Abstract
INTRODUCTION
We compared three operational case definitions of mild behavioral impairment (MBI) in the context of MBI prevalence estimates and dementia risk modeling.
METHODS
Participants were dementia‐free older adults (n = 13701) from the National Alzheimer's Coordinating Center. Operational case definitions of MBI were generated based on neuropsychiatric symptoms at one (OV), two‐consecutive (TCV), or more than two‐thirds (TTV) of dementia‐free study visits. Definitions were compared in prevalence and in Cox regressions using MBI to predict incident dementia.
RESULTS
OV MBI was the most prevalent (54.4%), followed by TCV (32.3%) and TTV (26.7%) MBI. However, OV MBI had the lowest rate of incident dementia (hazard ratio [HR] = 2.54, 95% confidence interval [CI]: 2.33–2.78) and generated poorer model metrics than TCV MBI (HR = 4.06, 95% CI: 3.74–4.40) and TTV MBI (HR = 5.77, 95% CI: 5.32–6.26).
DISCUSSION
Case ascertainment with longer timeframe MBI operational case definitions may more accurately define groups at risk of dementia in datasets lacking tools designed to detect MBI.
Highlights
Mild behavioral impairment (MBI) can identify older adults at risk of dementia.
Neuropsychiatric symptom (NPS) assessment tools can be proxy measures for MBI.
Hazard for dementia was highest for MBI defined by NPS presence at more than two‐thirds of visits.
Keywords: Alzheimer disease, dementia, mild behavioral impairment, neuropsychiatric symptoms
1. BACKGROUND
Mild behavioral impairment (MBI) identifies older adults at high risk for neurodegenerative disease using neuropsychiatric symptoms (NPS). 1 These NPS include decreased motivation (apathy), affective dysregulation (mood and anxiety), impulse dyscontrol (agitation and aggression), social inappropriateness, and abnormal perception/thought content (delusions and hallucinations; psychosis). NPS qualify as MBI symptoms when they (1) emerge de novo in later life and persist at least intermittently for ≥6 months; (2) cannot be explained by psychiatric conditions; and (3) occur in older adults without dementia. MBI should be thought of as a subset of NPS that meet the aforementioned criteria (Figure S1), which improve specificity for the NPS identified by MBI representing early‐stage neurodegenerative disease as a complementary behavioral analog to late‐life emergent cognitive symptoms. 2 As such, MBI can improve early dementia risk detection, thereby facilitating research into early neurodegenerative disease mechanisms and the development of preventative or disease‐modifying treatments. 3 , 4
The Mild Behavioral Impairment Checklist (MBI‐C) was developed to capture NPS that meet MBI criteria. 5 Studies that used the MBI‐C demonstrated poorer cognition and greater β‐amyloid (Aβ) and tau pathology, medial temporal lobe atrophy, and Alzheimer's disease (AD) genetic risk in MBI older adults. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 However, given the novelty of the Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART‐AA) MBI criteria and the MBI‐C, which were published in 2016 and 2017, respectively, datasets with abundant MBI‐C data are not yet widely available. To address this issue, other existing measures of NPS, most commonly the Neuropsychiatric Inventory (NPI) and its derivatives, have been used as proxy measures of MBI. 14 , 15 , 16 , 17 , 18 These general NPS assessment tools are considered proxy measures of MBI because, although they identify and measure NPS, MBI criteria must be applied afterwards as a filter to identify those at higher risk for dementia. In contrast to the MBI‐C, where MBI criteria are inherent, other algorithms and specific data cleaning procedures have been used to apply MBI criteria to measurements made by other NPS assessment tools. This approach has generated novel insight into MBI and its relationship to dementia that would have been overlooked until more frequent MBI‐C endorsement. Longitudinally, MBI, defined using the NPI or NPI Questionnaire (NPI‐Q), has consistently been associated with a greater risk for cognitive decline and incident dementia, 15 , 17 , 19 , 20 and a lower rate of reversion from mild cognitive impairment (MCI) to normal cognition in participants from the National Alzheimer's Coordinating Center (NACC). 18 In the Alzheimer's Disease Neuroimaging Initiative and MEMENTO cohorts, where only NPI/NPI‐Q data are available, MBI was linked to more severe neurodegenerative disease pathology, including lower plasma Aβ42/Aβ40 and higher phosphorylated tau and neurofilament light, in addition to greater white matter hyperintensity volume and entorhinal cortex atrophy. 21 , 22 , 23 , 24 Finally, in the Canadian Comprehensive Assessment of Neurodegeneration and Dementia study, MBI derived from the NPI‐Q was associated with worse gait, hearing loss, and frailty, all of which are non‐cognitive markers of dementia. 25 , 26 , 27 , 28
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (e.g., PubMed, Google Scholar) to identify studies of mild behavioral impairment (MBI). These studies, which have been cited, demonstrate the link between MBI and dementia, but may be limited in their case operationalization of MBI.
Interpretation: MBI operational case definitions that align more closely with established MBI criteria are more strongly associated with incident dementia and enhance dementia prognostication. MBI defined based on the presence of persistent neuropsychiatric symptoms (NPS), defined as more than two‐thirds of a participant's study visits, yielded larger effect sizes and better model metrics than operational case definitions based on one or two consecutive visits.
Future directions: Studies of MBI in longitudinal datasets without tools designed specifically to detect MBI can evaluate NPS presence at more than two‐thirds of visits to classify older adults with MBI with greater accuracy.
The use of other NPS assessment tools as a proxy measure for MBI will likely continue to produce important findings about MBI, especially in datasets that preceded the MBI‐C. Yet, current methods used to operationally define cases of MBI using proxy tools face limitations, potentially hindering accurate differentiation between MBI and non‐MBI NPS. In older adults with MCI, only MBI was associated with a lower likelihood of reversion to normal cognition, whereas non‐MBI NPS showed no difference compared to no NPS. 18 This finding suggests that distinguishing MBI from non‐MBI NPS, the latter of which is often captured by the NPI and its derivatives, is essential to fully understand and make use of MBI as a tool for neurodegenerative disease research and for sample enrichment. 4 One issue with using the NPI and its derivatives for MBI is the reference timeframe by which the instruments assess NPS, which spans 1 month. 29 , 30 , 31 In contrast, the MBI‐C reference frame is 6 months, consistent with the MBI symptom persistence criterion. 5 Consequently, studies that operationally define MBI using NPI/NPI‐Q data from a single visit risk misclassifying as MBI, NPS that are transient and potentially arising from non‐neurodegenerative etiologies. Furthermore, estimates of MBI prevalence in samples of older adults without dementia are considerably higher when using the NPI/NPI‐Q (28%–85%) 14 , 16 , 27 compared to the MBI‐C (6%–14%) 6 , 7 , 32 , 33 at a single visit, suggesting low specificity when operationally defining MBI this way. More recent studies have operationally defined MBI based on the presence of NPS across two consecutive visits, often separated by approximately 6 or 12 months, to meet the persistence criterion for MBI. 18 , 19 , 21 , 23 However, due to the 1‐month timeframes of the NPI and NPI‐Q instruments, this method still risks misclassifying participants with transient NPS as having MBI. A new operational case definition to identify MBI more accurately, and to distinguish MBI from non‐MBI NPS, is warranted.
This study proposes a new operational case definition of MBI in datasets with retrospective longitudinal data that have not implemented the MBI‐C. To demonstrate its utility, we compare the novel method to previously used MBI operational case definitions in terms of prevalence estimates and dementia prognostication in NACC data.
2. METHODS
2.1. Study design
Data were obtained from the NACC Uniform Dataset (NACC‐UDS). Forty‐five Alzheimer's Disease Research Centers (ADRCs) funded by the National Institute on Aging contributed to this dataset between 2005 and 2022. Each ADRC recruited and collected data, including for NPS, on participants with or without dementia approximately annually. All ADRCs obtained informed consent from their participants and received ethics approval from their institutions prior to submitting data to NACC. More detailed information about the NACC‐UDS and its data collection procedures has been published elsewhere. 34 , 35 , 36 , 37
2.2. Participants
Longitudinal data for 45100 participants were available. Participants had normal cognition (CN), subjective cognitive decline (SCD), MCI, or dementia. Participants were excluded if they: (1) were <50 years old, consistent with MBI criteria, 1 n = 1069; (2) reported a history of relevant psychiatric/neurological conditions, n = 14556; (3) were missing data for statistical model covariates (i.e., years of education), n = 244; or (4) were missing NPI‐Q data, n = 910 (Figure 1). Applying the exclusion criteria resulted in 28321 participants who were eligible for analysis. Relevant psychiatric conditions included posttraumatic stress disorder, bipolar disorder, schizophrenia, obsessive‐compulsive disorder, remote anxiety or depression, which preclude MBI diagnosis. 1 Excluded neurological conditions included Down syndrome, Parkinson's disease, and Huntington disease, in which NPS may arise independent of dementia.
FIGURE 1.
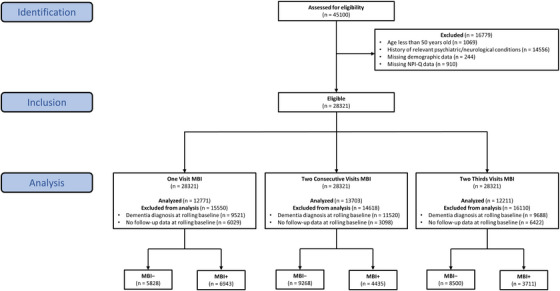
Participant flow diagram. Data came from the National Alzheimer's Coordinating Center (NACC). Relevant psychiatric conditions used for exclusion included posttraumatic stress disorder, bipolar disorder, schizophrenia, obsessive‐compulsive disorder, remote anxiety, or depression, Down syndrome, Huntington disease, and Parkinson's disease. NPI‐Q, Neuropsychiatric Inventory Questionnaire; MBI, mild behavioral impairment.
2.3. MBI operational case definitions
The presence and severity of NPS were measured using the NPI‐Q. 29 , 31 Briefly, informants rated the presence and severity of NPS on a scale from 1 to 3 over the previous month. A published algorithm was used to derive MBI symptom severity score from 10/12 NPI‐Q items (excluding sleep and appetite) based on the ISTAART‐AA diagnostic criteria for MBI. 14 The decreased motivation domain was derived from the NPI‐Q apathy item; emotional dysregulation from the NPI‐Q depression, anxiety, and elation items; impulse dyscontrol from the NPI‐Q agitation, irritability, and aberrant motor behavior items; social inappropriateness from the NPI‐Q disinhibition item; and psychosis from the NPI‐Q delusions and hallucinations items. The total MBI symptom severity (range = 0–30) was computed as the sum of all five MBI domain scores, with higher scores indicating greater severity.
Three MBI operational case definitions were explored. One visit (OV) MBI classified participants as having MBI if they had a total MBI symptom severity score ≥1 at a single dementia‐free study visit. Two consecutive visits (TCV) MBI classified participants as having MBI if they had a total MBI symptom severity score ≥1 for two consecutive dementia‐free study visits. Two‐thirds visits (TTV) MBI classified participants as having MBI if the participant had a total MBI symptom severity score ≥1 at a dementia‐free study visit and for more than two‐thirds of all subsequent dementia‐free study visits, when including the initial visit with NPS. To account for participants who developed MBI over the course of their observational period in NACC, participants were categorized MBI+ if they developed MBI at any visit and MBI– if they never developed MBI at any visit. A participant‐specific baseline was applied to MBI+ participants; their baseline was shifted to the first visit they were classified as MBI+ and all prior visits were considered pre‐MBI. Correspondingly, time to dementia was calculated as the difference in years between the first dementia visit and the participant‐specific baseline visit. As the baseline was shifted for MBI+ participants, additional exclusion criteria were applied to each set of analyses involving the three MBI operational case definitions: having a clinical dementia diagnosis (OV n = 9521, TCV n = 11520, TTV n = 9586) or no follow‐up data (OV n = 6029, TCV n = 3098; TTV n = 5034) at the participant‐specific baseline. These steps resulted in a final sample of 12771 participants for the OV analysis, 13703 for the TCV analysis, and 13701 for the TTV analysis. Example scenarios illustrating differences between the MBI operational case definitions in the MBI classification of participants and how dementia‐free survival time was calculated using the participant‐specific baseline, if appropriate, are shown in Figure 2. Simulated data accompanied by a data dictionary and the R code used to generate each MBI operational case definition in this study are available at https://osf.io/k75rg for those interested in exploring MBI case ascertainment scenarios or implementing these operational case definitions in their own studies.
FIGURE 2.
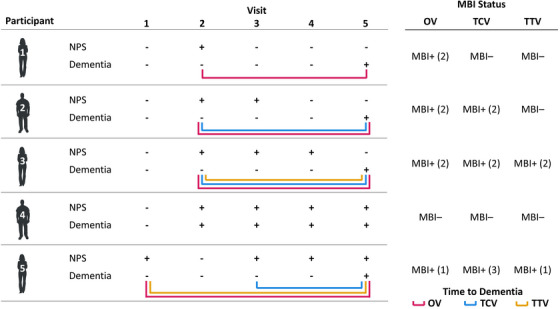
Example case ascertainment for three MBI operational case definitions. Positive/negative signs indicate the presence/absence, respectively, of NPS or a dementia diagnosis at a given study visit for a participant. Numbers in parentheses in the right table indicate the study visit at which a participant was first defined as having MBI, if applicable. Colored lines indicate the time between MBI classification, if applicable, and the first dementia visit. NPS, neuropsychiatric symptoms; MBI, mild behavioral impairment; OV, one visit MBI operational case definition; TCV, two consecutive visits MBI operational case definition; two‐thirds visits MBI operational case definition.
2.4. Statistical analysis
Descriptive statistics including means, standard deviations (SDs), ranges, medians, interquartile ranges (IQRs), and percentages were used to describe participant demographic and clinical characteristics. Between‐group comparisons for MBI– and MBI+ participants, defined using each of the three operational case definitions, were conducted using general linear models. To determine potential trends in missing NPI‐Q data, demographic comparisons were also conducted between eligible participants and those excluded for missing NPI‐Q data.
Dementia‐free survival was compared between MBI groups using Kaplan–Meier survival estimates. To extract hazard ratios (HRs), Cox proportional hazards regression models were conducted with MBI status as the exposure and incident dementia as the outcome. These Cox regressions were first run as univariable models to compare the effect size corresponding to each MBI operational case definition independently of covariates. Subsequently, multivariable Cox regression models adjusted for age, sex, education, and cognitive status (CN/SCD or MCI) to control for other factors that may contribute to incident dementia. Schoenfeld and Martingale residuals were evaluated to verify that the proportional hazards and linearity assumptions, respectively, were met for predictors in each Cox regression model, as appropriate.
To compare the three MBI operational case definitions for dementia modeling performance, we extracted the concordance index (C‐index) and Akaike's information criterion (AIC) for each model. The C‐index is a commonly used evaluation metric for the predictive ability of survival models with higher values indicating greater prediction accuracy, 38 and the AIC may be used to compare model performance between non‐nested models in the same data with lower values indicating better model fit. 39 Sensitivity analyses were used to compare MBI operational case definitions using higher MBI total score cutoffs of ≥2 and ≥3. All analyses employed a statistical significance threshold of P < .05 and were conducted on R version 4.0.2. 40 Data access was provided by NACC after submitting an approved research proposal.
3. RESULTS
Baseline participant characteristics for each sample are summarized in Table 1. Across all three cohorts, participants were approximately 57% female, 73 years old (SD = 9, range = 50–104), and had completed 16 years of education (SD = 3, range = 0–30). The most common cognitive statuses were CN (63%), followed by MCI (31%) and SCD (6%). The median follow‐up time was approximately 3 years (IQR = 2–6) and 18% of participants progressed to dementia during follow‐up. AD accounted for approximately 82% of the dementia diagnoses. Comparisons between MBI– and MBI+ participants, according to each MBI operational case definition, can be found in Tables S1–S3. Participants that were missing NPI‐Q data were more likely to be older and less likely to have cognitive impairment, than eligible participants (Table S4).
TABLE 1.
Baseline participant characteristics.
| Variable | OV | TCV | TTV |
|---|---|---|---|
| n | 12771 | 13703 | 12211 |
| Age (years) | 73.4 (9.0), 50–104 | 73.2 (9.0), 50–104 | 73.1 (9.0), 50–104 |
| Education (years) | 15.7 (3.1), 0–30 | 15.7 (3.1), 0–30 | 15.7 (3.1), 0–30 |
| Sex (female) | 7313 (57.3) | 7895 (57.6) | 7007 (57.4) |
| Diagnosis | |||
| CN | 7967 (62.4) | 8616 (62.9) | 7627 (62.5) |
| SCD | 723 (5.7) | 764 (5.6) | 670 (5.5) |
| MCI | 4081 (32.0) | 4323 (31.5) | 3914 (32.1) |
| MBI prevalence | |||
| Global | 6943 (54.4) | 4435 (32.3) | 3711 (30.4) |
| Decreased motivation | 1365 (10.7) | 1069 (7.8) | 965 (7.9) |
| Affective dysregulation | 4146 (32.5) | 2738 (20.0) | 2313 (18.9) |
| Impulse dyscontrol | 3934 (30.8) | 2746 (20.0) | 2282 (18.7) |
| Social inappropriateness | 765 (6.0) | 612 (4.5) | 542 (4.4) |
| Psychosis | 293 (2.3) | 240 (1.8) | 225 (1.8) |
| MBI severity | |||
| Global | 1.3 (1.9), 0–21 | 0.9 (1.9), 0–21 | 0.9 (1.9), 0–21 |
| Decreased motivation | 0.1 (0.4), 0–3 | 0.1 (0.4), 0–3 | 0.1 (0.4), 0–3 |
| Affective dysregulation | 0.5 (0.9), 0–8 | 0.3 (0.8), 0–8 | 0.3 (0.8), 0–8 |
| Impulse dyscontrol | 0.5 (1.0), 0–9 | 0.4 (0.9), 0–9 | 0.3 (0.9), 0–9 |
| Social inappropriateness | 0.1 (0.3), 0–3 | 0.1 (0.3), 0–3 | 0.1 (0.3), 0–3 |
| Psychosis | 0 (0.2), 0–6 | 0 (0.2), 0–5 | 0 (0.2), 0–5 |
| Conversion to dementia | 2437 (19.1) | 2441 (17.8) | 2369 (19.4) |
| Follow‐up duration (years) | 3.3 (1.9–6.0) | 3.4 (2.0–6.1) | 3.4 (2.0–6.2) |
Notes: Continuous variables are in mean (standard deviation), range, except for follow‐up duration which is shown in median (interquartile range). Categorical variables are shown in n (%). All values have been rounded to one decimal place, if appropriate.
Abbreviations: CN, cognitively normal; MCI, mild cognitive impairment; MBI, mild behavioral impairment; OV, one visit MBI operational case definition; TCV, two consecutive visits MBI operational case definition; TTV, two‐thirds visits MBI operational case definition; SCD, subjective cognitive decline.
The prevalence of MBI was highest using the OV operational case definition (54.4%), followed by TCV (32.3%), and lowest using TTV (30.4%). Regardless of operational case definition, affective dysregulation and impulse dyscontrol tended to be the most prevalent MBI domains followed by decreased motivation, social inappropriateness, and psychosis. Kaplan–Meier survival estimates revealed that 25.9% of older adults with OV MBI progressed with an estimated median time to dementia of 11.77 years (95% CI: 11.01–13.02), as shown in Figure 3. In contrast, 33.0% of older adults with TCV MBI progressed with a median time to dementia of 7.28 years (95% CI: 6.78–8.51), and 39.8% of older adults with TTV MBI progressed with a median time to dementia of 4.82 years (95% CI: 4.48–5.06). Regardless of operational case definition, MBI older adults were more likely to progress to dementia than non‐MBI older adults (all P < .001). Only 11.1% of those who did not meet the criteria for any MBI progressed to dementia. Sensitivity analysis showed that the prevalence of MBI decreased when using higher MBI cutoff scores (Table S5).
FIGURE 3.
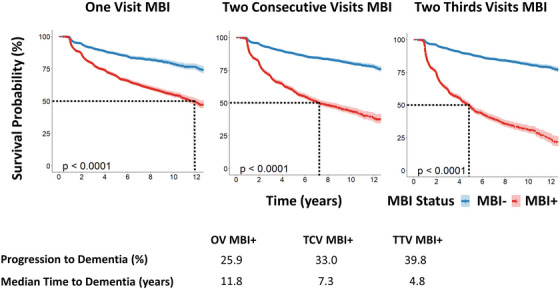
Kaplan–Meier survival curves for three operational case definitions of MBI. Blue lines indicate the survival curves for older adults without MBI (MBI–), whereas red lines indicate survival curves for older adults with MBI (MBI+) defined using one of three MBI operational case definition. Dotted lines in each survival curve indicate the median time to dementia for the MBI+ group in years. All P‐values indicated the statistical significance of the relationship between MBI status, using one of the three MBI operational case definitions, and incident dementia. MBI, mild behavioral impairment; OV, one visit MBI operational case definition; TCV, two consecutive visits MBI operational case definition; two‐thirds visits MBI operational case definition.
Univariable Cox proportional hazard regression models showed that dementia risk was lowest in participants with OV MBI, intermediate for TCV MBI, and highest for TTV MBI (Table 2). Specifically, the HR for OV MBI 2.54 (95% CI: 2.33–2.78, P < .001) compared to participants without MBI, whereas the HRs for TCV and TTV MBI were 4.06 (95% CI: 3.74–4.40, P < .001) and 6.16 (95% CI: 5.66–6.70, P < .001), respectively. This pattern remained after controlling for age, sex, education, and cognitive status: the adjusted HRs were 1.48 (95% CI: 1.35–1.62, P < .001) for OV MBI, 2.13 (95% CI: 1.96–2.32, P < .001) for TCV MBI, and 2.97 (95% CI: 2.72–3.25, P < .001) for TTV MBI.
TABLE 2.
Comparison between three MBI operational case definitions and their relationship with incident dementia.
| Model | HR | 95% CI | P | C‐index | AIC |
|---|---|---|---|---|---|
| Univariable | |||||
| One visit MBI | 2.54 | 2.33–2.78 | <.001 | 0.61 | 42412 |
| Two consecutive visits MBI | 4.06 | 3.74–4.40 | <.001 | 0.67 | 42140 |
| Two‐thirds visits MBI | 6.16 | 5.66–6.70 | <.001 | 0.72 | 39709 |
| Multivariable | |||||
| One visit MBI | 1.48 | 1.35–1.62 | <.001 | 0.83 | 39355 |
| Two consecutive visits MBI | 2.13 | 1.96–2.32 | <.001 | 0.85 | 39357 |
| Two‐thirds visits MBI | 2.97 | 2.72–3.25 | <.001 | 0.86 | 37357 |
Notes: All values have been rounded to two decimal places, except for P‐values which have been rounded to three decimal places, if appropriate. The outcome variable for all models was incident dementia. The main predictor for all models was MBI status (0 = MBI–, 1 = MBI+) defined using one of three MBI operational case definitions. Multivariable models were adjusted for age, sex, education, and cognitive diagnosis (CN/SCD or MCI). C‐index and AIC values apply to the corresponding models as a whole.
Abbreviations: AIC, Akaike's information criterion; C‐index, concordance index; CN, cognitively normal; HR, hazard ratio; MBI, mild behavioral impairment, MCI, mild cognitive impairment, SCD, subjective cognitive decline; 95% CI, 95% confidence interval.
In both univariable and multivariable Cox regression models, C‐index and AIC model metrics indicated that the TTV MBI operational case definition was linked to better model performance than TCV and OV operational case definitions: C‐indices were highest for TTV MBI models, intermediate for TCV MBI models, and lowest for OV MBI models. Correspondingly, AIC was lowest for TTV MBI, intermediate for TCV MBI, and highest for OV MBI models (Table 2). A sensitivity analysis revealed that these patterns in HR and model performance across MBI operational case definitions remained consistent when using higher MBI cutoff scores of ≥2 and ≥3, with marginally larger HRs for higher cutoff scores at the cost of lower similar or slightly worse model performance (Table S5) when compared to an MBI cutoff score of ≥1.
4. DISCUSSION
Valuable insight into MBI can be obtained from datasets without MBI‐C data by using other NPS assessment tools with the appropriate operational case definitions and data cleaning procedures. Here, we outlined and compared three operational case definitions for MBI that varied in how they addressed the symptom persistence criterion of MBI, while all incorporating the de novo symptom emergence criterion by including only those without past psychiatric conditions. The most stringent operational case definition of MBI (i.e., TTV) led to lower prevalence estimates of MBI than previously used operational case definitions of MBI based on the presence of NPS at one or two consecutive visits. Attendant with TTV‐associated group refinement, models produced larger effect sizes and better model metrics for dementia prognostication compared to OV and TCV MBI. These differences suggest that the TTV operational case definition yields the greatest specificity for MBI, though comparisons with a gold standard diagnosis of MBI by the MBI‐C are warranted.
NPS are common features of dementia, with a 5‐year period prevalence of 97% in AD patients. 41 However, when NPS manifest in older adults without dementia and under certain circumstances, they may be an indicator of dementia risk. The MCI criteria may be applied to distinguish chronic cognitive impairment or benign lapses in cognition from cognitive changes suggestive of dementia risk. 42 Similarly, the MBI criteria play a pivotal role to distinguish chronic and/or recurrent psychiatric conditions and benign alterations in behavior or personality from dementia‐related NPS, the latter being sequelae of neurodegenerative disease. 1 Importantly, MBI represents another tool that can be applied to stratify populations according to dementia risk in clinical and research settings. Clinicians may use MBI to screen patients for dementia risk, with the combination of cognitive and behavioral markers providing greater information on risk than either alone. If appropriate, patients may then be referred to further specialty care, potentially along with more in‐depth assessments involving biomarker or neuroimaging tests to confirm a diagnosis. In research studies such as clinical trials, MBI may enrich sample selection by identifying those with a higher likelihood of biomarker positivity, and at greater risk of incident dementia, thereby reducing screening failures and minimizing variability in outcomes within treatment and control groups. 18 Altogether, MBI has the potential to be incorporated into patient stratification strategies, which are essential to advance research into dementia, whose causes are multifactorial and whose phenotypes, especially during preclinical or prodromal stages, are heterogeneous. 43 , 44 Further research into MBI and its relationship to aspects of dementia is key, therefore, in order to fully utilize MBI in clinical and research settings.
Careful participant exclusion criteria may be applied to address MBI criteria when using NPS assessment tools as proxy measures of MBI. Participants <50 years of age or with dementia or psychiatric conditions may be excluded during participant selection to ensure that any observed NPS have emerged in later life before a dementia diagnosis, not explained by psychiatric conditions. Satisfying the criterion that requires NPS to persist at least intermittently for ≥6 months is more elusive, especially when using NPS assessment tools with 1‐month reference frames. Our findings highlight the importance of incorporating longitudinal information into MBI case ascertainment, yielding greater effect sizes and better dementia prognostication modeling. The TTV operational case definition offers several other advantages over previous MBI operational case definitions. By considering a given proportion of visits rather than an absolute number of visits, the TTV definition enables flexible case ascertainment that can become more accurate as more data become available. A dementia‐free participant with NPS for both of their only two study visits would be classified as having MBI according to all three operational case definitions. However, their classification would change to TTV MBI– if they failed to show NPS for the next three study visits, despite remaining TCV and OV MBI+. Furthermore, the TTV operational case definition allows participants who fail to report NPS at a study visit due to a variety of potential factors (e.g., a change in informant) to still be classified as MBI+, provided there is sufficient evidence from subsequent study visits that their NPS represents a change from longstanding patterns of behavior (i.e., NPS at more than two‐thirds of visits). These operational case definitions are intended for research to help obtain valuable information about MBI from legacy datasets with measures of NPS that identify the broader category of NPS but do not identify MBI explicitly, the latter of which holds greater prognostic value.
This study is not without limitations. First, in the absence of a gold standard clinical diagnosis of MBI in the NACC‐UDS, we cannot definitively conclude whether the TTV operational case definition enhances specificity for identifying MBI+ older adults. Second, given the nature of the NPI and its derivatives that have a 1‐month reference frame, the TTV operational case definition cannot guarantee the persistence of NPS in‐between study visits. Third, although the use of all available visit information is a strength of the TTV operational case definition, it also means that the amount of information will vary across individuals in studies where participants were followed for various lengths of time. Fourth, the TTV operational case definition holds little utility in cross‐sectional datasets and in clinical practice, where case ascertainment must be performed using information collected at a single visit. In other words, while the prognostic value of MBI improves with more stringent operational case definitions, practicality for clinical use improves in the opposite direction, where a single timepoint assessment is most efficient. In this case, a measure such as the MBI checklist, which incorporates the emergence and persistence criteria explicitly remains the optimal tool for identifying persons with MBI. The MBI‐C is free to use (www.mbitest.org), has been validated in community and clinical samples, and takes approximately 7 min to administer in‐person, by telephone, or online by participants, informants, or clinicians, 5 , 32 , 33 , 45 , 46 , 47 making it an accessible and scalable tool that can be applied to the growing number of people at risk for dementia worldwide. 48 The TTV operational case definition should be used as an alternative to study MBI in datasets where sufficient MBI‐C data are not available.
In conclusion, MBI may be implemented in an accessible and scalable manner to help identify older adults at risk of dementia, and can facilitate research and therapies targeting early stages of neurodegenerative disease. While the MBI‐C remains the most efficient method to detect and measure MBI in older adults, datasets without MBI‐C data may still be used to gain insight into MBI and its relationship with dementia, which is needed to fully utilize MBI in practice. We recommend the TTV operational case definition of MBI for datasets that have not implemented the MBI‐C. However, for studies based on a comprehensive assessment of risk at a defined time point, the TTV operational case definition may not be appropriate or should be modified to be based on the presence of MBI at >2/3 of study visits up to that time point.
CONFLICT OF INTEREST STATEMENT
Z.I. has received consulting honoraria from Lundbeck/Otsuka and his institution has received funds from Biogen and Roche. G.B.P. is supported by the Canadian Institutes for Health Research – Foundation Grant #FDN‐143290. D.X.G. and E.E.S. report no conflicts of interest. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA‐funded ADCs: P50 AG005131 (PI James Brewer, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG005138 (PI Mary Sano, PhD), P50 AG005142 (PI Helena Chui, MD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005681 (PI John Morris, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG008051 (PI Thomas Wisniewski, MD), P50 AG008702 (PI Scott Small, MD), P30 AG010124 (PI John Trojanowski, MD, PhD), P30 AG010129 (PI Charles DeCarli, MD), P30 AG010133 (PI Andrew Saykin, PsyD), P30 AG010161 (PI David Bennett, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG013854 (PI Robert Vassar, PhD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P30 AG019610 (PI Eric Reiman, MD), P50 AG023501 (PI Bruce Miller, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG028383 (PI Linda Van Eldik, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P30 AG035982 (PI Russell Swerdlow, MD), P50 AG047266 (PI Todd Golde, MD, PhD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG049638 (PI Suzanne Craft, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Marwan Sabbagh, MD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), and P30 AG072959 (PI James Leverenz, MD). NACC funding sources were not involved in the analysis and interpretation of the data, in the writing of the report, or in the decision to submit the article for publication.
Guan DX, Smith EE, Pike GB, Ismail Z. Persistence of neuropsychiatric symptoms and dementia prognostication: A comparison of three operational case definitions of mild behavioral impairment. Alzheimer's Dement. 2023;15:e12483. 10.1002/dad2.12483
REFERENCES
- 1. Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Creese B, Ismail Z. Mild behavioral impairment: measurement and clinical correlates of a novel marker of preclinical Alzheimer's disease. Alzheimers Res Ther. 2022;14(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mortby ME, Black SE, Gauthier S, et al. Dementia clinical trial implications of mild behavioral impairment. Int Psychogeriatr. 2018;30(2):171‐175. [DOI] [PubMed] [Google Scholar]
- 4. Ismail Z, Ghahremani M, Chen H‐Y, Wang M, Zetterberg H, Smith EE, Mild behavioral impairment is associated with plasma p‐tau181 in a dementia‐free population. Paper presented at: Alzheimer's Association International Conference, 2022.
- 5. Ismail Z, Agüera‐Ortiz L, Brodaty H, et al. The Mild Behavioral Impairment Checklist (MBI‐C): a rating scale for neuropsychiatric symptoms in pre‐dementia populations. J Alzheimers Dis. 2017;56(3):929‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kassam F, Chen H, Nosheny R, et al. Cognitive profile of people with mild behavioral impairment in Brain Health Registry participants. Int Psychogeriatr. 2022:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Creese B, Brooker H, Ismail Z, et al. Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. 2019;27(8):823‐834. [DOI] [PubMed] [Google Scholar]
- 8. Johansson M, Stomrud E, Insel PS, et al. Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer's disease. Transl Psychiatry. 2021;11(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lussier FZ, Pascoal TA, Chamoun M, et al. Mild behavioral impairment is associated with β‐amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement. 2020;16(1):192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shu J, Qiang Q, Yan Y, et al. Distinct patterns of brain atrophy associated with mild behavioral impairment in cognitively normal elderly adults. Int J Med Sci. 2021;18(13):2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsunoda K, Yamashita T, Osakada Y, et al. Positive baseline behavioral and psychological symptoms of dementia predict a subsequent cognitive impairment in cognitively normal population. Neurol Clin Neurosci. 2021;9(3):218‐222. [Google Scholar]
- 12. Creese B, Arathimos R, Brooker H, et al. Genetic risk for Alzheimer's disease, cognition, and mild behavioral impairment in healthy older adults. Alzheimers Dement (Amst). 2021;13(1):e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matuskova V, Ismail Z, Nikolai T, et al. Mild behavioral impairment is associated with atrophy of entorhinal cortex and hippocampus in a memory clinic cohort. Front Aging Neurosci. 2021;13:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheikh F, Ismail Z, Mortby ME, et al. Prevalence of mild behavioral impairment in mild cognitive impairment and subjective cognitive decline, and its association with caregiver burden. Int Psychogeriatr. 2018;30(2):233‐244. [DOI] [PubMed] [Google Scholar]
- 15. Ismail Z, McGirr A, Gill S, Hu S, Forkert ND, Smith EE. Mild behavioral impairment and subjective cognitive decline predict cognitive and functional decline. J Alzheimers Dis. 2021;80(1):459‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mortby ME, Ismail Z, Anstey KJ. Prevalence estimates of mild behavioral impairment in a population‐based sample of pre‐dementia states and cognitively healthy older adults. Int Psychogeriatr. 2018;30(2):221‐232. [DOI] [PubMed] [Google Scholar]
- 17. Rouse HJ, Small BJ, Schinka JA, Loewenstein DA, Duara R, Potter H. Mild behavioral impairment as a predictor of cognitive functioning in older adults. Int Psychogeriatr. 2021;33(3):285‐293. [DOI] [PubMed] [Google Scholar]
- 18. McGirr A, Nathan S, Ghahremani M, Gill S, Smith E, Ismail Z. Progression to dementia or reversion to normal cognition in mild cognitive impairment as a function of late onset neuropsychiatric symptoms. Neurology. 2022;98(21):e2132‐2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kan CN, Cano J, Zhao X, Ismail Z, Chen CL‐H, Prevalence XuX. Clinical correlates, cognitive trajectories, and dementia risk associated with mild behavioral impairment in Asians. J Clin Psychiatry. 2022;83(3):40123. [DOI] [PubMed] [Google Scholar]
- 20. Ruthirakuhan M, Ismail Z, Herrmann N, Gallagher D, Lanctot KL. Mild behavioral impairment is associated with progression to Alzheimer's disease: a clinicopathological study. Alzheimers Dement. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miao R, Chen HY, Gill S, Naude J, Smith EE, Ismail Z. Plasma β‐amyloid in mild behavioural impairment – neuropsychiatric symptoms on the Alzheimer's continuum. J Geriatr Psychiatry Neurol. 2021:08919887211016068. [DOI] [PubMed] [Google Scholar]
- 22. Naude JP, Gill S, Hu S, et al. Plasma neurofilament light: a marker of neurodegeneration in mild behavioral impairment. J Alzheimers Dis. 2020;76:1017‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miao R, Chen HY, Robert P, Smith EE, Ismail Z. White matter hyperintensities and mild behavioral impairment: findings from the MEMENTO cohort study. Cereb Circ Cogn Behav. 2021;2:100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gill S, Wang M, Mouches P, et al. Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int J Geriatr Psychiatry. 2021;36(9):1398‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guan DX, Chen H‐Y, Camicioli R, Montero‐Odasso M, Smith EE, Ismail Z. Dual‐task gait and mild behavioral impairment: the Interface between non‐cognitive dementia markers. Exp Gerontol. 2022:111743. [DOI] [PubMed] [Google Scholar]
- 26. Gosselin P, Guan DX, Chen HY, et al. The relationship between hearing and mild behavioral impairment and the influence of sex: a study of older adults without dementia from the COMPASS‐ND study. J Alzheimers Dis Rep. 2022;6:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guan D, Rockwood K, Smith E, Ismail Z. Sex moderates the association between frailty and mild behavioral impairment. J Prev Alzheimers Dis. 2022;9(4):692‐700. [DOI] [PubMed] [Google Scholar]
- 28. Montero‐Odasso M, Pieruccini‐Faria F, Ismail Z, et al. CCCDTD5 recommendations on early non cognitive markers of dementia: a Canadian consensus. Alzheimer's & Dementia: Translational Research & Clinical Interventions. 2020;6(1):e12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233‐239. [DOI] [PubMed] [Google Scholar]
- 30. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308‐2308. [DOI] [PubMed] [Google Scholar]
- 31. Cummings J. The Neuropsychiatric Inventory: development and applications. J Geriatr Psychiatry Neurol. 2020;33(2):73‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mallo SC, Ismail Z, Pereiro AX, et al. Assessing mild behavioral impairment with the mild behavioral impairment‐checklist in people with mild cognitive impairment. J Alzheimers Dis. 2018;66:83‐95. [DOI] [PubMed] [Google Scholar]
- 33. Mallo SC, Ismail Z, Pereiro AX, et al. Assessing mild behavioral impairment with the mild behavioral impairment checklist in people with subjective cognitive decline. Int Psychogeriatr. 2019;31(2):231‐239. [DOI] [PubMed] [Google Scholar]
- 34. Beekly DL, Ramos EM, van Belle G, et al. The national Alzheimer's coordinating center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18(4):270‐277. [PubMed] [Google Scholar]
- 35. Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Assoc Disord. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210‐216. [DOI] [PubMed] [Google Scholar]
- 37. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's disease centers’ uniform data set (UDS): the neuropsychological test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543‐2546. [PubMed] [Google Scholar]
- 39. Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261‐304. [Google Scholar]
- 40. R: A Language and Environment for Statistical Computing [computer program]. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 41. Vik‐Mo AO, Giil LM, Ballard C, Aarsland D. Course of neuropsychiatric symptoms in dementia: 5‐year longitudinal study. Int J Geriatr Psychiatry. 2018;33(10):1361‐1369. [DOI] [PubMed] [Google Scholar]
- 42. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183‐194. [DOI] [PubMed] [Google Scholar]
- 43. Young AL, Marinescu RV, Oxtoby NP, et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun. 2018;9(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdelnour C, Agosta F, Bozzali M, et al. Perspectives and challenges in patient stratification in Alzheimer's disease. Alzheimers Res Ther. 2022;14(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Creese B, Griffiths A, Brooker H, et al. Profile of mild behavioral impairment and factor structure of the mild behavioral impairment checklist in cognitively normal older adults. Int Psychogeriatr. 2020;32(6):705‐717. [DOI] [PubMed] [Google Scholar]
- 46. Cui Y, Dai S, Miao Z, et al. Reliability and validity of the Chinese version of the mild behavioral impairment checklist for screening for Alzheimer's disease. J Alzheimers Dis. 2019;70(3):747‐756. [DOI] [PubMed] [Google Scholar]
- 47. Hu S, Patten S, Charlton A, et al. Validating the mild behavioral impairment checklist in a cognitive clinic: comparisons with the Neuropsychiatric Inventory Questionnaire. J Geriatr Psychiatry Neurol. 2022:08919887221093353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prince MJ, Wimo A, Guerchet MM, et al. World Alzheimer Report 2015 – The Global Impact of Dementia: an analysis of prevalence, incidence, cost and trends. Alzheimer's Dis Int. 2015. https://www.alzint.org/resource/world-alzheimer-report-2015/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


