Abstract
In this review, the current status of the worldwide experience on different catheter-directed treatment systems utilized as alternative reperfusion methods in acute pulmonary embolism was evaluated, and the risk stratification algorithms in which catheter-directed treatments may be implemented, source of evidence in this setting, adjudication of benefits and risks of available techniques, and innovative multi-disciplinary frameworks for referral patterns and care delivery were discussed. Moreover, our perspectives on risk-based catheter-directed treatment utilization strategies in acute pulmonary embolism were summarized.
Keywords: Ultrasound-assisted thrombolysis, rheolytic thrombectomy, catheter-directed thrombolysis, catheter-directed thrombectomy, pulmonary embolism
Highlights
Catheter-directed treatment (CDT) of acute pulmonary embolism (PE) is rapidly evolving; however, the vast majority of techniques in CDT have been poorly evaluated, and pooled data have been mainly based on case reports, retrospective analysis of small series, non-randomized studies, or inadequately powered randomized control trials (RCTs).
The decisions for urgency reperfusion strategies and mode of treatments in acute PE seem to require novel, innovative risk algorithms which consider dynamic changes in vital parameters under initial therapies as a benefit or failure and costs of major bleedings, beyond the 4-risk statuses as high-, intermediate-high-, intermediate-low-, and low risk at initial assessment.
The promising evidence for efficacy and safety of CDTs with reduced fibrinolytic dose/shorter infusion duration or pure mechanical extraction/thrombo-aspiration systems seems to change treatment algorithms in patients with acute PE at high-risk and intermediate-high-risk statuses.
New prospective RCTs are needed to standardize the timing of intervention, patient and CDT technique selection, and peri-procedural lytic and anticoagulation regimens and to improve the benefit of pulmonary embolism response team framework in PE management.
Introduction
Acute pulmonary embolism (PE) has been documented as one of the most frequent lethal cardiovascular diseases in the Western part of the World.1 Acute-onset hemodynamic instability suggests thrombotic obliteration in 30%-50% of the pulmonary arteries (PA),1,2 and a pressure mismatch resulting in right ventricle (RV) failure evidenced by increased RV diameter to left ventricle diameter ratio (RV/LVr) either assessed by echocardiography (Echo) or computed tomographic pulmonary angiography (CTPA) has been shown to predict clinical worsening regardless of the initial clinical status.1-6 European Society of Cardiology/European Respiratory Society (ESC/ERS) 2019 PE Guidelines have recommended an updated risk-based treatment strategy.1 Patients at high-risk status have been regarded to require urgent reperfusion therapy.1 Moreover, rescue reperfusion therapies or catheter-directed treatments (CDTs) should be considered in cases of rapidly developing hemodynamic instability even in patients initially in intermediate- or low-risk (IR or LR) subgroups.1
Although global trends in PE have demonstrated an increasing incidence with a decreasing all-cause or PE-related 30-day mortality, small but significant increases in the utilization rates of low-molecular-weight heparin and systemic thrombolytic therapies (TTs) and interventional or surgical strategies have remained unsatisfactory.1 This underutilization of TT in acute PE, even in the presence of clear indications,1 seems to be associated with increased bleeding risk despite the proven reductions in the pooled rate of mortality and/or PE recurrence with TT as compared to anticoagulant therapies shown in 2 meta-analyses.7,8 Hence, various CDTs have been developed to relieve thrombotic pulmonary blood flow obstruction quickly and to improve hemodynamic status with minimal thrombolytic dosages or even without thrombolytics.9-43 However, these CDT methods have faced the challenge of trying to aspirate, extract, or dissolve massive, sometimes partially organized clots from a large territory characterized by numerous fractal branchings with multiple angles.9-43 Furthermore, the vast majority of these techniques have been poorly evaluated, and pooled data have been mainly based on case reports or retrospective analysis of small series or non-randomized studies in which different CDT methods have been utilized.9-43
In this article, we aimed to overview the current status of percutaneous reperfusion methods utilized in acute PE treatments regarding the definitive criteria for risk stratification algorithms in which CDTs may be implemented, adjudication of benefits and risks of available techniques, and innovative multidisciplinary frameworks for referral patterns and care delivery. Moreover, we summarized our single-center experience on risk-based CDT utilization strategies in acute PE care.
Currently available CDTs developed for acute PE treatment have been based on 2 mechanisms: i.e., catheter-directed thrombolysis (CDTL) or catheter-directed thrombectomy (CDTE).1,9,10
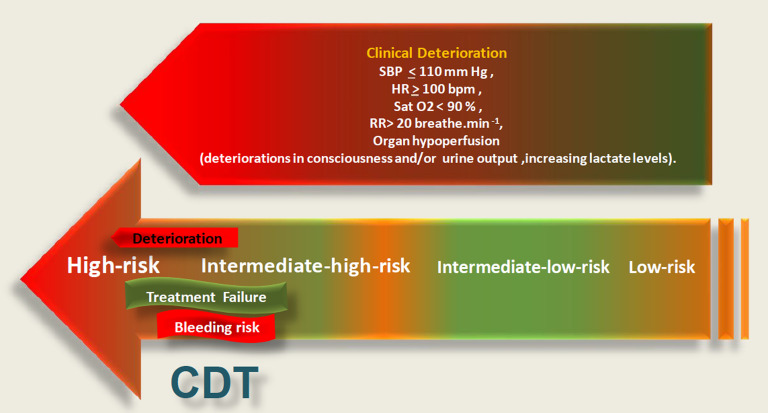
The decisions for urgency reperfusion strategies and the mode of treatments in acute PE seem to require novel, innovative risk algorithms which consider dynamic changes in vital parameters under initial therapies as a benefit or failure and costs of major bleedings, beyond the 4 risk statuses as high, intermediate-high, intermediate-low, and low risk (HR, IHR, ILR, and LR) at initial assessment.1-4,9,10 From the perspective of reperfusion therapies, definitions of treatment failure and predictors of deterioration to a higher risk status as assessed by cardiopulmonary, metabolic, and systemic organ decompensation need to be more clearly evaluated (Figure 1).1-4,9,10 Because treatment goals of CDTs are quite different between HR and IHR statuses, the terms of treatment success or failure with lack of improvement or with deterioration to life-threatening scenario should also be defined on the basis of initial risk status, and different weighting of treatment failure in patients at IHR and HR should be taken into consideration in a multistate survival analysis model.1,9,10 Moreover, overall bleeding risk and co-morbidities should be implemented in the decision-making for CDTE or CDTL.1,9,10 Post hoc analysis of IR subgroup in the Pulmonary EmbolIsm THrOmbolysis (PEITHO) trial revealed novel predictors of early hemodynamic deterioration or death, in addition to RV/LVr >1.0 and troponin elevation.3 These were systolic blood pressure <110 mm Hg for >15 minutes, respiratory rate >20/minutes or SpO2 <90% at room air and heart rate >100 beats per minute not due to hypovolemia, arrhythmia, or sepsis.3 The indicators of impending deterioration in acute PE have been summarized in a recently published consensus paper on percutaneous treatment options for acute PE: a clinical consensus statement of the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and European Association of Percutaneous Cardiovascular Interventions.10
Figure 1.
Essentials of decision-making for catheter-directed treatment in acute pulmonary embolism comprising the predictors of cardiopulmonary, metabolic and systemic deterioration, and risk of major bleeding.
Figure 1 demonstrated the current perspective for reperfusion therapies based on predictors of cardiopulmonary, metabolic, and systemic deterioration in acute PE with considering the risk of major bleeding.10
Since the approval of the Greenfield suction embolectomy catheter as first CDT for acute PE by Food and Drug Administration (FDA) Committee, many CDT techniques have been introduced into the clinical practice over 4 decades (Figure 2).11-43 However, the need for risk-adjusted CDT treatments balancing efficacy and safety outcomes has remained unmet until the approval of an ultrasound-associated thrombolysis (USAT) technology (EkoSonic® Endovascular System, Boston Scientific, USA) by FDA for the treatment of acute pulmonary embolism.15,16 In a meta-analysis of earlier series, CDT was associated with a clinical success of 86.5% with minor and major complication rates of 8% and 2.4%, respectively. In a prospective multicenter registry in which fragmentation, embolectomy, and catheter thrombolysis techniques were utilized, success rates of CDT in massive and submassive PE were 85.7% and 97.3%, respectively.13 Moreover, CDT was reported to be associated with a significant reduction in pulmonary artery mean pressure (PAMP) and improvement in RV function without major complication.13
Figure 2.
CDT trials in acute pulmonary embolism.
EkoSonic® Endovascular System (Boston Scientific) consists of a removable microsonic device containing multiple small ultrasound transducers within an intelligent drug delivery catheter having cooling lumina and multiple side holes distributed over the treatment zone (usually 12 cm) connecting a central EkoSonic® control unit.15 This system produces high frequency (2.2 Mhz) and low power (0.5 W per element) pulses of varying waveforms which facilitates penetrance of fibrinolytics to deeper layers of clot and dissolution of fibrin cross-links.15 The efficacy and safety issues of ultrasound-assisted thrombolysis (USAT) with adjunctive moderate-dose tissue plasminogen activator (t-PA) regimens have been confirmed by an RCT,15 retrospective and prospective studies, and meta-analyses.16-28 We have reported serial results of our single-center experience on USAT in patients with PE at HR and IHR statuses.18,20,21 Moreover, based on our meta-analysis on USAT series published before December 2015, RV/LVr, PAMP, and CT obstruction scores from 11 trials revealed that overall PAMP, RV/LVr, and CT obstruction measures were reduced significantly.19 All-cause and cardiovascular mortality, major and minor bleeding episodes, and recurrent PE from 15 trials showed that the pooled incidence of all-cause and cardiovascular mortality was 3.2% and 2.2%, and the incidence of major and minor bleeding episodes was 5.5% and 6.9%, respectively.19 Furthermore, as compared to those in TT arms of 2 meta-analyses evaluating TT versus anticoagulation in acute PE, USAT provided similar overall mortality rates with a significantly reduced overall rate of major bleeding (5.5% vs. 9.9% and 9.2%, P < .001 and P = .002).7,8,19
Although USAT has been approved as a novel treatment method in acute PE, optimal ranges of t-PA dose and infusion durations remain to be determined. Therefore, OPTALYSE-PE RCT comparing 4 cohorts of t-PA dosage and infusion durations in USAT treatments was designed to evaluate the efficacy and safety of lower doses and shorter infusion durations in acute PE.22 The improvements in RV/LVr ranging from 23% to 26% were comparable across the cohorts.22 However, increasing the dose and infusion duration of t-PA seemed to be related to a higher resolution of thrombotic burden.22 Long-term follow-up over a 1-year period also demonstrated the sustained benefit in terms of RV function, functional status, and quality of life.24 Nevertheless, the low enrolment rate seems to suggest a possible selection bias due to the long list of exclusion criteria ignoring unmet needs in real-life PE practice.22,23 Moreover, one-fifth of the patients were at ILR status in which the superiority of USAT over anticoagulants has never been confirmed, and age, risk status, and other therapies were not taken into account in the analysis.22,23 Thereafter, KNOCOUT-PE multicenter registry has been designed to evaluate the impact of OPTALYSE-PE results on decision-making for low-dose/short-infusion duration regimens in USAT therapies. Interim analysis at 2019 comprising 260 patients from pre-OPTALYSE period and 138 patients post-OPTALYSE period revealed a 6-fold increase in the utilization of low-dose (<12 mg) and short-infusion duration regimens, but lowering the t-PA dose and shortening the infusion durations did not reduce the frequency of major bleeding, intracranial bleeding, and mortality.25 More interestingly, in the final analysis of the prospective arm in KNOCOUT-PE study presented in 2021, mean t-PA doses <20 mg and <12 mg were noted in 70.6% and 32.4% of patients, respectively.26 Mean t-PA infusion duration was 10.4 ± 5.2 and intensive care stay was 48.9 ± 47.4 hours.26 The reduction in RV/LVr was 22.6% in post-procedural period followed by 41.8% reduction at 30-day control.26 The improvements in PE-specific quality of life and patient-reported outcomes by 3 months were also reported to be significant.26 However, 9 out of 498 patients in prospective cohort have not been included in the safety analysis, and 1 intracranial hemorrhage (0.9%) in the subgroup of 12.1-20 mg t-PA and 2 deaths (3.1%) in the subgroup of 4-12 mg t-PA reported in interim analysis of 2019 have not been addressed in 2021 analysis in which no intracranial bleeding or death was reported in the prospective arm.25,26 Therefore, these inconclusive KNOCOUT PE results should not be considered as convincing evidence for benefits from low-dose, short-infusion regimens in the real-life practice of USAT therapies. An ongoing RCT, with an updated design of ULTIMA, HI-PEITHO (the higher-risk PE thrombolysis study) has been designed to compare the efficacy and safety of USAT plus parenteral heparin versus parenteral heparin alone therapies in patients with PE at IHR.27 The primary endpoint was defined as a 7-day composite of PE-related mortality, PE recurrence or cardiorespiratory decompensation, or collapse.27
In our 10-year single-center CDT practice, USAT was the treatment of choice in 236 patients (39.33%) with acute PE at HR or IHR. Our series comprising patients with multiple comorbidities have represented the largest single-center and long-term data on USAT ever published.21 Moderate dose (35.4 ± 13.3 mg) and slow-infusion (26.6 ± 7.7 hours) t-PA regimen resulted in statistically significant and clinically relevant reductions in RV/LVr, Qanadli score (QS) of thrombotic obliteration, and PAMP, and HR versus IHR was associated with more pronounced improvements in these measures (Figure 3).21 In-hospital major and minor bleeding and mortality rates were 6.2%, 12.4%, and 6.2%, respectively. Bleeding and unresolved PE accounted for 50% and 42.8% of in-hospital deaths, respectively.21 Despite the higher lytic dosages and longer infusion times in our study compared with those in the OPTALYSE-PE trial, major bleeding or mortality risk was not related to dose or infusion duration of t-PA.21 This difference might be due to the higher frequency of comorbidities in our study in comparison to OPTALYSE-PE trial.21,22 The HR versus IHR status related to a significantly higher 30-day mortality rate, whereas age >65 years was associated with long-term mortality.21 However, retrospective analysis and operator-driven selection of the t-PA regimen were the main limitations of our study. Prospective trial designs which permit comparisons among risk-based anticoagulant and full-dose or reduced-dose systemic fibrinolytic therapies, different doses and infusion regimens of t-PA with USAT, and USAT versus other CDTs might provide more relevant data for efficacy and safety concerns in this setting.
Figure 3.
Absolute reductions in RV/LV diameter ratio and percentage reductions in obstruction scores reported in catheter-directed thrombolysisand catheter-directed thrombolysis series.
Standard Catheter-Directed Thrombolysis Devices
Cragg–Mcnamara (Medtronic, Minneapolis, Minn, USA), Unifuse (Angiodynamics, Latham, NY, USA), and Fountain (Merit Medical, South Jordan, UT, USA) catheters are other devices available for CDTL.28,29,35-37 These catheters have multiple side slits to facilitate the distribution of the fibrinolytic agent within the thrombus.28,29,35-37 The main advantage of these catheters over the USAT is the lower cost. In the SUNSET-sPE (Standard vs. Ultrasound-Assisted Catheter Thrombolysis for Submassive Pulmonary Embolism) trial, primary end-point, which is the reduction in the PA obstruction score at 48 hours, was comparable between patients randomized to USAT versus standard CDTL arms (21% and 22%, respectively).37 The improvements in RV/LVr, intensive care unit and hospital stay, bleeding, and adverse events up to 90 days as secondary outcomes were also similar between the 2 cohorts.37 However, the lack of standardization in t-PA regimen across the groups, ignoring the possible confounders, the small sample size and low power of the study, and the assumed effect size of 50% in favor of USAT based on the results of a prior deep venous thrombosis study comparing the USAT and standard CDTL have been considered as important limitations of this phase II trial evaluating clot resolution.37 Moreover, whether relevant clinical benefits could be obtained with more limited reductions in clot burden remains to be determined. Nevertheless, a signal may imply a probable superiority of USAT over standard CDTL in 20 patients treated with a low-dose/short-duration t-PA regimen after the publication of the OPTALYSE trial.37
The novel Bashir endovascular device (BEC, Thrombolex, New Britain, Pa, USA) is composed of a 7F venous access sheath and a spiral-cut nitinol basket at the tip of the catheter having 6 mini-infusion catheters and a total of 48 laser-drilled holes.30 The longer (12.5 cm) and shorter (10 cm, Bashir Short-Basket) versions of the basket are available.30 The basket has the ability to expand up to a maximum diameter of 45 mm, making direct contact with the wall of the PA. Serial collapse, re-deployment, and re-expansion sequences of basket enhance the penetration of t-PA to fibrin-binding sites, and pulse sprays also generate microbubbles of t-PA entrapped inside the deep layer of clot and maintain lysis even after the removal of the device. The t-PA by Endovascular Administration for the Treatment of Submassive PE Using CDT for the Reduction of Thrombus Burden (RESCUE) multicenter prospective trial (NCT03927508) was designed to evaluate the efficacy and safety issues of the BEC system in IR PE, and 109 patients at 18 sites in the US were enrolled.30 The primary efficacy endpoint was the core lab-assessed change in the CT angiography-derived mean RV/LVr at 48 hours. The primary safety endpoint was serious adverse events, including major bleeding at 72 hours. The median device placement time was 15 minutes, and 7 mg of t-PA was delivered into each PA over a 5-hour infusion period. At 48 hours after delivering t-PA, the RV/LVr decreased from baseline by 0.56 (33.3%, P < .0001), and the core lab-assessed refined Modified Miller Index reduced by 35.9% (P < .0001). Compared to the other core lab-assessed CDT trials, this reduction was more than 2-fold greater. The major bleeding rate was less than 1%, and the length of hospital stay was 2.8 days.30 Based on these findings, the BEC catheter received FDA clearance for the treatment of acute PE in April 2023.
Angiojet rheolytic thrombectomy (ART) (Boston Scientific, Marlborough, MA, USA) system is another CDTL device that generates high pressure and circumferential saline jet expulsions at the catheter tip (2500 psi or 1.7-107 Pa) creating a local low-pressure zone for suction, fragmentation, and aspiration of the clot. On-the-wire design of this system allows selective aspiration of clot along the all subsegmentary PA branches. However, the mortality rate in the unstable settings remains high, and a controversy exists regarding the safety concerns of ART.13,14,31-34 In an early meta-analysis of 68 patients treated with ART, the rates of major and minor complications and procedure-related deaths were 28% and 40%, and 7.35%, respectively.12 More importantly, 76% of all major complications were reported to be attributable to ART, and the authors concluded that this device should not be used as the initial mechanical treatment in future CDT protocols for patients with acute massive PE.12 Accordingly, the FDA has issued a black-box warning on the commercially available ART device label pertaining to its use in patients with acute PE. In recent ART series, significant improvements in clinical, hemodynamic and angiographic measures, and favorable in-hospital and long-term courses were reported.31-35
In our series, ART was performed in 56 patients (9.33%) with acute PE at HR or IHR status.34 Patients had absolute or relative contraindication for TT such as active bleeding from esophageal varices, recent or active major bleeding, early post-surgical PE, and intracranial metastasis or bleeding.34 The ART activation duration (median 304 seconds) was markedly longer and either the utilization rate of adjunctive t-PA (33.9%) and t-PA dose (median 15 mg) were lower in our series as compared to those in the previous studies.34 The ART is related to significant improvements in PA obstruction, RV/LVr, and PA pressures (P < .001 for all) (Figure 3). Transient bradyarrhythmias were observed in 32.1% of patients and terminated spontaneously immediately after the deactivation of ART. Gross hemoglobinuria due to hemolysis was uniformly observed immediately after ART and cleared within the first day in all the patients with saline overhydration. Major and minor bleeding, and in-hospital death rates were 37.5%, 7.1%, 12.5%, and 8.9%, respectively.34 In-hospital deaths were caused by major bleeding and unresolved PE in 20% and 80% of patients, respectively. Although post-procedural renal worsening was observed in 39.3% of our patients, 1-session dialysis was required in only 1 (1.8%) patient, and all patients recovered. Older age, but not risk status or ART activation duration, was associated with post-procedural renal worsening, whereas HR status is related to in-hospital and cumulative long-term deaths.34 However, retrospective nature of the analysis and operator-driven decisions for the total duration of ART activation runs and adjuvant t-PA regimen were the main limitations of our study.
In our unpublished systematic review and meta-analysis based on 427 patients from 24 PE studies in which ART was utilized, according to different definitions, the reported frequencies of HR, IR, and IHR status were 51.52%, 48.47%, and 40.51%, and massive and sub-massive PEs were 60.65% and 39.35%, respectively. Overall, ART duration was available in 3 series, and adjunctive fibrinolytic was noted in 45% of ART procedures. Improvements in the RV/LVr, PA pressures, and obstruction scores were significant (P < .0001 for all). Overall pooled proportion of major and minor bleeding was around 10%. Overall pooled rate of renal worsening was 15% (95% CI 10-21.8%), and PE-related death and all-cause death were 12.7% (95% CI 9.1-17.3%) and 15% (95% CI 11-20%), respectively. However, except for PE-related death and all-cause death, significant heterogeneity and some evidence of funnel plot asymmetry and publication bias were noted for other outcome measures. Male versus female sex is related to the increase the risk of worsening renal function. Aging significantly increased, and the male gender decreased the risks for PE-related and all-cause death, but HR status did not. All these results suggest that a reappraisal for black-box warning on ART is necessary.
Mechanical Thrombectomy
These systems have been developed to accelerate the resolution of obstructive clots from PA branches without the need for adjunctive fibrinolytic agents if possible. However, the requirement for large-bore venous access in some systems and the risk of dislodgement of thrombi to more distal PA branches have been considered as major drawbacks of previously used mechanical thrombectomies (MTs). The FlowTriever Retrieval/Aspiration system (Inari Medical, Inc., Irvine, Calif, USA) and the Penumbra Indigo aspiration catheter system (Penumbra, Alameda, Calif, USA) are 2 important MT devices having FDA approval for percutaneous PE treatments. The FlowTriever Retrieval/Aspiration device consists of aspiration cannulas available in three sizes: 16F, 20F, and 24F, along with three self-expanding nitinol mesh disks designed to capture and retrieve large thrombi into the aspiration catheter.38 The safety and effectiveness of this device were documented in the prospective, single-arm FLARE (FlowTriever Pulmonary Embolectomy Clinical Study) trial in which patients with acute IR PE and RV/LVr ≥0.9 were enrolled.38 Baseline characteristics were consistent with a stable status, and more than one-half of the population seemed to be at ILR status. Although the primary effectiveness end point was met by a mean absolute or % reduction in RV/LVr (0.38% or 25.1%, P < .0001) compared with baseline, the mean final RV/LVr of 1.15 ± 0.25 was still unsatisfactory.38,39 Essentially, an average reduction in mean PA pressure was mainly driven by a reduction in patients with mPAP >25 mm Hg (P < .0001), and the statistical reduction in the obstruction score (20.8 ± 2.4 vs. 18.9 ± 2.9; P < .001) seemed to be not clinically relevant.38,39 The multiple passes of 20-F catheter within an average duration of 94 minutes and the need for 2 or 3 devices in 58.7% of procedures implicate the aggressive aspect of this strategy for IR PE which may be managed with anticoagulant therapies.38,39
The questions regarding the limitations of FLARE study were cleared with the prospective, multi-center FLASH registry (FlowTriever All-Comer Registry for Patient Safety and Hemodynamics) enrolling 800 patients from 50 United States sites.40 Thrombolytic contraindication was reported in one-third of patients, and the risk status was intermediate and high in 92.1% and 7.9%, respectively. On-table PA pressure drop and improvement in cardiac index and normalization in RV function were significant.40 But baseline systolic PA pressure >70 mm Hg was associated with a less reduction in PA pressure. Major adverse event and all-cause death rates in the first 48 hours were 1.8% and 0.3%, respectively. The t-PA was needed in only 2.3% of patients, and no device-related serious adverse event was reported. Post-procedural overnight intensive unit care was required in less than 40% of them and the mean length of hospital stay was 3 days. The 30-day all-cause mortality and all-cause re-admission rates were also impressive (0.8% and 6.2%, respectively).40 The FLAME (Flowtriever for massive PE) trial was designed as the largest prospective study of interventional treatment in HR PE, and Flowtriever compared with control arm cohort including systemic thrombolysis, surgical embolectomy, other CDTs, or anticoagulant treatment options was associated with a significant reduction in the rates of primary composite end-point and more than 90% reduction in in-hospital mortality.41 Finally, for addressing the gaps in this setting, the PEERLESS study was designed as a prospective, multicenter RCT to compare the efficacy and safety outcomes of FlowTriever system versus CDTL in patients with PE at IHR.
The Penumbra Indigo aspiration device is another MT system that is capable of a 28.5 mm Hg negative continuous suction pressure.42 The safety and efficacy of this device were evaluated in the multicenter EXTRACT PE trial in which patients with IR acute PE were enrolled, and the primary efficacy end point was met by absolute or % reduction in RV/LVr from baseline to 48 hours post-procedure (0.43, 95% CI: 0.38-0.47% or 27.5%, P < .0001).42 Median procedural time was 37 minutes, and adjunctive thrombolytic was needed only in 1.7% of the patients. The median length of stay in the intensive care unit was only 1 day. Pulmonary vascular injury, clinical deterioration, and major bleeding were reported in less than 2%, and the device-related death rate was 0.8%.42 The STRIKE-PE multicenter study was designed to evaluate the technical performance, efficacy, and safety of this device in real-world practice, and enrolment of the 600 patients with acute PE was planned. Interim analysis at 90-day follow-up showed a significant reduction in RV/LVr (27.5%) identical to those in EXTRACT PE study and significant improvements in mean PA pressure, Borg dyspnea score, 6-minute walk distance, and quality of life measures.43 No death was reported, and major adverse events and device-related adverse events within the first 48 hours were observed in 2.1% and 1.1% of patients, respectively.43
The AngioVac system is a veno-venous or veno-arterial filtration circuit composed of the aspiration and reinfusion cannulas, a centrifugal high-flow pump producing up to 80 mm Hg of suction pressure and an extracorporeal filter.44 This system has been reported to be utilized in PE, right-sided intracardiac thrombi or vegetations, or ilio-caval thrombus and received FDA approval at 2014 for the filtration of intravascular thrombi and emboli. Two meta-analyses on AngioVac series revealed that complete success rates were significantly higher and mortality risk was lower in right atrial/ilio-caval thrombi versus thrombi in PA.45,46 Our experience on AngioVac thrombectomy is limited to complete the extraction of a large and mobile right atrial thrombus attached to sutures of atrial septal defect repair patch which was resistant to previous heparin treatments.44
In a network meta-analysis comparing the CDT, systemic TT, surgical embolectomy, and anticoagulant therapies in patients at IR to HR groups, CDT was found to be associated with significant reductions in in-hospital mortality, PE recurrence, and major bleeding rates as compared to the other 3 treatment modalities and reductions in long-term mortality equivalent to surgical embolectomy.47
Veno-arterial Extracorporeal Membrane Oxygenation in Massive Pulmonary Embolism-Related Cardiac Arrest: In a systematic review, regardless of the systemic TT prior to cannulation, 61% of the patients survived to discharge and 88% were neurologically intact at follow-up.48 Age >65 years old and cannulation during cardiopulmonary resuscitation were associated with 3-fold and 6-fold increases in the risk of death, respectively.48
Pulmonary embolism response team (PERT): There is no controversy regarding the reasonability of the PERT activation in referral patterns of patients with acute PE as a kind of multidisciplinary triage in urgent conditions. However, this could not be translated to validation of benefit in the absence of the RCT data favoring the advantages of PERT framework versus traditional decision-making with multidisciplinary consultations in the referral centers experienced for PE. Time constraints in the decision for appropriate reperfusion methods in acute PE seem to be less important as compared to critical time window for initiating the urgent reperfusion in acute ST-elevation coronary syndromes. Important variations in the utilization of different CDTs across the centers of the PERT network have also been documented.1,9,10 Approximately 10-20% of patients at IR PE have been reported to be treated with CDT methods, and PERT implementation seems to increase the rates of CDT use in IHR group and TT in HR group.49 Since 2015, the PERT teamwork was reported to be associated with a significant decrease in major bleeding rates with a non-significant trend for reduction in all-cause mortality.49 In a systematic review and meta-analysis evaluating PERT implementation across countries, 30% of all PE cases were found to be evaluated by PERT, and relative risk reduction for mortality was more pronounced in patients at HR.50 However, heterogeneity was significant. This analysis also showed a significant increase in the utilization of advanced therapies with PERT implementation. Moreover, whether the artificial intelligence may provide potential contributions to PERT activation networks remains to be evaluated.50
In the clinical consensus statement of the ESC for percutaneous treatment options for acute PE, CDT is proposed for patients at HR and patients at IHR in the absence of stabilization in vital parameters despite the initial anticogulation with unfractioned or low-molecular-weight heparin if contraindications for systemic TT are present (Figure 4). Starting the CDT is recommended maximum 60-90 minutes after establishing the indication for intervention. Moreover, in cases of the treatment failure to systemic TT, starting CDT 2-4 hours after completion of systemic TT is recommended as a rescue option. If hemodynamic stabilization is not achieved, individual management including extracorporeal membrane oxygenation (ECMO) should be considered.
Figure 4.
Flowchart for catheter-directed treatment in with acute pulmonary embolism at high risk and intermediate-high risk.
Conclusions
The promising evidence for the efficacy and safety of CDTs with reduced fibrinolytic dose/shorter infusion duration or pure mechanical extraction/thrombo-aspiration systems seems to change treatment algorithms in patients with acute PE at HR and IHR. The efficacy and safety issues of low-dose/short-duration t-PA regimen with USAT have remained to be confirmed in real-life settings. From our perspective, mild-to-moderate dose and slow-infusion regimen in USAT seems to be more realistic in real-life practice facing multiple co-morbidities. Neither t-PA dose nor infusion duration is related to bleeding or mortality, while HR status increases 30-day mortality. Angiojet rheolytic thrombectomy with adequate hydration is also useful lytic-free reperfusion tool in HR and IHR, especially in cases with massive and extensive PA obstruction, unstable hemodynamic status and high bleeding risk or active bleeding. New prospective RCTs are needed to standardize the timing of intervention, patient and CDT technique selection, peri-procedural lytic, and anticoagulation regimens and to improve benefit from PERT framework in PE management.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – C.K., H.C.T., B.Kültürsay; Design – H.C.T., B.Keskin, A.S.; Supervision – B.Kültürsay, A.H., A.S., A.K.; Analysis and/or Interpretation – A.H., B.Keskin; Literature Search – H.C.T., A.H., B.Keskin; Writing – C.K., B.Kültürsay, A.K.; Critical Review – C.K., A.S., A.K.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54(3):1901647. ( 10.1183/13993003.01647-2019) [DOI] [PubMed] [Google Scholar]
- 2. Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176(6):1415 1420. ( 10.2214/ajr.176.6.1761415) [DOI] [PubMed] [Google Scholar]
- 3. Barco S, Vicaut E, Klok FA, et al. Improved identification of thrombolysis candidates amongst intermediate-risk pulmonary embolism patients: implications for future trials. Eur Respir J. 2018;51(1):1701775. ( 10.1183/13993003.01775-2017) [DOI] [PubMed] [Google Scholar]
- 4. Türkday Derebey S, Tokgöz HC, Keskin B, et al. A new index for the prediction of in-hospital mortality in patients with acute pulmonary embolism: the modified shock index. Anatol J Cardiol. 2023;27(5):282 289. ( 10.14744/AnatolJCardiol.2023.2530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hakgör A, Tokgöz Demircan HC, Keskin B, et al. A novel composed index to evaluate the right ventricle free-wall adaptation against ventricular wall stress in acute pulmonary embolism [published online ahead of print, 2023 Jun 7]. Anatol J Cardiol. 2023;27(7):423 431. ( 10.14744/AnatolJCardiol.2023.2677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keskin B, Tokgöz HC, Akbal ÖY, et al. Clinical, imaging and hemodynamic correlates and prognostic impact of syncope in acute pulmonary embolism: a single-center study. Turk Gogus Kalp Damar Cerrahisi Derg. 2022;30(3):317 326. ( 10.5606/tgkdc.dergisi.2022.22798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311(23):2414 2421. ( 10.1001/jama.2014.5990) [DOI] [PubMed] [Google Scholar]
- 8. Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015;36(10):605 614. ( 10.1093/eurheartj/ehu218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giri J, Sista AK, Weinberg I, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation. 2019;140(20):e774 e801. ( 10.1161/CIR.0000000000000707) [DOI] [PubMed] [Google Scholar]
- 10. Pruszczyk P, Klok FA, Kucher N, et al. Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2022;18(8):e623 e638. ( 10.4244/EIJ-D-22-00246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenfield LJ, Bruce TA, Nichols NB. Transvenous pulmonary embolectomy by catheter device. Ann Surg. 1971;174(6):881 886. ( 10.1097/00000658-197112000-00001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20(11):1431 1440. ( 10.1016/j.jvir.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 13. Kuo WT, Banerjee A, Kim PS, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148(3):667 673. ( 10.1378/chest.15-0119) [DOI] [PubMed] [Google Scholar]
- 14. Dumantepe M, Uyar I, Teymen B, Ugur O, Enc Y. Improvements in pulmonary artery pressure and right ventricular function after ultrasound-accelerated catheter-directed thrombolysis for the treatment of pulmonary embolism. J Card Surg. 2014;29(4):455 463. ( 10.1111/jocs.12354) [DOI] [PubMed] [Google Scholar]
- 15. Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479 486. ( 10.1161/CIRCULATIONAHA.113.005544) [DOI] [PubMed] [Google Scholar]
- 16. Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8(10):1382 1392. ( 10.1016/j.jcin.2015.04.020) [DOI] [PubMed] [Google Scholar]
- 17. Ozcinar E, Cakici M, Dikmen Yaman N, et al. Thrombus resolution and right ventricular functional recovery using ultrasound-accelerated thrombolysis in acute massive and submassive pulmonary embolism. Int Angiol. 2017;36(5):428 437. ( 10.23736/S0392-9590.17.03775-0) [DOI] [PubMed] [Google Scholar]
- 18. Kaymaz C, Öztürk S, Akbal Ö, et al. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: results from a single-center cohort. Angiology. 2017;68(5):433 440. ( 10.1177/0003319716661446) [DOI] [PubMed] [Google Scholar]
- 19. Kaymaz C, Akbal OY, Tanboga IH, et al. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: a meta-analysis. Curr Vasc Pharmacol. 2018;16(2):179 189. ( 10.2174/1570161115666170404122535) [DOI] [PubMed] [Google Scholar]
- 20. Kaymaz C, Akbal OY, Hakgor A, et al. A five-year, single-centre experience on ultrasound-assisted, catheter-directed thrombolysis in patients with pulmonary embolism at high risk and intermediate to high risk. EuroIntervention. 2018;14(10):1136 1143. ( 10.4244/EIJ-D-18-00371) [DOI] [PubMed] [Google Scholar]
- 21. Kaymaz C, Akbal OY, Keskin B, et al. An eight-year, single-center experience on ultrasound assisted thrombolysis with moderate-dose, slow-infusion regimen in pulmonary embolism. Curr Vasc Pharmacol. 2022;20(4):370 378. ( 10.2174/1570161120666220428095705) [DOI] [PubMed] [Google Scholar]
- 22. Tapson VF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11(14):1401 1410. ( 10.1016/j.jcin.2018.04.008) [DOI] [PubMed] [Google Scholar]
- 23. Kaymaz C, Akbal ÖY, Hakgör A, Karagöz A, Tanyeri S. Does the OPTALYSE PE trial cover unmet need in the real-life practice of pulmonary embolism? JACC Cardiovasc Interv. 2018;11(22):2342 2343. ( 10.1016/j.jcin.2018.09.003) [DOI] [PubMed] [Google Scholar]
- 24. Piazza G, Sterling KM, Tapson VF, et al. One-year echocardiographic, functional, and quality of life outcomes after ultrasound-facilitated catheter-based fibrinolysis for pulmonary embolism. Circ Cardiovasc Interv. 2020;13(8):e009012. ( 10.1161/CIRCINTERVENTIONS.120.009012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterling K, et al. Interi m analysis of the KNOCOUT PE registry: a multicenter international registry to describe the impact of the OPTALYSE PE study on EKOSONIC™ variations in practice, equipoise regarding dose and duration of t-PA and associated outcomes. Presented at the Pulmonary Embolism Response Team (PERT) Consortium in 2019. [Google Scholar]
- 26. Sterling K, et al. KNOC OUT PE: international EkoSonic registry of the treatment and clinical outcomes of patients with pulmonary embolism. Presented at the Vascular InterVentional Advances Meeting [VIVA] in 2021. [Google Scholar]
- 27. Klok FA, Piazza G, Sharp ASP, et al. Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: rationale and design of the HI-PEITHO study. Am Heart J. 2022;251:43 53. ( 10.1016/j.ahj.2022.05.011) [DOI] [PubMed] [Google Scholar]
- 28. Rao G, Xu H, Wang JJ, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute pulmonary embolism: a multicenter comparison of patient-centered outcomes. Vasc Med. 2019;24(3):241 247. ( 10.1177/1358863X19838334) [DOI] [PubMed] [Google Scholar]
- 29. Rothschild DP, Goldstein JA, Ciacci J, Bowers TR. Ultrasound-accelerated thrombolysis (USAT) versus standard catheter-directed thrombolysis (CDT) for treatment of pulmonary embolism: a retrospective analysis. Vasc Med. 2019;24(3):234 240. ( 10.1177/1358863X19838350) [DOI] [PubMed] [Google Scholar]
- 30. Bashir R. A Prospective Multicenter Trial of Pharmaco-Mechanical Catheter-Directed Thrombolysis with the BASHIR™ Endovascular Catheter for Intermediate-Risk Acute Pulmonary Embolism – the RESCUE Study. T Ranscatheter Cardiovascular Therapeutics (TCT) Conference, September 16-19, 2022. [Google Scholar]
- 31. Das S, Das N, Serota H, Vissa S. A retrospective review of patients with massive and submassive pulmonary embolism treated with AngioJet rheolytic thrombectomy with decreased complications due to changes in thrombolytic use and procedural modifications. Vascular. 2018;26(2):163 168. ( 10.1177/1708538117722728) [DOI] [PubMed] [Google Scholar]
- 32. Villalba L, Nguyen T, Feitosa RL, Gunanayagam P, Anning N, Dwight K. Single-session catheter-directed lysis using adjunctive power-pulse spray with AngioJet for the treatment of acute massive and submassive pulmonary embolism. J Vasc Surg. 2019;70(6):1920 1926. ( 10.1016/j.jvs.2019.03.038) [DOI] [PubMed] [Google Scholar]
- 33. Chechi T, Vecchio S, Spaziani G, et al. Rheolytic thrombectomy in patients with massive and submassive acute pulmonary embolism. Catheter Cardiovasc Interv. 2009;73(4):506 513. ( 10.1002/ccd.21858) [DOI] [PubMed] [Google Scholar]
- 34. Akbal ÖY, Keskin B, Tokgöz HC, et al. A seven-year single-center experience on AngioJet rheolytic thrombectomy in patients with pulmonary embolism at high risk and intermediate-high risk. Anatol J Cardiol. 2021;25(12):902 911. ( 10.5152/AnatolJCardiol.2021.28303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Gregorio MA, Guirola JA, Kuo WT, et al. Catheter-directed aspiration thrombectomy and low-dose thrombolysis for patients with acute unstable pulmonary embolism: prospective outcomes from a PE registry. Int J Cardiol. 2019;287:106 110. ( 10.1016/j.ijcard.2019.02.061) [DOI] [PubMed] [Google Scholar]
- 36. Bayiz H, Dumantepe M, Teymen B, Uyar I. Percutaneous aspiration thrombectomy in treatment of massive pulmonary embolism. Heart Lung Circ. 2015;24(1):46 54. ( 10.1016/j.hlc.2014.06.014) [DOI] [PubMed] [Google Scholar]
- 37. Avgerinos ED, Jaber W, Lacomis J, et al. Randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism: the SUNSET sPE trial. JACC Cardiovasc Interv. 2021;14(12):1364 1373. ( 10.1016/j.jcin.2021.04.049) [published correction appears in JACC Cardiovasc Intv. 2021;14(19): 2194]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tu T, Toma C, Tapson VF, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. 2019;12(9):859 869. ( 10.1016/j.jcin.2018.12.022) [DOI] [PubMed] [Google Scholar]
- 39. Kaymaz C, Akbal ÖY, Karagöz A, Hakgör A, Tanyeri S. Is a flare at the dawn of pulmonary embolism? JACC Cardiovasc Interv. 2019;12(17):1743 1744. ( 10.1016/j.jcin.2019.06.008) [DOI] [PubMed] [Google Scholar]
- 40. Toma C, Jaber WA, Weinberg MD, et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention. 2023;18(14):1201 1212. ( 10.4244/EIJ-D-22-00732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silver MJ, Giri J, Gibson CM. Outcomes in High-risk Pulmonary Embolism Patients Undergoing FlowTriever Mechanical Thrombectomy: Results From The FLAME Study. Clinical Trial Results; presented at ACC March 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sista AK, Horowitz JM, Tapson VF, et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv. 2021;14(3):319 329. ( 10.1016/j.jcin.2020.09.053) [DOI] [PubMed] [Google Scholar]
- 43. Perkowski P, Moriarty JM, Keeling WB, Sterling KM, Dohad SY, Weinberg I. Computer-aided mechanical aspiration thrombectomy with the indigo Lightning 12 aspiration system for the treatment of acute pulmonary embolism: interim analysis of the STRIKE-PE study. J Vasc Surg. 2023;77(4):52S -53S. ( 10.1016/j.jvs.2023.01.145) [DOI] [Google Scholar]
- 44. Akbal ÖY, Tokgöz HC, Bayram Z, Özdemir N, Kaymaz C. Successful aspiration thrombectomy of large right atrial thrombus attached to atrial septal defect repair patch. Anatol J Cardiol. 2022;26(1):63 65. ( 10.5152/AnatolJCardiol.2021.00557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basman C, Rashid U, Parmar YJ, Kliger C, Kronzon I. The role of percutaneous vacuum-assisted thrombectomy for intracardiac and intravascular pathology. J Card Surg. 2018;33(10):666 672. ( 10.1111/jocs.13806) [DOI] [PubMed] [Google Scholar]
- 46. Hameed I, Lau C, Khan FM, et al. AngioVac for extraction of venous thromboses and endocardial vegetations: a meta-analysis. J Card Surg. 2019;34(4):170 180. ( 10.1111/jocs.14009) [DOI] [PubMed] [Google Scholar]
- 47. Ishisaka Y, Watanabe A, Fujisaki T, et al. Comparison of interventions for intermediate to high-risk pulmonary embolism: A network meta-analysis. Catheter Cardiovasc Interv. 2023;102(2):249 265. ( 10.1002/ccd.30745) [DOI] [PubMed] [Google Scholar]
- 48. Chopard R, Nielsen P, Ius F, et al. Optimal reperfusion strategy in acute high-risk pulmonary embolism requiring extracorporeal membrane oxygenation support: a systematic review and meta-analysis. Eur Respir J. 2022;60(5):2102977. ( 10.1183/13993003.02977-2021) [DOI] [PubMed] [Google Scholar]
- 49. Chopard R, Campia U, Morin L, et al. Trends in management and outcomes of pulmonary embolism with a multidisciplinary response team. J Thromb Thrombolysis. 2022;54(3):449 460. ( 10.1007/s11239-022-02697-3) [DOI] [PubMed] [Google Scholar]
- 50. Hobohm L, Farmakis IT, Keller K, et al. Pulmonary embolism response team (PERT) implementation and its clinical value across countries: a scoping review and meta-analysis [published online ahead of print, 2022 Aug 17]. Clin Res Cardiol. 2022:1 11. ( 10.1007/s00392-022-02077-0) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a