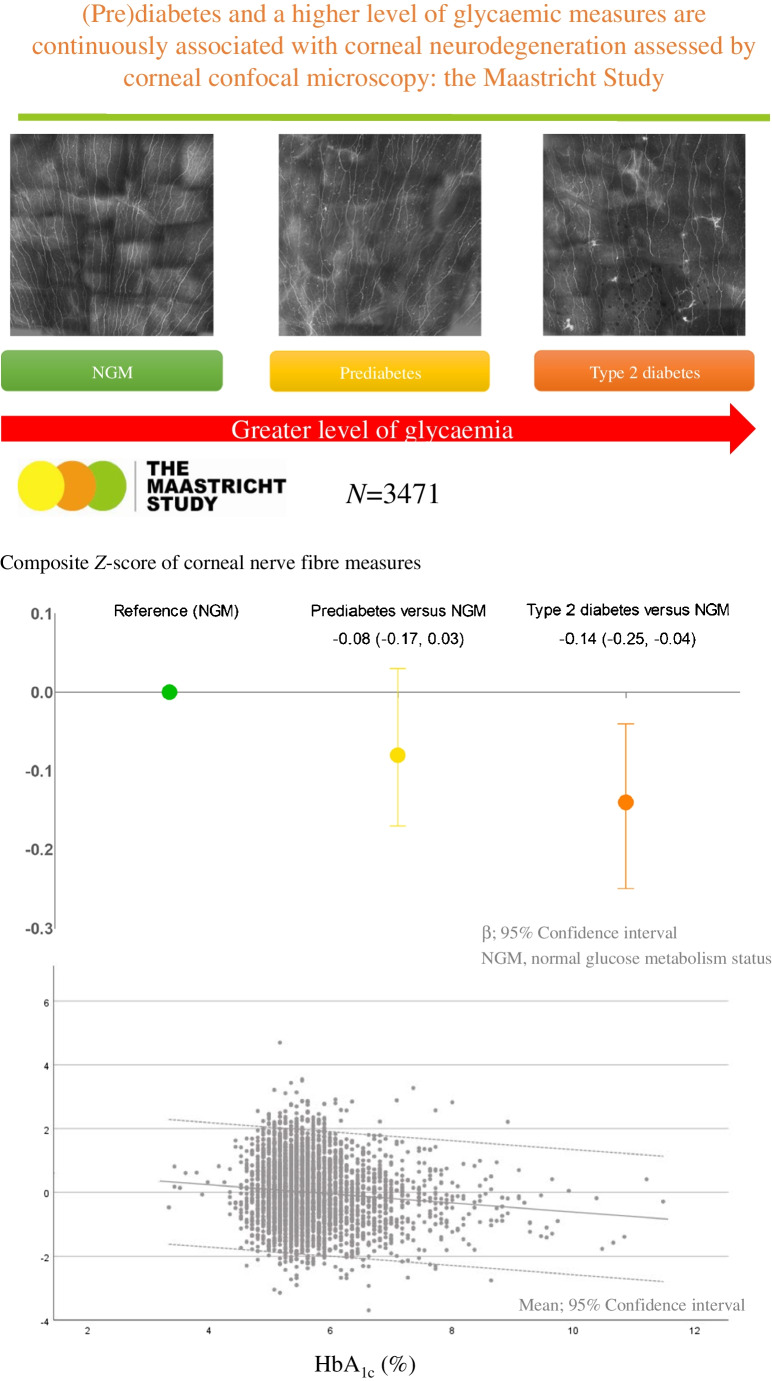
Abstract
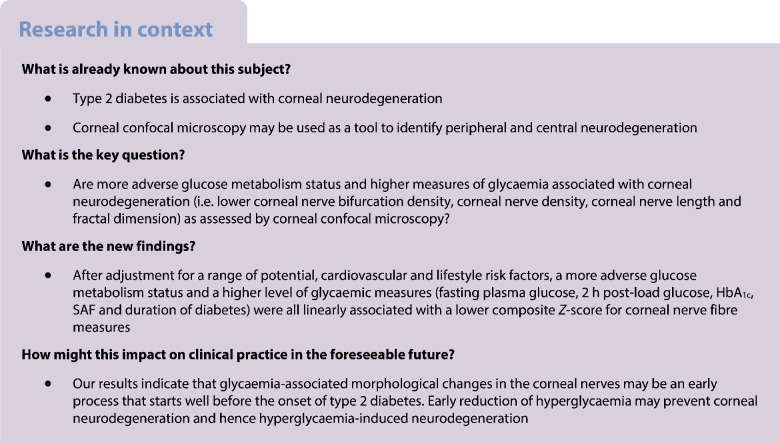
Aims/hypothesis
To assess the associations between glucose metabolism status and a range of continuous measures of glycaemia with corneal nerve fibre measures, as assessed using corneal confocal microscopy.
Methods
We used population-based observational cross-sectional data from the Maastricht Study of N=3471 participants (mean age 59.4 years, 48.4% men, 14.7% with prediabetes, 21.0% with type 2 diabetes) to study the associations, after adjustment for demographic, cardiovascular risk and lifestyle factors, between glucose metabolism status (prediabetes and type 2 diabetes vs normal glucose metabolism) plus measures of glycaemia (fasting plasma glucose, 2 h post-load glucose, HbA1c, skin autofluorescence [SAF] and duration of diabetes) and composite Z-scores of corneal nerve fibre measures or individual corneal nerve fibre measures (corneal nerve bifurcation density, corneal nerve density, corneal nerve length and fractal dimension). We used linear regression analysis, and, for glucose metabolism status, performed a linear trend analysis.
Results
After full adjustment, a more adverse glucose metabolism status was associated with a lower composite Z-score for corneal nerve fibre measures (β coefficients [95% CI], prediabetes vs normal glucose metabolism −0.08 [−0.17, 0.03], type 2 diabetes vs normal glucose metabolism −0.14 [−0.25, −0.04]; linear trend analysis showed a p value of 0.001), and higher levels of measures of glycaemia (fasting plasma glucose, 2 h post-load glucose, HbA1c, SAF and duration of diabetes) were all significantly associated with a lower composite Z-score for corneal nerve fibre measures (per SD: −0.09 [−0.13, −0.05], −0.07 [−0.11, −0.03], −0.08 [−0.11, −0.04], −0.05 [−0.08, −0.01], −0.09 [−0.17, −0.001], respectively). In general, directionally similar associations were observed for individual corneal nerve fibre measures.
Conclusions/interpretation
To our knowledge, this is the first population-based study to show that a more adverse glucose metabolism status and higher levels of glycaemic measures were all linearly associated with corneal neurodegeneration after adjustment for an extensive set of potential confounders. Our results indicate that glycaemia-associated corneal neurodegeneration is a continuous process that starts before the onset of type 2 diabetes. Further research is needed to investigate whether early reduction of hyperglycaemia can prevent corneal neurodegeneration.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00125-023-05986-5.
Keywords: Corneal neurodegeneration, Glycaemic measures, (Pre)diabetes
Introduction
Diabetes can lead to a vast spectrum of systemic complications including central and peripheral neurodegeneration [1, 2]. Techniques that allow early detection and regular follow-up of hyperglycaemia-induced neurodegeneration are thus of major importance.
As postulated in the ‘ticking clock hypothesis’, hyperglycaemia-mediated damage is a continuous (i.e. linear) process that starts before the onset of type 2 diabetes [3, 4]. Indeed, we have previously demonstrated linear associations of adverse glucose metabolism status and higher levels of glycaemic measures with lower retinal nerve fibre layer thickness [5], more brain structural abnormalities [6, 7], worse peripheral nerve function [8] and lower heart rate variability [9], which are all measures of neurodegeneration and/or neural dysfunction. Additionally, chronic hyperglycaemia accelerates the formation of advanced glycation end-products (AGEs), which are considered a major initiator of neurodegeneration [10]. AGEs are protein-bound compounds that exhibit intrinsic fluorescence properties, which can be measured non-invasively as skin autofluorescence (SAF) [11]. We have shown previously that AGEs assessed as SAF are associated with lower retinal nerve fibre layer thickness [5].
Various instruments and techniques are currently available to allow scientists to monitor central and peripheral nervous system activity. However, these methods are often time-consuming and have relatively high costs [12, 13], making them impractical for routine and large-scale use. There thus is a need for a sensitive and practical method that allows detection of early hyperglycaemia-mediated neurodegeneration.
Here we focus on hyperglycaemia as a determinant of morphological changes in small nerve fibres (unmyelinated C-fibres) assessed by corneal confocal microscopy, an in vivo, non-invasive and sensitive measure [14] to assess corneal nerve degeneration. The current literature supports the concept that hyperglycaemia affects corneal nerve fibres [15], and that corneal confocal microscopy may be used as a tool to identify peripheral [15, 16] and central neurodegeneration [16]. Moreover, studies have suggested that alterations in corneal nerves morphology may serve as an early indicator of diabetic neuropathy, offering a potential opportunity for timely diagnosis and treatment [17, 18]. However, these studies had several important limitations. Specifically, previous studies were not population-based, did not adjust for cardiovascular risk and lifestyle factors, and did not investigate whether various measures of glycaemia are linearly associated with measures of corneal nerve fibre damage.
Hence, we investigated, in a large, well-characterised population-based cohort study, whether a more adverse glucose metabolism status and higher measures of glycaemia are associated with neurodegeneration assessed using measures of corneal nerve fibre damage, i.e. lower corneal nerve bifurcation density, corneal nerve density, corneal nerve length and fractal dimension, as measured by corneal confocal microscopy.
Methods
Study population and design
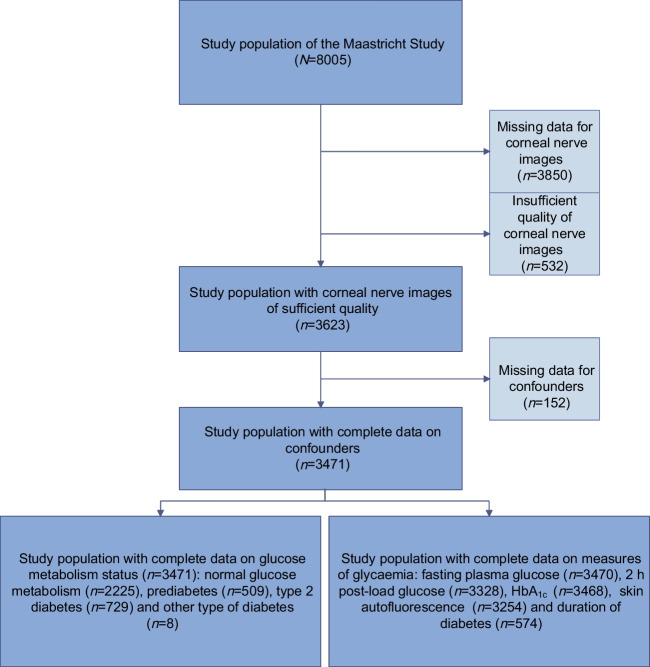
We used data from the Maastricht Study, an observational prospective population-based cohort study enriched with type 2 diabetes individuals. Individuals with type 2 diabetes were over-sampled for efficiency reasons, i.e. to increase the statistical power to identify any potential contrasts between individuals with and without type 2 diabetes. The rationale and methodology have been described previously [19]. In brief, the study focuses on the aetiology, pathophysiology, complications and comorbidities of type 2 diabetes mellitus, and is characterised by an extensive phenotyping approach. Eligible for participation were all individuals aged between 40 and 75 years living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known type 2 diabetes status [19]. The present report includes cross-sectional data for N=3471 participants who completed the baseline survey between November 2010 and December 2017. The examinations for each participant were performed within a time window of 3 months. Corneal confocal microscopy measurements were performed between April 2013 and January 2017. Individuals who participated in the Maastricht Study before the start of corneal confocal microscopy measurement (i.e. before April 2013) were re-invited (n=974) (‘catch-up visit’). For these participants, there was a median time interval (‘visit interval or lag time’) of 5.2 years between corneal confocal microscopy measurements and all other measurements. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (permit 131088–105234-PG). All participants gave written informed consent [19].
Assessment of corneal confocal microscopy measurements (outcome)
We used corneal confocal microscopy using a Heidelberg retina tomograph III (HRT3), Rostock cornea module (Heidelberg Engineering, Germany) to image corneal nerves [14] of the left eye only for logistical reasons. Before the measurement, we anaesthetised both eyes using oxybuprocaine hydrochloride 0.4 mg/ml eye drops (Minims; Bausch & Lomb, France) to eliminate all causes of blinking, and wetted both eyes using carbomer 2 mg/g eye gel (Vidsic Bausch & Lomb) to ensure optimal contact between the cornea and the applanation cap. Further methodological details are provided in the electronic supplementary material (ESM) Methods.
Individuals who had a corneal transplant or had a corneal infection of the left eye were excluded from the measurements. Participants were instructed to fix their vision on a white light throughout the scan. Trained research assistants imaged the sub-basal nerve plexus layer in the central part of the cornea according to a standard operating procedure. We assessed multiple recordings of 400×400 μm (384×384 pixels, 8-bit) assembled using a composite algorithm implemented in the HRT3 user interface (Heidelberg Engineering), as previously described [14]. Real-time mapping was performed on an area up to 1600×1600 µm (1536×1536 pixels, 8-bit) that partially included the inferior whorl in some of the composite images. No measures were taken to include or exclude this region. The advantage of performing large scale corneal confocal microscopy imaging (1600 × 1600 μm) is described in the ESM Methods.
We used the U-net-based convolutional neural network to fully automatically trace the corneal nerves [20] (ESM Fig. 1) and analysed the following indices: corneal nerve bifurcation density (number of bifurcation points [branching points] per mm2), corneal nerve density (total number of corneal nerve fibres [including both main fibres and branches] per mm2, with ‘main nerve fibres’ referring to the largest and most prominent nerve fibres), corneal nerve length (total length of corneal nerve fibres in mm, both main and branches, per mm2) and corneal nerve fractal dimension (quantification of the nerve structure complexity). Composite segmented images were reviewed manually, and images were considered to be usable (n=3623) if 50% or more of the total captured area was of good quality (see ESM Methods for more details). ESM Fig. 2 provides examples of included and excluded images. We also assessed the location of the captured images based on the orientation of the corneal nerves (ESM Fig. 3). The intra-class and inter-observer correlation coefficients, both indices of reliability, were ≥ 0.97 and ≥ 0.89, respectively [21].
Assessment of determinants
Glucose metabolism status
After an overnight fast, participants underwent a standardised seven-point OGTT, as part of which venous samples were collected at 15, 30, 45, 60, 90 and 120 min post-ingestion of a 75 g glucose drink. All participants underwent an OGTT except those who used insulin or had a fasting plasma glucose concentration above 11.0 mmol/l. Based on fasting plasma glucose and 2 h post-load glucose, glucose metabolism status was determined as normal glucose metabolism, prediabetes (defined as impaired fasting glucose, impaired glucose tolerance or both), type 2 diabetes or other types of diabetes (including type 1 diabetes) according to the WHO 2006 criteria [22]
Measures of glycaemia
Fasting plasma glucose (mmol/l) and HbA1c (mmol/mol, %) were determined in venous plasma samples collected after an overnight fast. The value for 2 h post-load glucose (mmol/l) was determined in venous plasma collected at 120 min after ingestion of the glucose drink. The levels of AGEs in the skin were assessed using an AGE Reader (DiagnOptics Technologies, the Netherlands). In brief, the AGE Reader is a desktop device that uses the characteristic fluorescent properties of certain AGEs to quantify their accumulation in the skin as SAF (arbitrary units) [23]. The AGE Reader illuminates a skin surface of 4 cm2, shielded from other light, and uses the ratio of the reflection of fluorescent light (wavelength 420–600 nm) to non-fluorescent light (300–420 nm) to calculate SAF (see ESM Methods for more details). The duration of diabetes in individuals with type 2 diabetes was assessed using a questionnaire.
Covariates
As described previously [19], we assessed educational level (low, intermediate, high), socioeconomic status (income level and occupational status) [24], smoking status (never, former, current), alcohol use (none, low, high), history of ocular disorders (corneal diseases or uveitis), and use of contact lenses by questionnaire, assessed the presence of retinopathy in both eyes via fundus photography (see ESM Methods for more details), assessed dietary habits (‘dietary intake’) using the Dutch Healthy Diet index sum score, which is a measure of adherence to the Dutch dietary guidelines 2015 (see ESM Methods for more details) [25], based on a validated food frequency questionnaire [26], assessed use of lipid-modifying, antihypertensive and glucose-lowering medication as part of a medication interview, assessed weight (kg), height (cm) and waist circumference (cm) during a physical examination, calculated BMI (kg/m2) based on body weight and height, measured office and 24 h ambulatory BP (mmHg), measured total daily physical activity (h/day) using an accelerometer [27], measured lipid profile and plasma biomarkers of low-grade inflammation [28] (i.e. high-sensitive C-reactive protein, serum amyloid A, IL-6, IL-8 and TNF-α) in fasting venous blood samples, measured urinary albumin excretion in two 24 h urine collections, calculated the eGFR based on serum creatinine only, as values for cystatin C were not available for all study participants, and assessed sensory neuropathy as having neuropathic pain using the Dutch version of the DN4 interview [29], via impaired uni- or bilateral vibration perception, measured three times using a Horwell neurothesiometer (Scientific Laboratory Supplies, UK) at the distal phalanx of the hallux of the right and left foot [19], and/or on the basis of use of medication prescribed for neuropathic pain (gabapentin, pregabalin, duloxetine, amitriptyline, nortriptyline or carbamazepine, the latter only in individuals without a diagnosis of epilepsy).
Statistical analyses
Population characteristics and measures of the corneal nerves were described for the total study population and by tertiles of unweighted composite Z-score for corneal nerve fibre measures using the appropriate descriptive statistics (Table 1). Before we calculated the composite Z-score, we checked whether associations of potential determinants with the individual corneal nerve fibre measures were not directionally different, and this was the case. Additional general study population characteristics and general study population characteristics of the included and excluded participants are provided in ESM Table 1 and ESM Table 2. All analyses were performed using Statistical Package for Social Sciences version 25.0 (IBM SPSS, USA). For all analyses, a p value < 0.05 was considered statistically significant.
Table 1.
General study population characteristics according to tertiles of composite Z-score for corneal nerve fibre measures in the study population with complete data on glucose metabolism status
| Composite Z-score for corneal nerve fibre measures | ||||
|---|---|---|---|---|
| Characteristic | Total study population (n=3471) | Tertile 1 (high) (n=1157) | Tertile 2 (middle) (n=1157) | Tertile 3 (low) (n=1157) |
| Age (years) | 59.4±8.7 | 58.4±8.7 | 59.4±8.6 | 60.6±8.6 |
| Men | 1681 (48.4) | 490 (42.4) | 579 (50.0) | 612 (52.9) |
| Educational level | ||||
| High | 1332 (38.4) | 440 (38.0) | 441 (38.1) | 451 (39.0) |
| Medium | 956 (27.5) | 336 (29.0) | 324 (28.0) | 296 (25.6) |
| Low | 1183 (34.1) | 381 (32.9) | 392 (33.9) | 410 (35.4) |
| Glucose metabolism status | ||||
| Normal glucose metabolism | 2225 (64.1) | 799 (69.1) | 742 (64.1) | 684 (59.1) |
| Prediabetes | 509 (14.7) | 164 (14.2) | 165 (14.3) | 180 (15.6) |
| Type 2 diabetes | 729 (21.0) | 193 (16.7) | 246 (21.3) | 290 (25.1) |
| Other type of diabetes | 8 (0.2) | 1 (0.1) | 4 (0.3) | 3 (0.3) |
| Measures of glycaemia | ||||
| Fasting plasma glucose (mmol/l) | 5.7±1.4 | 5.5±1.1 | 5.8±1.5 | 5.9±1.5 |
| 2 h post-load glucose (mmol/l) | 6.0 (4.9–8.3) | 5.9 (4.8–7.9) | 6.0 (4.9–8.3) | 6.3 (5.0–9.0) |
| HbA1c (mmol/mol) | 38.6±8.4 | 37.4±6.8 | 38.8±9.0 | 39.5±9.1 |
| HbA1c (%) | 5.7±0.8 | 5.6±0.6 | 5.7±0.8 | 5.8±0.8 |
| SAF (AU) | 2.1±0.5 | 2.1±0.4 | 2.1±0.5 | 2.2±0.5 |
| Duration of type 2 diabetes (years) | 3.0 (0.0–8.3) | 2.0 (0.0–7.0) | 3.0 (0.0–9.0) | 4.0 (0.3–9.0) |
| Waist circumference (cm) | 94.3±13.2 | 92.8±12.6 | 94.8±13.2 | 95.4±13.2 |
| Total to HDL-cholesterol ratio | 3.4 (2.8–4.2) | 3.3 (2.7–4.2) | 3.4 (2.8–4.2) | 3.4 (2.8–4.2) |
| Use of lipid-modifying medication | 1009 (29.1) | 312 (27.0) | 327 (28.3) | 370 (32.0) |
| Office systolic BP (mmHg) | 132.8±17.6 | 130.8±16.9 | 133.4±17.7 | 134.2±17.9 |
| Office diastolic BP (mmHg) | 75.5±9.9 | 74.9±9.7 | 75.3±9.9 | 75.6±10.0 |
| Use of antihypertensive medication | 1220 (35.1) | 372 (32.2) | 403 (34.8) | 445 (38.5) |
| Smoking status | ||||
| Never | 1341 (38.6) | 474 (41.0) | 472 (40.8) | 395 (34.1) |
| Former | 1711 (49.3) | 540 (46.7) | 566 (48.9) | 605 (52.3) |
| Current | 419 (12.1) | 143 (12.4) | 119 (10.3) | 157 (13.6) |
| Alcohol consumption | ||||
| None | 632 (18.2) | 219 (18.9) | 203 (17.5) | 210 (18.2) |
| Low | 2077 (59.8) | 697 (60.2) | 698 (60.3) | 682 (58.9) |
| High | 762 (22.0) | 241 (20.8) | 256 (22.1) | 265 (22.9) |
| Sensory neuropathya, yes vs. no | 520 (15.1) | 142 (12.4) | 184 (16.1) | 194 (16.9) |
| Neuropathic painb | 308 (8.9) | 94 (8.2) | 108 (9.4) | 106 (9.2) |
| Impaired unilateral vibration perception | 244 (7.0) | 51 (4.4) | 89 (7.7) | 104 (9.0) |
| Impaired bilateral vibration perceptionc | 100 (3.1) | 18 (1.6) | 41 (3.8) | 41 (3.9) |
| Mean neurothesiometer outcome (V)d | 10 (6.8–15.6) | 9.4 (6.4–14.5) | 10.0 (7.0–15.7) | 10.8 (7.2–17.3) |
| No corneal confocal microscopy scan at baseline | 974 (28.1) | 274 (23.7) | 293 (25.3) | 407 (35.2) |
| Corneal confocal microscopy interval (years) | 5.2 (4.9–5.8) | 5.1 (4.8–5.5) | 5.1 (4.8–5.8) | 5.2 (5.0–5.8) |
| Corneal nerve bifurcation density | 73.9±39.9 | 118.1±31.4 | 67.4±13.9 | 36.3±13.9 |
| Corneal nerve density | 79.6±24.3 | 105.0±17.1 | 77.7±10.7 | 56.0±12.6 |
| Corneal nerve length | 14.9±4.4 | 19.6±2.5 | 14.8±1.6 | 10.3±2.3 |
| Corneal nerve fractal dimension | 1.3±0.1 | 1.4±0.0 | 1.4±0.1 | 1.2±0.1 |
Data are presented as means ± SD, median (IQR) or n (%)
aSensory neuropathy was defined as having neuropathic pain, impaired uni- or bilateral vibration perception, and/or use of medication prescribed for neuropathic pain (gabapentin, pregabalin, duloxetine, amitriptyline, nortriptyline or carbamazepine, the latter only in individuals without a diagnosis of epilepsy). In the analysis of sensory neuropathy, it is important to note that there were missing data. The sample size for total study population n=3439, for each tertile is as follows: Tertile 1 (high) n=1146, Tertile 2 (middle) n=1146 and Tertile 3 (low) n=1147
bIn the analysis of neuropathic pain, it is important to note that there were missing data. The sample size for total study population n=3450, for each tertile is as follows: Tertile 1 (high) n=1149, Tertile 2 (middle) n=1151 and Tertile 3 (low) n=1150
cIn the analysis of impaired bilateral vibration perception it is important to note that there were missing data. The sample size for total study population n=3256, for each tertile is as follows: Tertile 1 (high) n=1108, Tertile 2 (middle) n=1092 and Tertile 3 (low) n=1056
dOn right and left first toe
AU, arbitrary units
Main analyses
We used multivariable linear regression analyses to investigate the associations of determinants (i.e. glucose metabolism status [entered as dummy variables for prediabetes, type 2 diabetes, or other types of diabetes vs normal glucose metabolism] and measures of glycaemia [standardised fasting plasma glucose, 2 h post-load glucose, HbA1c, SAF and duration of diabetes]) with outcomes (i.e. composite Z-score for corneal nerve fibre measures [main outcome] and standardised corneal nerve fibre measures [i.e. corneal nerve bifurcation density, corneal nerve density, corneal nerve length and corneal nerve fractal dimension]).
We performed an analysis of the linear trend for the association with glucose metabolism status as a determinant. To estimate the linear trend, we entered glucose metabolism status into the model as an ordinal variable (i.e. glucose metabolism status was coded as normal glucose metabolism = 0, prediabetes = 1, type 2 diabetes = 2). In a p for trend analysis, we excluded participants with other types of diabetes (such as type 1 diabetes) because other types of diabetes do not constitute part of the spectrum of deterioration of glucose metabolism status from normal glucose metabolism to prediabetes and type 2 diabetes. Then, we checked whether we could assume a linear trend by comparing the statistical variance explained by the statistical model in which glucose metabolism status was entered as dummy variables to that obtained using the statistical model in which glucose metabolism status was entered as an ordinal variable. We used a likelihood ratio test to assess whether the difference in the amount of variance explained by both models was statistically significant. A p value > 0.05 indicates that both models are not different, and thus a linear trend can be assumed.
We used several sets of adjustments. Model 1 shows crude results adjusted for corneal confocal microscopy lag time. Model 2, in addition to the adjustment in model 1, was adjusted for age, sex and educational status (high, medium, low). We chose these variables because they are key potential confounders. Model 3, in addition to the adjustments in model 2, was adjusted for office systolic BP, use of antihypertensive medication (yes/no), waist circumference, total cholesterol to HDL-cholesterol ratio, use of lipid-modifying medication (yes/no), smoking status (never, former, current) and alcohol consumption status (none, low [for women, ≤7 alcohol units per day; for men, ≤14 alcohol units per day], high [for women, >7 alcohol units per day; for men, >14 alcohol units per day]). Biologically, these risk factors may be associated with hyperglycaemia and corneal neurodegeneration. All associations are expressed as standardised regression coefficients and the corresponding 95% CI. To facilitate interpretation, we show unstandardised regression coefficients (β) in ESM Table 3. The associations between glucose metabolism status and measures of glycaemia with corneal nerve fractal dimension had long strings of digits, near to zero, that we multiplied by 100 for convenient expression (in ESM Table 3).
We also analysed potential interaction with sex by adding an interaction term (i.e. potential determinant × sex) [30] to model 3 (ESM Table 4).
Additional analyses
To ensure the robustness of our findings, we performed several additional analyses as described in ESM Methods.
Results
Selection and characteristics of the study population
Figure 1 shows an overview of selection of the study population.
Fig. 1.
Selection of participants for inclusion in analyses
Table 1 and ESM Table 1 show the general participant characteristics according to tertiles of composite Z-score for corneal nerve fibre measures. Overall, participants with a lower composite Z-score for corneal nerve fibre measures were older and had a more adverse cardiovascular risk profile. The general characteristics of participants included in the study were comparable to those with missing data (ESM Table 2).
Glucose metabolism status and corneal nerve fibre measures
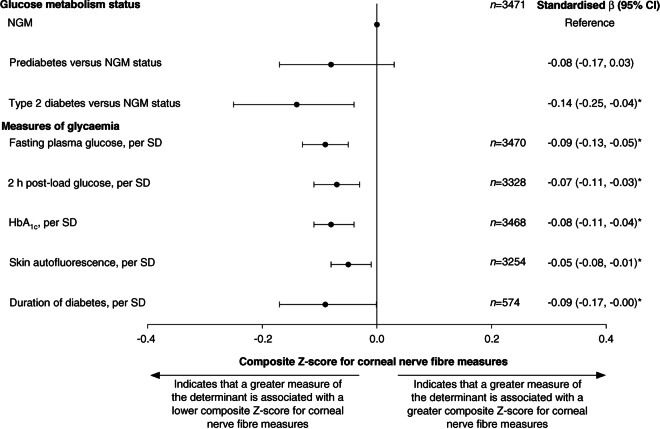
According to model 3, a more adverse glucose metabolism status was associated with a lower composite Z-score for corneal nerve fibre measures (β [95% CI]: type 2 diabetes vs normal glucose metabolism −0.14 [−0.25, −0.04], prediabetes vs normal glucose metabolism −0.08 [−0.17, 0.03]; linear trend analysis showed a p value of 0.001) (Table 2 and Fig. 2). In general, directionally similar associations were observed for individual corneal nerve fibre measures (Table 2).
Table 2.
Associations of glucose metabolism status and measures of glycaemia with corneal nerve fibre measures
| Determinant | Number of participants | Model | Composite Z-score for corneal nerve fibre measures | Corneal nerve bifurcation density | Corneal nerve density | Corneal nerve length | Corneal nerve fractal dimension |
|---|---|---|---|---|---|---|---|
| Glucose metabolism status | 3471 | ||||||
| Prediabetes vs normal glucose metabolism | 1 | −0.13 (−0.22, −0.03)* | −0.09 (−0.19, 0.01) | −0.12 (−0.22, −0.03)* | −0.13 (−0.23, −0.04)* | −0.12 (−0.21, −0.02)* | |
| 2 | −0.08 (−0.17, 0.02) | −0.06 (−0.15, 0.04) | −0.08 (−0.18, 0.02) | −0.07 (−0.17, 0.02) | −0.07 (−0.17, 0.03) | ||
| 3 | −0.08 (−0.17, 0.03) | −0.07 (−0.17, 0.03) | −0.09 (−0.19, 0.01) | −0.07 (−0.17, 0.03) | −0.05 (−0.15, 0.05) | ||
| Type 2 diabetes vs normal glucose metabolism | 1 | −0.21 (−0.30, −0.13)* | −0.13 (−0.21, −0.05)* | −0.16 (−0.25, −0.08)* | −0.22 (−0.30, −0.13)* | −0.28 (−0.36, −0.19)* | |
| 2 | −0.14 (−0.23, −0.05)* | −0.08 (−0.16, 0.01) | −0.10 (−0.18, −0.01)* | −0.13 (−0.22, −0.05)* | −0.21 (−0.30, −0.12)* | ||
| 3 | −0.14 (−0.25, −0.04)* | −0.11 (−0.21, −0.001)* | −0.13 (−0.24, −0.03)* | −0.12 (−0.23, −0.02)* | −0.17 (−0.27, −0.06)* | ||
| Measures of glycaemia | |||||||
| Fasting plasma glucose, per SD | 3470 | 1 | −0.11 (−0.14, −0.08)* | −0.08 (−0.12, −0.05)* | −0.09 (−0.12, −0.05)* | −0.11 (−0.14, −0.08)* | −0.12 (−0.16, −0.09)* |
| 2 | −0.08 (−0.12, −0.05)* | −0.06 (−0.10, −0.03)* | −0.06 (−0.10, −0.03)* | −0.08 (−0.11, −0.04)* | −0.10 (−0.13, −0.06)* | ||
| 3 | −0.09 (−0.13, −0.05)* | −0.08 (−0.12, −0.04)* | −0.08 (−0.12, −0.04)* | −0.08 (−0.12, −0.04)* | −0.08 (−0.12, −0.05)* | ||
| 2 h post-load glucose, per SD | 3328 | 1 | −0.09 (−0.12, −0.06)* | −0.06 (−0.09, −0.03)* | −0.07 (−0.10, −0.04)* | −0.09 (−0.13, −0.06)* | −0.11 (−0.14, −0.07)* |
| 2 | −0.06 (−0.10, −0.03)* | −0.04 (−0.08, −0.01)* | −0.05 (−0.08, −0.01)* | −0.06 (−0.10, −0.03)* | −0.08 (−0.12, −0.05)* | ||
| 3 | −0.07 (−0.11, −0.03)* | −0.06 (−0.10, −0.02)* | −0.07 (−0.11, −0.03)* | −0.07 (−0.11, −0.03)* | −0.07 (−0.11, −0.03)* | ||
| HbA1c, per SD | 3468 | 1 | −0.10 (−0.13, −0.07)* | −0.06 (−0.09, −0.03)* | −0.07 (−0.10, −0.03)* | −0.10 (−0.13, −0.07)* | −0.12 (−0.16, −0.09)* |
| 2 | −0.07 (−0.11, −0.04)* | −0.04 (−0.08, −0.01)* | −0.05 (−0.08, −0.01)* | −0.07 (−0.11, −0.04)* | −0.10 (−0.14, −0.07)* | ||
| 3 | −0.08 (−0.11, −0.04)* | −0.06 (−0.10, −0.02)* | −0.06 (−0.10, −0.02)* | −0.07 (−0.11, −0.03)* | −0.09 (−0.13, −0.05)* | ||
| SAF, per SD | 3254 | 1 | −0.09 (−0.12, −0.05)* | −0.06 (−0.09, −0.02)* | −0.08 (−0.11, −0.04)* | −0.09 (−0.13, −0.06)* | −0.10 (−0.13, −0.06)* |
| 2 | −0.05 (−0.09, −0.01)* | −0.03 (−0.07, 0.01) | −0.05 (−0.09, −0.01)* | −0.05 (−0.09, −0.01)* | −0.06 (−0.10, −0.02)* | ||
| 3 | −0.05 (−0.08, −0.01)* | −0.03 (−0.07, 0.01) | −0.05 (−0.09, −0.01)* | −0.04 (−0.08, −0.001)* | −0.05 (−0.09, −0.01)* | ||
| Duration of diabetes, per SD | 574 | 1 | −0.09 (−0.17, −0.01)* | −0.09 (−0.17, −0.01)* | −0.09 (−0.17, −0.01)* | −0.10 (−0.18, −0.02)* | −0.06 (−0.14, 0.03) |
| 2 | −0.08 (−0.16, −0.001)* | −0.09 (−0.17, −0.001)* | −0.09 (−0.17, −0.01)* | −0.09 (−0.17, −0.01)* | −0.05 (−0.13, 0.04) | ||
| 3 | −0.09 (−0.17, −0.001)* | −0.10 (−0.18, −0.01)* | −0.09 (−0.18, −0.01)* | −0.09 (−0.18, −0.01)* | −0.04 (−0.13, 0.04) |
Values are standardised regression coefficients (95% CI) representing the differences in corneal nerve fibre measures in SD for prediabetes or type 2 diabetes vs normal glucose metabolism or per SD greater for measures of glycaemia
For the glucose metabolism status, fasting plasma glucose, 2 h post-load glucose, HbA1c, SAF and duration of diabetes (type 2 diabetes only) study populations, 1 SD corresponds to 0.9 (unit-less) for the composite Z-score for corneal nerve fibre measures, 39.9 branches/mm2 for corneal nerve bifurcation density, 24.3 main and branches/mm2 for corneal nerve density, 4.4 mm/mm2 for corneal nerve length, 0.1 (unit-less) for corneal nerve fractal dimension, 1.4 mmol/l for fasting plasma glucose, 3.9 mmol/l for 2 h post-load glucose, 0.8% or 8.4 mmol/mol for HbA1c, 0.5 arbitrary units for SAF and 7.4 years for the duration of diabetes
Variables entered into models: model 1, adjusted only for corneal confocal microscopy visit interval; model 2, model 1 + age, sex and educational status (high, medium, low); model 3, model 2 + office systolic BP, total cholesterol to HDL-cholesterol ratio, use of antihypertensive and/or lipid-modifying medication (yes/no), waist circumference, smoking status (never, former, current) and alcohol consumption status (none, low, high)
Asterisks indicate values that are statistically significant (p<0.05)
Fig. 2.
Associations between glucose metabolism status and measures of glycaemia with the composite Z-score for corneal nerve fibre measures. Standardised regression coefficients (β) represent the differences in corneal nerve fibre measures in SD, for prediabetes or type 2 diabetes vs NGM, or per SD greater for measures of glycaemia. For the glucose metabolism status, fasting plasma glucose, 2 h post-load glucose, HbA1c, SAF and duration of diabetes (for type 2 diabetes only) study populations, 1 SD corresponds to 0.9 (unit-less) for the composite Z-score for corneal nerve fibre measures, 1.4 mmol/l for fasting plasma glucose, 3.9 mmol/l for 2 h post-load glucose, 0.8% or 8.4 mmol/mol for HbA1c, 0.5 arbitrary units for SAF and 7.4 years for duration of diabetes. Variables entered into the models in addition to glucose metabolism status or measures of glycaemia were corneal confocal microscopy visit interval, age, sex educational status (high, medium, low), office systolic BP, total cholesterol to HDL-cholesterol ratio, use of antihypertensive or lipid-modifying medication (yes/no), waist circumference, smoking status (never, former, current) and alcohol consumption status (none, low, high). Asterisks indicate values that are statistically significant (p<0.05). NGM, Normal glucose metabolism
Measures of glycaemia and corneal nerve fibre measures
According to model 3, higher levels of measures of glycaemia (fasting plasma glucose, 2 h post-load glucose, HbA1c, SAF and duration of diabetes) were all significantly associated with a lower composite Z-score for corneal nerve fibre measures (per SD: −0.09 [−0.13, −0.05], −0.07 [−0.11, −0.03], −0.08 [−0.11, −0.04], −0.05 [−0.08, −0.01], −0.09 [−0.17, −0.001], respectively) (Table 2 and Fig. 2). In general, directionally similar associations were observed for individual corneal nerve fibre measures (Table 2).
Interaction analyses
Sex modified the association of prediabetes vs normal glucose metabolism and composite Z-score for corneal nerve fibre measures (p value for the interaction term prediabetes × sex = 0.01). In women, but not in men, we found a significant association of prediabetes vs normal glucose metabolism with the composite Z-score for corneal nerve fibre measures (−0.18 [−0.33, −0.04] in women and 0.04 [−0.10, 0.19] in men). Stratified analyses for all associations are shown in ESM Table 4. However, the sex interaction stratified analyses did not consistently demonstrate significant sex differences in the associations between (pre)diabetes, measures of glycaemia and corneal nerve measures.
Additional analyses
Quantitatively similar results were observed for a range of additional analyses (ESM Results and ESM Tables 3–13).
Discussion
The present population-based observational study had the following main findings. First, after adjustment for a range of potential confounders, a more adverse glucose metabolism status and higher levels of glycaemic measures (fasting plasma glucose, 2 h post-load glucose, HbA1c, SAF and duration of diabetes) were all linearly associated with a lower composite Z-score for corneal nerve fibre measures. Second, associations were linear across glucose metabolism categories, implying that glycaemia-associated corneal nerve damage starts well before the onset of type 2 diabetes. Third, to put our findings into perspective, the β coefficients for prediabetes and type 2 diabetes correspond to 10 and 17 years of additional ageing, respectively (ESM Table 13).
To our knowledge, our study is the first large population-based study that investigated glycaemia-associated corneal nerve damage across glucose metabolism categories with adjustment for an extensive set of potential confounders. Our results are in line with observations from previous research [21, 31–40]. Novel associations that this study reports are the associations of 2 h post-load glucose and SAF with corneal nerve indices. In addition, the present study is, to our knowledge, the first to demonstrate that fasting plasma glucose, 2 h post-load glucose, SAF and duration of diabetes are all linearly associated with corneal nerve indices.
Our findings imply that the process of glycaemia-induced corneal neurodegeneration starts well before the onset of type 2 diabetes. Thus, these findings are in line with the ‘ticking clock hypothesis’ [5–9], which states that glycaemia-induced microvascular and neuronal deterioration is a continuous process that starts long before the onset of type 2 diabetes and gradually worsens during prediabetes and early and advanced type 2 diabetes [4]. Our results show a gradual decrease in the composite Z-score for corneal neurodegeneration from the reference group (with normal glucose metabolism) to prediabetes and towards type 2 diabetes, which is directionally comparable to previous findings on retinal nerve fibre layer thickness [5], brain structural abnormalities [6, 7], peripheral nerve function [8] and heart rate variability [9], which are all measures of neurodegeneration and/or neural dysfunction.
Additionally, our findings suggest that AGEs may be implicated in the pathobiology of corneal neurodegeneration. Indeed, we demonstrated that SAF, which reflects the accumulation of AGEs in the skin, was significantly associated with a lower composite Z-score for corneal nerve fibre measures even after adjustment for fasting plasma glucose, 2 h post-load glucose or HbA1c (ESM Table 11).
We used a composite score (i.e. composite Z-score for corneal nerve fibre measures) to determine corneal neurodegeneration. The advantage of this approach is that the influence of the technical or biological variability is reduced [41] provided that the associations between the key exposure (here glucose) and all elements of the Z-score are directionally similar, which is the case here: when we investigated the associations between glucose metabolism status and measures of glycaemia with the corneal nerve fibre measures individually (corneal nerve bifurcation density, corneal nerve density, corneal nerve length and fractal dimension), we found directionally similar associations (Table 2).
Note that the corneal nerve measures in our study population differ from those in other studies [42], possibly because (1) we assessed the corneal nerve variables in larger images, with an area up to 1600×1600 µm, and therefore partially including the inferior whorl in some of the composite images, which is characterised by a different architecture, distribution and density of the corneal nerve fibres [43], (2) we used a different deep learning model (U-Net-based convolutional neural network) to fully automatically trace and analyse the indices of corneal nerves [44], and (3) the corneal nerve density and length were also defined differently from other studies (total number and length of corneal nerves, both main and branches, per mm2). However, these outcomes were assessed in all sub-groups of participants and therefore the differences do not detract from the consistency of our findings.
Although the association between prediabetes and a lower composite Z-score for corneal nerve measures, in model 3, was not significant when compared with normal glucose metabolism, this is probably due to insufficient statistical power. Therefore, we tested the linear trend with glucose metabolism status deterioration, using the statistically more powerful p value to estimate the linear trend, and observed that a more adverse glucose metabolism status was consistently associated with a linear decrease in the composite Z-score for corneal nerve fibre measures. These associations are consistent with our findings that showed linear associations of continuous glycaemia measures with corneal nerve fibres (Table 2).
Our findings may have implications for clinical practice. First, early glycaemic control, before the onset of type 2 diabetes, is likely to be important in prevention of early neurodegeneration. Second, because corneal neurodegeneration may be a biomarker for diabetic neuropathy [45], detection of early stages of neuropathy is relatively inexpensive and easy to perform.
Our study had several strengths. First, it is, to our knowledge, the first large population-based observational study with oversampling of individuals with type 2 diabetes that studied the associations between (pre)diabetes and measures of glycaemia with corneal neurodegeneration. Second, we adjusted for a large series of potential confounders. Finally, additional analyses generally yielded consistent findings.
We suggest that the composite Z-score for corneal nerve measures may be a potential predictor for neuropathy, independent of glucose metabolism status. Our results showed numerically similar correlation coefficients for individuals in all glucose metabolism strata, although the smaller sample size of individual groups led to lower statistical power than for the main study population. In addition, we performed an additional analysis observing the associations of prediabetes and type 2 diabetes vs normal glucose metabolism with the composite Z-score for corneal nerve fibre measures after exclusion of participants with sensory neuropathy. After adjustment (model 3), a more adverse glucose metabolism status was associated with a lower composite Z-score for corneal nerve fibre measures (β [95% CI]: type 2 diabetes vs normal glucose metabolism −0.16 [−0.27, −0.04], prediabetes vs normal glucose metabolism −0.08 [−0.19, 0.03]) (data not shown). These associations suggest that corneal neurodegeneration starts before the development of clinical sensory neuropathy.
Our study also had limitations. First, because of the observational cross-sectional nature of the study, any causal inferences should be made with caution. Nevertheless, reverse causation appears unlikely, and we adjusted for many potential confounders. Second, underestimation of the association of prediabetes vs normal glucose metabolism with composite Z-score for corneal nerve fibre measures may have occurred due to misclassification of individuals with prediabetes, as the classification was based on one OGTT only. Third, we may have underestimated associations due to range restriction for analyses involving 2 h post-load glucose, as individuals who used insulin or had a fasting plasma glucose concentration above 11.0 mmol/l did not undergo an OGTT. Fourth, even though we considered an extensive number of confounders, we cannot fully exclude bias due to unmeasured confounding, for example by environmental factors such as air pollution. Fifth, because corneal confocal microscopy measurements started from April 2013, individuals who participated in the Maastricht Study before the start of corneal confocal microscopy measurement (i.e. before April 2013; n=974; 28.1%) had a median visit interval of 5.2 years (IQR 4.9–5.8). However, to provide a more appropriate estimate of the true association, we adjusted for visit interval in model 1 for all associations. We also analysed potential interaction with visit interval. We added an interaction term (i.e. fasting plasma glucose × visit interval) [30] to model 3 (data not shown). The interval did not modify the association between fasting plasma glucose and composite Z-score for corneal nerve fibre measures (p value of the interaction term fasting plasma glucose × visit interval = 0.16). We also performed additional analyses in which we excluded participants with a catch-up visit to assess the association between fasting plasma glucose and Z-score for corneal nerve fibre measures. Similar results were observed (data not shown). Finally, this study comprised individuals of European descent aged between 40 and 75 years old with access to high-quality diabetes care. Therefore, further study is required to determine whether our results may be generalised to other populations.
Conclusions
In summary, the present population-based study demonstrated that a more adverse glucose metabolism status and a higher level of glycaemic measures are continuously associated with lower composite Z-score for corneal nerve fibre measures. These associations were independent of demographics, cardiovascular or lifestyle risk factors. Hence, our results indicate that glycaemia-associated morphological changes in the corneal nerves may be an early process that starts well before the onset of type 2 diabetes. Whether early intervention to reduce hyperglycaemia can prevent corneal neurodegeneration requires further study.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AGE
Advanced glycation end-product
- SAF
Skin autofluorescence
Acknowledgements
The authors would like to acknowledge the ZIO Foundation (Vereniging Regionale HuisartsenZorg Heuvelland) for their contribution to the Maastricht Study. The researchers are indebted to the participants for their willingness to participate in the study. Some of the data were presented as an abstract at the 58th EASD Annual Meeting in 2022.
Data availability
Data from the Maastricht Study are available to any researcher who meets the criteria for access to confidential data. The corresponding author may be contacted to request access.
Funding
This study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31O.041), Stichting de Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), the Cardiovascular Center (Maastricht, the Netherlands), the School for Cardiovascular Diseases (Maastricht, the Netherlands), the School of Mental Health and Neuroscience (Maastricht, the Netherlands), the School for Public Health and Primary Care (Maastricht, the Netherlands), the School for Nutrition and Translational Research in Metabolism (Maastricht, the Netherlands), Stichting Annadal (Maastricht, the Netherlands), Health Foundation Limburg (Maastricht, the Netherlands), Perimed (Järfälla, Sweden) and unrestricted grants from Janssen-Cilag (Tilburg, the Netherlands), Novo Nordisk (Alphen aan den Rijn, the Netherlands) and Sanofi-Aventis(Gouda, the Netherlands).
Authors’ relationships and activities
BEdG is an Associate Editor for Diabetologia; however, he was not involved in the handling of this paper during the editorial process. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
SBAM, FCTvdH, CDAS, CABW, CJHvdK and TTJMB contributed to the conception and design of this study, participated in acquisition of data, analysed and interpreted data, drafted the manuscript, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published. SBAM is also the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. KAMO, FS, AC, BEdG, MMJvG, PCD, CGS, RMMAN, NCS, AAK and MTS contributed to the conception and design of the study, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sara B. A. Mokhtar and Frank C. T. van der Heide contributed equally to this study.
References
- 1.Umegaki H. Neurodegeneration in diabetes mellitus. Adv Exp Med Biol. 2012;724:258–265. doi: 10.1007/978-1-4614-0653-2_19. [DOI] [PubMed] [Google Scholar]
- 2.Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? Jama. 1990;263(21):2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 4.Wong MS, Gu K, Heng D, Chew SK, Chew LS, Tai ES. The Singapore Impaired Glucose Tolerance Follow-up Study: does the ticking clock go backward as well as forward? Diabetes Care. 2003;26(11):3024–3030. doi: 10.2337/diacare.26.11.3024. [DOI] [PubMed] [Google Scholar]
- 5.van der Heide FCT, Foreman YD, Franken IWM, et al. (Pre)diabetes, glycemia, and daily glucose variability are associated with retinal nerve fiber layer thickness in the Maastricht Study. Sci Rep. 2022;12(1):17750. doi: 10.1038/s41598-022-22748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Agtmaal MJM, Houben A, de Wit V, et al. Prediabetes is associated with structural brain abnormalities: the Maastricht Study. Diabetes Care. 2018;41(12):2535–2543. doi: 10.2337/dc18-1132. [DOI] [PubMed] [Google Scholar]
- 7.Vergoossen LW, Schram MT, de Jong JJ, et al. White matter connectivity abnormalities in prediabetes and type 2 diabetes: the Maastricht Study. Diabetes Care. 2020;43(1):201–208. doi: 10.2337/dc19-0762. [DOI] [PubMed] [Google Scholar]
- 8.van der Velde J, Koster A, Strotmeyer ES, et al. Cardiometabolic risk factors as determinants of peripheral nerve function: the Maastricht Study. Diabetologia. 2020;63(8):1648–1658. doi: 10.1007/s00125-020-05194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coopmans C, Zhou TL, Henry RMA, et al. Both prediabetes and type 2 diabetes are associated with lower heart rate variability: the Maastricht Study. Diabetes Care. 2020;43(5):1126–1133. doi: 10.2337/dc19-2367. [DOI] [PubMed] [Google Scholar]
- 10.Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1037–1045. doi: 10.1016/j.bbadis.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Moura Semedo C, Balu Webb M, Waller H, Khunti K, Davies M. Skin autofluorescence, a non-invasive marker of advanced glycation end products: clinical relevance and limitations. Postgrad Med J. 2017;93(1099):289. doi: 10.1136/postgradmedj-2016-134579. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt G, Dinter D, Reiser MF, Schoenberg SO. The uses and limitations of whole-body magnetic resonance imaging. Dtsch Arztebl Int. 2010;107(22):383–389. doi: 10.3238/arztebl.2010.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petropoulos IN, Ponirakis G, Khan A, Almuhannadi H, Gad H, Malik RA. Diagnosing diabetic neuropathy: something old, something new. Diabetes Metab J. 2018;42(4):255–269. doi: 10.4093/dmj.2018.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allgeier S, Zhivov A, Eberle F, et al. Image reconstruction of the subbasal nerve plexus with in vivo confocal microscopy. Investig Ophthalmol Vis Sci. 2011;52(9):5022–5028. doi: 10.1167/iovs.10-6065. [DOI] [PubMed] [Google Scholar]
- 15.Cosmo E, Midena G, Frizziero L, Bruno M, Cecere M, Midena E (2022) Corneal confocal microscopy as a quantitative imaging biomarker of diabetic peripheral neuropathy: a review. J Clin Med 11(17). 10.3390/jcm11175130 [DOI] [PMC free article] [PubMed]
- 16.Malik RA, Efron N (2022) Corneal confocal microscopy and the nervous system: introduction to the special issue. J Clin Med 11(6). 10.3390/jcm11061475 [DOI] [PMC free article] [PubMed]
- 17.Zhou T, Lee A, Lo ACY, Kwok JSWJ (2022) Diabetic corneal neuropathy: pathogenic mechanisms and therapeutic strategies. Front Pharmacol 13. 10.3389/fphar.2022.816062 [DOI] [PMC free article] [PubMed]
- 18.So WZ, Qi Wong NS, Tan HC, et al. Diabetic corneal neuropathy as a surrogate marker for diabetic peripheral neuropathy. Neural Regen Res. 2022;17(10):2172–2178. doi: 10.4103/1673-5374.327364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schram MT, Sep SJ, van der Kallen CJ, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29(6):439–451. doi: 10.1007/s10654-014-9889-0. [DOI] [PubMed] [Google Scholar]
- 20.Scarpa F, Colonna A, Ruggeri A. Multiple-image deep learning analysis for neuropathy detection in corneal nerve images. Cornea. 2020;39(3):342–347. doi: 10.1097/ICO.0000000000002181. [DOI] [PubMed] [Google Scholar]
- 21.De Clerck EEB, Schouten J, Berendschot T, et al. Reduced corneal nerve fibre length in prediabetes and type 2 diabetes: the Maastricht Study. Acta Ophthalmol. 2020;98(5):485–491. doi: 10.1111/aos.14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.van Eupen MG, Schram MT, van Sloten TT, et al. Skin autofluorescence and pentosidine are associated with aortic stiffening: the Maastricht Study. Hypertension. 2016;68(4):956–963. doi: 10.1161/hypertensionaha.116.07446. [DOI] [PubMed] [Google Scholar]
- 24.Qi Y, Koster A, van Boxtel M et al (2019) Adulthood socioeconomic position and type 2 diabetes mellitus-a comparison of education, occupation, income, and material deprivation: the Maastricht Study. Int J Environ Res Public Health 16(8). 10.3390/ijerph16081435 [DOI] [PMC free article] [PubMed]
- 25.Looman M, Feskens EJ, de Rijk M, et al. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017;20(13):2289–2299. doi: 10.1017/s136898001700091x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Dongen MC, Wijckmans-Duysens NEG, den Biggelaar LJ, et al. The Maastricht FFQ: development and validation of a comprehensive food frequency questionnaire for the Maastricht Study. Nutrition. 2019;62:39–46. doi: 10.1016/j.nut.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 27.van der Berg JD, Willems PJ, van der Velde JH, et al. Identifying waking time in 24-h accelerometry data in adults using an automated algorithm. J Sports Sci. 2016;34(19):1867–1873. doi: 10.1080/02640414.2016.1140908. [DOI] [PubMed] [Google Scholar]
- 28.van Dooren FE, Schram MT, Schalkwijk CG, et al. Associations of low grade inflammation and endothelial dysfunction with depression - The Maastricht Study. Brain Behav Immun. 2016;56:390–396. doi: 10.1016/j.bbi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114(1–2):29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 30.de Ritter R, Sep SJS, van der Kallen CJH, et al. Sex differences in the association of prediabetes and type 2 diabetes with microvascular complications and function: the Maastricht Study. Cardiovasc Diabetol. 2021;20(1):102. doi: 10.1186/s12933-021-01290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao C, Wang R, Jones M, et al. The relationship between corneal nerve density and hemoglobin A1c in patients with prediabetes and type 2 diabetes. Invest Ophthalmol Vis Sci. 2020;61(12):26. doi: 10.1167/iovs.61.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih KC, Lam KS, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes. 2017;7(3):e251. doi: 10.1038/nutd.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansoor H, Tan HC, Lin MT, Mehta JS, Liu YC (2020) Diabetic corneal neuropathy. J Clin Med 9(12). 10.3390/jcm9123956 [DOI] [PMC free article] [PubMed]
- 34.Lagali NS, Allgeier S, Guimarães P, et al. Reduced corneal nerve fiber density in type 2 diabetes by wide-area mosaic analysis. Invest Ophthalmol Vis Sci. 2017;58(14):6318–6327. doi: 10.1167/iovs.17-22257. [DOI] [PubMed] [Google Scholar]
- 35.Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med. 2014;31(4):431–438. doi: 10.1111/dme.12324. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Graham J, Petropoulos IN, et al. Corneal nerve fractal dimension: a novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Invest Ophthalmol Vis Sci. 2018;59(2):1113–1118. doi: 10.1167/iovs.17-23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petropoulos IN, Al-Mohammedi A, Chen X, et al. The utility of corneal nerve fractal dimension analysis in peripheral neuropathies of different etiology. Transl Vis Sci Technol. 2020;9(9):43. doi: 10.1167/tvst.9.9.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7):2454–2463. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 39.Petropoulos IN, Alam U, Fadavi H, et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36(11):3646–3651. doi: 10.2337/dc13-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom. 2012;95(3):348–354. doi: 10.1111/j.1444-0938.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 41.Andrade C. Z Scores, standard scores, and composite test scores explained. Indian J Psychol Med. 2021;43(6):555–557. doi: 10.1177/02537176211046525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gad H, Petropoulos IN, Khan A, et al. Corneal confocal microscopy for the diagnosis of diabetic peripheral neuropathy: a systematic review and meta-analysis. J Diabetes Investig. 2022;13(1):134–147. doi: 10.1111/jdi.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel DV, McGhee CN. Mapping of the normal human corneal sub-basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005;46(12):4485–4488. doi: 10.1167/iovs.05-0794. [DOI] [PubMed] [Google Scholar]
- 44.Colonna A, Scarpa F, Ruggeri A (2018) Segmentation of corneal nerves using a U-Net-based convolutional neural network: first international workshop, COMPAY 2018, and 5th International Workshop, OMIA 2018, Held in Conjunction with MICCAI 2018, Granada, Spain, September 16–20, 2018, Proceedings. In: Stoyanov D, Taylor Z, Ciompi F et al (eds) Computational Pathology and Ophthalmic Medical Image Analysis. Springer, London, pp 185–192
- 45.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–688. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the Maastricht Study are available to any researcher who meets the criteria for access to confidential data. The corresponding author may be contacted to request access.