Abstract
We have evaluated the clinical potential of TXU (anti-CD7)-pokeweed antiviral protein (PAP) immunoconjugate (TXU-PAP) as a new biotherapeutic anti-human immunodeficiency virus (anti-HIV) agent by evaluating its anti-HIV type 1 (anti-HIV-1) activity in vitro, as well as in a surrogate human peripheral blood lymphocyte-severe combined immunodeficient (Hu-PBL-SCID) mouse model of human AIDS. The present report documents in a side-by-side comparison the superior in vitro anti-HIV-1 activity of TXU-PAP compared to the activities of zidovudine, 2′,3′-didehydro-2′,3′-dideoxythymidine, unconjugated PAP, and B53-PAP, an anti-CD4-PAP immunoconjugate. Notably, TXU-PAP elicited potent anti-HIV activity in the Hu-PBL-SCID mouse model of human AIDS without any side effects and at doses that were very well tolerated by cynomolgus monkeys. Furthermore, plasma samples from TXU-PAP-treated cynomolgus monkeys showed potent anti-HIV-1 activity in vitro.
Pokeweed antiviral protein (PAP), a ribosome inhibitory protein isolated from the leaves or seeds of Phytolacca americana (3, 10, 12, 13), was discovered due to its ability to inhibit the transmission of tobacco mosaic virus in plants (20). It was subsequently demonstrated that the purified protein displays broad-spectrum antiviral activity against seven different viruses each representing a different plant virus group (20). The antiviral activity profile of PAP extends to mammalian viruses as well (1, 2, 8, 19–21). A series of studies provided evidence that PAP is an effective inhibitor of influenza virus (20), poliovirus (21), herpes simplex virus (1), and human immunodeficiency virus (HIV) type 1 (HIV-1) (4, 23).
The antiviral activity of PAP can be greatly enhanced and made highly cell selective by conjugation of PAP to antibodies specific for cell-surface receptors that are capable of being internalized upon ligand occupation (4, 8, 23). Inhibition of HIV-1 replication occurs at picomolar concentrations of PAP immunoconjugates, whereas inhibition of proliferation of normal CD4+ T cells occurs only at about 1,000 times higher concentrations (23). Studies with clinical isolates of zidovudine (AZT)-sensitive and AZT-resistant HIV-1 demonstrated that PAP immunoconjugates exhibit potent anti-HIV activity, with 50% inhibitory concentrations (IC50s) being below 100 pM for all isolates (4). In a more recent report, we described the large-scale manufacturing of TXU-PAP, an immunoconjugate prepared by covalently linking PAP to the anti-CD7 monoclonal antibody (MAb) TXU, for clinical trials (17). The preclinical toxicity of TXU-PAP in mice and cynomolgus monkeys was also reported (22). In cynomolgus monkeys, TXU-PAP showed favorable pharmacokinetics, with an elimination half-life of 8.1 to 8.7 h. The monkeys treated with TXU-PAP at dosages of 50 μg/kg of body weight/day for 5 days or 100 μg/kg/day for 5 days tolerated the therapy very well, without any significant clinical compromise or side effects, and at necropsy no gross or microscopic lesions were found (22).
The present report documents in a side-by-side comparison the superior in vitro anti-HIV-1 activity of TXU-PAP compared to the activities of AZT, 2′,3′-didehydro-2′,3′-dideoxythymidine (d4T), unconjugated PAP, and B53-PAP (14), an anti-CD4-PAP immunoconjugate. Furthermore, by using a surrogate severe combined immunodeficient (SCID) mouse model of human AIDS, we demonstrate that TXU-PAP is a potent and nontoxic anti-HIV agent in vivo. Notably, plasma samples from TXU-PAP-treated cynomolgus monkeys demonstrated potent anti-HIV-1 activity in vitro.
MATERIALS AND METHODS
Preparation of PAP immunoconjugates.
Affinity-purified MAbs B53/TXU-5 (immunoglobulin G1 [IgG1]; anti-CD4) and TXU (IgG1; anti-CD7) were conjugated to 2-iminothiolane-modified PAP from spring leaves of P. americana with the heterobifunctional cross-linking agent N-succinimidyl 3-(2-pyridyldithio)propionate by previously described procedures (4, 17, 23). PAP immunoconjugates were then purified from unconjugated MAb and free PAP by size-exclusion high-performance liquid chromatography and cation-exchange chromatography as previously described in detail (17, 23). The purities, compositions, and immunoreactivities of B53 (anti-CD4) and TXU (anti-CD7)-PAP immunoconjugates were reported previously (4, 17). Controls included unconjugated PAP, B43 (anti-CD19)-PAP directed against B cells, and unconjugated MAb B53 (anti-CD4) and TXU (anti-CD7). These control reagents were prepared by previously published procedures (4, 17, 23). Additional controls included AZT and d4T, which were provided by Neal T. Wetherall and Cheryl Hodges-Savola from VIROMED Laboratories, Inc.
Stock HTLVIIIB virus.
HIV-1 HTLVIIIB (kindly provided by Neal T. Wetherall, VIROMED Laboratories, Inc.), which was propagated in CCRF-CEM cells, was used in in vitro assays of the anti-HIV-1 activities of the PAP immunoconjugates. Cell-free supernatants of HTLVIIIB-infected CCRF-CEM cells were harvested, dispensed into 1-ml aliquots, and frozen at −70°C. Periodic titration of stock virus was performed by examining its cytopathic effects in MT-2 cells.
In vitro assays of anti-HIV-1 activity.
Normal human peripheral blood mononuclear cells (PBMNCs) from HIV-negative donors were cultured for 72 h in RPMI 1640 supplemented with 20% (vol/vol) heat-inactivated fetal bovine serum, 3% interleukin-2, 2 mM l-glutamine, 25 mM HEPES, 2 g of NaHCO3 per liter, 50 μg of gentamicin per ml, and 4 μg of phytohemagglutinin (PHA) per ml prior to exposure to HIV-1 at a multiplicity of infection of 0.1 during a 1-h adsorption period at 37°C in a humidified 5% CO2 atmosphere. Subsequently, cells were cultured in 96-well microtiter plates (100 μl/well; 2 × 106 cells/ml) in the presence of various concentrations of PAP immunoconjugates or standard anti-HIV drugs, and aliquots of culture supernatants were removed from the wells on the 7th day after infection for p24 antigen and reverse transcriptase (RT) assays as described previously (4, 17, 23). The applied p24 enzyme immunoassay was the unmodified kinetic assay commercially available from Coulter Corporation/Immunotech, Inc. (Westbrooke, Maine). The assay uses a murine MAb to the HIV core protein coated onto microwell strips to which the antigen present in the test culture supernatant samples binds. Percent viral inhibition was calculated by comparing the p24 values for the test substance-treated infected cells with the p24 values for untreated infected cells (i.e., virus controls). An unmodified procedure commercially available from Amersham Lifescience, which uses a DNA-RNA primer-template attached to scintillant-filled microspheres, was used to assess the RT activity. Incorporation of radiolabeled nucleotides by reverse transcription results in extension of the primer and stimulation of the scintillant within the microspheres. The resulting signals of RT activity were detected and quantified with a scintillation counter and are recorded as counts per minute. In some experiments, we examined the anti-HIV activities of 1:2-, 1:10-, 1:20-, and 1:100-diluted plasma samples obtained 1 h posttherapy from TXU-PAP-treated cynomolgus monkeys. The intravenous TXU-PAP doses were 50 μg/kg of body weight for monkey 52E and 100 μg/kg for monkey 52D and monkey 410C (22). In parallel, the effects of various treatments on cell viability were also examined as described previously (4, 23). In brief, noninfected PBMNCs were treated with PAP immunoconjugates for 7 days under identical experimental conditions. A microculture tetrazolium assay with 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazolium hydroxide was performed to quantitate cellular proliferation.
Preparation of viral stocks of clinical HIV-1 isolates.
HIV-1 isolates were recovered from peripheral blood specimens from HIV-1-infected patients participating in National Institutes of Health-sponsored AIDS clinical trials at the University of Minnesota AIDS Clinical Trials Unit by a culture technique previously described in detail (5, 14, 15). In brief, 10 × 106 Ficoll-Hypaque-separated mononuclear cells from seropositive patients were cocultured with 5 × 106 PHA-stimulated peripheral blood mononuclear cells from an HIV-1-seronegative healthy volunteer donor for 42 days at 37°C in 5% CO2 in 50-ml tissue culture flasks containing 15 ml of RPMI 1640 supplemented with 20% fetal calf serum, 5% interleukin-2 (Cellular Products, Buffalo, N.Y.), 160 U of penicillin per ml, and 160 μg of streptomycin per ml. The cocultured supernatants were assayed every 3 to 4 days for the presence of HIV-1 p24 gag antigen with a commercially available enzyme-linked immunosorbent assay p24 antigen detection kit (Abbott Laboratories, North Chicago, Ill.) as reported previously (9). p24 antigen-positive cultures were expanded by a standard protocol, and aliquots of cell-free stock viruses were prepared from the supernatants of the expanded cultures when the RT activity in the supernatant exceeded 20,000 cpm/50 μl. Some isolates were recovered from frozen supernatants of p24 antigen-positive cultures or from frozen cells from patients positive for HIV-1 by culture. In these cases, PBMNCs (2 × 106 to 5 × 106 cells/ml) were exposed for 2 h at 37°C in 5% CO2 to 1 ml of the p24-positive culture supernatant or 1 × 106 thawed peripheral blood mononuclear cells from patients positive for HIV-1 by culture and were cultured in 50-ml tissue culture flasks. Subsequently, positive cultures were expanded as described above.
SCID mouse model of human AIDS.
All SCID mice used in the efficacy study were produced by SPF CB-17 scid/scid breeders (originally obtained from Melvin Bosma, Fox Chase Cancer Center, Philadelphia, Pa.) in the AAALAC-approved and -accredited Research Animal Resources SCID Mouse Facility of the University of Minnesota (Minneapolis, Minn.). All husbandry and experimental contact made with the mice maintained specific-pathogen-free conditions. The mice were housed in Micro-Isolator cages containing autoclaved food, water, and bedding. Trimethoprim-sulfamethoxazole (Bactrim) was added to the drinking water of the mice three times a week.
Human peripheral blood lymphocyte-SCID (Hu-PBL-SCID) mice (16) were generated by reconstituting SCID mice by intraperitoneal injection of 10 × 106 peripheral blood mononuclear cells from a single Epstein-Barr virus-seronegative volunteer donor. Two weeks after inoculation of the cells, mice were challenged by intraperitoneal injection of 1.4 × 104 to 7.7 × 104 median tissue culture infectious doses of cell-free virus. Three different clinical HIV-1 strains (strains AT-101, AT-328, and AT-332) were used. These isolates were recovered from peripheral blood leukocytes of HIV-1-infected individuals participating in National Institutes of Health-sponsored AIDS clinical trials at the University of Minnesota as described previously (5, 14, 15). SCID mice were infected with HIV-1 isolates in a biosafety level 3 containment facility, and all manipulations were performed in a biosafety cabinet. The TXU-PAP immunoconjugate was administered intraperitoneally by injecting half of the total dose as an intraperitoneal bolus dose and delivering the remainder of the total dose over 2 weeks by using Alzet micro-osmotic pumps or by administering the total dose by daily intraperitoneal injections over a 5-day treatment period. Treatments were initiated immediately prior to HIV-1 inoculation. Throughout the experimental period, mice were monitored daily for overall health and survival. Two weeks after infection with HIV-1, Hu-PBL-SCID mice were electively killed, and their peritoneal lavage cells as well as spleen cells were examined for evidence of infection by an HIV-1 culture assay as well as by PCR amplification of a 115-bp DNA sequence in the gag region of the HIV-1 genome, as detailed below. For histopathologic studies, tissues were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin by routine methods. Glass slides with affixed 6-μm tissue sections were prepared, stained with hematoxylin-eosin, and submitted to the veterinary pathologist for examination. Fresh peritoneal lavage cells as well as spleen cells were isolated and cocultured with PHA-stimulated human peripheral blood mononuclear cells from an HIV-1 antibody-negative donor, and culture supernatants were tested every 3 to 4 days for a maximum of 28 days for the presence of HIV-1 antigen by a commercially available enzyme immunoassay (Abbott Laboratories) that detects primarily the core p24 antigen of HIV-1. In addition to this culture method, we also isolated the DNA from the peritoneal lavage cells as well as splenocytes for the detection of HIV-1 DNA by PCR amplification of a 115-bp sequence in the gag region of the HIV-1 genome using two 29-base oligonucleotide primers (primers SK38 and SK39) that flank the region to be amplified (18). DNA samples were also examined for the presence of human DNA by PCR amplification of a 110-bp fragment from the first exon of the human β-globin gene by using two 20-base oligonucleotide primers (primers PCO3 and PCO4) that flank the region to be amplified, as previously described in detail (11). Oligonucleotide primers (SK38 [5′-ATA ATC CAC CTA TCC CAG TAG GAG AAA T-3′] and SK39 [5′-TTT GGT CCT TGT CTT ATG TCC AGA ATG C-3′]) were synthesized by the University of Minnesota Microchemical Facility with a synthesizer (Applied Biosystems, Foster City, Calif.). HIV DNA was amplified with 1.0 μg of genomic DNA with 2.5 U of Taq DNA polymerase (Perkin-Elmer Cetus, Norwalk, Conn.) in 1× PCR buffer (50 mM KCl, 10 mM Tris-Cl [pH 8.3], 2.5 mM MgCl2, 0.01% [wt/vol] gelatin) containing 0.5 μM (each) primer and 200 μM deoxynucleoside triphosphates (Pharmacia, Piscataway, N.J.) in a total volume of 100 μl. Before amplification, the samples were overlaid with 100 μl of mineral oil (Sigma, St. Louis, Mo.). Thirty cycles were performed by incubating the samples at 95°C for 1 min and 60°C for 1 min. Oligomer hybridization was used to detect PCR-amplified HIV DNA. Briefly, 30 μl of amplified DNA was added to 10 μl of a probe mixture consisting of 0.2 pmol of 32P-labeled SK19 (5′-ATC CTG GGA TTA AAT AAA ATA GTA AGA ATG TAT AGC CCT AC-3′), 24 mM NaCl, and 4 mM EDTA (pH 8.0) (18). Samples were denatured in a 95°C bath for 5 min, followed by a 15-min incubation at 55°C to anneal the probe and target sequences. A total of 10 μl of a bromophenol blue-xylene cyanol dye mixture was added to each tube, and 25 μl of each sample was analyzed on a 10% polyacrylamide gel in 1× TBE buffer (0.089 M Tris-borate, 0.002 M EDTA). Following electrophoresis, the gel was dried and exposed to Kodak XAR-5 film for 2 h with an intensifying screen. Controls included (i) the PCR buffer without the genomic DNA, (ii) PCR products of DNA from HIV-1-infected but nonreconstituted SCID mice as well as from uninfected Hu-PBL-SCID mice as negative background controls, and (iii) HIV-1 control plasmid DNA (Perkin-Elmer Cetus) as well as DNA from HIV-infected and phosphate-buffered saline (PBS)-treated (i.e., sham-treated) Hu-PBL-SCID mice as positive DNA controls.
RESULTS
In vitro anti-HIV activities of B53 (anti-CD4)-PAP and TXU (anti-CD7)-PAP immunoconjugates.
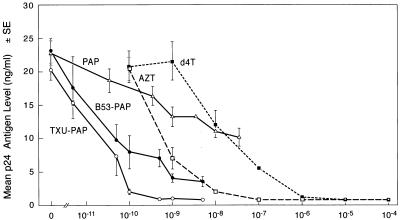
The antiviral activities of PAP immunoconjugates against strain HTLVIIIB were evaluated by using HIV-1 p24 core antigen production as a marker of viral replication. As shown in Fig. 1, both B53-PAP and TXU-PAP inhibited viral replication in a dose-dependent fashion. The 50% inhibitory doses (ID50s) for HIV-1 p24 production were 30 pM (5.9 ng/ml) for B53 (anti-CD4)-PAP and 20 pM (4.4 ng/ml) for TXU (anti-CD7)-PAP, whereas unconjugated PAP inhibited p24 production 270 to 400 times less efficiently, with an ID50 of 8 nM (228 ng/ml). Both PAP-containing immunoconjugates were two to three orders of magnitude more potent than AZT (ID50 = 1 nM) or d4T (ID50 = 18 nM). A similar efficacy profile was produced when RT activity served as an indicator for viral replication (data not shown). Thus, the antiviral effects of PAP-containing immunoconjugates influence both structural and functional proteins of HIV-1, without eliciting significant cytotoxicity. Overall, TXU (anti-CD7)-PAP was a slightly more potent anti-HIV-1 agent than B53 (anti-CD4)-PAP. The anti-HIV-1 activity of TXU (anti-CD7)-PAP was highly reproducible and was not associated with significant cytotoxicity to T cells in MTA cell proliferation assays when the immunoconjugate was used at concentrations ranging from 1 ng/ml (4.5 pM) to 1,000 ng/ml (4.5 nM) (Fig. 2).
FIG. 1.
In vitro anti-HIV-1 activity of TXU (anti-CD7)-PAP. The antiviral activity of TXU-PAP against HIV-1 HTLVIIIB was evaluated in a side-by-side comparison with the activities of B53 (anti-CD4)-PAP, unconjugated PAP, AZT, and d4T by an in vitro p24 enzyme immunoassay and RT assays (data not shown) as described in Materials and Methods.
FIG. 2.
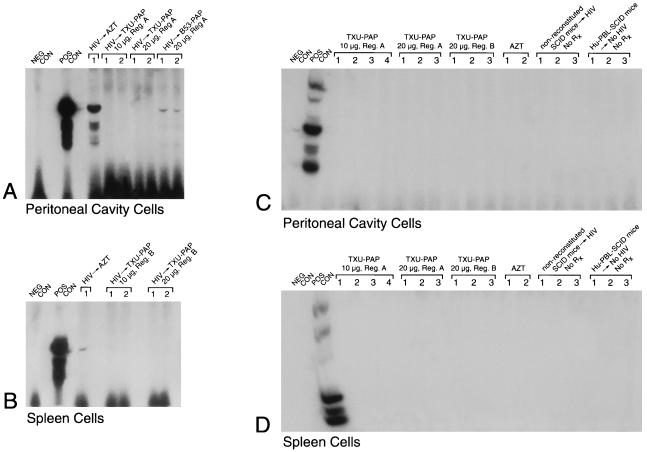
In vivo anti-HIV-1 activity of TXU (anti-CD7)-PAP. Hu-PBL-SCID mice were inoculated with clinical HIV-1 isolates in a biosafety level 3 containment facility as described in Materials and Methods. PAP immunoconjugates were administered intraperitoneally by injecting half of the total dose as an intraperitoneal bolus dose and delivering the remainder of the total dose over 2 weeks with Alzet micro-osmotic pumps (regimen A) or by administering the total dose by daily intraperitoneal injections over a 5-day treatment period (regimen B). In the AZT-treated mice, AZT was added to their water at a final concentration of 1 mg/ml, resulting in an average consumption of 200 mg of AZT per kg/day. All treatments were initiated immediately prior to the inoculation of HIV-1. Two weeks after infection with HIV-1, Hu-PBL-SCID mice were electively killed and their peritoneal lavage cells as well as spleen cells were examined for evidence of infection by a culture assay for HIV-1 (Table 1) as well as by PCR amplification of a 115-bp DNA sequence in the gag region of the HIV-1 genome. Polyacrylamide gels of the PCR-amplified HIV-1 DNA hybridized with the 32P-labeled SK19 probe are shown. No PCR evidence of HIV-1 infection was found in any of the TXU-PAP-treated mice. The controls included (i) the PCR buffer without the genomic DNA (NEG CON), (ii) PCR product of DNA from HIV-1-injected but unreconstituted SCID mice as well as from uninfected Hu-PBL-SCID mice as negative background controls, and (iii) HIV-1 control plasmid DNA (POS CON) (Perkin-Elmer Cetus) as well as DNA from infected but untreated (data not shown) Hu-PBL SCID mice as positive DNA controls. (A and C). Data for PCR detection of HIV in peritoneal cavity cells. (B and D). Data for PCR detection of HIV in spleen cells. Rx, treatment. Each lane corresponds to a Hu-PBL-SCID mouse sample or the indicated controls.
In vivo anti-HIV-1 activities of B53 (anti-CD4)-PAP and TXU (anti-CD7)-PAP in a surrogate SCID mouse model of human AIDS.
We examined the in vivo anti-HIV-1 activities of B53 (anti-CD4)-PAP and TXU (anti-CD7)-PAP in a Hu-PBL-SCID mouse model of human AIDS. As shown in Table 1, of the 23 Hu-PBL-SCID mice infected with HIV-1 and treated with PBS, (i) 11 were analyzed by both culture for HIV and PCR for HIV and 10 were positive by both assays, while 1 was positive only by PCR; (ii) 6 were analyzed by culture for HIV only, and all 6 were positive; and (iii) 6 were analyzed by PCR for HIV only, and all 6 were positive (Fig. 2 and 3). Similarly, 5 Hu-PBL-SCID mice infected with HIV-1 and treated with the B-cell-directed control B43-PAP immunoconjugate were analyzed by PCR for HIV, and all 5 tested positive, whereas no false-positive results by culture for HIV or PCR for HIV were observed for any of the 17 control Hu-PBL-SCID mice that were not injected with HIV-1 (Table 1).
TABLE 1.
Anti-HIV-1 activity of TXU (anti-CD7)-PAP in Hu-PBL-SCID micea
| Treatment (dose [μg]) | Total no. of SCID Mice | No. of Hu-PBL-SCID mice with following HIV status by PCR and culture/total no. of Hu-PBL-SCID mice:
|
||||||
|---|---|---|---|---|---|---|---|---|
| PCR+ | Cx+ | PCR+ Cx+ | PCR+ Cx− | PCR+ CxND | PCR− Cx− | PCRND Cx+ | ||
| PBS | 23 | 16/16 | 16/17 | 10/11 | 1/11 | 6/6 | 0/11 | 6/6 |
| TXU (anti-CD7)-PAP | ||||||||
| 10 | 10 | 0/10 | ND | ND | ND | 0/10 | ND | ND |
| 20 | 10 | 0/10 | ND | ND | ND | 0/10 | ND | ND |
| B53 (anti-CD4)-PAP | ||||||||
| 10 | 5 | 5/5 | ND | ND | ND | 5/5 | ND | ND |
| 20 | 4 | 3/4 | 0/3 | 0/3 | 3/3 | 1/1 | 0/3 | ND |
| 40 | 18 | 3/18 | 0/11 | 0/11 | 2/11 | 1/7 | 9/11 | ND |
| 60 | 5 | 0/3 | 0/5 | 0/3 | 0/3 | ND | 3/3 | 0/2 |
| B43 (anti-CD19)-PAP, 20 | 5 | 5/5 | ND | ND | ND | 5/5 | ND | ND |
| AZT | 10 | 4/10 | 4/8 | 4/8 | 4/8 | 0/2 | 0/8 | ND |
| Control Hu-PBL-SCID mice (no HIV infection, no treatment) | 17 | 0/9 | 0/16 | 0/8 | 0/8 | 0/1 | 8/8 | 0/8 |
| Control SCID mice (No Hu-PBL, HIV infection, no treatment) | 3 | 0/3 | ND | ND | ND | 0/3 | ND | ND |
Hu-PBL-SCID mice were inoculated with clinical HIV-1 isolates in a biosafety level 3 containment facility. TXU-PAP immunoconjugate was administered intraperitoneally by injecting half of the total dose as an intraperitoneal bolus dose and delivering the remainder of the total dose over 2 weeks by using Alzet micro-osmotic pumps (regimen A) (n = 5) or by administering the total dose by daily intraperitoneal injections over a 5-day treatment period (regimen B) (n = 5). B53-PAP was administered by daily intraperitoneal injection over a 5-day treatment period when it was used at a dose of 10 or 20 μg. Mice receiving 40 or 60 μg of B53-PAP received 20 μg of the dose as an intraperitoneal bolus injection and the remainder over 2 weeks by using the Alzet micro-osmotic pumps. Two weeks after infection with HIV-1, Hu-PBL-SCID mice were electively killed and their peritoneal lavage cells as well as spleen cells were examined for evidence of infection by a culture assay for HIV-1 as well as by PCR amplification of a 115-bp DNA sequence in the gag region of the HIV-1 genome. In PBS-treated control Hu-PBL-SCID mice, culture assays for HIV were performed with spleen cells (n = 4) and with a mixture of peritoneal lavage and spleen cells (n = 12). PCR assays for HIV were performed with both spleen cells and peritoneal lavage cells except for two PBS-treated Hu-PBL-SCID mice, whose spleens were not examined by PCR. PCR+, PCR positive; Cx+, culture positive; PCR−, PCR negative; Cx−, culture negative; PCRND, PCR result was not determined; CxND, culture result was not determined; ND, not determined.
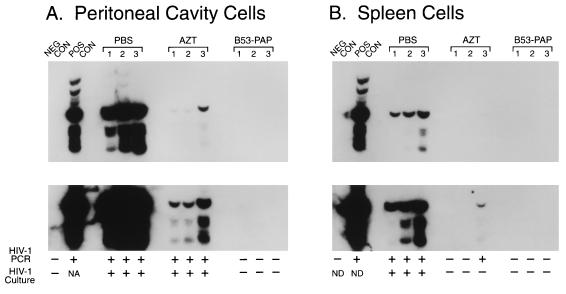
FIG. 3.
In vivo anti-HIV-1 activity of B53(anti-CD4)-PAP. The antiviral activity of the anti-CD4 antiviral immunoconjugate B53-PAP (60 μg/mouse) was examined in the Hu-PBL-SCID mouse model of human AIDS by PCR assays for the evaluation of the HIV status of treated mice as described in Materials and Methods. AZT was added to their water at a final concentration of 1 mg/ml, resulting in an average consumption of 200 mg of AZT per kg/day. Two weeks after infection with HIV-1, Hu-PBL-SCID mice were electively killed and their peritoneal cavity cells as well as spleen cells were examined for evidence of HIV infection by a culture assay as well as by PCR amplification of a 115-bp DNA sequence in the gag region of the HIV genome. The controls in the PCR assays included (i) the PCR buffer without the genomic DNA (NEG CON) and (ii) HIV-1 control plasmid DNA (POS CON) (Perkin-Elmer Cetus). Polyacrylamide gels of PCR-amplified DNA from peritoneal cavity cells (A) and spleen cells (B) hybridized with 32P-labeled SK19 probe (18) are shown. The results of the HIV culture assays are also indicated (+, culture positive; −, culture negative). ND, not determined. The results depicted in panel A demonstrate that peritoneal cavity cells from PBS- or AZT-treated control mice were positive for HIV by PCR as well as by culture, whereas cells from B53-PAP-treated mice were negative for HIV by PCR as well as by culture assays. The results depicted in panel B demonstrate that spleen cells from PBS-treated mice were positive for HIV by PCR as well as culture, whereas only PCR evidence of HIV infection was found in one of the AZT-treated mice. No PCR or culture evidence of HIV infection was found in spleen cells from B53-PAP-treated mice. Each lane corresponds to a Hu-PBL-SCID mouse sample or the indicated controls.
TXU (anti-CD7)-PAP elicited more potent anti-HIV-1 activity than B53 (anti-CD4)-PAP in the Hu-PBL-SCID mouse model. No PCR evidence of HIV-1 infection was found in any of the 20 Hu-PBL-SCID mice treated with 10 or 20 μg of TXU (anti-CD7)-PAP administered according to the 14-day (regimen A) or 5-day (regimen B) treatment schedules mentioned above (Fig. 2; Table 1). By comparison, viral genomes were detected by PCR in all four Hu-PBL-SCID mice treated with B53 (anti-CD4)-PAP at a total dose of 20 μg, even though no virus was recovered by culture from any of these mice. At higher doses, B53 (anti-CD4)-PAP also elicited potent anti-HIV-1 activity (Fig. 3). HIV-1 DNA was detected by PCR in only 3 of 18 Hu-PBL-SCID mice treated with B53 (anti-CD4)-PAP at a total dose of 40 μg, and none of the 11 mixed peritoneal lavage plus splenocyte cultures from these mice were culture positive for HIV (Fig. 3; Table 1). Similarly, no culture or PCR evidence of HIV-1 infection was found in any of the five Hu-PBL-SCID mice treated with 60 μg of B53 (anti-CD4)-PAP.
Importantly, CD4+ CD7+ CD45+ gp120− T cells were detected by multiparameter flow cytometry in the peritoneal lavage fluids of Hu-PBL-SCID mice treated with 60 μg of B53 (anti-CD4)-PAP or 20 μg of TXU (anti-CD7)-PAP, and the presence of human DNA in the spleens as well as the peritoneal cavities of these Hu-PBL-SCID mice was confirmed by β-globin gene PCR (data not shown). Thus, the absence of HIV-1 in B53 (anti-CD4)-PAP- or TXU(anti-CD7)-PAP-treated Hu-PBL-SCID mice was not caused by the absence of human T cells due to poor engraftment or PAP immunoconjugate-induced indiscriminate cytotoxicity. All mice treated with B53 (anti-CD4)-PAP or TXU (anti-CD7)-PAP remained healthy throughout the test period. No overt signs of ill health or unusual responses were observed. In contrast to B53 (anti-CD4)-PAP- or TXU (anti-CD7)-PAP-treated mice, only 3 of 10 Hu-PBL-SCID mice treated with AZT added to their water at a final concentration of 1 mg/ml, resulting in an average consumption of 200 mg of AZT per kg/day, tested HIV-1 negative. Of the remaining seven mice, four were culture positive and PCR positive, and three mice were culture negative but PCR positive (Table 1).
In vitro anti-HIV-1 activities of plasma samples from TXU-PAP-treated cynomolgus monkeys.
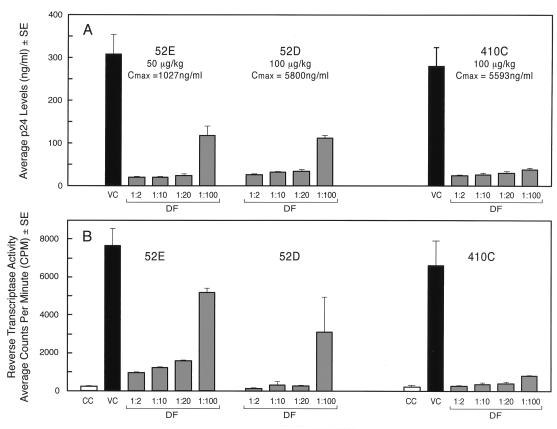
We have previously reported that monkeys treated with TXU-PAP experienced no significant side effects (14). The TXU-PAP concentrations in the plasma samples at 1 h postinfusion were 1,027 ng/ml in monkey 52E treated with 50 μg of TXU-PAP per kg, 5,800 ng/ml in monkey 52D treated with 100 μg of TXU-PAP per kg, and 5,593 ng/ml in monkey 410C treated with 100 μg of TXU-PAP per kg. As shown in Fig. 4, these plasma samples showed potent antiviral activity against HTLVIIIB in vitro even at a 1:100 dilution.
FIG. 4.
In vitro anti-HIV-1 activities of plasma samples from TXU-PAP-treated cynomolgus monkeys. The toxicity and pharmacokinetics of TXU-PAP in nonhuman primates were described previously (22). Monkey 52E had received a 1-h intravenous infusion of 50 μg of TXU-PAP per kg and monkeys 52D and 410C had received a 1-h intravenous infusion of 100 μg TXU-PAP per kg 1 h prior to collection of the peripheral blood samples. The solid-phase enzyme-linked immunosorbent assay-based TXU-PAP levels were 1,027 ng/ml in the plasma of monkey 52E, 5,800 ng/ml in the plasma of monkey 52D, and 5,593 ng/ml in the plasma of monkey 410C. The in vitro effects of serially diluted plasma samples on HIV-1 replication were examined as described in Materials and Methods by p24 enzyme immunoassay and RT assays. The activity data are presented according to the plasma dilution factors (DF) used. Cmax, maximum concentration of drug in plasma; CC, cell control (media plus noninfected cells); VC, virus control (media plus HIV-1-infected cells).
DISCUSSION
We have evaluated the clinical potential of the TXU (anti-CD7)-PAP immunoconjugate as a new biotherapeutic anti-HIV agent by evaluating its anti-HIV-1 activity in vitro, as well as in a surrogate Hu-PBL-SCID mouse model of human AIDS. The present report documents in a side-by-side comparison the superior in vitro anti-HIV-1 activity of TXU-PAP compared to the activities of AZT, d4T, unconjugated PAP, and B53-PAP, an anti-CD4-PAP immunoconjugate. Notably, TXU-PAP elicited potent anti-HIV activity in the Hu-PBL-SCID mouse model of human AIDS without any side effects and at doses that were very well tolerated by cynomolgus monkeys. Furthermore, plasma samples from TXU-PAP-treated cynomolgus monkeys showed potent anti-HIV-1 activity in vitro.
On the basis of its potent anti-HIV-1 activity, we postulate that the incorporation of this immunoconjugate into clinical treatment protocols may improve the prognosis for AIDS patients. In July 1997 a phase I trial of TXU-PAP (trial BB-IND-6985) was initiated at a dosage of 0.001 mg/kg/day for 5 days, which is 100-fold lower than the well-tolerated dose level of 0.1 mg/kg/day for 5 days in cynomolgus monkeys and 25,000-fold lower than the 50% lethal dose for BALB/c mice (i.e., 50 μg/mouse = 2.5 mg/kg).
Humoral immune responses to the MAb as well as toxin portions of immunotoxins have contributed to their limited clinical utility. TXU-PAP-treated monkeys developed anti-PAP as well as an anti-mouse IgG antibodies (22). The immunogenicity of TXU-PAP might be reduced by replacing the mouse antibody with a chimeric or humanized version of TXU as well as by attaching allergens, haptens, or chemical agents such as polyethylene glycol that suppress immune responses. Antitoxin immune responses might be alleviated by rotating varieties of the plant toxin PAP, which may be harvested in three different forms on the basis of plant structure and maturity, or by rotating different species of toxin (13).
Our strategy of targeting PAP to uninfected or latently infected CD4+ cells by using an MAb against the normal antigens on CD4+ cells, such as the CD7 antigen, does not rely on the expression of HIV-1 envelope proteins on infected cells. This approach also avoids potential problems caused by envelope antigen heterogeneity among different HIV-1 isolates or the presence of plasma anti-envelope antibodies. It has been suggested that concomitant infections with other viruses such as cytomegalovirus and herpes simplex virus may induce HIV expression in latently infected CD4+ cells (6, 7, 11). The reported ability of PAP to inhibit the replication of many viruses including cytomegalovirus (8) and herpes simplex virus (1) may therefore provide another unique advantage for the treatment of HIV-1 infections.
ACKNOWLEDGMENTS
We are indebted to A. Erice and H. Balfour for providing patient-derived HIV strains and for performing culture assays of cells from Hu-PBL-SCID mice for HIV as well as helpful discussions.
REFERENCES
- 1.Aron G M, Irvin J D. Inhibition of herpes simplex virus multiplication by the pokeweed antiviral protein. Antimicrob Agents Chemother. 1980;17:1032. doi: 10.1128/aac.17.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z C, Antoniw J F, White R F, Lin Q. Effect of pokeweed antiviral protein (PAP) on the infection of plant viruses. Plant Pathol. 1992;40:612. [Google Scholar]
- 3.Endo Y, Tsurugi K, Lambert J M. The site of action of six different ribosome-inactivating proteins from plants on eukaryotic ribosomes: the RNA N-glycosidase activity of the proteins. Biochem Biophys Res Commun. 1988;150:1032–1036. doi: 10.1016/0006-291x(88)90733-4. [DOI] [PubMed] [Google Scholar]
- 4.Erice A, Lieler C L, Meyers D E, Sannerund K J, Irvin J D, Balfour H H, Uckun F M. Inhibition of zidovudine (AZT)-sensitive strains of human immunodeficiency virus type 1 by pokeweed antiviral protein targeted to CD4+ cells. Antimicrob Agents Chemother. 1993;37:835. doi: 10.1128/aac.37.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erice A, Sannerud K J, Leske V L, Aeppli D, Balfour H H. Sensitive microculture method for isolation of human immunodeficiency virus type 1 from blood leukocytes. J Clin Microbiol. 1992;30:444–448. doi: 10.1128/jcm.30.2.444-448.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauci A S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 7.Fauci A S, Schnittman S M, Poli G, Koenig S, Pantaleo G. Immunopathogenic mechanisms in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1991;114:678–693. doi: 10.7326/0003-4819-114-8-678. [DOI] [PubMed] [Google Scholar]
- 8.Gehrz R C, Wilson C, Eckhardt J, Myers D, Irvin J D, Uckun F M. Treatment of human cytomegalovirus (HCMV) with novel antiviral immunoconjugates. In: Landin M P, editor. Progress in cytomegalovirus research. Amsterdam, The Netherlands: Elsevier Science Publishers BV; 1991. pp. 353–356. [Google Scholar]
- 9.Goudsmit J, Lange J M A, Paul D A, Dawson G J. Antigenemia and antibody titers to core and envelope antigens in AIDS, AIDS-related complex, and subclinical human immunodeficiency virus infection. J Infect Dis. 1987;155:558–560. doi: 10.1093/infdis/155.3.558. [DOI] [PubMed] [Google Scholar]
- 10.Hartley M R, Legname G, Osborn R, Chen Z, Lord J M. Single-chain ribosome inactivating proteins from plants depurinate Escherichia coli 23S ribosomal RNA. FEBS Lett. 1991;290:65–68. doi: 10.1016/0014-5793(91)81227-y. [DOI] [PubMed] [Google Scholar]
- 11.Ho D D, Pomerantz R J, Kaplan J C. Pathogenesis of infection with human immunodeficiency virus. N Engl J Med. 1987;317:278–286. doi: 10.1056/NEJM198707303170505. [DOI] [PubMed] [Google Scholar]
- 12.Irvin J. Pokeweed antiviral protein. Pharmacol Ther. 1983;21:371–387. doi: 10.1016/0163-7258(83)90061-x. [DOI] [PubMed] [Google Scholar]
- 13.Irvin J D, Uckun F M. Pokeweed antiviral protein: ribosome inactivation and therapeutic applications. Pharmacol Ther. 1992;55:279–302. doi: 10.1016/0163-7258(92)90053-3. [DOI] [PubMed] [Google Scholar]
- 14.Jackson J B, Kwok S Y, Sninsky J J, Hopsicker J S, Sannerud K J, Rhame F S, Henry K, Simpson M, Balfour H H., Jr Human immunodeficiency virus type 1 detected in all seropositive symptomatic and asymptomatic individuals. J Clin Microbiol. 1990;28:16–19. doi: 10.1128/jcm.28.1.16-19.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy J A, Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985;152:734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- 16.Mosier D E, Gulizia R Y, Baird S M, Wilson D B, Spector D H, Spector S A. Human immunodeficiency virus infection of human PBL-SCID mice. Science. 1991;251:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 17.Myers D E, Jun X, Clementson D, Donelson R, Sicheneder A, Hoffman N, Bell K, Sarquis M, Langlie M, Uckun F M. Large scale manufacturing of TXU (anti-CD7)-pokeweed antiviral protein (PAP) immunoconjugate for clinical trials. Leukemia Lymphoma. 1997;27:275–302. doi: 10.3109/10428199709059683. [DOI] [PubMed] [Google Scholar]
- 18.Ou C-Y, Kwok S, Mitchell S W, Mackm D H, Sninsky J J, Krebs J W, Feorino P, Warefield D, Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988;239:295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- 19.Teltow G J, Irvin J D, Aron G M. Inhibition of herpes simplex virus DNA synthesis by pokeweed antiviral protein. Antimicrob Agents Chemother. 1983;23:390. doi: 10.1128/aac.23.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson J A, Walker V M, Flewett T H, Barclay G R. The inhibition of infection of cucumber mosaic virus and influenza virus by extracts from Phytolacca americana. J Gen Virol. 1974;22:225–232. doi: 10.1099/0022-1317-22-2-225. [DOI] [PubMed] [Google Scholar]
- 21.Ussery M A, Irvin J D, Hardesty B. Inhibition of poliovirus replication by a plant antiviral peptide. Ann N Y Acad Sci. 1977;284:431. doi: 10.1111/j.1749-6632.1977.tb21979.x. [DOI] [PubMed] [Google Scholar]
- 22.Waurzyniak B, Schneider E A, Tumer N, Yanishevski Y, Gunther R, Chelstrom L M, Wendorf H, Myers D E, Irvin J D, Messinger Y, Ek O, Zeren T, Chandan-Langlie M, Evans W E, Uckun F M. In vivo toxicity, pharmacokinetics, and antileukemic activity of TXU (anti-CD7)-pokeweed antiviral protein immunotoxin. Clin Cancer Res. 1997;3:881–890. [PubMed] [Google Scholar]
- 23.Zarling J M, Moran P A, Haffar O, Sias J, Richman D D, Spina C A, Myers D E, Kuebelbeck V, Ledbetter J A, Uckun F M. Inhibition of HIV replication by pokeweed antiviral protein targeted to CD4+ cells by monoclonal antibodies. Nature. 1990;347:92–95. doi: 10.1038/347092a0. [DOI] [PubMed] [Google Scholar]