PE, Curtin W. Anterior cruciate ligament graft tensioning. Is the maximal sustained one-handed pull technique reproducible? BMC Res Notes. 2011;4:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Onggo JR, Nambiar M, Pai V. Fixed- versus adjustable-loop devices for femoral fixation in anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2019;35(8):2484-2498. [DOI] [PubMed] [Google Scholar]
- 39. Pasquali M, Plante MJ, Monchik KO, Spenciner DB. A comparison of three adjustable cortical button ACL fixation devices. Knee Surg Sports Traumatol Arthrosc. 2017;25(5):1613-1616. [DOI] [PubMed] [Google Scholar]
- 40. Petre BM, Smith SD, Jansson KS, et al. Femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(2):416-422. [DOI] [PubMed] [Google Scholar]
- 41. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803. [DOI] [PubMed] [Google Scholar]
- 42. Rylander L, Brunelli J, Taylor M, et al. A biomechanical comparison of anterior cruciate ligament suspensory fixation devices in a porcine cadaver model. Clin Biomech (Bristol, Avon). 2014;29(2):230-234. [DOI] [PubMed] [Google Scholar]
- 43. Singh S, Ramos-Pascual S, Czerbak K, et al. Biomechanical testing of fixed and adjustable femoral cortical suspension devices for ACL reconstruction under high loads and extended cyclic loading. J Exp Orthop. 2020;7(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith PA, Piepenbrink M, Smith SK, Bachmaier S, Bedi A, Wijdicks CA. Adjustable- versus fixed-loop devices for femoral fixation in ACL reconstruction: an in vitro full-construct biomechanical study of surgical technique-based tibial fixation and graft preparation. Orthop J Sports Med. 2018;6(4):2325967118768743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor KA, Cutcliffe HC, Queen RM, et al. In vivo measurement of ACL length and relative strain during walking. J Biomech. 2013;46(3):478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Kampen A, Wymenga AB, van der Heide HJ, Bakens HJ. The effect of different graft tensioning in anterior cruciate ligament reconstruction: a prospective randomized study. Arthroscopy. 1998;14(8):845-850. [DOI] [PubMed] [Google Scholar]
- 47. Woo SL-Y, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. Am J Sports Med. 1991;19(3):217-225. [DOI] [PubMed] [Google Scholar]